Abstract
Acquired resistance to cisplatin (DDP)-based chemotherapy greatly hinders the treatment of gastric cancer (GC). LINC00665 serves as an oncogene in GC. Hence, the current study was designed to investigate the regulatory effects of LINC00665 on DDP-resistance of GC. LINC00665 and miR-379-5p expression levels were detected by real-time quantitative polymerase chain reaction (RT-qPCR) and Glucose regulated protein 78 (GRP78) protein level was measured by western blot assay. Interactions between LINC00665 and miR-379-5p or between miR-379-5p and GRP78 were verified by dual luciferase reporter assay. Cell counting kit 8 (CCK-8) assay and flow cytometry assay respectively determine the proliferative ability and apoptosis of GC cells. Western blot analysis was also performed to detect the protein levels of C/EBP-homologous protein (CHOP), X box binding protein (XBP1) and apoptosis-related proteins. In addition, GRP78 expression was evaluated by immunofluorescence. It was observed that the expression levels of LINC00665 and GRP78 were upregulated, and the expression level of miR-379-5p was downregulated in DDP-sensitive and DDP-resistant GC cell lines. What’s more, GRP78 expression and the cell growth inhibition rates of DDP-sensitive and DDP-resistant GC cells had a negative correlation. Additionally, miR-379-5p was a target miRNA of LINC00665, and GRP78 was a target mRNA of miR-379-5p. Functional studies revealed that knockdown of LINC00665 inhibited DDP-resistant GC cell proliferation, induced apoptosis as well as suppressed Endoplasmic reticulum (ER) stress. Mechanistically, knockdown of LINC00665 downregulated GRP78 expression by strengthening miR-379-5p. LINC00665 silencing could overcome DPP-resistance of GC cells by downregulating GRP78 via sponging miR-379-5p, indicating that LINC00665 might be a potential therapeutic target for DDP- resistant GC patients.
Keywords: LINC00665, MiR-379-5p, GRP78, Gastric cancer, Cisplatin resistance
Introduction
Gastric cancer (GC) is one of the most common malignant tumors in the world. It is often diagnosed in the late stage and progresses rapidly with easy recurrence (Casamayor et al. 2018). Cisplatin (DDP)-based chemotherapy is the main clinical method for the treatment of advanced gastric cancer patients (Wang et al. 2019a, b). However, acquired resistance to DDP-based chemotherapy has been one of the major obstacles in the clinical therapies of gastric cancer (Wagner et al. 2017). The exploration of factors influencing the resistance of gastric cancer to chemotherapy and its mechanism is helpful for improving the prognosis of gastric cancer patients.
Endoplasmic reticulum (ER) stress is an important signaling pathway, which belongs to the mechanism of cell self-protection (King and Wilson 2020). ER stress activates the unfolded protein response (UPR) and initiates a cluster of downstream signals to maintain ER homeostasis (Hetz et al. 2020). During the progress of growth, invasion and metastasis, tumor cells undergo ER stress under hypoxia, hypoglycemia and other environmental stresses (Zheng et al. 2019). More importantly, studies have also shown that ER stress plays an extremely important role in the chemoresistance of tumors (Wang et al. 2019a, b; Zhu et al. 2019).
Glucose regulated protein 78 (GRP78) has long been recognized as a molecular chaperone in ER and can be induced by ER stress response (Machihara and Namba 2020). The elevated expression of GRP78 usually correlates with a variety of tumor microenvironmental stresses (Li and Li 2012). GRP78 is implicated in tumor cell proliferation, apoptosis and drug resistance (Ran et al. 2017). Therefore, GRP78 has the potential to be an appealing target for a more selective chemotherapy.
Long non-coding RNA (lncRNA), as a new multifunctional molecule with complex mechanisms, plays a key role in the biological processes of gastric cancer and participates in the complex mechanism of drug resistance through abnormal regulation of gene expression (Wei et al. 2020; Yuan et al. 2020). LINC00665 knockdown can restore gefitinib sensitivity by suppressing cancer cell proliferation and inducing apoptosis (Liu et al. 2019). Additionally, LINC00665 acts as an oncogene in gastric cancer (Qi et al. 2019; Yang et al. 2020).
microRNAs (miRNAs) are short endogenous noncoding RNAs that exist in eukaryotes and widely involve in various physiological processes such as proliferation and apoptosis (Wu et al. 2020). Zhang et al. (Zhang et al. 2020) found that miR-379-5p can inhibit the migration and invasion of nasopharyngeal carcinoma cells. Furthermore, miR-379-5p has been shown to play critical roles in the progression of some other tumors (Wu et al. 2017; Xu et al. 2019).
