Abstract
We investigated the effect of visual impairment (VI) on dementia development in a national cohort. In this 12-year nationwide population-based retrospective cohort study, national data were collected from National Health Insurance Cooperation of South Korea from 2002 to 2017, comprising 799,074 subjects selected from the dementia-free cohort representative of the Korean population. Crude hazard ratios (HRs) as well as age- and sex-adjusted HRs and confidence intervals (CIs) for the development of dementia were estimated using multivariable Cox regression models. VI significantly increased the risk of dementia with a HR of 2.726 (95% CI 2.251–3.300, p < 0.0001) after adjusting for age, sex, and interaction between age, sex, and VI. HR of interaction between VI and age for dementia was 0.539 (95% CI 0.436–0.667, p < 0.0001). In the sensitivity analysis after adjustment for age, sex, household income level, BMI and other comorbidities, VI showed higher risk for all the type of dementia (p < 0.0001). In subgroup analysis of VI, young males showed the highest risk for development of dementia with a HR of 2.687 (95% CI 2.219–3.254, p < 0.0001). VI significantly increased the risk of dementia in the study cohort, and young males with VI appeared to be the most susceptible to the development of dementia.
Subject terms: Dementia, Vision disorders, Risk factors
Visual impairment (VI) is well known to be associated with cognitive decline1–4. Age-Related Eye Disease Study (AREDS) reported that reduced vision associated with age-related macular degeneration was associated with cognitive impairment in older subjects4. The Singapore Malay Eye Study also reported an association between reduced vision with cataract and cognitive dysfunction in an older Asian population3. Subjective vision dysfunction based on self-reports was associated with poor cognitive function in a study based in the USA2. Furthermore, visual acuity may be an indicator of the neurodegeneration in brain aging5.
Likewise, there have been several reports on the associations between VI and dementia6–8. Several authors have reported that VI might contribute to a decline in cognition and the development of dementia based on national cohort data8,9. Significant associations have been found between visual structures10–15 or clinical ophthalmological disorder such as cataract and glaucoma16–20 and Alzheimer’s disease (AD). Previous research reported amyloid β, the pathological hallmark of AD, deposits in the crystalline lenses of AD patients21. Some studies suggested that retinal pathologies, such as deposits in the macular region, retinal nerve fiber layer thinning10, optic disc cupping and retinal microvascular abnormalities22 may be related to AD and cognitive impairment23. In addition, VI is related to physical and psychological disorders24, which themselves are related to the risk of dementia via interaction effects9,25.
On the other hands, Hong et al. suggested that the presence of VI was not related with cognitive decline over 5–10 years26. Other study reported that open angle glaucoma was not related with increased risk of developing AD27. Comprehensively, the relationship between VI and dementia still remains controversial.
Many epidemiological studies in South Korea have been conducted with the nationwide cohorts facilitated by the Korean National Health Insurance Service (NHIS)24,28,29. This mandatory universal health insurance system provided the longitudinal database including diagnostic and treatment codes, individual disability information, and socioeconomic data for the entire population of approximately 50 million people. In this study, we used the qualified longitudinal database and constructed a nationwide disease-free cohort to investigate the risk of dementia in VI subjects.
Patients and methods
Study population and data collection
For this national cohort study, we used customized health information database from the National Health Insurance Cooperation that can be modified as requested for the purpose of policy and academic research (http://nhiss.nhis.or.kr). The cohort data included personal information, health insurance claim codes (procedures and prescriptions), diagnostic codes from the Korean Standard Classification of Diseases, 7th Revision (KCD-7), which is based on the International Classification of Diseases, 10th Revision (ICD-10)30 but with a few changes specific to Korea, death records from the Korean National Statistical Office, medical data and socioeconomic data (residence and income) for each subject over the period from 2002 to 2017.
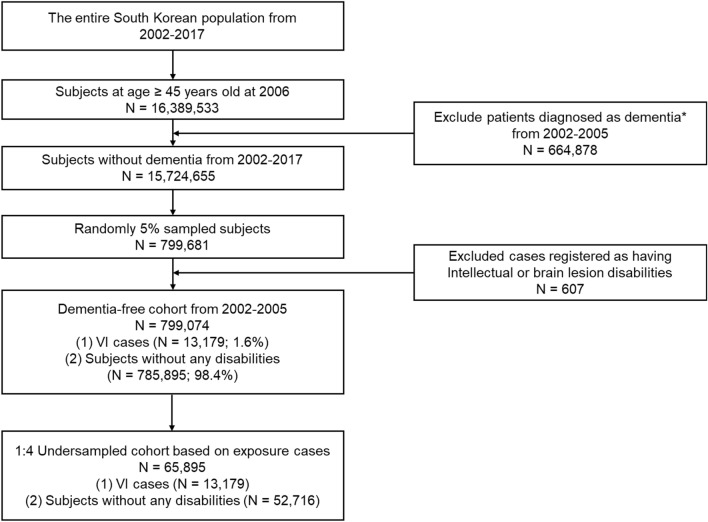
We identified a dementia-free cohort representative of the Korean population by washout of dementia cases among individuals aged 45 years and older diagnosed from 2002 to 2005. Diagnosis of dementia was based on both ICD10 diagnosis codes (F00, F01, F02, F03, G30, F05, or G31) and medication prescriptions [acetylcholinesterase inhibitors (donepezil, galantamine, or rivastigmine) or NMDA receptor antagonists (memantine)]. Approximately 5% of subjects were randomly sampled, and subjects with intellectual or brain lesion disabilities that could affect diagnosis of dementia were excluded. Among a total of 799,074 subjects remained in the dementia-free cohort, large differences were observed in the number of participants with VI and without any other legally recognized disabilities: 13,179 subjects had VI without any disabilities (corresponding to only 1.6%), while 785,895 subjects had never been diagnosed with VI or other disabilities (corresponding to 98.4%) from 2006 to 2017. To overcome this imbalance in sample sizes and avoid possible confounding with age and sex, we constructed a reduced dementia-free cohort by age-sex exact matching with a ratio of 1:4. Finally, a total of 65,895 subjects were included in the age- and sex- matched dementia-free cohort, and used in subsequent analyses. Figure 1 shows the processes used to obtain the dementia-free cohort.
