Abstract
Ovarian cancer is one of the leading lethal gynecological cancers, causing serious harm to the health of female populations. Growing studies emphasize that lncRNAs serve as significant regulators in the tumorigenesis and evolution of numerous malignancies, including ovarian cancer. Recently, the oncogenic activity of lncRNA ARAP1-AS1 has been justified in a variety of cancers. However, the potential function of ARAP1-AS1 in ovarian cancer development is still unclear. Herein, we firstly revealed the expression profile of ARAP1-AS1 in ovarian cancer. Compared to normal samples and cells, upregulation of ARAP1-AS1 was observed in tissues and cells of ovarian cancer. Therewith, it was disclosed that knockdown of ARAP1-AS1 alleviated the carcinogenicity of ovarian cancer cells. Besides, our findings delineated that ARAP1-AS1 silence inhibited the expression of oncogene PLAGL2. Considering that ARAP1-AS1 was principally expressed in the the cytoplasm of ovarian cancer cells, we speculated that ARAP1-AS1 facilitated ovarian cancer progression via functioning as a ceRNA. Further investigations indicated that ARAP1-AS1 promoted PLAGL2 expression by competitively binding with miR-4735-3p. Of note, ARAP1-AS1 contributed to the malignant phenotypes of ovarian cancer cells through modulation of miR-4735-3p/PLAGL2 axis, revealing ARAP1-AS1 as a promising therapeutic target for ovarian cancer patients.
Keywords: Ovarian cancer, ARAP1-AS1, PLAGL2, MiR-4735-3p
Introduction
Ovarian cancer is considered as the most prevailing gynecological malignancy and occupies about 4% of all diagnosed cases of female cancers, leading to increasing numbers of fatalities in women globally and seriously threating women’s health (Gumusoglu et al. 2021 ; Siegel et al. 2016). On account of great advance in clinical therapeutic regimens for ovarian cancer, such as surgical resection, chemotherapy and radiotherapy, the lifespan of patients has been significantly improved (Murali et al. 2018). Nevertheless, the 5-year survival rate of patients with ovarian cancer is still less than 35% due to extensive metastasis and frequent recurrence (Pradeep 2014; Wang and López-Ozuna 2020). In addition, it is estimated that 70% of new cases are diagnosed at advanced stage of ovarian cancer because of the absence of effective screening methods (Moufarrij 2019). Hence, identifying the mechanism governing ovarian cancer is important for the development of potent therapeutic strategies for this disease.
Recently, increasing investigators have focused on the potential of long non-coding RNA (lncRNA) in the tumorigenesis and development of ovarian cancer (Yao 2018; Xu et al. 2018). LncRNAs are a set of RNA transcripts with over 200 nucleotides in length and possess no protein-coding capacity (Fritah et al. 2014). Surging evidence has illustrated that lncRNAs are involved in the multiple physiological and pathological processes of human malignancies, including ovarian cancer (Yang et al. 2014). For instance, lncRNA PVT1 serves as a diagnostic biomarker and modulates tumor development in prostate cancer (Yang 2017). LncRNA FBXL19-AS1 facilitates the proliferation and invasion of breast cancer cells through functioning as a molecular sponge for miR-718 (Ding et al. 2019). Low level of lncRNA GAS5 aggravates ovarian cancer progression via targeting miR-196-5p and thereby modulating HOXA5 (Zhao 2018). The abnormal expression of lncRNA ARAP1-AS1 has been found to be strongly associated with the initiation and occurrence of various malignant tumors, including gastric cancer (Jiang 2020), cervical cancer (Zhang et al. 2020) and bladder cancer (Teng 2019). However, the functional role and latent regulatory mechanism of ARAP1-AS1 in ovarian cancer are largely to be elucidated.
The current study was aimed to explore the participation of ARAP1-AS1 in ovarian cancer and clarify its underlying molecular mechanism. We confirmed that ARAP1-AS1 contributed to the tumorigenesis of ovarian cancer via promoting cell proliferation, migration and invasion. More importantly, the oncogenic property of ARAP1-AS1 in ovarian cancer was mediated by miR-4735-3p/PLAGL2 axis, which offered an innovative strategy for the treatment of ovarian cancer.
Materials and methods
Cell culture
Human normal ovarian surface epithelial cell line (IOSE-80) and four human ovarian cancer cell lines (A2780, OVCAR3, CP70 and SKOV3) were procured from American Type Culture Collection (ATCC, Maryland, USA). All the cells were cultivated in RPMI 1640 medium supplied by Gibco (Grand Island, USA) containing 10% FBS (Gibco) and 100 U/mL of streptomycin/penicillin (Invitrogen, Carlsbad, USA) under a humid atmosphere of 5% CO2 at 37 °C.
