Abstract
Stress urinary incontinence (SUI) is a common type of urinary incontinence adversely affecting the quality of life of women. For mild SUI, life style changes, pelvic floor exercises and medical treatment with duloxetine may help. Most patients of moderate to severe SUI usually require surgical treatment. Various surgical treatment options include Kelly’s plication, Burch colposuspension, bulking agents and sling surgeries. Although, suburethral fascial slings including the autologous rectus fascia slings were in vogue before 1990, they were overtaken by minimally invasive, faster and easier artificial midurethral slings (tension free vaginal tape and transobturator tape). However, observation of serious long-term and life changing complications of synthetic midurethral slings like mesh erosion, chronic pelvic pain and dyspareunia led to their adverse publicity and medico legal implications for the operating surgeons. This led US FDA (Food and Drug Administration) to issue a warning against their use. Currently, their use has significantly decreased in many countries, and they are no longer available in some countries. This has led to renaissance of use of natural autologous fascial sling, especially rectus fascia for surgical management of SUI. Although performing rectus fascia sling surgery is technically more challenging, takes longer, has more short-term morbidity like voiding dysfunction, their long-term success is high with very little risk of serious complications like mesh erosion, chronic pelvic pain and dyspareunia. However, multicentric trials and longer follow ups are needed before it’s routine recommendation This review discusses the role of autologous fascial sling (especially rectus fascia) for the surgical management of SUI in the current time and the need of ongoing training of this procedure to gynecology residents and urogynecology fellows.
Keywords: Stress urinary incontinence (SUI), Surgical treatment, Autologous fascial sling surgery, Midurethral sling, Burch colposuspension
Introduction
Stress urinary incontinence (SUI) is defined as involuntary passage of urine with raised intra- abdominal pressure and is a common problem affecting 18–26.4% of women [1, 2]. The predisposing factors include child birth trauma, obesity, conditions causing persistent raised intra-abdominal pressure like abdominal masses, chronic constipation and chronic cough [1–4]. Diagnosis of SUI is made by detailed history, thorough physical and gynecological examination including the cough stress test (passage of urine on coughing) [1, 2]. The severity should be gauged using validated questionnaires assessing quality of life and impact of SUI like International Consultation on Incontinence Questionnaire (ICIQ-SF) score [1, 2]. Although not mandatory, urodynamic studies are helpful to confirm the diagnosis of SUI and more importantly, to exclude detrusor overactivity and pre-existing voiding dysfunction as these can jeopardize the outcomes of surgical management of SUI [5, 6].
The first line of management for mild to moderate SUI is conservative with life style modifications like weight loss, fluid and diet modifications, supervised pelvic floor exercises, weighted vaginal cones and mechanical devices and inserts [2–8]. Sometimes medical management is done using selective serotonin and norepinephrine reuptake inhibitors (SNRI) like Duloxetine for a period of 8–12 weeks in patients not responding to conservative treatment and those awaiting surgery [1, 9–11].
However, most patients of moderate to severe SUI need surgical treatment [1, 2]. In addition to sling surgeries which are described below other surgical options include the Kelly’s plication performed during vaginal hysterectomy with poor long-term success [2], open or laparoscopic Burch colposuspension in which vagina at bladder neck and periurethral area is suspended and sutured to ipsilateral Cooper’s ligament on both sides has high success, but needs great expertise and is associated with significant morbidity [2, 12]. It can also be performed laparoscopically with high success but needs expertise in laparoscopy [12]. Bulking agents in which collagen injection is given in wall of urethra still has scope in failed cases but is not a primary treatment [13].
The Evolution of Sling Surgeries
Historically, autologous fascial pubovaginal slings (AFPVS) were introduced almost a century back by Goebell in 1910 and Aldridge in 1942 but were popularized by McGuire and Lytton in 1978 who standardized the technique of use of rectus fascia sling as pubovaginal sling with 80% success rate [14, 15]. The technique was further modified by Ghoneim et al. and other authors [16, 17].
Autologous fascial sling surgery was often criticized for its invasiveness, increased perioperative morbidity and extended hospital stay. After the year 1996 with the advent of synthetic midurethral slings, the use of autologous PVS declined dramatically and was largely replaced by the synthetic midurethral slings (SMUS) to the extent that synthetic slings became the most common procedure done for SUI globally [17]. A survey conducted in 2013 and 2014 showed that 99% of gynecologists and 87% of urologists considered midurethral slings as the treatment of choice for uncomplicated SUI [18, 19].
However, the safety profile of synthetic midurethral slings has recently been challenged as the long-term serious complications of artificial meshes became apparent all over the world [20]. With the US Food and Drug Administration warning in 2011 regarding artificial meshes, there has again been an increase in the use of native tissue surgeries like Burch colposuspension and autologous PVS [21].
