Highlights
-
•
Chemically diverse dietary phytochemicals exhibit anticancer activity owing to their antioxidant potential.
-
•
Dietary phytochemicals act as blocking and suppressing agents for exposition of chemoprevention.
-
•
Potent apoptosis and autophagy modulation is chief contributor for exhibition of anticancer efficacy.
Keywords: Apoptosis, Autophagy, Cancer, Chemoprevention, Dietary phytochemical
Abstract
Despite the advancement in prognosis, diagnosis and treatment, cancer has emerged as the second leading cause of disease-associated death across the globe. With the remarkable application of synthetic drugs in cancer therapy and the onset of therapy-associated adverse effects, dietary phytochemicals have been materialized as potent anti-cancer drugs owing to their antioxidant, apoptosis and autophagy modulating activities. With dynamic regulation of apoptosis and autophagy in association with cell cycle regulation, inhibition in cellular proliferation, invasion and migration, dietary phytochemicals have emerged as potent anti-cancer pharmacophores. Dietary phytochemicals or their synthetic analogous as individual drug candidates or in combination with FDA approved chemotherapeutic drugs have exhibited potent anti-cancer efficacy. With the advancement in cancer therapeutics, dietary phytochemicals hold high prevalence for their use as precision and personalized medicine to replace conventional chemotherapeutic drugs. Hence, keeping these perspectives in mind, this review focuses on the diversity of dietary phytochemicals and their molecular mechanism of action in several cancer subtypes and tumor entities. Understanding the possible molecular key players involved, the use of dietary phytochemicals will thrive a new horizon in cancer therapy.
1. Introduction
Despite the advancement in prognosis, diagnosis and treatment, cancer has emerged as the second leading cause of disease-associated death across the globe [1,2]. According to the reports given by WHO, cancer-associated mortalities have been estimated to be 8.8 million that instituting about 16.66 % of the total death cases as of 2015 [1]. With a primary focus on radiotherapy, chemotherapy, surgery and synergistic drug treatment approaches, cancer treatment has emerged as a high cost regulating approach with several adverse treatment related adverse modalities [3,4]. Moreover, the conventional approaches also have adverse consequences of disease recurrence. With the dire need of abiding by the negative consequences, phytochemicals have emerged as potent non-toxic, chemotherapeutic agents in cancer therapeutics [3,4].
Diet plays an important role in cancer prevention as more than 33 % of the cancer-associated mortalities can be avoided by a change in lifestyle and dietary habits with proper nutritional supplements [[5], [6], [7], [8]]. Dietary phytochemicals, the non-nutritive disease-preventing bioactive compounds constituting polyphenols, carotenoids, glucosinolates, organosulphides, nitrogen-containing compounds and terpenoids are abundantly found in daily dietary supplements have immense potential as chemotherapeutic agents [5,9,10]. These bioactive dietary phytochemicals exert non-toxic and target specific chemotherapeutic action with effective bioactivity and enhanced bioavailability as individual drug candidates or in synergism with conventional chemotherapeutic drugs. Mechanistically, these dietary phytochemicals scavenge reactive oxygen species (ROS) produced in the cellular compartments due to defective cellular metabolism that in turn inhibit cancer progression [5,6,10,11]. With the regulation of several cell death pathways such as apoptosis and autophagy in colossal association with cell cycle regulation, inhibition in cellular proliferation, invasion and migration, dietary phytochemicals exhibit enhanced anticancer activity [[12], [13], [14], [15], [16], [17]].
With more focus on identification and evaluation of anti-cancer efficacy of these dietary phytochemicals, their mode of action, molecular pathways associated with such activity, bioavailability and bioactivity, several studies have been made on the anti-cancer propensity of dietary phytochemicals in numerous cell lines and tumor entities [7,[18], [19], [20]]. Hence with the explosion in cancer incidences globally and the escalating use of dietary phytochemicals, this review has focused on the chemical diversity of dietary phytochemicals and their molecular mode of action in several cancer subtypes.