Figure 1.
Flow chart of eligible subjects in the dementia-free cohort. VI = visual impairment. *Diagnosis of dementia was based on both International Classification of Diseases-10 diagnosis codes (F00, F01, F02, F03, G30, F05, or G31) and medication prescriptions [acetylcholinesterase inhibitors (donepezil, galantamine, rivastigmine) or NMDA receptor antagonists (memantine)].
This study was approved by the Samsung Medical Center Institutional Review Board (SMC IRB 2018-08-017). The requirement for written informed consent was waived for all subjects by the Samsung Medical Center Institutional Review Board because of the retrospective design and fully anonymised national data. The research was conducted according to the tenets of the Declaration of Helsinki.
Definitions of exposure and outcomes
Individuals registered as visually impaired persons by the Ministry of Health and Welfare were considered the exposure group. In Korea, legal VI is defined as the presence of any of the following four conditions that show stabilization after at least 6 months of treatment and are not reversed by medication or surgery, with the exception of keratoplasty: 1) best-corrected visual acuity (BCVA) < 20/1000 in the worse eye, 2) BCVA < 20/100 in the better eye, 3) visual field (VF) < 10˚ from the visual axis for both eye, and 4) a binocular VF < 1/224. For the registration of VI status in Korea, an individual must submit a medical certificate issued by an ophthalmologist regarding BCVA, VF and possible reason for VI, and then an assessment committee discusses the feasibility of VI registration24. The degree of VI is typically divided into six grades according to the severity of impairment; data are then divided into two grades (severe VI, grades I–II; mild VI, grades III–VI)24.
Dementia occurrence was defined by the first prescription of acetylcholinesterase inhibitors (donepezil, galantamine, or rivastigmine) or NMDA receptor antagonists (memantine) and diagnosis with an ICD-10 dementia code (F00, F01, F02, F03, G30, F05, or G31) as described above. We also considered subtypes of dementia: AD (F00, G30), vascular dementia (VaD) (F01) and frontotemporal dementia (FTD) (G31)31.
Other variables
Two age groups were used in the analyses: < 65 years old and ≥ 65 years old. According to body mass index (BMI), individuals were classified as normal (18.5–22.9), underweight (< 18.5), overweight (23–24.9), or obese (≥ 25). We defined household income level based on the ventiles of medical insurance fee as follows: low (1–5 ventiles), middle-low (6–10 ventiles), middle-high (11–15 ventiles), and high (16–20 ventiles). Comorbidities (i.e., depression, dyslipidemia, stroke, coronary heart disease (CHD), hypertension (HTN), and diabetes mellitus (DM)) diagnosed in 2006 using ICD-10 codes were recorded, as these may be associated with an increased risk of dementia.
Statistical analysis
Chi-square tests or Fisher’s exact tests were used to compare the general characteristics between the exposure and non-exposure groups. Subjects were described as censored if they did not develop dementia until the end of the study or if they were dropped-out from the cohort for some reasons, such as death and insurance disqualification. The cumulative probability of dementia occurrence was estimated by Kaplan–Meier method. Hazard ratios (HRs) for the development of dementia in individuals with versus without VI were assessed via Cox-proportional hazard models in which VI was considered as a time-varying variable to reflect the change of VI status during the follow-up time. Univariable and multivariable analyses were conducted to estimate crude and age-sex adjusted HRs and 95% confidence intervals (CIs), respectively. Subgroup analyses were performed for each age and sex group. The interaction effects between age, sex and VI on dementia were examined. Sensitivity analysis was done to investigate the VI effect with the further adjustment of BMI, household income level, and systemic comorbidities, i.e., depression, dyslipidemia, stroke, CHD, HTN and DM. P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS (version 9.3; SAS Institute, Inc., Cary, NC, U.S.A.) and R Statistical Software (version 3.5; Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Among a total of 65,895 subjects in the dementia-free cohort, 13,179 were registered as visually impaired subjects while the remaining 52,716 did not have any disabilities. Dementia occurred in 7607 subjects: 1817 cases (13.8%) among the 13,179 VI subjects and 5790 cases (11%) among the 52,716 subjects without any disabilities.
Of all dementia patients in VI, there were 882 (11.6%) early-onset patients who were diagnosed with dementia when they were younger than 65 years, and 6725 (88.4%) late-onset patients diagnosed when they were 65 years or older. More females (4606; 60.5%) than males (3001; 39.5%) developed dementia. The mean follow-up period and the mean time to dementia diagnosis were 10.28 ± 3.22 years (median: 12.01 years [min, 0.00; max, 12.01]) and 7.30 ± 3.04 years (median: 7.57 years [min, 0.00; max, 12.01]), respectively in the whole cohort. For the subjects with VI, the mean time to diagnosis of dementia was shortened to 5.93 ± 3.26 years (median: 5.96 years [min, 0.01; max, 11.99]).
Table 1 shows the characteristics of the dementia-converter group and the dementia-nonconverter group. There were significant differences in age, female predominance, depression, dyslipidemia, stroke, CHD, HTN, and DM between the dementia and non-dementia groups (p < 0.0001).
Table 1.