In other countries also, there have been major concerns about the use of meshes, mainly for prolapse surgeries but also for SUI surgery putting the synthetic midurethral slings under scrutiny [22]. Adverse publicity and patient litigations about the adverse effects of synthetic mesh like mesh extrusion or erosion, chronic pelvic pain and dyspareunia, has caused fear and panic among women and doctors. Though, most of the cases of mesh complications were reported after vaginal mesh kits used for prolapse surgery, similar complications have also been observed after synthetic midurethral slings. With the result, midurethral slings are not available in many countries like United Kingdom and Scotland now, and the manufacturers are also reluctant to produce more slings [23, 24]. Although, midurethral artificial tapes are still available and used in India, there is a real chance of their non-availability and discontinuation in near future by extrapolation of results and panic in other countries about their use, necessitating use of alternative procedures using native tissue.
Complications of the Synthetic midurethral Slings: The Downfall
The synthetic midurethral slings are minimally invasive. The technique of insertion was easy to learn, could be done as a day care procedure with good surgical outcomes. Due to these reasons, there was an exponential rise in its popularity. The most commonly used synthetic sling material was polypropylene, which is non-degradable and hence has the innate disadvantage of sling erosion and other mesh complications. The incidence of mesh complications depends on patient factors such as thin atrophic vaginal wall, history of radiotherapy, surgical or technical factors like dissection in a plane that is too close to the urethra, or occult perforation into the bladder or urethra during dissection and excessive sling tensioning and the sling composition. Synthetic slings are 15 times more likely to extrude into the urethra and 14 times more likely to erode into the vagina compared to autologous slings [22].
Patients with mesh related complications often present with complaints of long lasting pain or chronic pelvic pain/dyspareunia, recurrent vaginal discharge/UTI, urinary incontinence and in rare cases perforation of the mesh through vagina, urethra, bladder or rectum [23, 24].
Due to the serious long-term side effects, malpractice litigations, patient concern and adverse publicity artificial slings are gradually losing sheen. This has created a vacuum in surgical treatment options for SUI. There has been renaissance in the use of natural tissue pubovaginal slings especially rectus fascia slings in the surgical management of SUI [17].
Natural Tissue Sling Surgery
Natural tissue sling surgery helps in avoiding the mesh related complications of SMUS. Various tissue materials which have been used for natural tissue sling surgery for SUI are given in Fig. 1 [14, 25, 26]. Autologous fascial slings are the most common and described in detail below.
Fig. 1.
Different types of tissues used for natural slings
Autologous Fascial Pubovaginal Sling Surgery(AFPVS)
These procedures involve using the patients own tissue for making the sling. The two most commonly used tissues are the rectus fascia and fascia lata. The former being used more commonly due to the ease of harvesting and greater familiarity of the anatomy of the abdominal region than that of the thigh. Both the fascia otherwise have shown equal efficacy [22].
Mechanism of Action of AFPVS
Fascial slings were traditionally applied at the bladder neck and proximal urethra thereby restoring the normal urethrovesical junction support and causing mechanical compression and kinking of proximal urethra especially during stress. Videourodynamic studies have confirmed that during raised intraabdominal pressure (like coughing) sling moves anteriorly due to contraction of rectus abdominis muscle. It causes rotation of bladder base posteroinferiorly with associated kinking of posterior urethra and raised bladder outlet pressure preventing SUI. Therefore, due to their compressive action, historically these slings were utilized in patients with severe stress urinary incontinence (SUI) such as patients with neurogenic bladder, history of radiotherapy, urethral reconstruction, etc. [22, 23].
Considering that AFPVS are to replace the SMUS for the management of uncomplicated SUI with urethral hypermobility, they will have to be applied at the level of the mid urethra, where they will provide a stable platform or hammock to anchor the urethra during times of increased intraabdominal pressure. Mechanical compression of the bladder neck and proximal urethra in a patient with uncomplicated SUI can lead to long-term voiding difficulties, de novo urgency and other adverse effects [22].
Indications of AFPVS Surgery
Conventionally applied AFPVS are the procedure of choice for [15]:
Complicated SUI
Recurrent SUI/ previous failed SMUS
- SUI with conditions where midurethral sling (artificial mesh) is less preferred or contraindicated.
- Intentional urethral mucosal opening during surgery like for excision of urethral diverticulum or prolapse repair or urethro-vaginal fistula
- Excision of synthetic eroded midurethral sling mesh
- History of pelvic irradiation in past/long-term steroid treatment
- Extensive tissue fibrosis and scarring
- Chronic pelvic pain and dyspareunia
When applied at the level of mid urethra, it may be used as the primary procedure for women with uncomplicated SUI [1].
Surgical Procedure
For the traditional placement of the AFPVS, the surgical procedure described by McGuire et al. [1] should be followed, which states that the sling should be placed at the urethrovesical junction. This placement is preferred in patients with low urethral closing pressure, and/or scarring and fixation of the urethra because of a previous operation.
In patients with uncomplicated SUI, we follow the surgical procedure given below.