2. Chemical diversity and classification of dietary phytochemicals
Dietary phytochemicals are non-nutritive disease preventive bioactive plant chemicals that can be used directly as food, food additives or as food adjuvants [[5], [6], [7], [8]]. It has been investigated that over 5000 distinct dietary phytochemicals have been recognized, isolated and screened for their biological activity. The variable biological activity of these dietary phytochemicals against cancer are due to the diverse chemical classes, huge molecular confirmations and structural complexities [[21], [22], [23], [24], [25]]. Moreover, the diverse chemical classifications are also responsible for the enhanced antioxidative and pro-oxidative activity displayed by these dietary phytochemicals. The various class of dietary phytochemicals can be grouped into major divisions of polyphenolics, carotenoids, glucosinolates, organosulfur compounds, nitrogen-containing compounds and terpenoids. An account of various types of dietary phytochemicals has been described below (Fig. 1, Fig. 2, Table 1).
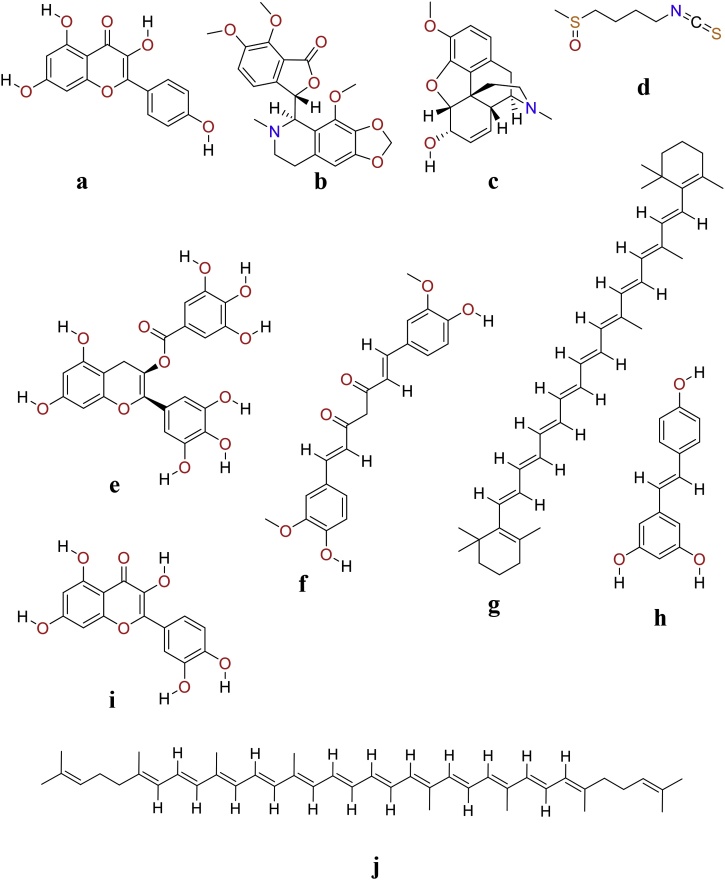
Fig. 1.
Chemical structure of chemo-preventive dietary phytochemicals.
Kaempferol (a), Noscapine (b), Codeine (c), Sulforaphane (d), EGCG (e), Curcumin (f), β-carotene (g), Resveratrol (h), Quercetin (i) and Lycopene (j).
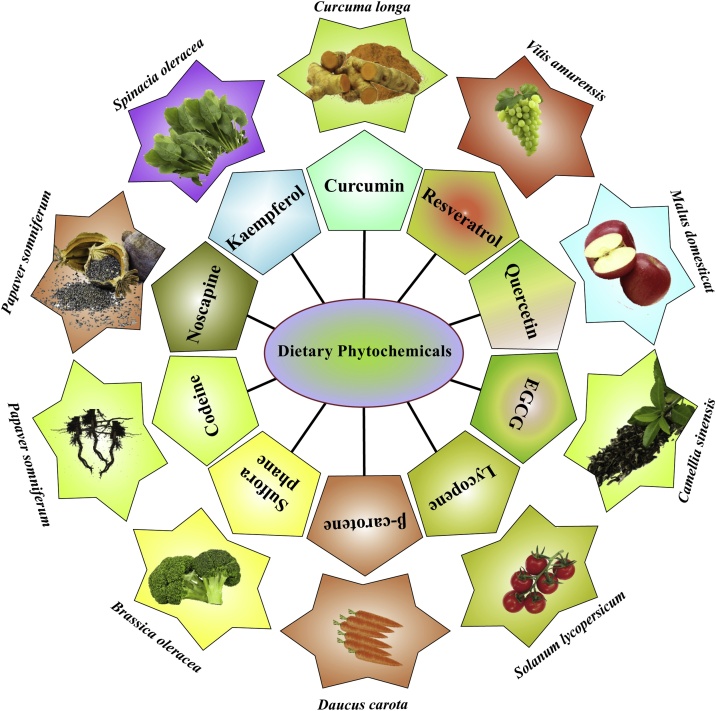
Fig. 2.