Cell transfection
To knockdown ARAP1-AS1 or PLAGL2, shRNA targeting ARAP1-AS1 (sh-ARAP1-AS1) or PLAGL2 (sh-PLAGL2) as well as negative control shRNA (sh-NC) were synthesized by GenePharma Corporation (Shanghai, China). For overexpression or inhibition of miR-4735-3p, the mimic and inhibitor of miR-4735-3p as well as corresponding controls were also obtained from GenePharma Corporation. Cell transfection was implemented using Lipofectamine 2000 reagents (Invitrogen) in the light of product manuals.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Cellular RNA isolation was conducted with Trizol solution (Invitrogen). Then, RNA was reverse-transcribed into cDNA by using PrimeScriptTM RTMaster Mix (TaKaRa, Ohtsu, Japan). RT-qPCR was implemented with SYBR Premix Ex Taq II (TaKaRa, Dalian, China) on a Light Cycler480 instrument (Roche, Basel, Switzerland) referring to the supplier’s directions. Comparative Ct method was adopted to quantify gene expression. GAPDH and U6 served as the endogenous references for normalization, respectively.
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay
Following transfection, 3 × 103 cells were plated into each well of 96-well plates and incubated for various time-points from 0 to 72 h. At the end of indicated incubation time, each well was supplemented with 15 μl MTT reagent (Sigma-Aldrich, Northbrook, USA), followed by additional 4 h of incubation, treated with 150 µl DMSO. The absorbance at 570 nm was detected with a microplate spectrophotometer.
Colony formation assay
Following trypsinization, transfected cells were suspended in DMEM medium containing 10% FBS, then plated in 6-well plates and incubated at 37 °C in a humid atmosphere of 5% CO2 for 2 weeks. The colonies were fixed by methanol, dyed with 0.1% crystal violet and thereafter counted.
Cell migration and invasion assays
Wound healing assay was conducted to estimate cell migration. In short, cells were plated into a 6-well plate and grown to confluence. Subsequently, artificial scratches were produced by a 20 μl pipette tip. Cells were cultured at 37 °C and monitored with an Olympus 1X71 camera system at 0 and 48 h after scratching. Transwell assay was employed to evaluate the invasive ability of ovarian cancer cells using transwell membranes covered with Matrigel (Millipore, Billerica, USA). Transfected cells were placed to the upper transwell chamber, and DMEM medium containing 20% FBS was added into the lower chamber as a chemoattractant. 24 h of incubation later, non-invaded cells were wiped out with a cotton swab and then invaded cells were fixed by 4% paraformaldehyde, dyed with haematoxylin and counted by a microscope.
Western blot
Total protein from cells were extracted with RIPA buffer (Solarbio, Beijing, China), electrophoresed on 10% SDS-PAGE and subsequently transferred to PVDF membrane. Following blocking with 5% fat-free milk for 2 h at room temperature, membrane was treated with the primary antibodies against PLAGL2 at 4 °C all the night, incubated with secondary antibodies for 1 h at room temperature and detected by the ECL detection kit (Pierce Chemical, Rockford, USA).
RNA immunoprecipitation (RIP) assay
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) was adopted for the RIP experiment pursuant to the manufacturer’s protocols. In short, transfected cells were harvested and lysed with RIP lysis buffer. Then, cell extracts were hatched with magnetic beads conjugated to Ago2 antibody (Millipore) with IgG (Millipore) as negative control (Millipore, MA, USA). After elution from beads, immunoprecipitated RNAs were subjected to RT-qPCR assay.
Luciferase reporter assay
The full length of ARAP1-AS1 and its mutant without the predicted miR-4735-3p binding sites were synthesized and ligated into pGL3 vectors (Promega, Fitchburg, USA), termed as ARAP1-AS1-WT and ARAP1-AS1-Mut. Likewise, the 3′UTR of PLAGL2 containing the putative miR-4735-3p binding sites or mutant sites was cloned into reporter plasmids to construct PLAGL2-WT or PLAGL2-Mut. The ovarian cancer cells were co-transfected with indicated reporter plasmids and miR-4735-3p mimic or NC mimic by utilizing LipofectamineTM 2000 referring to product manuals. Cells were collected at 48 h after transfection, and then measurement of luciferase activity was conducted with the Dual Luciferase Reporter Assay Kit (Promega) according to the vender’s instructions.
Subcellular fractionation analysis
The cytoplasmic and nuclear fractions were drawn out from ovarian cancer cells by using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Waltham, USA). The isolated RNAs were subjected to RT-qPCR analysis. U6 was taken as nuclear control and GAPDH served as cytoplasmic control.