Salient Features of Autologous Rectus Fascia Sling Surgery (ARFS)
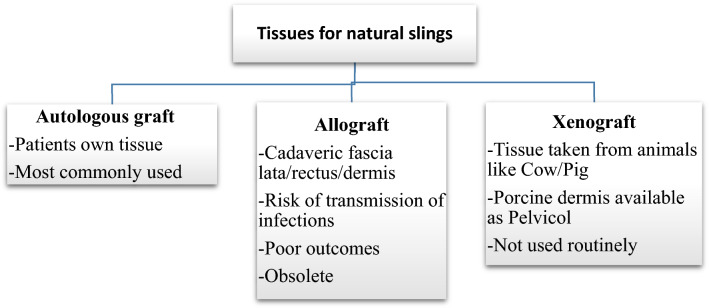
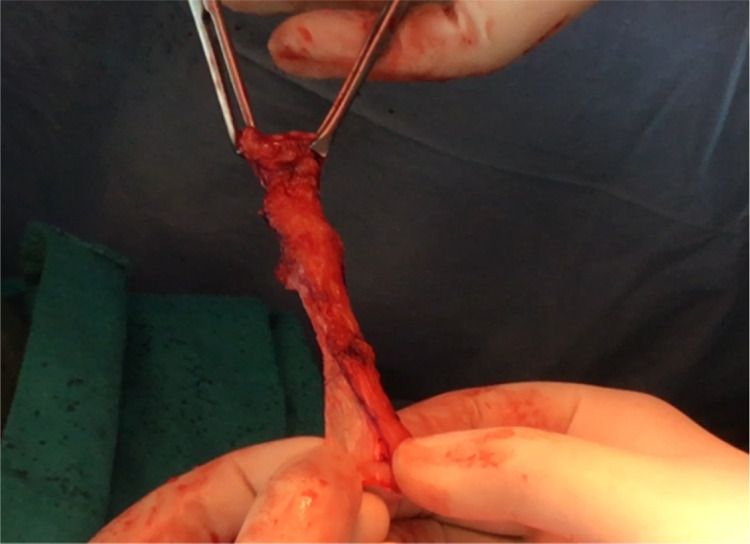
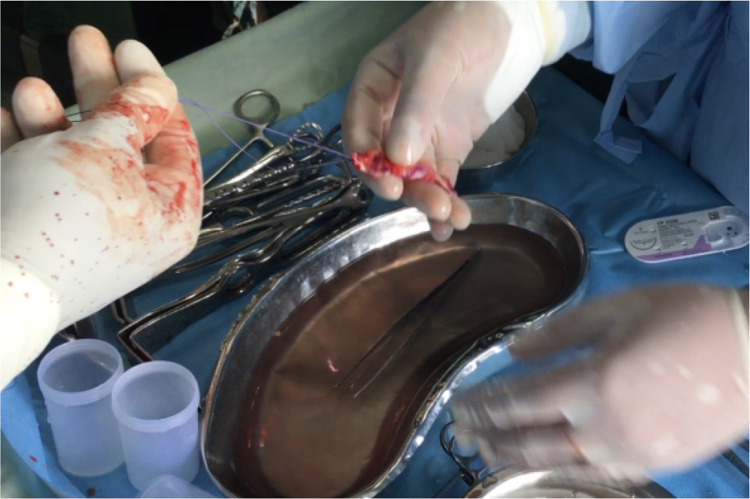
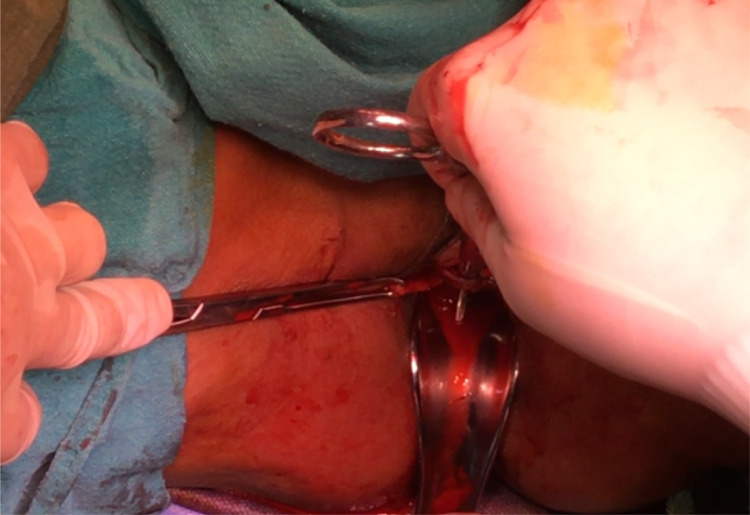
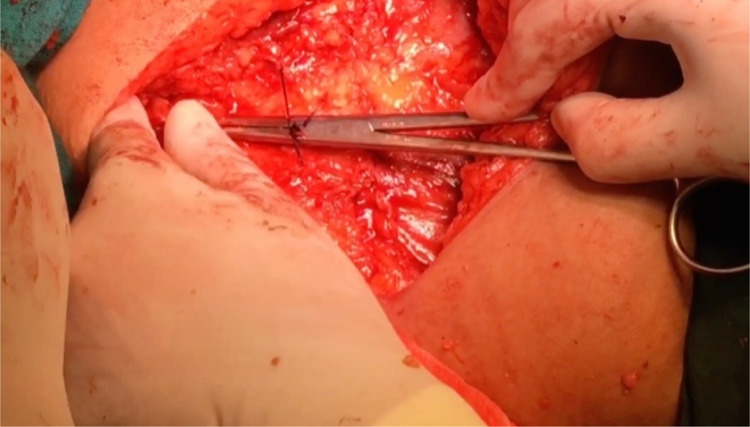
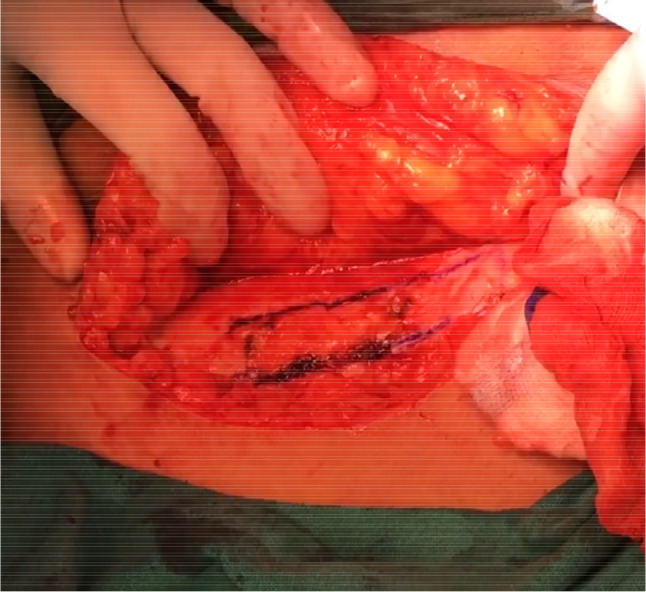
After routine preoperative preparations and antibiotics, the patient is positioned in dorsal lithotomy position, and bladder is catheterised with 14 Fr Foleys double lumen catheter. A combined abdominal–vaginal approach is used. A low transverse abdominal incision is given 2 cm above the pubic symphysis, and abdomen is opened in layers till rectus fascia is reached. (Fig. 2). A graft of rectus fascia 8 cm in length and 2 cm in breadth is taken (Fig. 3). Stay sutures are placed at both its ends with No.1 Proline suture (Fig. 4). It is kept in a solution containing dexamethasone, heparin and gentamycin in normal saline. (Fig. 4). Thereafter, dissection is done transabdominally in the space of Retzius. Simultaneously, at the vaginal end, midline vertical vaginal incision of 2 cm given just below the urethra about 1 cm distal to the bladder neck and vaginal wall dissected from underlying peri-urethral tissue and urethra and extended till inferior pubic ramus on each side. A long Kelly’s clamp is inserted through the abdominal incision in the space of Retzius and brought out at the vaginal end by piercing the perineal membrane and each sling arm is passed from vaginal end to abdominal end avoiding injury to bladder or urethra (Fig. 5) and central portion of the sling is