Characteristics of study population by presence of dementia (n = 65,895).
| Variables, n (%) | Dementia group (n = 7607) | Control group (n = 58,288) | p value |
|---|---|---|---|
| VI | < 0.0001 | ||
| Noa | 5790 (76.1%) | 46,926 (80.5%) | |
| Yes | 1817 (23.9%) | 11,362 (19.5%) | |
| Age | < 0.0001 | ||
| < 65 years | 882 (11.6%) | 32,973 (56.6%) | |
| ≥ 65 years | 6725 (88.4%) | 25,315 (43.4%) | |
| Sex | < 0.0001 | ||
| Male | 3001 (39.5%) | 33,489 (57.5%) | |
| Female | 4606 (60.5%) | 24,799 (42.5%) | |
| VI severity | |||
| Mild VI | 1356 (17.8%) | 9269 (15.9%) | < 0.0001 |
| Severe VI | 461 (6.1%) | 2093 (3.6%) | < 0.0001 |
| VI with other impairment | |||
| Hearing impairment | 32 (0.4%) | 186 (0.3%) | < 0.0001 |
| Kidney impairment | 23 (0.3%) | 193 (0.3%) | 1.0000 |
| Comorbidity | |||
| Depression | 1060 (13.9%) | 4736 (8.1%) | < 0.0001 |
| Dyslipidemia | 1924 (25.3%) | 12,112 (20.8%) | < 0.0001 |
| Stroke | 896 (11.8%) | 3087 (5.3%) | < 0.0001 |
| Coronary heart disease | 1525 (20%) | 8078 (13.9%) | < 0.0001 |
| Hypertension | 4314 (56.7%) | 22,404 (38.4%) | < 0.0001 |
| Diabetes mellitus | 2805 (36.9%) | 15,055 (25.8%) | < 0.0001 |
| BMIb,c | < 0.0001 | ||
| Underweight | 311 (4.1%) | 1442 (2.5%) | |
| Normal | 2112 (27.8%) | 16,558 (28.4%) | |
| Overweight | 1323 (17.4%) | 12,862 (22.1%) | |
| Obese | 1583 (20.8%) | 16,991 (29.2%) | |
| Household income levelb,d | < 0.0001 | ||
| Low | 1296 (17%) | 10,678 (18.3%) | |
| Middle-low | 1093 (14.4%) | 10,436 (17.9%) | |
| Middle-high | 1618 (21.3%) | 14,186 (24.3%) | |
| High | 2509 (33%) | 19,079 (32.7%) | |
VI = visual impairment; BMI = body mass index.
aSubjects who had never been diagnosed with V1 or other disabilities.
bThere exist missing values in data: 12,713 (19.3%) and 5000 (7.6%) of the entire population for BMI and household income level, respectively.
cBMI was classified as normal (18.5–22.9), underweight (< 18.5), overweight (23–24.9), and obese (≥ 25).
dAccording to medical insurance fee, household income level was classified as low (1–5 ventiles), middle-low (6–10 ventiles), middle-high (11–15 ventiles), and high (16–20 ventiles).
VI as a risk factor for dementia
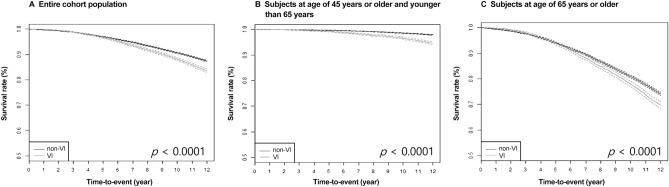
Kaplan–Meier survival analysis revealed significantly higher cumulative incidence of dementia in the VI group than the non-VI group (log-rank test, p < 0.0001) (Fig. 2). This difference in cumulative incidence of dementia was also observed in both age groups (log-rank test, all p < 0.0001).
Figure 2.
Kaplan–Meier curves for cumulative probability of dementia occurrence according to VI status in (A) Entire cohort population, (B) Younger age population (< 65 years old), and (C) Older age population (≥ 65 years old). Those with VI depicted on the lower line had a higher rate of incident dementia across all age groups by log-rank tests (all p < 0.0001).
In univariable analyses, VI increased the risk of dementia (HR 1.563, 95% CI 1.485–1.646, p < 0.0001). Additionally, age over 65 years, female sex, having depression, dyslipidemia, stroke, CHD, HTN and/or DM, being underweight, and being in the highest household income level increased the risk of dementia (Table e-1).
In the multivariable Cox regression analysis with a time-varying effect of VI, we found significantly increased risk of dementia in participants with VI (HR 1.559, 95% CI 1.479–1.644) after adjusting age and sex (Model 1 in Table 2). Individuals 65 years or older and female subjects had a higher risk of developing dementia (HR 10.587, 95% CI 9.867–11.360, and HR 1.522, 95% CI 1.453–1.594, respectively). When the interaction effects of age and sex with VI on dementia were further considered, VI was still a significant risk factor for dementia (HR 2.726, 95% CI 2.251–3.300) (Model 2 in Table 2). While other interactions were not significant, a significant interaction between VI and age (p < 0.0001) implied that the effect of VI according to categorized age was significantly different. In subgroup analyses, young males showed the highest risk for development of dementia with a HR of 2.687 (95% CI 2.219–3.254, p < 0.0001) compared to old females with a HR of 1.410 (95% CI 1.310–1.517, p < 0.0001). We observed that younger subjects with VI at baseline (HR 2.664, 95% CI 2.321–3.058) were more vulnerable to dementia than older subjects with VI (HR 1.432, 95% CI 1.353–1.517) (Fig. 3). The effect of VI did not show difference (p for interaction = 0.9219) between males (HR 1.638, 95% CI 1.508–1.780) and females (HR 1.509, 95% CI 1.409–1.616).
Table 2.
