Abstract
Background
We report a case of afibrinogenemia in a lady, which was detected for the first time during her pregnancy.
Case
A 24-year-old G4A3 was referred as a case of vaginal bleeding, after a cervical cerclage at 14 weeks of gestation. Elastometry targeted correction of coagulopathy was done initially, and targeted cryoprecipitate transfusion was done to maintain her gestation. She underwent induced vaginal delivery at 34 weeks of gestation. Fourteen days postpartum, the mother and child were discharged home well.
Conclusion
Coagulation factor deficiency should be considered as a rare cause for RPL. Serum fibrinogen level of 50–100 mg/dl during pregnancy seems to be a safe and adequate target to maintain in pregnant patients with afibrinogenemia.
Keywords: Afibrinogenemia, Pregnancy, Fibrinogen, Cryoprecipitate, Viscoelastometry, Rotational thromboelastometry
Introduction
Afibrinogenemia and hypofibrinogenemia, respectively, refer to the absence and reduced levels of fibrinogen (also known as coagulation factor I) in the blood, a protein that is essential in the coagulation process [1].
Afibrinogenemia is rare disorder affecting one in a million pregnancy [2]. Affected individuals may be susceptible to severe bleeding episodes, particularly during infancy and childhood [2].
We report a case of afibrinogenemia, diagnosed during pregnancy, in a woman with recurrent pregnancy loss.
Case Report
A 24-year-old third gravida, with a history of three abortions in the past, was referred from another hospital with complaints of vaginal bleeding since 2 days after a cervical cerclage procedure. She had undergone encirclage suspecting cervical incompetence, at 14 weeks of gestation. She was started on progesterone, aspirin and low molecular weight heparin (LMHW) for her current pregnancy, in view of her three prior abortions.
All prior pregnancies lasted less than 6 weeks of gestation and were aborted due to bleeding. She was not diabetic and hypothyroid, and her antiphospholipid antibody (APLA) done was negative.
She had normal menstrual cycles with up to 5 days of bleeding. She had no menorrhagia in the past, no family history of bleeding disorders or any other relevant history of bleeding manifestations. She was born out of a non-consanguineous marriage.
She reached our Emergency Department and was pale (Hb 5.1 g/dl) and slightly tachycardic (106/min).
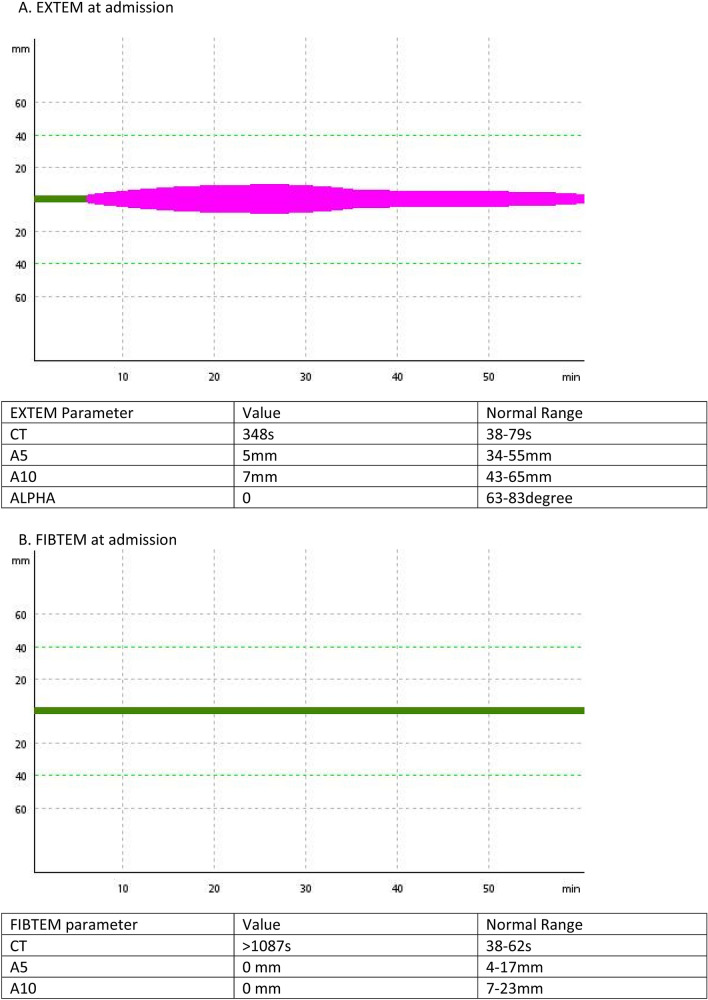
Her initial laboratory investigations showed low fibrinogen (< 10 mg/dl, Clauss method), prolonged prothrombin time (PT > 180 s) and activated partial thromboplastin time (aPTT > 180 s). Rotational thromboelastometry (ROTEM) showed poor clot formation in CT EXTEM (384 s) and prolonged CTFIBTEM (> 1087 s) (Fig. 1).