Statistical analysis
All quantitative data were displayed as mean ± standard deviation (SD) and all experiments were executed in triplicate. Student’s t-test and one-way analysis of variance (ANOVA) were employed for comparisons between two or more groups. Differences were regarded to be statistically significant when P < 0.05.
Results
ARAP1-AS1 and PLAGL2 were overtly upregulated in ovarian cancer
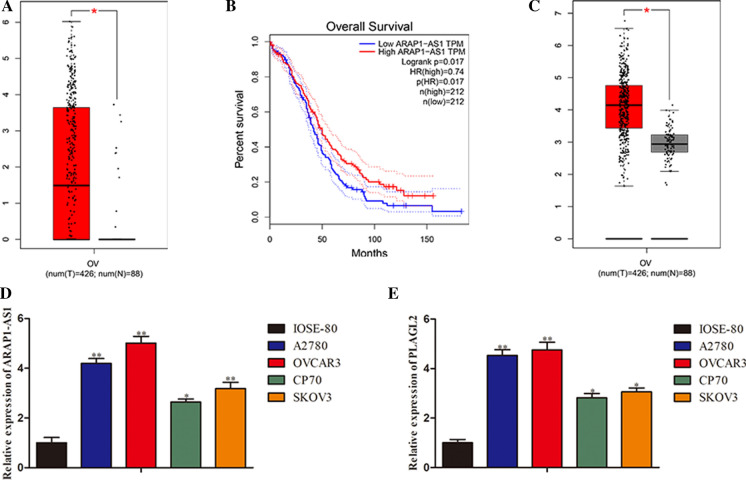
To identify the expression pattern of ARAP1-AS1 and PLAGL2 in ovarian cancer, we first browsed the GEPIA website. By analysis of the TCGA data, we found that the expression of ARAP1-AS1 in tumor samples was much higher than in normal tissues (Fig. 1a). As regards the overall survival, patients with a high ARAP1-AS1 expression exhibited a significantly poorer prognosis compared with those with a low ARAP1-AS1 expression (Fig. 1b). Moreover, it was disclosed that PLAGL2 level was prominently elevated in cancer specimens compared to adjacent non-cancer samples (Fig. 1c). Subsequently, RT-qPCR assay was performed to confirm the expression levels of ARAP1-AS1 and PLAGL2 in ovarian cancer cells. Results illustrated that ARAP1-AS1 expressed at a high level in ovarian cancer cell lines (A2780, OVCAR3, CP70 and SKOV3) in contrast with normal ovarian epithelia IOSE-80 (Fig. 1d). Likewise, upregulation of PLAGL2 was observed in ovarian cancer cells (Fig. 1e). Taken together, these findings indicated that the expression of ARAP1-AS1 and PLAGL2 were significantly enhanced in ovarian cancer.
Fig. 1.
ARAP1-AS1 and PLAGL2 were overtly upregulated in ovarian cancer. a–c The TCGA data analyses of ARAP1-AS1 and PLAGL2l expression in ovarian cancer samples and normal tissues. d, e The RT-qPCR detection of ARAP1-AS1 and PLAGL2l levels in ovarian cancer cell lines (A2780, OVCAR3, CP70 and SKOV3) and normal ovarian endothelial cell line IOSE-80. Data represent the mean ± SD. from three independent experiments. *P < 0.05, **P < 0.01 vs. IOSE-80
Depletion of ARAP1-AS1 dampened ovarian cancer cell proliferation, migration and invasion
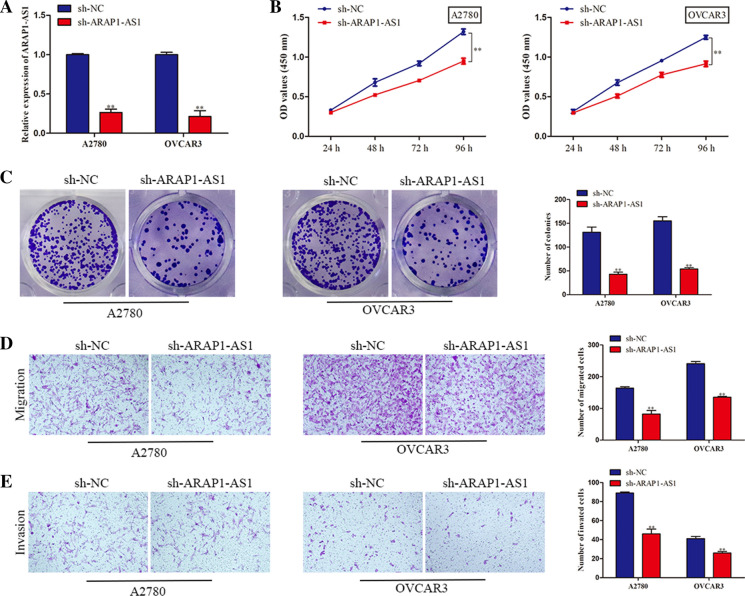
Based on the foregoing findings, we intended to explore the function of ARAP1-AS1 in the malignant behaviors of ovarian cancer cells. Given that A2780 and OVCAR3 cells showed the highest expression of ARAP1-AS1, these two cell lines were selected for loss-of-function assays. RT-qPCR analysis demonstrated that ARAP1-AS1 was knocked down after transfection with sh-ARAP1-AS1 plasmid (Fig. 2a). MTT assay illuminated that silencing of ARAP1-AS1 contributed to the remarkable decrease of A2780 and