Impact of VI on the risk of dementia via multivariable time-varying Cox regression analyses.
| Variables | Model 1a | Model 2b | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| VI (yes) | 1.559 (1.479–1.644) | < 0.0001 | 2.726 (2.251–3.300) | < 0.0001 |
| Age group (≥ 65 years) | 10.587 (9.867–11.360) | < 0.0001 | 12.069 (10.674–13.646) | < 0.0001 |
| Sex (female) | 1.522 (1.453–1.594) | < 0.0001 | 1.406 (1.193–1.657) | < 0.0001 |
| VI* age group (yes, ≥ 65 years) | 0.539 (0.436–0.667) | < 0.0001 | ||
| VI* sex (yes, female) | 0.986 (0.749–1.300) | 0.9219 | ||
| Age group* sex (≥ 65 years, female) | 1.106 (0.929–1.316) | 0.2578 | ||
| VI* age group* sex (yes, ≥ 65 years, female) | 0.972 (0.720–1.312) | 0.8540 | ||
VI = visual impairment; CI = confidence interval; HR = hazard ratio.
aAdjusted for age and sex.
bAdjusted for age, sex and all interactions between age, sex and VI.
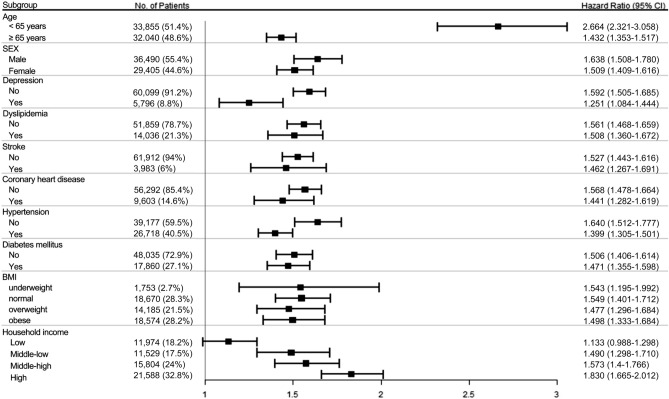
Figure 3.
Forest plot for subgroup analyses based on age, sex, systemic comorbidities, BMI and household income level to examine the effect of VI on dementia. Forest plots were generated using the R forest plot package (https://cran.r-project.org/web/packages/forestplot/forestplot.pdf)57.
Sensitivity analysis of the effect of VI on dementia
Subgroup analyses were performed to determine whether VI increased the risk for development of dementia according to household income level, BMI, and the presence or absence of various comorbidities, including depression, dyslipidemia, stroke, CHD, HTN and DM. In all subgroups, VI significantly increased the risk of dementia with HRs ranged from 1.133 to 1.830 (Fig. 3). The effect of VI on dementia development differed among some subgroups: subjects with depression (HR 1.592, 95% CI 1.505–1.685) vs without depression (HR 1.251, 95% CI 1.084–1.444); and subjects with HTN (HR 1.640, 95% CI 1.512–1.777) vs without HTN (HR 1.399, 95% CI 1.305–1.501).
Furthermore, we conducted a multivariable analysis to adjust age, sex, household income level, BMI and comorbid confounders, such as such as depression, dyslipidemia, stroke, CHD, HTN and DM. Even after the adjustment, VI was still a significant risk factor for dementia (HR 2.290, 95% CI 1.946–2.694) (Table 3). These sensitivity analyses suggested that our findings were robust.
Table 3.
Sensitivity analyses by additional adjustment with comorbidities, BMI and household income level according to sub-types of dementia.
| Variables | Any dementia (n = 65,985) | AD (n = 66,640) | VaD (n = 61,890) | FTD (n = 60,340) |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| VI (yes) | 2.290 (1.946–2.694) | 2.261 (1.869–2.735) | 3.810 (2.859–5.076) | 2.272 (1.352–3.817) |
| Age group (≥ 65 years) | 9.926 (8.995–10.953) | 11.616 (10.368–13.013) | 11.000 (8.959–13.505) | 12.756 (9.321–17.458) |
| Sex (female) | 1.430 (1.352–1.513) | 1.503 (1.412–1.600) | 1.297 (1.161–1.449) | 1.594 (1.343–1.891) |
| Comorbidity (yes) | ||||
| Depression | 1.405 (1.299–1.521) | 1.422 (1.306–1.548) | 1.409 (1.208–1.643) | 1.226 (0.952–1.578) |
| Dyslipidemia | 0.886 (0.827–0.949) | 0.919 (0.853–0.991) | 0.856 (0.749–0.978) | 0.760 (0.615–0.938) |
| Stroke | 1.517 (1.388–1.658) | 1.604 (1.457–1.765) | 2.112 (1.806–2.470) | 1.519 (1.152–2.002) |
| Coronary heart disease | 1.099 (1.021–1.182) | 1.067 (0.985–1.155) | 1.081 (0.940–1.244) | 1.125 (0.903–1.402) |
| Hypertension | 1.374 (1.290–1.464) | 1.315 (1.226–1.409) | 1.576 (1.387–1.791) | 1.313 (1.085–1.587) |
| Diabetes mellitus | 1.273 (1.198–1.353) | 1.236 (1.156–1.322) | 1.451 (1.288–1.635) | 1.369 (1.140–1.645) |
| BMIa,b | ||||
| Underweight | 1.489 (1.316–1.686) | 1.566 (1.367–1.794) | 1.231 (0.936–1.619) | 1.443 (0.972–2.142) |
| Normal | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) |
| Overweight | 0.797 (0.742–0.856) | 0.787 (0.727–0.851) | 0.782 (0.680–0.899) | 0.844 (0.682–1.044) |
| Obese | 0.676 (0.631–0.724) | 0.703 (0.652–0.757) | 0.639 (0.558–0.731) | 0.662 (0.538–0.814) |
| Household income levela,c | ||||
| Low | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) |
| Middle-low | 0.883 (0.806–0.967) | 1.028 (0.928–1.139) | 1.027 (0.854–1.235) | 0.829 (0.626–1.098) |
| Middle-high | 0.855 (0.788–0.929) | 0.977 (0.889–1.074) | 1.018 (0.861–1.205) | 0.822 (0.638–1.057) |
| High | 0.876 (0.811–0.946) | 1.054 (0.966–1.149) | 0.995 (0.851–1.165) | 0.931 (0.741–1.170) |
VI = visual impairment; HR = hazard ratio; CI = confidence interval; AD = Alzheimer’s disease; VaD = vascular dementia; FTD = frontotemporal dementia; BMI = body mass index.