Despite the knowledge we have amassed above, the deep research about the function of LINC00665/miR-379-5p/GRP78 axis in the chemoresistance of gastric cancer is still limited. In the present study, we verified the interactions between LINC00665 and miR-379-5p or miR-379-5p and GRP78, investigated the role of LINC00665/miR-379-5p/GRP78 axis in GC cell proliferation, apoptosis and DPP- resistance and further explored the molecular mechanism.
Materials and methods
Cell culture, treatment and transfection
The normal gastric mucosal epithelial cell line (GES-1) and three human GC cell lines (SGC-7901, AGS, HST2) were purchased from Cell Bank of Chinese Academy of Sciences (Shanghai, China). Cells were incubated with Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco, MA, USA) at 37 °C in a 5% CO2 incubator.
DDP-resistant cell lines were established by regulating DDP concentration. Human GC cell lines were exposed into RPMI-1640 medium containing DDP (0.5 mg/L). Then, GC cell lines acquired resistance to 5 mg/L through this process of gradually increasing DDP concentration.
When cell confluency reached 80%, transfection was performed using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s instructions.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA in GC cells was extracted utilizing TRIzol reagent (Invitrogen, CA, USA). In strict line with the manufacturer’s protocol, total RNA was reverse transcribed into cDNA using a PrimeScript™ RT reagent kit (TaKaRa, Tokyo, Japan). RT-qPCR analysis for LINC00665 and miR-379-5p detection was performed by 7500 Fast PCR instrument (Applied Biosystems, CA, USA). The relative level of LINC00665 was normalized to the endogenous control GAPDH and the relative level of miR-379-5p was normalized to the endogenous control U6. The following thermocycling conditions were used for qPCR: an initial heating at 95 °C for 5 min; followed by 40 cycles of initiation at 95 °C for 30 s, annellation at 60 °C for 30 s and elongation at 72 °C for 80 s. The sequences of primers are as follows: LINC00665, forward: 5′-GGTGGATCACGAGGTCAGGAGA-3′ and reverse: 5′- GCTCACTGCAAGCTCTGCCTAC-3′; miR-379-5p, forward: 5′- GCGCTGGTAGACTATGGAA-3′ and reverse: 5′- GTGCAGGGTCCGAGGT-3′; U6, forward: 5′-CTCGCTTCGGCAGCACATATACTA-3′ and reverse: 5′-ACGAATTTGCGTGTCATCCTTGCG-3′; GAPDH, forward: 5′- TGCACCACCAACTGCTTAGC-3′ and reverse: 5′- GGCATGGACTGTGGTCATGAG-3′. The relative levels of LINC00665 and miR-379-5p were calculated by 2−ΔΔCT method (Livak and Schmittgen 2001).
Western blotting analysis
Total cellular proteins were extracted using radio-immunoprecipitation assay (RIPA) buffer (Beyotime, Shanghai, China) supplemented with proteinase inhibitor and phosphatase inhibitor. The lysate was centrifuged at 20,000 g for 5 min and protein concentrations were quantified using a BCA assay kit (Beyotime, Shanghai, China). Equal amounts of proteins were separated by sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA). Following blockage by 5% non-fat milk, the membranes were incubated with specific primary antibodies against GRP78 (Abcam, ab21685, 1:1000), cleaved-caspase-3 (Abcam, ab2302, 1:500), caspase-3 (Abcam, ab32351, 1:5000), Bcl-2 (Abcam, ab182858, 1:2000), Bax (Abcam, ab32503, 1:2000), CHOP (Abcam, ab11419, 1:200), XBP1 (Abcam, ab37152, 1:2000) and GAPDH (Abcam, ab9485, 1:2500) at 4 °C overnight. Subsequently, the membranes were incubated with the corresponding IgG-HRP secondary antibody (Abcam, ab205718, 1:50,000) at room temperature for 1 h. The protein signals were visualized and analyzed using Enhanced Chemiluminescence Kit (Invitrogen, CA, USA) under Image J software.