placed at the midurethral level. Cystoscopy is done after the procedure to rule out any bladder or urethral injury. The prolene sutures at the end of sling (sling arm) are brought out through the lower leaf of rectus fascia on both the sides. Rectus is closed using loop nylon after mobilization in a tension free manner. At the vaginal end, sling tensioning is done using a Kelly’s clamp (Fig. 6), which is placed between the urethra and the sling and sling pulled through the abdominal end. The two prolene sutures are tied to each other and tightened over a Kelly’s clamp (Fig. 7) or assistant’s horizontally placed two fingers to avoid overtightening. The sling is then anchored to the periurethral tissue using 2–0 Vicryl suture, and the vaginal incision is closed using 1–0 Vicryl in a continuous fashion. At the abdominal end, after ensuring haemostasis, subcutaneous drain no.16 is placed and fat closed using 2–0 Vicryl. Drain is kept under negative suction pressure. Skin is closed using 3–0 Nylon, and sterile aseptic dressing is applied.
Fig. 2.
A low transverse abdominal incision is given 2 cm above the pubic symphysis and abdomen is opened in layers till rectus fascia is reached and 8 X 2 cm strip marked out
Fig. 3.
8 × 2 cm rectus fascia cut out
Fig. 4.
Graft is kept in a solution of dexamethasone. Gentamycin and heparin. Stay sutures taken at both the ends of the graft using No.1 Prolene suture
Fig. 5.

Each sling arm is then passed from vaginal end to abdominal end using the Kelly’s clamp
Fig. 6.
At the vaginal end, sling tensioning is done using a Kelly’s clamp
Fig. 7.