aThere exist missing values in data: 12,713 (19.3%) and 5000 (7.6%) of the entire population for BMI and household income level, respectively.
bBMI was classified as normal (18.5–22.9), underweight (< 18.5), overweight (23–24.9), and obese (≥ 25).
cAccording to medical insurance fee, household income level was classified as low (1–5 ventiles), middle-low (6–10 ventiles), middle-high (11–15 ventiles), and high (16–20 ventiles).
VI as a risk factor for dementia according to type of dementia
After adjustment for age and sex, VI increased the risk for all types of dementia, namely AD (HR 2.813, 95% CI 2.371–3.339), VaD (HR 4.052, 95% CI 3.109–5.281), and FTD (HR 2.689, 95% CI 1.684–4.294). With further adjustment for household income level, BMI and comorbid confounders, the effect of VI on dementia was still significant for all types of dementia, and greater in VaD (HR 3.810, 95% CI 2.859–5.076) than AD (HR 2.261, 95% CI 1.869–2.735) and FTD (HR 2.272, 95% CI 1.352–3.817) (Table 3).
Risk of dementia according to the severity of VI and other impairments accompanied
According to the severity of VI, subjects were classified as no VI, mild VI and severe VI. After adjusting for age and sex, we found that severe VI (HR 5.558, 95% CI 4.374–7.062) increased the risk of dementia more than mild VI (HR 2.539, 95% CI 2.167–2.974). We divided the exposure group into three subgroups: VI only, VI with hearing impairment, and VI with kidney impairment. The risk of dementia was greatest in the subjects with VI and kidney impairment (HR 7.501, 95% CI 3.832–14.682), compared to those with VI and hearing impairment group (HR 3.652, CI 1.346–9.907) and those with VI only (HR 2.682, CI 2.307–3.118) (Table e-2).
Discussion
This nationwide cohort study showed that VI increased the risk of dementia, especially in young males, while dementia was most prevalent among older females. This was consistently observed even after adjustment for age, sex, household income level, BMI and other comorbidities. In subgroup analyses, younger males with VI had the highest risk of dementia, and VaD was the type of dementia most closely associated with VI. Subjects with VI and kidney impairments had a significantly greater risk of dementia than those with VI only or those with VI and hearing impairment. Subjects with severe VI were more at risk of developing dementia than those with mild VI.
Several studies have investigated the association between VI and cognitive function and the impact of VI is still controversial. In the Singapore Malay Eye Study, older people with VI, particularly those with VI due to cataracts, were more likely to have cognitive dysfunction3. In a nationally representative sample of the US population, VI based on self-reports was significantly associated with worse cognitive function after adjusting for demographic factors, health, and other possible confounders2. In the Fujiwara-kyo eye study, subjects with mild VI had a 2.4-fold higher odds ratio of having cognitive impairment than those without VI after adjusting for age, sex, and length of education32. However, in another longitudinal study, no relationship between sensory impairments and decline in cognition has been reported, and only age was found to be significantly associated with possible cognitive decline26.
Regarding the effect of VI on the development of dementia, few longitudinal studies were reported6,9. Poor vision was reported to be related to the dementia development in the USA; individuals with good vision at baseline had a 63% cut the risk of dementia down over a mean follow-up period of 8.5 years. Moreover, participants with poor vision who did not visit an ophthalmologic clinic had 9.5 times increased the risk of AD6. A recent cohort study investigated the relationship between vision loss and 12-year risk of dementia in individuals from three cities in France9. This population-based cohort study demonstrated that moderate to severe near VI could be a marker of dementia risk in following years, particularly in participants with depression. Furthermore, self-reported loss of distance visual function was related with an increased risk of dementia. These results are similar with our findings; however, self-reported vision loss possibly differs according to cognitive function capabilities, and no information about BCVA was provided in the previous study. In our study, VI was diagnosed by a certified ophthalmologist, and VI registration was assessed by the government, which would have increased the accuracy and reliability of our findings.
Pathological changes in the visual system, which is composed of the anterior and posterior segments of the eye, optic nerve, and visual cortex, are correlated with dementia. Goldstein et al. reported that Aβ fibrils, which accumulate in the brain of AD patients, are also present in the cytosol of lens fiber cells in individuals with AD, which could promote supranuclear cataract formation16. There are several reports of intra-retinal thinning in AD patients based on optical computed tomography (OCT) analysis12,33,34. Retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL) thickness are reduced in AD patients compared with healthy controls. A reduction in the number of ganglion cells and in the thickness or the RNFL have also been reported based on histological analysis35. In a recent in vivo study, profound tau pathology in the visual system leading to early retinal neuron damage was observed in a mouse model of AD36. Studies have also shown degeneration of optic nerve axons in patients with AD35,37. As described earlier, senile plaques and neurofibrillary tangles present in the brain lesions of AD patients have also been found in the visual cortex of these patients38. The density of these plaques and tangles is greater in area V2, which is associated with visual function, than in V1, particularly in early-onset cases21,39. It is possible that VI could be a result of dementia pathology and thus a consequence of dementia rather than a risk factor. There are several reports that VI is associated with AD7,40,41. Uhlmann et al. found that the prevalence of VI was higher in the late-onset AD patients than in their age-matched controls, and that the degree of VI was correlated with the severity of cognitive dysfunction7. A range of complex visual disturbances were common in AD, which might reflect visuospatial dysfunction related to bi-parietal atrophy, a vulnerable area in AD42. Our study showed significantly higher incidence of VI in dementia group, and subjects with severe VI had a 2.19-fold more developing dementia than those with mild VI. This point is expected to be further developed as a remarkable research topic in the future.