Cell counting kit 8 (CCK-8) assay
The human GC cell lines and DDP-resistant cell lines were seeded into 96-well plates at a density of 104 cells per well. After adherence, cells were treated with 10 μL DDP (5 mg/ml) for 72 h. Then, 10 μL of CCK-8 solution (Beyotime, Shanghai, China) was added to each well and incubated for 2 h at 37 °C. The absorbance was measured at 450 nm by a microplate reader (BioTek, VT, USA).
Flow cytometric analysis
Following designed treatment, GC cells were collected to assess cell apoptotic condition. Briefly, cells were trypsinized and double-stained with fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide (PI) for 20 min away from light. Cell apoptosis was analyzed by a flow cytometer (FACScan; BD Biosciences, CA, USA).
Luciferase reporter assay
The binding relationship between LINC00665 and miR-379-5p as well as miR-379-5p and GRP78 were investigated using luciferase reporter assay. Mutant (MUT) and wild-type (WT) sequences of LINC00665 containing the binding sites of miR-379-5p were amplified and cloned into a pmirGLO plasmid (Promega, WI, USA). Similarly, mutant (MUT) and wild-type (WT) sequences of GRP78 3′UTR containing its seed region of miR-379-5p were amplified and cloned into a pmirGLO plasmid (Promega, WI, USA). Cells were co-transfected with constructed plasmids in addition to miR-379-5p mimic or mimic NC. 48 h post transfection, luciferase activities were examined using Dual-Luciferase Assay System (Promega, WI, USA).
Immunofluorescence
GC cells were fixed in 4% PFA solution for 30 min. After washing twice with PBS, cells were permeabilized by incubation with 0.2% (w/v) Triton X-100 for 10 min. Then, the fixed cells were blocked with 10% BSA for 30 min and incubated at 4 °C overnight with primary antibodies (anti-CHOP; Abcam, ab11419, 1:200). Subsequently, cells were washed twice with PBS and incubated with florescence-labeled secondary antibodies (Abcam, ab150113, 1:1000) at 37 °C for 1 h. After cells were stained with 0.1% DAPI (Solarbio, Beijing, China) at 37 °C for 30 min, images were taken and analyzed using a fluorescence microscope (Leica, Wetzlar, Germany).
Statistical analysis
All experiments were repeated three times independently. Statistical analyses were conducted using SPSS 21.0 software. Significant differences among multiple groups were evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s test. Differences with * p < 0.05, ** p < 0.01, or *** p < 0.001 were considered statistically significant.
Results
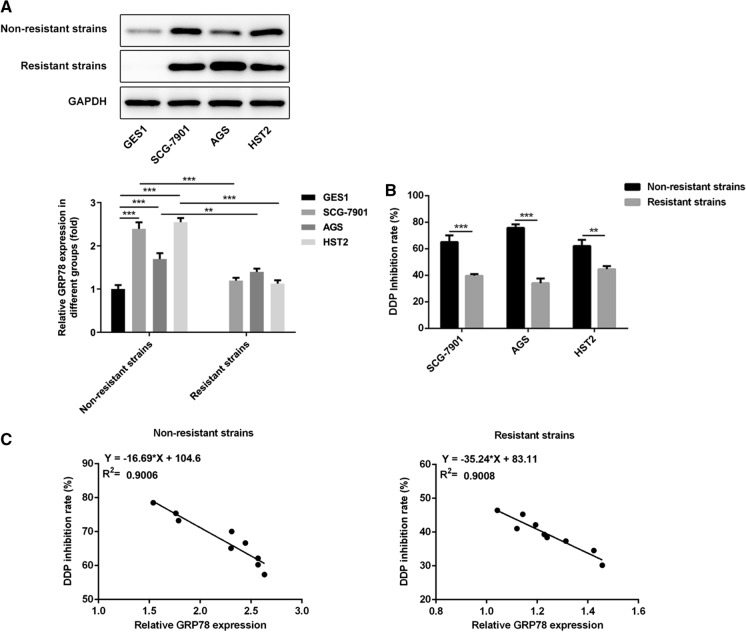
Negative correlation between GRP78 expression and the cell growth inhibition rate of DDP in DDP-sensitive and DDP-resistant GC cell lines
GRP78 expression was determined in normal gastric mucosal epithelial cell line (GES-1) and three human GC cell lines (SGC-7901, AGS, HST2). GRP78 levels were significantly up-regulated in GC cell lines compared to normal gastric mucosal epithelial cell (Fig. 1a). In addition, in comparison with that in DDP-sensitive GC cell lines, the cell growth inhibition rate of DDP markedly decreased in DDP-resistant GC cell lines (Fig. 1b). The correlation between GRP78 expression and viability of GC cell lines was further analyzed. In line with our hypothesized, it was observed that the cell growth inhibition rate of DDP negatively correlated with GRP78 expression in GC cell lines, especially in DDP-resistant GC cells (Fig. 1c).