Dietary sources of chemo-preventive dietary phytochemicals.
Dietary polyphenols, carotenoids, terpenoids, glucosinolates, organosulphides and nitrogen containing compounds exhibit potent chemo-preventive activity against several cancers. Dietary polyphenols such as curcumin, resveratrol, quercetin, kaempferol, EGCG and carotenoids like β-carotene and lycopene exhibit potent chemo-prevention. In addition to this, glucosinolates and organosulphides such as sulforaphane also demonstrate potent anti-cancer efficacy. Nitrogen containing compounds like codeine and noscapine also display potent anti-cancer efficacy in several cancer cells.
Table 1.
Overview of chemical diversity of dietary phytochemicals.
| Phytochemicals |
Types and Example | |
|---|---|---|
| Group | Sub-Group | |
| Polyphenols | Flavonoids | Flavanols : Catechin, Gallate & Epigallocatechin |
| Flavonol : Quercetin, Gingerol & Kaempferol | ||
| Flavones : Apigenin & Fisetin | ||
| Isoflavonoids : Genistein | ||
| Anthocyanidin | ||
| Non-flavonoids | Phenolic acids: Hydroxybenzoic acid (Rosmarinic acid) & Hydroxycinnamic acids (Curcumin) | |
| Stilbenes : Resveratrol, Lignan | ||
| Carotenoids | β-Carotene, Lycopene, Crocetin | |
| Glucosinolates | Isothiocyanates, Indole and Sulforaphane | |
| Organosulphides | Allium compounds | |
| Nitrogen compounds | Alkaloids | Caffeine, Codeine, Noscopine & Quinidine |
| Capsaicinoids | Dihydrocapsaicin, Homocapsaicin, Capsaicin, Nonivamide | |
| Terpenoids | d-Limonene, d-Carvone, Perillyl alcohol, Andrographolide, Excisanin A, Gnidimacrin, Oridonin, Cucurbitacin B | |
2.1. Polyphenols
Polyphenols are the major secondary plant metabolites and dietary phytochemicals present in the human diets. Based on the numeral phenol rings and the structural essential elements in the side chain that bind to these phenol rings, polyphenol can be majorly grouped into two subdivisions such as flavonoids and non-flavonoids [5,9,10]. The flavonoids (or bioflavonoids) can be identified as a group of phenolic compounds with a 15-carbon skeleton structure. Flavonoids possess strong antioxidant activity that have a defined role in minimizing the risk of age-related chronic diseases like cancer [5,6,10,11]. The flavonoid group comprises of; flavones (fisetin and apigenin), flavonols (quercetin, kaempferol and gingerol), flavanols (catechin, gallate and epigallocatechin), isoflavonoids (genistein) and anthocyanidin [[26], [27], [28], [29], [30], [31]]. The non-flavonoids comprise phenolic acids and stilbenes. The phenolic acids are subdivided into two chief groups such as; hydroxycinnamic acids and hydroxybenzoic acids. The curcumin is included under hydroxycinnamic acid derivatives whereas rosmarinic acid is grouped under hydroxybenzoic acid derivatives. Resveratrol and lignan are majorly classified under stilbenes.