OVCAR3 cell viability (Fig. 2b). Similarly, colony formation assay revealed that inhibition of ARAP1-AS1 suppressed the proliferation of ovarian cancer cells (Fig. 2c). Furthermore, our observations manifested that the migratory capacity of A2780 and OVCAR3 cells was restrained due to knockdown of ARAP1-AS1 (Fig. 2d). In agreement with above results, transwell invasion assay delineated that ARAP1-AS1 downregulation led to the diminished number of invaded cells in ovarian cancer (Fig. 2e). To sum up, we concluded that ARAP1-AS1 acted as an oncogene in ovarian cancer.
Fig. 2.
Depletion of ARAP1-AS1 dampened ovarian cancer cell proliferation, migration and invasion. a The RT-qPCR assay was conducted to determine the efficiency of ARAP1-AS1 knockdown in A2780 and OVCAR3 cells. b Cell viability was measured by MTT assay. c Colony formation assay was also used to evaluate cell proliferative capacity. d, e Cell migration and invasion were detected with transwell assays. Data represent the mean ± SD. from three independent experiments. **P < 0.01 vs. sh-NC
ARAP1-AS1 regulated the expression of PLAGL2 via sponging miR-4735-3p
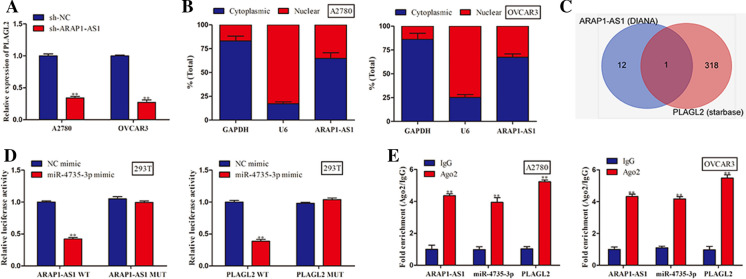
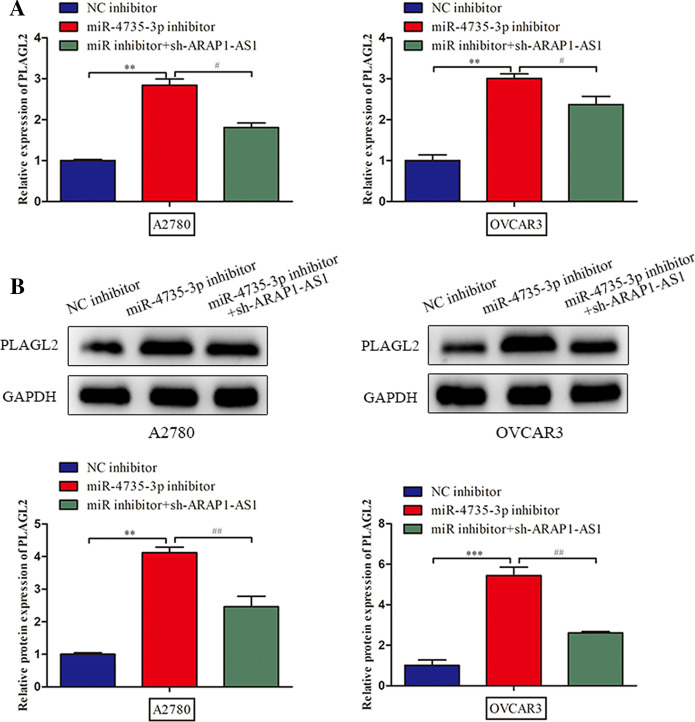
Thereafter, we discovered that suppression of ARAP1-AS1 markedly lessened the expression level of PLAGL2 (Fig. 3a). In order to further elucidate the regulatory mechanism underlying ARAP1-AS1, subcellular fractionation analysis was implemented. Results unraveled that ARAP1-AS1 was preferentially distributed in the cytoplasm of A2780 and OVCAR3 cells, which provided strong evidence that ARAP1-AS1 might serve as a competing endogenous RNA (ceRNA) to modulate PLAGL2 expression (Fig. 3b). Hence, we carried out bioinformatics analysis using starbase and DIANA databases and then found that miR-4735-3p possessed the speculated binding sites for both ARAP1-AS1 and PLAGL2 (Fig. 3c). After that, luciferase reporter assay suggested that miR-4735-3p mimic overtly reduced the luciferase activity of ARAP1-AS1-WT and PLAGL2-WT, while the mutant forms of ARAP1-AS1 and PLAGL2 had no noteworthy response to overexpression of miR-4735-3p (Fig. 3d). Consistently, RIP experiment revealed that ARAP1-AS1, miR-4735-3p and PLAGL2 were enriched by Ago2 antibody compared with IgG group, confirming the interaction among ARAP1-AS1, miR-4735-3p and PLAGL2 (Fig. 3e). Next, we performed RT-qPCR and western blot to evaluate the PLAGL2 mRNA and protein levels when transfected with miR-4735-3p inhibitor or sh-ARAP1-AS1. The results of Fig. 4a and b indicated that miR-4735-3p inhibitor increased PLAGL2 expression at mRNA