Fig. 1.
Negative correlation between GRP78 expression and the cell growth inhibition rate of DDP in DDP-sensitive and DDP-resistant GC cell lines. a Western blot analysis of GRP78 expression in normal gastric mucosal epithelial cell line (GES-1) and three human GC cell lines (SGC-7901, AGS, HST2). b CCK-8 assay for detection of cell growth inhibition rate of DDP in DDP-sensitive and DDP-resistant GC cell lines (SGC-7901, AGS, HST2). c Analysis of correlation between GRP78 expression and the cell growth inhibition rate of DDP. **p < 0.01, ***p < 0.001
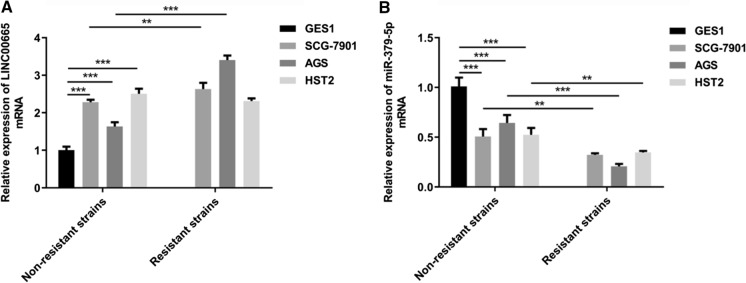
Upregulated LINC00665 expression and downregulated miR-379-5p expression in gastric cancer
LINC00665 and miR-379-5p levels in normal gastric mucosal epithelial cell line (GES-1) and three human GC cell lines (SGC-7901, AGS, HST2) were firstly detected. As compared to GES-1 cells, LINC00665 expression markedly increased (Fig. 2a) and miR-379-5p expression greatly decreased (Fig. 2b) in DDP-sensitive and DDP-resistant GC cell lines. Moreover, noticeably modulated expressions of LINC00665 and miR-379-5p were determined in DDP-resistant GC cells.
Fig. 2.
Upregulated LINC00665 expression and downregulated miR-379-5p expression in gastric cancer. a RT-qPCR for examination of the relative LINC00665 levels in normal gastric mucosal epithelial cell line (GES-1) and three human GC cell lines (SGC-7901, AGS, HST2). b RT-qPCR for examination of the relative miR-379-5p levels in normal gastric mucosal epithelial cell line (GES-1) and three human GC cell lines (SGC-7901, AGS, HST2). **p < 0.01, ***p < 0.001
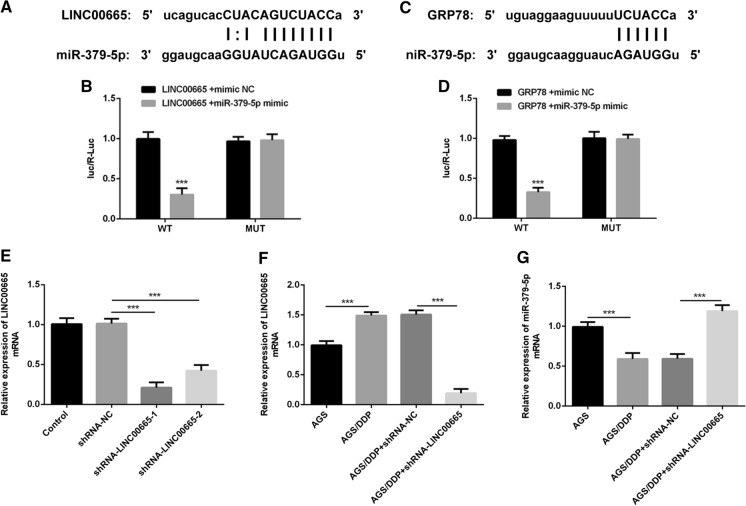
The targeted relationship between LINC00665 and miR-379-5p or miR-379-5p and GRP78
Bioinformatic analysis was performed to predict the binding regions between LINC00665 and miR-379-5p or miR-379-5p and GRP78 (Fig. 3a, c). Luciferase reporter assay revealed that miR-379-5p mimic significantly decreased the luciferase activity of LINC00665 while had no obvious inhibitory effects on the luciferase activity of LINC00665-MUT (Fig. 3b). It was also verified by luciferase reporter assay that miR-379-5p could sponge GRP78 in GC (Fig. 3d). 48 h post transfection, transfection efficiency was validated by employing RT-qPCR. Then, shRNA-LINC00665-1 was selected for the subsequent experiments due to the optimized transfection efficiency (Fig. 3e). It was observed that LINC00665 expression level was downregulated (Fig. 3f) while miR-379-5p expression level was upregulated (Fig. 3g) in DDP-resistant GC cells after introduction of shRNA-LINC00665. These data demonstrated that LINC00665 served as a molecular sponge for miR-379-5p and negatively regulated miR-379-5p expression in GC.