The two prolene sutures are tied to each other and tightened over a Kelly’s clamp
Autologous Fascia Lata Sling Surgery
In this surgery, pubovaginal sling is made with autologous fascia lata [25]. Patient is positioned in high lithotomy position, and fascia lata is harvested from thigh by giving a short transverse incision 2 fingers above the knee joint along the course of fascia lata. Fascia lata is cut from below and divided at upper end. Complete haemostasis is achieved. The skin edges are closed after putting a small drain, and compression bandage is applied on thigh. The non-absorbable (prolene) sutures are put at each end of harvested fascia lata. Rest of the surgical procedure is same as that of ARFS surgery mentioned above.
Post-Operative Care (1)
In the post-operative period, the patients are managed with iv fluids, analgesics and antibiotics. The abdominal drain is removed when the drain output becomes insignificant (< 10 ml). The urethral catheter is removed on 3rd post-operative day, and the patient is given a voiding trial. If she passes urine with a post void residue of < 1/3 of the pre-void then she is discharged. If unable to pass urine, catheter is reinserted and kept for another 5 days.
Outcomes
Outcomes and Efficacy of ARFS
RFS is the most commonly used autologous sling in clinical practice. This procedure is making a comeback due to mesh-related adverse effects of synthetic midurethral slings and associated medicolegal issues. ARFS have negligible long-term adverse effects, and their cure rates both short-term and long-term are comparable to SMUS. However, they are associated with few limitations of short-term morbidity and prolonged surgery [1, 27, 28].
Various studies have confirmed the short-term and long-term efficiency and safety of rectus fascia sling in clinical practice with overall success rate ranging between 31 and 100%. [14, 20, 22, 27–30]. These variations in outcomes have to be interpreted with caution due to the heterogeneity of the case selection, outcome measures and the short length of follow-up. Studies have compared results of synthetic midurethral tapes (tension free tapes) and rectus fascia sling surgery and observed almost equal success rates of the two procedures with lesser short-term morbidity of synthetic tapes but higher incidence of long-term mesh related complications [1, 25, 31–34].
Fusco et al. [35] in their large meta-analysis of 15,855 women observed equal objective cure rate with autologous rectus fascia sling and midurethral sling which were higher than Burch colposuspension. In the Cochrane database of systematic reviews, Rehman et al. [36] observed traditional rectus fascia slings to be as effective as artificial slings and Burch colposuspension but with slightly higher immediate adverse effects. Although traditionally rectus fascia sling is put at bladder neck, it can also be easily inserted at midurethra level with lesser chances of voiding dysfunction in the postoperative period as has been our experience and of other authors [1, 37, 38]. Thus, Osman et al. [37] loosely placed the rectus fascial graft at the midurethra rather than at bladder neck with 87.8% complete cure rate and 12.2% partial cure rate in primary SUI surgery and 72% complete cure rate, 17.5% partial cure rate and 10.5% failure rate in repeat rectus fascia sling surgery with much less denovo detrusor instability and voiding dysfunction.
Autologous rectus fascia sling surgery has proven benefits in cases with complicated SUI, previous failed SMUS or Burch colposuspension and patients with urethral reconstruction [20, 32]. It has also been used as salvage surgery after failed synthetic midurethral sling surgery or for complications of midurethral sling surgery with mesh erosion in which case either the mesh is removed with rectus fascia sling surgery in second stage or in the same sitting with excellent results in both methods [33, 34, 39, 40]. In such patients, it is conventionally placed at the bladder neck and has good long-term outcomes. McCoy et al. [41] in their repeat surgery used concomitant autologous rectus fascia sling in some cases and performed it in second sitting in cases based on surgeon’s preference and patients choice. They observed 93% success in concomitant ARFS group as compared to 88% success in two staged group (no difference).
Outcomes and Efficacy of Autologous Fascia Lata Sling
There is no difference in the outcome measures of fascia lata when compared to rectus fascia slings. Lee et al. [25] observed acceptable continence outcome with minimal morbidity in their follow-up of over 8 years after application of fascia lata pubovaginal sling surgery. However, it’s an attractive option for patients where good rectus fascia harvesting is difficult like previous multiple abdominal surgeries especially previous abdominoplasty or a ventral mesh incisional hernia repair and patients with morbid obesity [25]. There is also less risk of incisional hernia and abdominal seromas (hematomas) with fascia lata sling [25].However, in current practice it is rarely performed.
Complications
Voiding Dysfunction
It is one of the major complication after ARFS being seen in 1.5–7.8% cases in various studies [15, 42–44]. Voiding dysfunction after a sling procedure may present with either storage symptoms, voiding symptoms, or both. Exact reason for this voiding dysfunction is not known but it is seen more commonly in women with complicated SUI, where the sling is placed at the bladder neck than when it is placed midurethrally. Such patients may also have other factors like underactive bladder, prior radiotherapy, etc.