and protein levels, whereas the restoration of PLAGL2 mRNA and protein expression arose when ARAP1-AS1 was down-regulated, which indicating that the main mechanism for the regulation of PLAGL2 expression by miR-4735-3p or ARAP1-AS1 would be destabilization of target mRNA rather than translational affect. In other word, these findings illustrated that ARAP1-AS1 fortified PLAGL2 expression through functioning as miR-4735-3p sponge.
Fig. 3.
ARAP1-AS1 regulated the expression of PLAGL2 via sponging miR-4735-3p. a The RT-qPCR analysis of PLAGL2 expression level in A2780 and OVCAR3 cells transfected with sh-NC or sh-ARAP1-AS1. b Subcellular fractionation analysis was adopted to examine the subcellular localization of ARAP1-AS1 in ovarian cancer cells. c The bioinformatics analysis was preformed to search for potential miRNAs containing predicted binding sites with both ARAP1-AS1 and PLAGL2 by using DIANA and starBase databases. d, e The interaction among miR-4735-3p, ARAP1-AS1 and PLAGL2 was certified by luciferase reporter assay and RIP experiment. Data represent the mean ± SD. from three independent experiments. **P < 0.01 vs. sh-NC or NC mimic
Fig. 4.
ARAP1-AS1 regulated the expression of PLAGL2 via sponging miR-4735-3p. a, b RT-qPCR assay and western blot were applied to estimate the effects of miR-4735-3p and ARAP1-AS1 on the mRNA and protein expression of PLAGL2. Data represent the mean ± SD. from three independent experiments. **P < 0.01, ***P < 0.001 vs. NC inhibitor, #P < 0.05, ##P < 0.01 vs. miR-4735-3p inhibitor
ARAP1-AS1 played an oncogenic role in ovarian cancer through miR-4735-3p/PLAGL2 axis
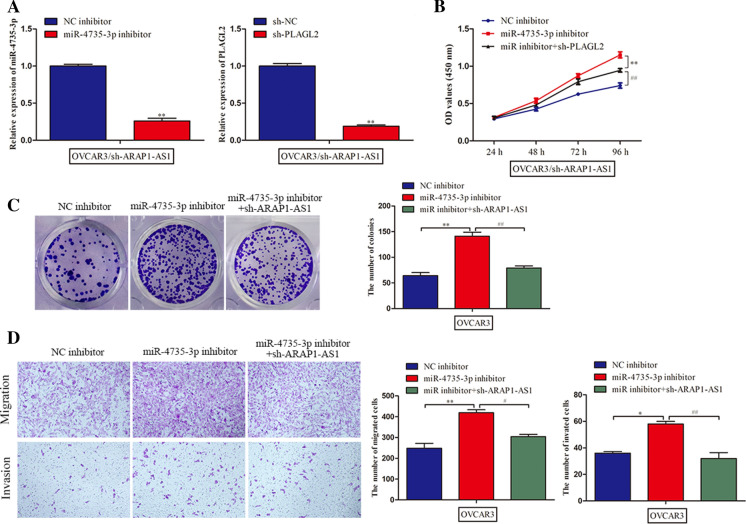
Finally, rescue assays were conducted to testify whether the effects of ARAP1-AS1 were mediated by miR-4735-3p/PLAGL2 pathway. We suppressed the expression of miR-4735-3p and PLAGL2 in ARAP1-AS1-downregulated OVCAR3 cells and transfection efficiency was verified by RT-qPCR analysis (Fig. 5a). MTT assay showed that the repression role of sh-ARAP1-AS1 on cell proliferation was aggravated by miR-4735-3p inhibitor and then retrieved when PLAGL2 was silenced (Fig. 5b). Likewise, the same result was observed about the number of colonies in the colony formation assay (Fig. 5c). Additionally, transwell assay demonstrated that ARAP1-AS1 knockdown-reduced migratory and invasive ability of OVCAR3 cells, and miR-4735-3p inhibitor promoted cell migration and invasion, while this effect was abrogated by PLAGL2 knockdown (Fig. 5d). By the large, ARAP1-AS1 regulated PLAGL2 to facilitate the deterioration of ovarian cancer by competing for miR-4735-3p.