Fig. 3.
The targeted relationship between LINC00665 and miR-379-5p or miR-379-5p and GRP78. a Bioinformatics analysis predicted the binding site of LINC00665 to miR-379-5p. b Luciferase reporter assay verified the binding relationship between LINC00665 and miR-379-5p. c Bioinformatics analysis predicted the binding site of miR-379-5p to GRP78. d Luciferase reporter assay verified the binding relationship between miR-379-5p and GRP78. e RT-qPCR for verification of transfection efficiency. f RT-qPCR for examination of the relative LINC00665 levels. g RT-qPCR for examination of the relative miR-379-5p levels. ***p < 0.001
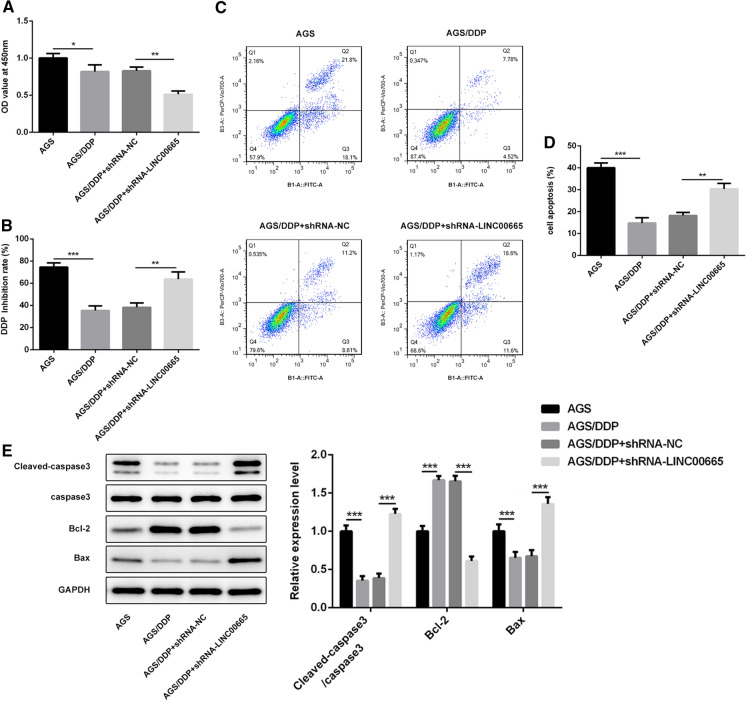
LINC00665 silencing improved DDP sensitivity of DDP-resistant GC cells
To clarify the role of LINC00665 in DDP-resistant GC, we examined DDP sensitivity in AGS/DDP cells with LINC00665 knockdown. First of all, CCK-8 assay uncovered that LINC00665 silencing greatly suppressed AGS/DDP cell proliferation and elevated the cell growth inhibition rate of DDP in AGS cells (Fig. 4a, b). Flow-cytometric analysis confirmed a higher apoptotic rate in AGS/DDP cells with LINC00665 knockdown (Fig. 4c). Moreover, decreased Bcl-2 expression and increased Bax and cleaved caspase-3 expressions also indicated that LINC00665 silencing promoted cell apoptosis, raising the cell growth inhibition rate of DDP in AGS cells (Fig. 4d). Data above proved that LINC00665 silencing could enhance the sensitivity to DDP in DDP-resistant GC cells.