Besides, elderly people with VI tend to engage less in cognitively stimulating activities or social activities, and a lack of sufficient sensory input could also lead to neurovascular and neurophysiological changes and thus result in cognitive impairment and development of dementia8,21,35. The effect of other sensory impairments on dementia in the elderly have been widely studied5,43. Hearing impairment in particular is a proven risk factor for the development of dementia1,26,43–46. In early stage hearing impairment, hearing loss increases cognitive load by auditory cortical re-organization in the form of decreased temporal and increased frontal activation47. Furthermore, in the elderly, proper listening conditions increase cognitive function and allow the formation of close relationships48. These resources may help compensate for the deleterious effects of AD pathology on other cognitive systems in older persons49. Hearing loss can also lead to social isolation, which can contribute to dementia50,51. VI likely increases the risk of dementia by similar pathomechanisms as detailed above for hearing impairment. In our cohort study, subjects with VI had a significantly increased risk of dementia after adjustment for confounding factors, and VI with hearing impairment had an additive effect on the risk of developing dementia; subjects with VI only had an HR of 2.682 (95% CI 2.307–3.118), while those with VI and hearing impairment had a HR of 3.652 (95% CI 1.346–9.907).
In our study, young males with VI were found to be most vulnerable to developing dementia. Young males had almost twice higher risk of developing dementia than older females. Older individuals and females showed higher risks of dementia development than the general population; however, among VI subjects, a younger age and male sex were factors associated with increased risk for the development of dementia. These results suggest that VI may accelerate the development of dementia among young males. It would contribute to develop or apply different health policies for people with VI to prevent dementia in each age-sex subgroup.
Furthermore, VI with kidney impairment had a stronger additive effect on the risk of developing dementia with a HR of 7.501 (95% CI 3.832–14.682) than VI with hearing impairment or VI without any other impairment. The effect of hearing loss or VI itself on dementia is related with a decline of sensory input, however, that of kidney impairment on dementia is thought to be another mechanism. The fact that kidney changes contribute to the development and rapid progression of dementia supports the strong additive effect of VI with kidney impairment52–54. It is also explained that changes in kidney function with increased toxic metabolites lead endothelial dysfunction and loss of vascular integrity55,56. Further study to analyze the influence of ischemia on the degree of dementia and VI to explain why VI is more associated with kidney changes than with reduced hearing would be important.
The present study had several limitations. First, there is a gap in time between the occurrence of a disability and registration as a legally disabled person. It may also take some time to receive a diagnosis of VI from the time visual acuity starts to decrease. Furthermore, to receive disability support services in South Korea, there is a waiting period of 6–12 months for registration depending on disability type. This means that a relatively long period of time could elapse from the time that VI develops to being classified as being visually disabled, except in acute events such as trauma. Because VI likely affected cognitive function before the disability was registered, we included all cases where dementia was diagnosed without a latent period after the disability was legally registered by the government into the case cohort group. Second, VI was treated with only for distant vision loss, so we were not able to determine the effect of near vision deterioration on dementia. Lastly, potential dementia cases with subjective memory impairment or amnestic mild cognitive impairment would have been excluded if neurologists did not diagnose dementia or prescribe medications; consequently, we likely underestimated the occurrence of dementia in our cohort.
Conclusion
In conclusion, individuals with VI exhibited a significantly higher risk for the development of all types of dementia based on the 12-year follow-up period. Notably, the risk was elevated almost twice higher in young males than in old females. VI increased the risk for developing VaD than that for AD. Individuals with severe VI had a higher risk of developing dementia than those with mild VI, and VI with hearing or kidney impairment had an additive effect on the risk of developing dementia. Ophthalmologists and neurologists should be aware that young males with VI are at increased risk for the development of dementia.
Supplementary Information
Author contributions
G.-I.L. wrote original draft and reviewed and edited. S.A.C. analyzed data and wrote original draft. K.K. analyzed the data and performed validation. S.W.S. contributed to the study concept and design and performed validation. H.J.K. reviewed and edited the manuscript. T.-Y.C. contributed to the study concept and supervised the study. D.H.L. contributed to the study concept, funding acquisition, validation, and reviewed and edited the manuscript. All authors read and approved the final manuscript. G.-I.L. and S.A.C. contributed equally to the article as the first authors. D.H.L. and T.-Y.C. are co-corresponding authors of this study.
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health &Welfare, Republic of Korea (Grant Numbers: HI19C0577 & HC19C0142), which was received by D.H.L.
Data availability
All datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ga-In Lee and Sang Ah Chi.
These authors jointly supervised this work: Tae-Young Chung and Dong Hui Lim.
Contributor Information
Tae-Young Chung, Email: tychung@skku.edu.
Dong Hui Lim, Email: ldhlse@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91026-4.