Fig. 1.
ROTEM assays. Two ROTEM assays were performed: extrinsically activated assay with tissue factor (EXTEM), and extrinsically activated test with tissue factor and the platelet inhibitor cytochalasin D (FIBTEM). a EXTEM: extrinsically activated assay with tissue factor (EXTEM) showed prolonged CT and shortened A5 and A10. b FIBTEM: extrinsically activated test with tissue factor and the platelet inhibitor cytochalasin D (FIBTEM) showed no clot formation. For the ROTEM assays, the following variables were measured: clotting time (CT [s]), time taken from the start of the test to the time for clot until 2 mm amplitude is reached; alpha is the angle between the central line and a tangent to the curve through 2 mm amplitude point, measured in degrees; A5, A10 (clot amplitude at respective min after the end of CT); and maximum clot firmness (MCF [mm], the maximum amplitude reached during the test). Finally, the lysis index at 60 min (LI60 [%], clot firmness at 60 min as percentage of MCF)
She underwent guided transfusion, initially with two units of packed red blood cells (PRBC) and 10 units of cryoprecipitate, her repeat fibrinogen was 86 mg/dl, and she was further transfused another 10 units of cryoprecipitate and two units of PRBC. Following the transfusion, her bleed clinically reduced. Her PT and aPTT were corrected (17.7 s and 24.4 s, respectively), and fibrinogen levels were 126 mg/dl. The serial fibrinogen, PT and aPTT values were monitored throughout pregnancy (Table 1).
Table 1.
Serial PT, aPTT and fibrinogen levels
| Days | Fibrinogen (mg/dl) | PT (s) | INR | aPTT (s) |
|---|---|---|---|---|
| 14 weeks 6 days | < 10 | > 180 | > 18 | > 180 |
| 15 weeks | 86 | 17.7 | 1.3 | 24.4 |
| 15 weeks 6 days | 57 | 13.3 | 1.03 | 25.7 |
| 16 weeks 1 day | 108 | 14 | 1 | 25.2 |
| 33 weeks 6 days | 158 | 14.1 | 1.02 | 24 |
| Day of delivery | 275 | 11.3 | 0.9 | 22.6 |
| PND2 | 260 | 13.6 | 0.93 | 23.9 |
PT Prothrombin time, INR international normalisation ratio, aPTT activated partial thromboplastin time, PND postnatal day, Sec seconds
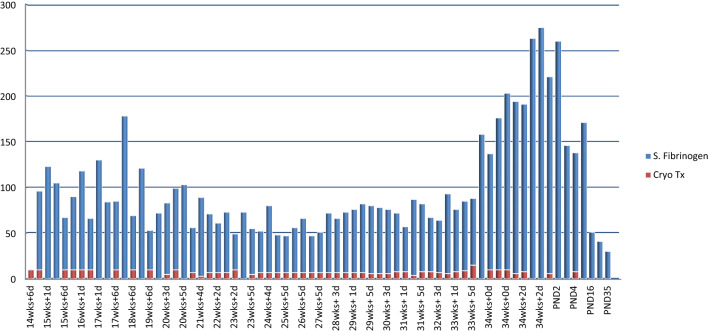
She was further investigated, and with a provisional diagnosis of afibrinogenemia in pregnancy, she was given seven units of cryoprecipitate twice a week from 14 weeks until 27 weeks of gestation, and thrice a week thereafter in the third trimester targeting serum fibrinogen levels around 100 mg/dl (Fig. 2).