De Novo Overactive Bladder
It is new onset urinary urgency developing after surgery which was not observed before. It is a common complication after ARFS reported in 15–20% of patients in various studies [15]. The mechanism of development of de novo urgency is probably secondary to increased bladder outlet pressure but can also be due to injury to autonomic nerves of bladder during surgery [15]. In our study [1], we observed de novo urgency in 13.3% cases of ARFS and 20% in MUS group (no statistical difference).
Wound Infection, Hematoma and Seroma
These may occur in 8–10% of cases due to excessive dissection. We observed slightly higher rates of 26.7% of wound infection and 13.3% of wound seromas, especially in our early cases. However, later with more meticulous haemostasis, use of abdominal wall drain and prolonged use of antibiotics and anti-inflammatory agents, the incidence of wound infection decreased significantly [1].
Urinary Retention
Inability to pass urine after removal of catheter can be seen in 5 to 20% cases after ARFS and is much higher in ARFS as compared to MUS group [15, 44]. In our study on ARFS and MUS, we observed higher urinary retention rate in ARFS than MUS but it got relieved with time with only one patient required sling revision. Patients should be given a meticulous voiding trial before discharge, if patient is unable to pass urine after removal of catheter or if there is significantly higher residual urine (> 1/3 of prevoid), then Foley’s catheter should be left for another one week. Antibiotics and anti-inflammatory drugs are given, and then catheter is removed. Usually with time, there is decrease in inflammation and edema with slight relaxation of sling, and patient is able to pass urine. If however, patient is unable to pass urine even after 6 weeks, then sling excision maybe needed as needed in 1 case in our study [1]. Patients should avoid straining to pass urine as the straining increases angulation of urethrovesical angle causing bladder outlet obstruction and worsening of voiding dysfunction. Hence, preoperative counseling of patients is important.
Urinary Tract Infections
It can also occur in some patients after ARFS. We observed UTI in 6.7% cases in our study in ARFS patients [1].
Urinary Tract Injury
There is a small risk of urethral injury, bladder injury and ureteral injury during passage of tape from vaginal end to abdominal end. However, adequate dissection and taking care of tissue planes and shifting bladder neck and urethra to opposite side while passing clamp can avoid the risk significantly.
Conclusion
The global literature confirms that ARFS surgery is a versatile procedure with satisfactory efficacy and safety for the surgical treatment of SUI as primary treatment as well as for secondary treatment after failure of other continence surgeries or after complications of SMUS. Although ARFS takes slightly longer, has increased short-term morbidity, its long-term safety and success makes it a surgical treatment of choice for SUI especially when MUS are being phased out due to long-term serious mesh related complications. The authors would like to emphasize the fact that short-term morbidity of ARFS surgery can be reduced by thorough pre-operative work up, correct operative technique, appropriate sling placement and tensioning and good post-operative care. Hence, there is need to train young generation of gynecologists through dedicated Urogynecology workshops and in residency programs.
About the Reviewer
Dr. JB Sharma is Professor of Obstetrics and Gynecology and Urogynecology at the prestigious All India Institute of Medical Sciences, New Delhi and runs the Urogynecology Fellowship Program to train gynecologists in Urogynecology. He is chairperson of Urogynecology committee of FOGSI. He has more than 30 years of experience. He has 360 papers and 3 books to his credit. He conducts MRCOG examination in India. He has research project with Oxford University, Indian Council of Medical Research, Department of Science and Technology and Ministry of Health, Govt. of India. He is Editor in Chief of Indian Obstetrics and Gynecology. He was awarded Dr. BC Roy award by honourable President of India for research in Obstetrics and Gynecology.
Compliance with Ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
J B Sharma is a Professor in the Department of Obstetrics, Gynecology and Urogynecology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India. Karishma Thariani is a Fellow, Urogynecology in the Department of Obstetrics, Gynecology and Urogynecology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India. Manasi Deoghare is a Resident in the Department of Obstetrics, Gynecology and Urogynecology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India. Rajesh Kumari is an Associate Professor in the Department of Obstetrics, Gynecology and Urogynecology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sharma JB, Deoghare MK, Bhatla N, et al. A comparative study of autologous rectus fascia pubovaginal sling surgery and synthetic transobturator vaginal tape procedure in treatment of women with urodynamic stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2020;1(252):349–354. doi: 10.1016/j.ejogrb.2020.06.062. [DOI] [PubMed] [Google Scholar]
- 2.Sharma JB. Urinary problems. In: Textbook of gynaecology. 1st ed. New Delhi: Avichal Publishing Company; 2018. p. 392–419.