Fig. 5.
ARAP1-AS1 played an oncogenic role in ovarian cancer through miR-4735-3p/PLAGL2 axis. a The RT-qPCR assay was employed to verify the effectiveness of transfection. b, c Cell proliferation was assessed by MTT and colony formation assays. d, e Transwell assays were carried out to estimate the role of miR-4735-3p/PLAGL in ovarian cancer cell migration and invasion. Data represent the mean ± SD. from three independent experiments. *P < 0.05, **P < 0.01 vs. sh-NC or NC inhibitor, #P < 0.05, ##P < 0.01 vs. miR-4735-3p inhibitor
Discussion
Ovarian cancer is one of the most prevalent tumors of the female reproductive system, and the leading cause of cancer mortality in women worldwide (Jayson 2014; Siegel et al. 2018). It has been reported that the death rate of ovarian cancer ranks first among all types of gynecological cancers (Fan 2020). The majority of patients with ovarian cancer have poor prognosis owing to the prevalence of postoperative recurrence (Kim et al. 2017). Furthermore, the efficacy of clinical treatments for advanced ovarian cancer remains unsatisfactory (Rustin 2011). Accordingly, deeply understanding the pathogenesis of ovarian cancer and finding out effective biomarkers are urgently needed to improve the therapeutic efficacy of ovarian cancer patients.
A growing number of studies have proven that lncRNAs play a vital role in human cancer through regulating the expression of genes at the epigenetic, transcriptional or post-transcriptional levels (Zhang and Ho 2019). Mounting evidence has testified that aberrantly expressed lncRNAs act as oncogenes or tumor suppressors in the tumorigenesis and progression of ovarian cancer (Wu et al. 2020; Xue 2020; Wang 2020). The oncogenic activity of ARAP1-AS1 has been demonstrated in a wide range of malignancies. For example, long non-coding RNA ARAP1-AS1 promotes proto-oncogene c-Myc translation to accelerate the tumorigenesis and metastasis of cervical cancer by dissociating PSF/PTB dimer (Zhang et al. 2020). YY1-induced upregulation of lncRNA ARAP1-AS1 contributes to colorectal cancer cell migration and invasion via the Wnt/β-catenin signaling pathway (Ye 2019). Long non-coding RNA ARAP1-AS1 induces cell proliferation and migration of breast cancer by regulation of miR-2110/HDAC2/PLIN1 axis (Lu 2020). For all this, the potential of ARAP1-AS1 participating in ovarian cancer progression remains to be characterized. In this study, we found that ARAP1-AS1 was highly expressed in the tissues and cells of ovarian cancer. Loss-of-function assays further unveiled that silencing of ARAP1-AS1 overtly suppressed the proliferation, migration and invasion of ovarian cancer cells.
Polymorphic adenoma-like protein 2 (PLAGL2) belongs to the PLAG gene family and has a N-terminal zinc finger structure which is highly conserved and can enable transcription factor PLAGL2 to promote the activation of specific gene transcription (Hensen 2002). Accumulating studies have demonstrated that PLAGL2 exhibits the carcinogenic function in diverse malignant tumors, such as colorectal cancer (Wu 2020), breast cancer (Xu 2018), prostate cancer (Guo 2016), glioma (Wu 2016) and ovarian cancer (Majem 2019). Herein, our findings illuminated that the expression level of PLAGL2 was higher in ovarian cancer tissues and cells than normal specimens and cells. Moreover, PLAGL2 expression was repressed by depletion of ARAP1-AS1. Results of subcellular localization suggested the main distribution of ARAP1-AS1 in cytoplasm, revealing that ARAP1-AS1 was likely to exert its role in ovarian cancer via a ceRNA mechanism (Zhan 2018; Liu 2017; Zhao et al. 2018). Subsequently, we discovered that ARAP1-AS1 upregulated PLAGL2 expression by competing for miR-4735-3p. Further experiments unraveled that ARAP1-AS1 acted as an oncogene in ovarian cancer through sponging miR-4735-3p to enhance PLAGL2 level.