Fig. 2.
Fibrinogen levels and cryoprecipitate transfusion. X axis: days of gestation. Y axis: S fibrinogen (mg/dl) and number of cryoprecipitate transfusion (unit). wks weeks, d days, S —serum, Cryo cryoprecipitate, Tx transfusion, PND postnatal day
In order to manage this particular case, a panel of doctors from the department of obstetrics, transfusion medicine, paediatrics, anaesthesia and critical care was convened to make crucial decisions with regard to patient care and management. Induced vaginal delivery was planned at around 34 weeks. From 3 days prior to her planned induction date, the fibrinogen levels were targeted above 200 mg/dl. At 32 weeks, intramuscular betamethasone was administered, two doses, 12 h apart. At 34 weeks and 1 day of gestation, she was induced with extra-amniotic saline using foley’s catheter. Twelve hours later, intracervical instillation of prostaglandin E2 gel was done. Once she had a soft cervix, adequate contractions were maintained by augmentation with oxytocin, and she underwent a preterm induced vaginal delivery with a right mediolateral episiotomy. She delivered a healthy female child weighing 2.150 kgs.
The third stage was actively managed with prophylactic oxytocics. Bleeding was minimal. Fibrinogen levels were maintained around 200 mg/dl up to 3 days postpartum (PP). Thereafter for 14 days of hospital stay, it was maintained above 100 mg/d by targeted cryoprecipitate transfusion.
Her fibrinogen levels at the day of delivery, first, second and fourth week postpartum were 275, 103, 40 and 31 mg/dl, respectively.
Discussion
Fibrinogen (coagulation factor 1) is a an important component in primary and secondary haemostatic system and is the key substrate for thrombin in establishment of the fibrin mesh that aids in coagulation [1]. Afibrinogenemia (ORPHA98880) is a rare, autosomal recessive inherited bleeding disorder of fibrinogen influencing the amount of fibrinogen in human blood [2–4].
The incidence is quite low that factor deficiencies are seldom considered as causes for recurrent pregnancy loss(RPL) [5, 6].
Factor XIII and fibrinogen are the only clotting factors associated with recurrent pregnancy loss [7].
The first reported case of a successful pregnancy outcome in a woman with afibrinogenemia was published in 1985 [8].
Owing to the rarity of the disease and the absences of controlled studies, management of pregnancy with hypofibrinogenemia is challenging.
It can manifest anytime during the individuals lifetime, rarely as early as the antenatal period or commonly soon after birth as umbilical stump bleed [9, 10].
Due to their specific physiological characteristics, female patients with congenital hypofibrinogenemia may present with spontaneous recurrent abortion, menorrhagia, antepartum and postpartum haemorrhage [11, 12].
On the other hand, the patient may also remain asymptomatic into their adulthood [13].
Hypofibrinogenemia is characterised by reduced amounts of immunoreactive fibrinogen. Heterozygous carriers of afibrinogenemia mutations are usually asymptomatic [13, 14].
For an obstetrician, it would be prudent to also consider hypo- or afibrinogenemia in an adult female as one of the rare causes for recurrent pregnancy loss.
Fibrinogen levels are physiologically elevated during pregnancy. It plays a critical role in normal development of foetal vascularity and in placental implantation [13, 15, 16].
Patients with afibrinogenemia have been shown to have successful pregnancies if treated with fibrinogen concentrate or cryoprecipitate [8, 11, 14, 17, 18].
In patients with afibrinogenemia, pregnancies invariably end in spontaneous abortion around the fifth to sixth week of gestation if no therapy is given [17–19].
Fibrinogen levels above 100 mg/dl are mentioned as an acceptable target during pregnancy [17, 20–22].
We targeted a slightly lower serum fibrinogen level 50–100 mg/dl, keeping in mind the risk of thrombophilia associated with higher levels in pregnancy [23].
The target levels were increased to above 200 mg/dl 2 days before labour and 3 days postpartum.