Fig. 4.
LINC00665 silencing improved DDP sensitivity of DDP-resistant GC cells. a CCK-8 assay for detection of cell viability of AGS/DDP cells. b CCK-8 assay for detection of cell growth inhibition rate of DDP in AGS/DDP cells. c Flow-cytometric analysis for detection of the apoptotic rate of AGS/DDP cells. d Western blot analysis of Bcl-2, Bax and cleaved caspase-3 expressions. *p < 0.05, **p < 0.01, ***p < 0.001
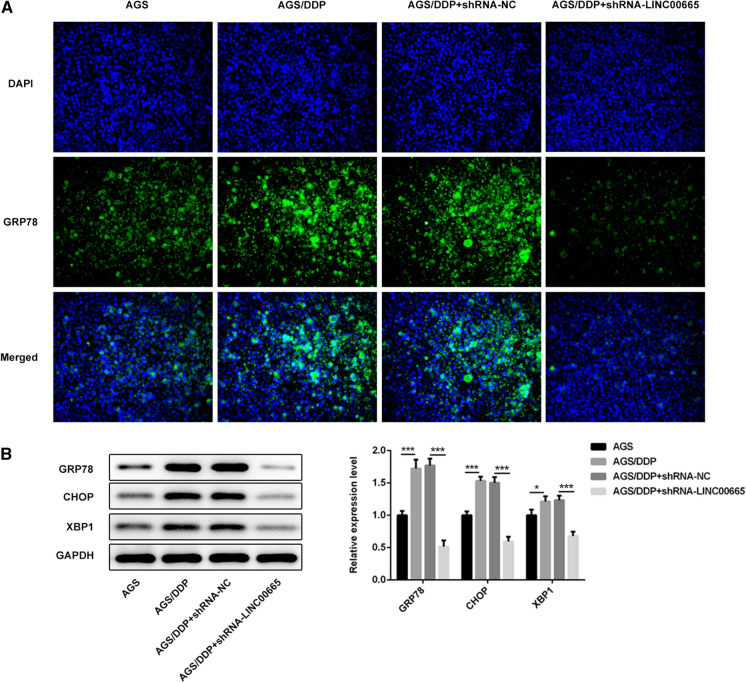
LINC00665 silencing increased DDP sensitivity by repressing endoplasmic reticulum stress
The influence that LINC00665 might have on endoplasmic reticulum stress was examined using immunofluorescence and western blot analysis. Silence of LINC00665 weakened the fluorescence intensity of GRP78 (Fig. 5a). Moreover, western blot analysis similarly presented that silence of LINC00665 reduced the protein expression levels of GRP78, CHOP and XBP1 (Fig. 5b). These findings suggested that LINC00665 silencing might improve DDP sensitivity of DDP-resistant GC cells by suppressing ER stress.
Fig. 5.
LINC00665 silencing increased DDP sensitivity by repressing endoplasmic reticulum stress. a Immunofluorescence assay for examination of GRP78 expression. b Western blot analysis of GRP78, CHOP and XBP1 expressions. *p < 0.05, ***p < 0.001
Discussion
Gastric cancer, as one of the most common gastrointestinal tumors, seriously threatens the healthy living of people worldwide (Casamayor et al. 2018). The onset of gastric cancer is insidious, and the disease is usually in late stage at the time of clinical consultation (Feng and Liu 2020). DDP-based chemotherapy is extensively used for the treatment of advanced malignant tumors, including gastric cancer (Wagner et al. 2017). However, resistance to chemotherapy largely leads to a reduction in the efficacy of cancer treatment (King and Wilson 2020). In this study, our purpose is to further clarify the underlying molecular mechanism of DDP-resistance.