To the best of our knowledge, this investigation was the first to shed light on the underlying function and molecular mechanism of ARAP1-AS1 involving in ovarian cancer. We expounded that ARAP1-AS1 induced the malignant behaviors of ovarian cancer cells by targeting miR-4735-3p/PLAGL2 pathway, which provided strong evidence that ARAP1-AS1 might serve as a novel indicator in the prognosis of ovarian cancer.
Acknowledgements
We deeply appreciate the supports by all participants.
Funding
This work was supported by Qiqihar science and technology plan innovation incentive project (No: CSFGG-2020002).
Declarations
Conflict of interest
The authors state that there is no conflict of interest.
Footnotes
The original online version of this article was revised: the funding number CSFGG202002 is updated as CSFGG2020002.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/21/2021
A Correction to this paper has been published: 10.1007/s10616-021-00512-0
References
- Ding Z, et al. LncRNA FBXL19-AS1 promotes breast cancer cells proliferation and invasion via acting as a molecular sponge to miR. Biosci Rep. 2019;39(4):12. doi: 10.1042/BSR20182018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fan Y, et al. LncRNA MIF-AS1 aggravates the progression of ovarian cancer by sponging miRNA-31-5p. Eur Rev Med Pharmacol Sci. 2020;24(5):2248–2255. doi: 10.26355/eurrev_202003_20490. [DOI] [PubMed] [Google Scholar]
- Fritah S, Niclou SP, Azuaje F. Databases for lncRNAs: a comparative evaluation of emerging tools. RNA. 2014;20(11):1655–1665. doi: 10.1261/rna.044040.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, et al. Overexpression of pleomorphic adenoma gene-like 2 is a novel poor prognostic marker of prostate cancer. PLoS ONE. 2016;11(8):e0158667. doi: 10.1371/journal.pone.0158667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensen K, et al. The tumorigenic diversity of the three PLAG family members is associated with different DNA binding capacities. Cancer Res. 2002;62(5):1510–1517. [PubMed] [Google Scholar]
- Jayson GC, et al. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- Jiang YH, et al. Increased long non-coding RNA ARAP1-AS1 expression and its prognostic significance in human gastric cancer: a preliminary study. Eur Rev Med Pharmacol Sci. 2020;24(4):1815–1820. doi: 10.26355/eurrev_202002_20359. [DOI] [PubMed] [Google Scholar]
- Kim JY, Cho CH, Song HS. Targeted therapy of ovarian cancer including immune check point inhibitor. Korean J Intern Med. 2017;32(5):798–804. doi: 10.3904/kjim.2017.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, et al. SP1-induced up-regulation of lncRNA SNHG14 as a ceRNA promotes migration and invasion of clear cell renal cell carcinoma by regulating N-WASP. Am J Cancer Res. 2017;7(12):2515–2525. [PMC free article] [PubMed] [Google Scholar]
- Lu C, et al. Long non-coding RNA ARAP1-AS1 accelerates cell proliferation and migration in breast cancer through miR-2110/HDAC2/PLIN1 axis. Biosci Rep. 2020 doi: 10.1042/BSR20191764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majem B, et al. MicroRNA-654-5p suppresses ovarian cancer development impacting on MYC WNT and AKT pathways. Oncogene. 2019;38(32):6035–6050. doi: 10.1038/s41388-019-0860-0. [DOI] [PubMed] [Google Scholar]
- Moufarrij S, et al. Epigenetic therapy for ovarian cancer: promise and progress. Clin Epigenetics. 2019;11(1):7. doi: 10.1186/s13148-018-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali R, Grisham RN, Soslow RA. The roles of pathology in targeted therapy of women with gynecologic cancers. Gynecol Oncol. 2018;148(1):213–221. doi: 10.1016/j.ygyno.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep S, et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell. 2014;26(1):77–91. doi: 10.1016/j.ccr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin G, et al. Early versus delayed treatment of relapsed ovarian cancer. Lancet. 2011;377(9763):380–381. doi: 10.1016/S0140-6736(11)60126-8. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA A Cancer J Clinician. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA A Cancer J Clinician. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Gumusoglu E, et al. The importance of dysregulated miRNAs on ovarian cysts and epithelial ovarian cancer. Eur J Gynaecol Oncol. 2016;42(1):66–72. [Google Scholar]