Recent Cochrane systematic review of maternal and foetal outcomes following natural vaginal versus caesarean section (c‐section) delivery in women with bleeding disorders and carriers, does not recommend one method over the other. Since a randomised controlled trial for higher levels of evidence was unlikely to come up in such rare diseases, the reviewers go on to recommend that the clinicians need to use their clinical judgement and lower level evidence (e.g. from observational trials, case studies) to decide upon the optimal mode of delivery to ensure the safety of both mother and foetus [24]. Incidence of venous thromboembolism (VTE) after caesarean sections(CS) is estimated four times higher than normal vaginal deliveries (VD) [25].
Independent of other VTE risk factors, it is greater following emergency CS than following elective CS [25]. Paradoxical prothrombotic states with embolic complications are also reported in certain phenotypes of afibrinogenemic pregnancies [26].
Review of the literature showed preterm delivery appears to be more frequent in these women despite replacement therapy [17].
The fibrinogen requirement increases as pregnancy progresses [14]. In order to prevent abruption, a higher level of fibrinogen is often targeted during delivery [14, 22, 23].
Fibrinogen has a half-life of approximately 4 days in a normal individual [27].
The clearance during pregnancy increases as gestational age increases [16, 19].
The transfusion requirement to maintain the target 50–100 mg/dl had increased from 1 transfusion a week to nearly 3 to 4 per week by 27 weeks of gestation in our patient.
Even though cryoprecipitate is a financially better option than fibrinogen concentrate, at approximately Rs 14,000(184 USD) per transfusion the cost is still prohibitive for the patient [monthly income Rs 9000 (118 USD)] [28, 29].
The financial burden of transfusion therapy in these individuals is often left unestimated and warrants studies in low resource settings.
Weighing the benefits of the substitution therapy against the possibility of inducing thrombosis, it was decided to electively induce her in a controlled setting at 34 weeks of gestation. This decision was made by the panel constituted for the patient treatment, after explaining the risks and possible benefits, in consensus with the patient and her relatives.
She underwent a successful vaginal delivery with negligible bleed peripartum.
Inherited disorders of fibrinogen affect either the quantity (afibrinogenemia and hypofibrinogenemia) or the quality (dysfibrinogenemia) of the circulating fibrinogen or both (hypodysfibrinogenemia) [27]. Hypofibrinogenemia is caused by homozygous or compound heterozygous mutation in one or another of the 3 fibrinogen genes: alpha (FGA), beta (FGB) or gamma (FGG) [1].
Further genetic studies are required to confirm the same in the patient and her family including the new-born child and were not done in our case due to lack of funding.
Conclusion
Coagulation factor deficiency should be considered as a rare cause for RPL.
Targeted cryoprecipitate transfusion to keep serum fibrinogen level between 50 and 100 mg/dL during pregnancy seems to be safe and adequate to maintain pregnancy in patients with afibrinogenemia as a cause of pregnancy loss.
Planned vaginal induction of labour is an option to consider over elective CS and spontaneous VD in these patients.
Acknowledgment
The authors would like to acknowledge the contribution of Dr Athira Sashidharan, Dr Aboobacker Mohammed Rafi, Dr Susheela J Innah from the Dept of Transfusion Medicine, Dr Mariam Thomas, Dept of Anaesthesia and Critical Care to patient care, and Dr V C Manoj, Department of Neonatology, for the care of the baby.
Athulya Shajan
is a resident in the department of Obstetrics and Gynecology.
Author Contributions
AS, NG and SG were involved in patient care and management. AS and SVA reviewed the literature and prepared the manuscript. AS, NG, SG and SVA reviewed and approved the manuscript
Funding
None.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
Written informed consent was procured from the patient for utilisation of the data for publication and educational purposes.