- 3.Singh U, Agarwal P, Verma ML, et al. Prevalence and risk factors of urinary incontinence in Indian women: a hospital-based survey. Indian J Urol Soc India. 2013;29(1):31–36. doi: 10.4103/0970-1591.109981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khullar V, Sexton CC, Thompson CL, et al. The relationship between BMI and urinary incontinence subgroups: results from EpiLUTS: BMI and urinary incontinence. Neurourol Urodyn. 2014;33(4):392–399. doi: 10.1002/nau.22428. [DOI] [PubMed] [Google Scholar]
- 5.Nambiar AK, Lemack GE, Chapple CR, Burkhard FC. The role of urodynamics in the evaluation of urinary incontinence: the European association of urology recommendations in 2016. Eur Urol. 2017;71(4):501–503. doi: 10.1016/j.eururo.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Rathi S, Patnaik P, et al. Does preoperative urodynamic testing improve surgical outcomes in patients undergoing the transobturator tape procedure for stress urinary incontinence? a prospective randomized trial. Korean J Urol. 2014;55(12):821. doi: 10.4111/kju.2014.55.12.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith A, Bevan D, Douglas HR, et al. Management of urinary incontinence in women: summary of updated NICE guidance. BMJ. 2013;347:f5170–f5170. doi: 10.1136/bmj.f5170. [DOI] [PubMed] [Google Scholar]
- 8.Faiena I, Patel N, Parihar JS, et al. Conservative management of urinary incontinence in women. Rev Urol. 2015;17(3):129–139. [PMC free article] [PubMed] [Google Scholar]
- 9.Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi: 10.1002/14651858.CD005654.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbison GP, Dean N. Weighted vaginal cones for urinary incontinence. Cochrane incontinence group, editor. Cochrane Database Syst Rev [Internet] 2013 doi: 10.1002/14651858.CD002114.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane incontinence group, editor. Cochrane Database Syst Rev [Internet]. 2014. [DOI] [PMC free article] [PubMed]
- 12.Lapitan MCM, Cody JD, Mashayekhi A. Open retropubic colposuspension for urinary incontinence in women. Cochrane incontinence group, editor. Cochrane Database Syst Rev [Internet]. 2017. [DOI] [PMC free article] [PubMed]
- 13.Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881. doi: 10.1002/14651858.CD003881.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayrak O, Osborn D, Reynolds WS, et al. Pubovaginal sling materials and their outcomes. Türk Ürol DergisiTurkish J Urol. 2014;40(4):233–239. doi: 10.5152/tud.2014.57778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahdy A, Ghoniem GM. Autologous rectus fascia sling for treatment of stress urinary incontinence in women: a review of the literature. Neurourol Urodyn [Internet]. 2019. [DOI] [PubMed]
- 16.Ghoniem GM, Rizk DEE. Renaissance of the autologous pubovaginal sling. Int Urogynecology J. 2018;29(2):177–178. doi: 10.1007/s00192-017-3521-2. [DOI] [PubMed] [Google Scholar]
- 17.Ghoniem G, Hammett J. Female pelvic medicine and reconstructive surgery practice patterns: IUGA member survey. Int Urogynecol J. 2015;26(10):1489–1494. doi: 10.1007/s00192-015-2734-5. [DOI] [PubMed] [Google Scholar]
- 18.Nager C, Tulikangas P, Miller D, et al. Position statement on mesh midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2014;20(3):123–125. doi: 10.1097/SPV.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 19.Chughtai BI, Elterman DS, Vertosick E, et al. Midurethral sling is the dominant procedure for female stress urinary incontinence: analysis of case logs from certifying american urologists. Urology. 2013;82(6):1267–1271. doi: 10.1016/j.urology.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Blaivas JG, Simma-Chiang V, Gul Z, et al. Surgery for stress urinary incontinence. Urol Clin North Am. 2019;46(1):41–52. doi: 10.1016/j.ucl.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Khan AA, Rosenblum N, Brucker B, et al. Changes in management of stress urinary incontinence following the 2011 FDA health notification. J Clin Urol. 2017;10(5):440–448. doi: 10.1177/2051415817691662. [DOI] [Google Scholar]
- 22.Bang S-L, Belal M. Autologous pubovaginal slings: back to the future or a lost art?. Res Rep Urol. 2016;11. [DOI] [PMC free article] [PubMed]
- 23.Chapple CR, Raz S, Brubaker L, et al. Mesh sling in an era of uncertainty: lessons learned and the way forward. Eur Urol. 2013;64(4):525–529. doi: 10.1016/j.eururo.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 24.RCOG statement in response to the announcement by the IMMDS review on use of surgical mesh [Internet]. Royal college of obstetricians & Gynaecologists. [cited 2019 Nov 21]. Available from: https://www.rcog.org.uk/en/news/rcog-statement-in-response-to-the-announcement-of-safety-review-of-mesh/.