Recently, it has been reported that lncRNAs play critical roles in the development and progression of gastric cancer, and that they were also involved in regulating the drug sensitivity to DDP (Wei et al. 2020; Yuan et al. 2020). Studies have shown that LINC00665 is closely related to the growth, progression and metastasis of many types of cancers, including gastric cancer (Chen et al. 2020; Yang et al. 2020). LINC00665 could confer gefitinib resistance by suppressing cell apoptosis (Liu et al. 2019). It was also demonstrated in our study that LINC00665 expression levels were distinctly up-regulated in DDP-resistant GC cells. In addition, LINC00665 knockdown arrested the proliferation and boosted the apoptosis of DDP-resistant cells.
miRNAs participate in gene expression regulation after transcription and are the key elements in the overall process of tumor development (Wu et al. 2020). Numerous members of miRNAs are closely associated with drug resistance to DDP (Wu et al. 2017; Zuo et al. 2020). miR-379-5p is confirmed to play a negative role in tumor growth and metastasis. Our research verified the interaction between LINC00665 and miR-379-5p and revealed that silence of LINC00665 significantly upregulated the level of miR-379-5p. Moreover, LINC00665 may promote DDP-resistance through negatively regulating miR-379-5p.
Researches in recent years have corroborated that ER stress plays an indispensable role in the pathogenesis of tumor and chemotherapy resistance (Zheng et al. 2019). GRP78 is a molecular chaperone and belongs to the HSP70 family. GRP78 on cell surface can be induced by ER stress response and participates in tumor cell proliferation, apoptosis and drug resistance (Ran et al. 2017; Machihara and Namba 2020).The present study discovered that GRP78 was expressed at high levels in DDP-sensitive and DDP-resistant GC cells and the sensitivity of GC cells to DDP was negatively related to GRP78 expression level. In addition to the target relationship between miR-379-5p and GRP78, silence of LINC00665 might arrest the resistance of GC cells to DDP by sponging miR-379-5p to downregulate GRP78 expression.
Conclusion
In summary, LINC00665 sponges miR-379-5p to upregulate GRP78, thereby playing a promotive role in the resistance of GC cells to DDP. Hence, inhibition of the abundance of LINC00665 might be a potential therapeutic method for chemotherapy resistance. These findings may provide new insights for the development of novel therapeutic strategies for GC clinically.
Authors' contributions
All the authors searched the literature, designed the study, performed the experiments, analyzed and interpreted the data and wrote the manuscript.
Funding
This work was supported by Jiangsu Provincial Natural Science Fund (No. BK20151017), Six Major Talent Peak Project of Jiangsu Province (No. WSW-050), Natural Science Foundation of China (No. 81502052), Youth Talent Program of Jiangsu Cancer Hospital (No. QL 201816), Jiangsu Provincial Medical Youth Talent (No.QNRC2016651), The TalentProgram of Jiangsu Cancer Hospital, 333 High-Level Talent Training Project in Jiangsu Province (No. LGY2018069).
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The paper refers to cell study in vitro and raises no ethical concerns.
Consent for publication
The final manuscript has been read and approved by all the authors for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The first authors: Chao Yue and Chen Yu have contributed equally to this work.
Contributor Information
Gang Li, Email: ligangdoctor591@163.com.
Lin Xu, Email: xulin471@126.com.
References
- Casamayor M, Morlock R, Maeda H, Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience. 2018;12:883. doi: 10.3332/ecancer.2018.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Yu Z, Huang W, Yang Y, Wang F, Huang H. LncRNA LINC00665 promotes prostate cancer progression via miR-1224-5p/SND1 axis. Onco Targets Ther. 2020;13:2527–2535. doi: 10.2147/OTT.S241578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Liu X. Interaction between ACOT7 and LncRNA NMRAL2P via methylation regulates gastric cancer progression. Yonsei Med J. 2020;61:471–481. doi: 10.3349/ymj.2020.61.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020 doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, Wilson JJ. Endoplasmic reticulum stress: an arising target for metal-based anticancer agents. Chem Soc Rev. 2020 doi: 10.1039/d0cs00259c. [DOI] [PubMed] [Google Scholar]