Footnotes
Athulya Shajan MBBS, Junior Resident in Department of Obstetrics and Gynaecology, Jubilee Mission Medical College and Research Institute, Thrissur, Kerala, India. Neetha George MS, FICOG, Associate Professor in the Department of Obstetrics and Gynaecology, Jubilee Mission Medical College and Research Institute, Thrissur, Kerala, India. Sareena Gilvaz MD DGO, Professor and Head of the Department of Obstetrics and Gynaecology, Jubilee Mission Medical College and Research Institute, Thrissur, Kerala, India. Siju V Abraham MD, Assistant Professor in the Department of Emergency Medicine, Jubilee Mission Medical College and Research Institute, Thrissur, Kerala, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lebreton A, Casini A. Diagnosis of congenital fibrinogen disorders. Ann Biol Clin (Paris) 2016;74(4):405–412. doi: 10.1684/abc.2016.1167. [DOI] [PubMed] [Google Scholar]
- 2.Stanciakova L, Kubisz P, Dobrotova M, Stasko J. Congenital afibrinogenemia: from etiopathogenesis to challenging clinical management. Expert Rev Hematol. 2016;9(7):639–648. doi: 10.1080/17474086.2016.1200967. [DOI] [PubMed] [Google Scholar]
- 3.Orphanet: Familial afibrinogenemia [Internet]. Available from: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=EN&Expert=98880. Accessed 4 Apr 2020.
- 4.Afibrinogenemia | Genetic and Rare Diseases Information Center (GARD): an NCATS Program [Internet]. Available from: https://rarediseases.info.nih.gov/diseases/5761/index. Accessed 4 Apr 2020.
- 5.Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open [Internet]. 2018;2018(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6276652/. Accessed 5 Apr 2020. [DOI] [PMC free article] [PubMed]
- 6.American College of Obstetricians and Gynecologists. ACOG practice bulletin. Management of recurrent pregnancy loss. Number 24, February 2001. (Replaces Technical Bulletin Number 212, September 1995). American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2002;78(2):179–90. [DOI] [PubMed]
- 7.Inbal A, Muszbek L. Coagulation factor deficiencies and pregnancy loss. Semin Thromb Hemost. 2003;29(2):171–174. doi: 10.1055/s-2003-38832. [DOI] [PubMed] [Google Scholar]
- 8.Inamoto Y, Terao T. First report of case of congenital afibrinogenemia with successful delivery. Am J Obstet Gynecol. 1985;153(7):803–804. doi: 10.1016/0002-9378(85)90354-0. [DOI] [PubMed] [Google Scholar]
- 9.Lak M, Keihani M, Elahi F, Peyvandi F, Mannucci PM. Bleeding and thrombosis in 55 patients with inherited afibrinogenaemia. Br J Haematol. 1999;107(1):204–206. doi: 10.1046/j.1365-2141.1999.01681.x. [DOI] [PubMed] [Google Scholar]
- 10.Hariharan G, Ramachandran S, Parapurath R. Congenital Afibrinogenemia presenting as antenatal intracranial bleed: a case report. Ital J Pediatr. 2010;5(36):1. doi: 10.1186/1824-7288-36-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zuo X, Teng Y. Women with congenital hypofibrinogenemia/afibrinogenemia: from birth to death. Clin Appl Thromb Off J Int Acad Clin Appl Thromb. 2020;26:1076029620912819. doi: 10.1177/1076029620912819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acharya SS, Dimichele DM. Rare inherited disorders of fibrinogen. Haemoph Off J World Fed Hemoph. 2008;14(6):1151–1158. doi: 10.1111/j.1365-2516.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 13.de Moerloose P, Casini A, Neerman-Arbez M. Congenital fibrinogen disorders: an update. Semin Thromb Hemost. 2013;39(6):585–595. doi: 10.1055/s-0033-1349222. [DOI] [PubMed] [Google Scholar]