- 25.Lee D, Alhalabi F, Zimmern PE. Long-term outcomes of autologous fascia lata sling for stress incontinence secondary to intrinsic sphincter deficiency in women. Urol Sci. 2017;28(3):135–138. doi: 10.1016/j.urols.2017.03.002. [DOI] [Google Scholar]
- 26.Khan ZA, Nambiar A, Morley R, et al. Long-term follow-up of a multicentre randomised controlled trial comparing tension-free vaginal tape, xenograft and autologous fascial slings for the treatment of stress urinary incontinence in women: long-term follow-up of TVT, pelvicoland AFS. BJU Int. 2015;115(6):968–977. doi: 10.1111/bju.12851. [DOI] [PubMed] [Google Scholar]
- 27.Lee D, Murray S, Bacsu CD, et al. Long-term outcomes of autologous pubovaginal fascia slings: is there a difference between primary and secondary slings?: long-term outcomes of autologous pubovaginal fascia slings. Neurourol Urodyn. 2015;34(1):18–23. doi: 10.1002/nau.22502. [DOI] [PubMed] [Google Scholar]
- 28.Mourad S, Elshawaf H, Ahmed M, et al. Autologous versus synthetic slings in female stress urinary incontinence: a retrospective study. Arab J Urol. 2018;16(4):397–403. doi: 10.1016/j.aju.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linder BJ, Elliott DS. Autologous transobturator urethral sling placement for female stress urinary incontinence. J Urol. 2015;193(3):991–996. doi: 10.1016/j.juro.2014.08.125. [DOI] [PubMed] [Google Scholar]
- 30.Preece PD, Chan G, O’Connell HE, et al. Optimising the tension of an autologous fascia pubovaginal sling to minimize retentive complications. Neurourol Urodyn. 2019;38(5):1409–1416. doi: 10.1002/nau.24000. [DOI] [PubMed] [Google Scholar]
- 31.Al-Azzawi IS. The first Iraqi experience with the rectus fascia sling and transobturator tape for female stress incontinence: a randomised trial. Arab J Urol. 2014;12(3):204–208. doi: 10.1016/j.aju.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plagakis S, Tse V. The autologous pubovaginal fascial sling: an update in 2019. LUTS Low Urin Tract Symptoms. 2020;12(1):2–7. doi: 10.1111/luts.12281. [DOI] [PubMed] [Google Scholar]
- 33.Petrou SP, Davidiuk AJ, Rawal B, et al. Salvage autologous fascial sling after failed synthetic midurethral sling: greater than 3-year outcomes. Int J Urol. 2016;23(2):178–181. doi: 10.1111/iju.13003. [DOI] [PubMed] [Google Scholar]
- 34.Milose JC, Sharp KM, He C, et al. Success of autologous pubovaginal sling after failed synthetic midurethral sling. J Urol. 2015;193(3):916–920. doi: 10.1016/j.juro.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 35.Fusco F, Abdel-Fattah M, Chapple CR, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2017;72(4):567–591. doi: 10.1016/j.eururo.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 36.Rehman H, Bezerra CA, et al. Traditional suburethral sling operations for urinary incontinence in women. Cochrane incontinence group, editor. Cochrane Database Syst Rev [Internet] 2017 doi: 10.1002/14651858.CD001754.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osman NI, Hillary CJ, Mangera A, et al. The midurethral fascial “sling on a string”: an alternative to midurethral synthetic tapes in the era of mesh complications. Eur Urol. 2018;74(2):191–196. doi: 10.1016/j.eururo.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 38.Schimpf MO, Rahn DD, Wheeler TL, et al. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211(1):71.e1–71.e27. doi: 10.1016/j.ajog.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 39.Clifton MM, Linder BJ, Lightner DJ, et al. Risk of repeat anti-incontinence surgery following sling release: a review of 93 cases. J Urol. 2014;191(3):710–714. doi: 10.1016/j.juro.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Shah K, Nikolavsky D, Gilsdorf D, et al. Surgical management of lower urinary mesh perforation after mid-urethral polypropylene mesh sling: mesh excision, urinary tract reconstruction and concomitant pubovaginal sling with autologous rectus fascia. Int Urogynecology J. 2013;24(12):2111–2117. doi: 10.1007/s00192-013-2146-3. [DOI] [PubMed] [Google Scholar]
- 41.McCoy O, Vaughan T, Nickles SW, et al. Outcomes of autologous fascia pubovaginal sling for patients with transvaginal mesh related complications requiring mesh removal. J Urol. 2016;196(2):484–489. doi: 10.1016/j.juro.2016.02.2976. [DOI] [PubMed] [Google Scholar]
- 42.Gammie A, Kirschner-Hermanns R, Rademakers K. Evaluation of obstructed voiding in the female: how close are we to a definition? Curr Opin Urol. 2015;25(4):292–295. doi: 10.1097/MOU.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 43.Wein AJ. Re: evaluation of obstructed voiding in the female: how close are we to a definition? J Urol. 2016;195:1041–1041. doi: 10.1016/j.juro.2016.01.082. [DOI] [PubMed] [Google Scholar]
- 44.Aponte MM, Shah SR, Hickling D, et al. Urodynamics for clinically suspected obstruction after anti-incontinence surgery in women. J Urol. 2013;190(2):598–602. doi: 10.1016/j.juro.2013.03.113. [DOI] [PubMed] [Google Scholar]