- Li Z, Li Z. Glucose regulated protein 78: a critical link between tumor microenvironment and cancer hallmarks. Biochim Biophys Acta. 2012;1826:13–22. doi: 10.1016/j.bbcan.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Liu X, Lu X, Zhen F, Jin S, Yu T, Zhu Q, Wang W, Xu K, Yao J, Guo R. LINC00665 induces acquired resistance to Gefitinib through recruiting EZH2 and activating PI3K/AKT pathway in NSCLC. Mol Ther Nucleic Acids. 2019;16:155–161. doi: 10.1016/j.omtn.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Machihara K, Namba T. Kuanoniamine C stimulates bortezomib-induced cell death via suppression of glucose-regulated protein 78 in osteosarcoma. Biochem Biophys Res Commun. 2020;527:289–296. doi: 10.1016/j.bbrc.2020.04.109. [DOI] [PubMed] [Google Scholar]
- Qi H, Xiao Z, Wang Y. Long non-coding RNA LINC00665 gastric cancer tumorigenesis by regulation miR-149-3p/RNF2 axis. Onco Targets Ther. 2019;12:6981–6990. doi: 10.2147/OTT.S214588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran D, Mao J, Shen Q, Xie C, Zhan C, Wang R, Lu W. GRP78 enabled micelle-based glioma targeted drug delivery. J Control Release. 2017;255:120–131. doi: 10.1016/j.jconrel.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang P, Xu X, Wang J, Wang D, Peng P, Zheng C, Meng QJ, Yang L, Luo Z. Knockdown of cytokeratin 8 overcomes chemoresistance of chordoma cells by aggravating endoplasmic reticulum stress through PERK/eIF2α arm of unfolded protein response and blocking autophagy. Cell Death Dis. 2019;10:887. doi: 10.1038/s41419-019-2125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Yang B, Fu Z, Wang X, Zhang Z. Efficacy and safety of oxaliplatin-based regimen versus cisplatin-based regimen in the treatment of gastric cancer: a meta-analysis of randomized controlled trials. Int J Clin Oncol. 2019;24:614–623. doi: 10.1007/s10147-019-01425-x. [DOI] [PubMed] [Google Scholar]
- Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv L, Liu J, Xu Y, Shen Y, Yang M. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. 2020;19:62. doi: 10.1186/s12943-020-01185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Niu X, Tao J, Li P, Lu Q, Xu A, Chen W, Wang Z. MicroRNA-379-5p plays a tumor-suppressive role in human bladder cancer growth and metastasis by directly targeting MDM2. Oncol Rep. 2017;37:3502–3508. doi: 10.3892/or.2017.5607. [DOI] [PubMed] [Google Scholar]
- Wu SR, Wu Q, Shi YQ. Recent advances of miRNAs in the development and clinical application of gastric cancer. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wang Y, Mojumdar K, Zhou Z, Jeong KJ, Mangala LS, Yu S, Tsang YH, Rodriguez-Aguayo C, Lu Y, Lopez-Berestein G, Sood AK, Mills GB, Liang H. A-to-I-edited miRNA-379-5p inhibits cancer cell proliferation through CD97-induced apoptosis. J Clin Invest. 2019;129:5343–5356. doi: 10.1172/JCI123396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Bai Q, Chen H, Su K, Gao C. LINC00665 induces gastric cancer progression through activating Wnt signaling pathway. J Cell Biochem. 2020;121:2268–2276. doi: 10.1002/jcb.29449. [DOI] [PubMed] [Google Scholar]
- Yuan L, Xu ZY, Ruan SM, Mo S, Qin JJ, Cheng XD. Long non-coding RNAs towards precision medicine in gastric cancer: early diagnosis, treatment, and drug resistance. Mol Cancer. 2020;19:96. doi: 10.1186/s12943-020-01219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Duan C, Yin S, Tian Y. MicroRNA-379-5p/YBX1 axis regulates cellular EMT to suppress migration and invasion of nasopharyngeal carcinoma cells. Cancer Manag Res. 2020;12:4335–4346. doi: 10.2147/CMAR.S253504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Shang Y, Tao J, Zhang J, Sha B. Endoplasmic reticulum stress signaling pathways: activation and diseases. Curr Protein Pept Sci. 2019;20:935–943. doi: 10.2174/1389203720666190621103145. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Xie M, Meng Z, Leung LK, Chan FL, Hu X, Chi K, Liu C, Yao X. Knockdown of TM9SF4 boosts ER stress to trigger cell death of chemoresistant breast cancer cells. Oncogene. 2019;38:5778–5791. doi: 10.1038/s41388-019-0846-y. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Zheng W, Liu J, Tang Q, Wang SS, Yang XS. MiR-34a-5p/PD-L1 axis regulates cisplatin chemoresistance of ovarian cancer cells. Neoplasma. 2020;67:93–101. doi: 10.4149/neo_2019_190202N106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.