- 14.Cai H, Liang M, Yang J, Zhang X. Congenital hypofibrinogenemia in pregnancy: a report of 11 cases. Blood Coagul Fibrinolysis. 2018;29(2):155–159. doi: 10.1097/MBC.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valiton V, Hugon-Rodin J, Fontana P, Neerman-Arbez M, Casini A. Obstetrical and postpartum complications in women with hereditary fibrinogen disorders: a systematic literature review. Haemoph Off J World Fed Hemoph. 2019;25(5):747–754. doi: 10.1111/hae.13825. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Deng D, Cheng P, Liao L, Luo M, Lin F. Management of dysfibrinogenemia in pregnancy: a case report. J Clin Lab Anal. 2017;32(3). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6816852/. Accessed 22 Apr 2020. [DOI] [PMC free article] [PubMed]
- 17.Tziomalos K, Vakalopoulou S, Perifanis V, Garipidou V. Treatment of congenital fibrinogen deficiency: overview and recent findings. Vasc Health Risk Manag. 2009;5:843–848. doi: 10.2147/VHRM.S5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi T, Asahina T, Maehara K, Itoh M, Kanayama N, Terao T. Congenital afibrinogenemia with successful delivery. Gynecol Obstet Invest. 1996;42(1):66–69. doi: 10.1159/000291892. [DOI] [PubMed] [Google Scholar]
- 19.Mensah PK, Oppenheimer C, Watson C, Pavord S. Congenital afibrinogenaemia in pregnancy. Haemoph Off J World Fed Hemoph. 2011;17(1):167–168. doi: 10.1111/j.1365-2516.2010.02363.x. [DOI] [PubMed] [Google Scholar]
- 20.de Moerloose P, Schved J-F, Nugent D. Rare coagulation disorders: fibrinogen, factor VII and factor XIII. Haemoph Off J World Fed Hemoph. 2016;22(Suppl 5):61–65. doi: 10.1111/hae.12965. [DOI] [PubMed] [Google Scholar]
- 21.Bolton-Maggs PHB, Perry DJ, Chalmers EA, Parapia LA, Wilde JT, Williams MD, et al. The rare coagulation disorders–review with guidelines for management from the United Kingdom Haemophilia Centre Doctors’ Organisation. Haemoph Off J World Fed Hemoph. 2004;10(5):593–628. doi: 10.1111/j.1365-2516.2004.00944.x. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Kanayama N, Tokunaga N, Asahina T, Terao T. Prenatal and peripartum management of congenital afibrinogenaemia. Br J Haematol. 2000;109(2):364–366. doi: 10.1046/j.1365-2141.2000.01993.x. [DOI] [PubMed] [Google Scholar]
- 23.Karimi M, Bordbar M, Aali M, Bazrafshan A, Tavoosi H, Gerdabi J. Successful delivery in an patient with afibrinogenemia after three abortions: a case report and review of the literature. Haemoph Off J World Fed Hemoph. 2018;24(2):e63–e66. doi: 10.1111/hae.13415. [DOI] [PubMed] [Google Scholar]
- 24.Karanth L, Kanagasabai S, Abas AB. Maternal and foetal outcomes following natural vaginal versus caesarean section (c-section) delivery in women with bleeding disorders and carriers. Cochrane Database Syst Rev. 2017 04;8:CD011059. [DOI] [PMC free article] [PubMed]
- 25.Blondon M, Casini A, Hoppe KK, Boehlen F, Righini M, Smith NL. Risks of venous thromboembolism after cesarean sections: a meta-analysis. Chest. 2016;150(3):572–596. doi: 10.1016/j.chest.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Roqué H, Stephenson C, Lee MJ, Funai EF, Popiolek D, Kim E, et al. Pregnancy-related thrombosis in a woman with congenital afibrinogenemia: a report of two successful pregnancies. Am J Hematol. 2004;76(3):267–270. doi: 10.1002/ajh.20110. [DOI] [PubMed] [Google Scholar]
- 27.Kaur J, Jain A. Fibrinogen. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from: http://www.ncbi.nlm.nih.gov/books/NBK537184/. Accessed 22 Apr 2020.
- 28.Okerberg CK, Williams LA, Kilgore ML, Kim CH, Marques MB, Schwartz J, et al. Cryoprecipitate AHF vs fibrinogen concentrates for fibrinogen replacement in acquired bleeding patients: an economic evaluation. Vox Sang. 2016;111(3):292–298. doi: 10.1111/vox.12417. [DOI] [PubMed] [Google Scholar]
- 29.Nascimento B, Goodnough LT, Levy JH. Cryoprecipitate therapy. BJA Br J Anaesth. 2014;113(6):922–934. doi: 10.1093/bja/aeu158. [DOI] [PMC free article] [PubMed] [Google Scholar]