References
- 1.Lin MY, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J. Am. Geriatr. Soc. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen SP, Bhattacharya J, Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. 2017;135:963–970. doi: 10.1001/jamaophthalmol.2017.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong SY, et al. Visual impairment, age-related eye diseases, and cognitive function: The Singapore Malay Eye study. Arch. Ophthalmol. (Chicago, Ill. : 1960) 2012;130:895–900. doi: 10.1001/archophthalmol.2012.152. [DOI] [PubMed] [Google Scholar]
- 4.Clemons TE, Rankin MW, McBee WL. Cognitive impairment in the Age-Related Eye Disease Study: AREDS report no. 16. Arch. Ophthalmol. (Chicago, Ill. : 1960) 2006;124:537–543. doi: 10.1001/archopht.124.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychol. Aging. 1994;9:339–355. doi: 10.1037/0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 6.Rogers MA, Langa KM. Untreated poor vision: A contributing factor to late-life dementia. Am. J. Epidemiol. 2010;171:728–735. doi: 10.1093/aje/kwp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlmann RF, Larson EB, Koepsell TD, Rees TS, Duckert LG. Visual impairment and cognitive dysfunction in Alzheimer's disease. J. Gen. Intern. Med. 1991;6:126–132. doi: 10.1007/BF02598307. [DOI] [PubMed] [Google Scholar]
- 8.Luo Y, et al. Association between sensory impairment and dementia in older adults: Evidence from China. J. Am. Geriatr. Soc. 2018;66:480–486. doi: 10.1111/jgs.15202. [DOI] [PubMed] [Google Scholar]
- 9.Nael V, et al. Vision loss and 12-year risk of dementia in older adults: The 3C cohort study. Eur. J. Epidemiol. 2019;34:141–152. doi: 10.1007/s10654-018-00478-y. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Ramos T, Benito-Leon J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson's disease, dementia with Lewy bodies, and Alzheimer's disease. JAD. 2013;34:659–664. doi: 10.3233/jad-121975. [DOI] [PubMed] [Google Scholar]
- 11.Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Clin. Neurol. Neurosurg. 2011;113:523–526. doi: 10.1016/j.clineuro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Marziani E, et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer's disease using spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2013;54:5953–5958. doi: 10.1167/iovs.13-12046. [DOI] [PubMed] [Google Scholar]
- 13.Iseri PK, Altinas O, Tokay T, Yuksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J. Neuro Ophthalmol. 2006;26:18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- 14.Moschos MM, et al. Structural and functional impairment of the retina and optic nerve in Alzheimer's disease. Curr. Alzheimer Res. 2012;9:782–788. doi: 10.2174/156720512802455340. [DOI] [PubMed] [Google Scholar]
- 15.Paquet C, et al. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Neurosci. Lett. 2007;420:97–99. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LE, et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer's disease. Lancet (London, England) 2003;361:1258–1265. doi: 10.1016/s0140-6736(03)12981-9. [DOI] [PubMed] [Google Scholar]
- 17.Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer's disease. Eur. Neurol. 2002;47:165–168. doi: 10.1159/000047976. [DOI] [PubMed] [Google Scholar]
- 18.Wostyn P, Audenaert K, De Deyn PP. Alzheimer's disease and glaucoma: Is there a causal relationship? Br. J. Ophthalmol. 2009;93:1557–1559. doi: 10.1136/bjo.2008.148064. [DOI] [PubMed] [Google Scholar]
- 19.Jefferis JM, Taylor JP, Clarke MP. Does cognitive impairment influence outcomes from cataract surgery? Results from a 1-year follow-up cohort study. Br. J. Ophthalmol. 2015;99:412–417. doi: 10.1136/bjophthalmol-2014-305657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuoka H, Sutu C, Afshari NA. The impact of cataract surgery on cognitive function in an aging population. Curr. Opin. Ophthalmol. 2016;27:3–8. doi: 10.1097/icu.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong R, Kergoat H. Oculo-visual changes and clinical considerations affecting older patients with dementia. Ophthalmic Physiol. Opt. 2015;35:352–376. doi: 10.1111/opo.12220. [DOI] [PubMed] [Google Scholar]
- 22.de Jong FJ, et al. Retinal vascular caliber and risk of dementia: The Rotterdam study. Neurology. 2011;76:816–821. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikram MK, Cheung CY, Wong TY, Chen CP. Retinal pathology as biomarker for cognitive impairment and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2012;83:917–922. doi: 10.1136/jnnp-2011-301628. [DOI] [PubMed] [Google Scholar]
- 24.Choi HG, Lee MJ, Lee SM. Visual impairment and risk of depression: A longitudinal follow-up study using a national sample cohort. Sci. Rep. 2018;8:2083. doi: 10.1038/s41598-018-20374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall CB, et al. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong T, Mitchell P, Burlutsky G, Liew G, Wang JJ. Visual impairment, hearing loss and cognitive function in an older population: Longitudinal findings from the Blue Mountains eye study. PLoS ONE. 2016;11:e0147646. doi: 10.1371/journal.pone.0147646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessing LV, Lopez AG, Andersen PK, Kessing SV. No increased risk of developing Alzheimer disease in patients with glaucoma. J. Glaucoma. 2007;16:47–51. doi: 10.1097/IJG.0b013e31802b3527. [DOI] [PubMed] [Google Scholar]
- 28.Jung YW, Pak H, Lee I, Kim EH. The effect of diagnosis-related group payment system on quality of care in the field of obstetrics and gynecology among Korean Tertiary Hospitals. Yonsei Med. J. 2018;59:539–545. doi: 10.3349/ymj.2018.59.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rim TH, Han J, Choi YS, Lee T, Kim SS. Stroke risk among adult patients with third, fourth or sixth cranial nerve palsy: A Nationwide Cohort Study. Acta Ophthalmol. 2017;95:e656–e661. doi: 10.1111/aos.13488. [DOI] [PubMed] [Google Scholar]