- Teng J, et al. Long non-coding RNA ARAP1-AS1 promotes the progression of bladder cancer by regulating miR-4735-3p/NOTCH2 axis. Cancer Biol Ther. 2019;20(4):552–561. doi: 10.1080/15384047.2018.1538613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, López-Ozuna VM. Biguanides in combination with olaparib limits tumorigenesis of drug-resistant ovarian cancer cells through inhibition of Snail. Cancer Med. 2020;9(4):1307–1320. doi: 10.1002/cam4.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Long non-coding RNA RHPN1-AS1 promotes tumorigenesis and metastasis of ovarian cancer by acting as a ceRNA against miR-596 and upregulating LETM1. Aging (Albany NY) 2020;12(5):4558–4572. doi: 10.18632/aging.102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, et al. Long non-coding RNA HAL suppresses the migration and invasion of serous ovarian cancer by inhibiting EMT signaling pathway. Biosci Rep. 2020 doi: 10.1042/BSR20194496. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu L, et al. PLAGL2 promotes epithelial-mesenchymal transition and mediates colorectal cancer metastasis via β-catenin-dependent regulation of ZEB1. Br J Cancer. 2020;122(4):578–589. doi: 10.1038/s41416-019-0679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, et al. MiR-218-5p inhibits the stem cell properties and invasive ability of the A2B5+CD133- subgroup of human glioma stem cells. Oncol Rep. 2016;35(2):869–877. doi: 10.3892/or.2015.4418. [DOI] [PubMed] [Google Scholar]
- Xu B, et al. MiR-449a suppresses cell migration and invasion by targeting PLAGL2 in breast cancer. Pathol Res Pract. 2018;214(5):790–795. doi: 10.1016/j.prp.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Xue F, et al. Non-coding RNA LOXL1-AS1 exhibits oncogenic activity in ovarian cancer via regulation of miR-18b-5p/VMA21 axis. Biomed Pharmacother. 2020;125:109568. doi: 10.1016/j.biopha.2019.109568. [DOI] [PubMed] [Google Scholar]
- Xu QF, Tang YX, Wang X. LncRNA EBIC promoted proliferation, metastasis and cisplatin resistance of ovarian cancer cells and predicted poor survival in ovarian cancer patients. Eur Rev Med Pharmacol Sci. 2018;22(14):4440–4447. doi: 10.26355/eurrev_201807_15495. [DOI] [PubMed] [Google Scholar]
- Yao N, et al. LncRNA GIHCG promotes development of ovarian cancer by regulating microRNA-429. Eur Rev Med Pharmacol Sci. 2018;22(23):8127–8134. doi: 10.26355/eurrev_201812_16504. [DOI] [PubMed] [Google Scholar]
- Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. LncRNA PVT1 predicts prognosis and regulates tumor growth in prostate cancer. Biosci Biotechnol Biochem. 2017;81(12):2301–2306. doi: 10.1080/09168451.2017.1387048. [DOI] [PubMed] [Google Scholar]
- Ye Y, et al. YY1-Induced upregulation of long noncoding RNA ARAP1-AS1 promotes cell migration and invasion in colorectal cancer through the Wnt/β-catenin signaling pathway. Cancer Biother Radiopharm. 2019;34(8):519–528. doi: 10.1089/cbr.2018.2745. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu D, Wang D. Long non-coding RNA ARAP1-AS1 promotes tumorigenesis and metastasis through facilitating proto-oncogene c-Myc translation via dissociating PSF/PTB dimer in cervical cancer. Cancer Med. 2020;9(5):1855–1866. doi: 10.1002/cam4.2860. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang X, Ho TT. Computational analysis of lncRNA function in cancer. Methods Mol Biol. 2019;1878:139–155. doi: 10.1007/978-1-4939-8868-6_8. [DOI] [PubMed] [Google Scholar]
- Zhan Y, et al. Long non-coding RNA DANCR promotes malignant phenotypes of bladder cancer cells by modulating the miR-149/MSI2 axis as a ceRNA. J Exp Clin Cancer Res. 2018;37(1):273. doi: 10.1186/s13046-018-0921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. Linc00511 acts as a competing endogenous RNA to regulate VEGFA expression through sponging hsa-miR-29b-3p in pancreatic ductal adenocarcinoma. J Cell Mol Med. 2018;22(1):655–667. doi: 10.1111/jcmm.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, et al. Lowly-expressed lncRNA GAS5 facilitates progression of ovarian cancer through targeting miR-196-5p and thereby regulating HOXA5. Gynecol Oncol. 2018;151(2):345–355. doi: 10.1016/j.ygyno.2018.08.032. [DOI] [PubMed] [Google Scholar]