- 30.Lee GI, et al. Risk factors for intraocular lens dislocation after phacoemulsification: A nationwide population-based cohort study. Am. J. Ophthalmol. 2020;214:86–96. doi: 10.1016/j.ajo.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Lee JE, et al. Association between timed up and go test and future dementia onset. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018;73:1238–1243. doi: 10.1093/gerona/glx261. [DOI] [PubMed] [Google Scholar]
- 32.Mine M, et al. Association of visual acuity and cognitive impairment in older individuals: Fujiwara-kyo Eye Study. BioResearch Open Access. 2016;5:228–234. doi: 10.1089/biores.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y, et al. Retinal nerve fiber layer structure abnormalities in early Alzheimer's disease: Evidence in optical coherence tomography. Neurosci. Lett. 2010;480:69–72. doi: 10.1016/j.neulet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 34.He XF, et al. Optical coherence tomography assessed retinal nerve fiber layer thickness in patients with Alzheimer's disease: A meta-analysis. Int. J. Ophthalmol. 2012;5:401–405. doi: 10.3980/j.issn.2222-3959.2012.03.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer's disease. N. Engl. J. Med. 1986;315:485–487. doi: 10.1056/nejm198608213150804. [DOI] [PubMed] [Google Scholar]
- 36.Chiasseu M, et al. Tau accumulation in the retina promotes early neuronal dysfunction and precedes brain pathology in a mouse model of Alzheimer's disease. Mol. Neurodegener. 2017;12:58. doi: 10.1186/s13024-017-0199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer's disease. Ophthalmology. 1990;97:9–17. doi: 10.1016/S0161-6420(90)32621-0. [DOI] [PubMed] [Google Scholar]
- 38.Hof PR, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease: II. Primary and secondary visual cortex. J. Comp. Neurol. 1990;301:55–64. doi: 10.1002/cne.903010106. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong RA. Neuropathological differences between areas B17 and B18: Implications for visual evoked responses in Alzheimer's disease. Dementia. 1994;5:247–251. doi: 10.1159/000106732. [DOI] [PubMed] [Google Scholar]
- 40.Ceccaldi M. Vision in Alzheimer's disease. Rev. Neurol. 1996;152:441–446. [PubMed] [Google Scholar]
- 41.Mendez MF, Tomsak RL, Remler B. Disorders of the visual system in Alzheimer's disease. J. Clin. Neuroophthalmol. 1990;10:62–69. [PubMed] [Google Scholar]
- 42.Mendez MF, Mendez MA, Martin R, Smyth KA, Whitehouse PJ. Complex visual disturbances in Alzheimer's disease. Neurology. 1990;40:439–443. doi: 10.1212/WNL.40.3_Part_1.439. [DOI] [PubMed] [Google Scholar]
- 43.Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261:1916–1919. doi: 10.1001/jama.1989.03420130084028. [DOI] [PubMed] [Google Scholar]
- 44.Gurgel RK, et al. Relationship of hearing loss and dementia: A prospective, population-based study. Otol. Neurotol. 2014;35:775–781. doi: 10.1097/mao.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomson RS, Auduong P, Miller AT, Gurgel RK. Hearing loss as a risk factor for dementia: A systematic review. Laryngosc. Investig. Otolaryngol. 2017;2:69–79. doi: 10.1002/lio2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ha J, et al. Hearing loss is associated with cortical thinning in cognitively normal older adults. Eur J Neurol. 2020;27:1003–1009. doi: 10.1111/ene.14195. [DOI] [PubMed] [Google Scholar]
- 47.Campbell J, Sharma A. Compensatory changes in cortical resource allocation in adults with hearing loss. Front. Syst. Neurosci. 2013;7:71. doi: 10.3389/fnsys.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erb J, Obleser J. Upregulation of cognitive control networks in older adults' speech comprehension. Front. Syst. Neurosci. 2013;7:116. doi: 10.3389/fnsys.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyle PA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Processing resources reduce the effect of Alzheimer pathology on other cognitive systems. Neurology. 2008;70:1534–1542. doi: 10.1212/01.wnl.0000304345.14212.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shankar A, Hamer M, McMunn A, Steptoe A. Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosom. Med. 2013;75:161–170. doi: 10.1097/PSY.0b013e31827f09cd. [DOI] [PubMed] [Google Scholar]
- 51.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol. Head Neck Surg. 2014;150:378–384. doi: 10.1177/0194599813518021. [DOI] [PubMed] [Google Scholar]
- 52.Sheladia S, Reddy PH. Age-related chronic diseases and Alzheimer's disease in Texas: A hispanic focused study. J Alzheimers Dis. Rep. 2021;5:121–133. doi: 10.3233/adr-200277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang M, et al. Kidney function and dementia risk in community-dwelling older adults: The Shanghai Aging Study. Alzheimers Res. Ther. 2021;13:21. doi: 10.1186/s13195-020-00729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seliger SL, et al. Moderate renal impairment and risk of dementia among older adults: The Cardiovascular Health Cognition Study. J. Am. Soc. Nephrol. 2004;15:1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 55.Mogi M, Horiuchi M. Clinical interaction between brain and kidney in small vessel disease. Cardiol. Res. Pract. 2011;2011:306189. doi: 10.4061/2011/306189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelleher RJ, Soiza RL. Evidence of endothelial dysfunction in the development of Alzheimer's disease: Is Alzheimer's a vascular disorder? Am. J. Cardiovasc. Dis. 2013;3:197–226. [PMC free article] [PubMed] [Google Scholar]
- 57.Max Gordon and Thomas Lumley. forestplot: Advanced Forest Plot Using 'grid' Graphics. R package version 1.9. https://CRAN.R-project.org/package=forestplot (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.