2.2. Carotenoids
Carotenoids, the natural fat-soluble pigments are classified as carotenes (pure hydrocarbons with no oxygen) and xanthophylls (containing oxygen) that possess strong antioxidant activity [32]. The major phytochemicals that come under xanthophylls are identified to be crocetin and lutein. Similarly, the carotenes constitute lycopene, β-cryptoxanthin, α, β and γ-carotene. Among α, β and γ carotene, β-carotene plays an important role in human health. β-carotene is a red-orange pigment generally found in plants and fruits [32].
2.3. Glucosinolates
Glucosinolates (GLS), are a class of plant thioglucosides (organic compounds containing sulfur and nitrogen and are derived from glucose and amino acid) [33]. Isothiocyanates, indole and sulforaphane belong to this group. In the human digestive system, glucosinolates are hydrolyzed to isothiocyanates by the action of the enzyme myrosinase [34]. The enzymatic degradation helps the release of chemopreventive agents into the host system. The major dietary phyto-constituents indole in the acidic environment of the stomach is condensed and changed to a digestion derivative named di-indolylmethane (DIM) that exhibit strong anti-cancer property [34]. Sulforaphane is generally derived from cruciferous vegetables display effective anti-cancer potency in various cancer cell lines both in vivo and in vitro [34].
2.4. Organosulfur compounds (organosulphides)
Alliin, a sulfur-containing dietary phytochemical compound has a major derivative of the amino acid cysteine [35]. It has been reported to be the first natural carbon-sulfur-centered stereometric compound found in garlic species. Alliin is majorly found to have a potential role in immune response in the host organism [36]. Three additional sulfoxides (methiin, propiin and isoalliin) are also present in the tissues of onion [37].
2.5. Nitrogen-containing compounds
Among the plant-based secondary metabolites, the nitrogen-containing phytochemicals (alkaloids) have emerged as very prominent class of disease preventive antioxidants [38,39]. The chief dietary phytochemicals such as caffeine, codeine, noscapine and quinidine are placed in the major group of alkaloids [39]. Another group of nitrogen-containing phytochemicals; capsaicinoids are medically used as analgesics [40]. The major phyto-constituents of capsaicinoids include dihydrocapsaicin, homocapsaicin, capsaicin and nonivamide [40].
2.6. Terpenoids
All living organisms synthesize several terpenes for indispensable physiological functions. The classification of these natural phyto-products are based on the numeral of isoprenoid units present. The major classes of terpenoids consist of compounds belonging to monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, triterpenes, and tetraterpenes. The dietary important terpenoids that have proven their potential as strong drug candidates include monotrepens (d-Limonene, d-carvone and perillyl alcohol) [41].
3. Diet, dietary phytochemicals and cancer
Diet plays a significant role in prevention of the cancer [42]. Though the interlink between diet and prevention of cancer has not yet been explicitly explained, there have been many pieces of evidence that suggest the importance of diet on limiting cancer risk. Studies have reported that cancers associated with the digestive tract were reduced almost half after daily consumption of dietary phytochemicals.
Consumption of polyphenol-rich fruits has been found to decrease the risk of cancer. Quercetin induces both apoptosis and autophagy in several tumor and cancer cell lines [43]. Anti-carcinogenic effects of resveratrol have efficiently reduced the skin and gastrointestinal tract tumor [7,8,11,44]. The chemo-preventive effect of curcumin isolated from turmeric is effective for the treatment of breast, lung and colon cancer, and brain tumor [45]. Epigallocatechin gallate (EGCG), the phyto-constituent present in green tea is favorable in treating prostate, bladder, cervical and brain cancers [42,[46], [47], [48]]. Similarly, Kaempferol is associated with reduction of pancreatic and lung cancer [49]. Gingerol, another active phyto-constituents isolated from fresh ginger is effective against tumors in colon, breast, ovary and pancreas origin [50].
Anthocyanidin, play an important role in reducing inflammation, thus limits tumor growth. Perillyl alcohol and d-limonene have displayed chemo-preventive activity against skin, liver, mammary and lung cancers. Owing to the effective antioxidant activity exhibited by β-carotene, it exhibits anti-cancer activity in several cancer cells and tumor cell lines [32]. The immunomodulatory function of β-carotene exhibits possible inhibition of carcinogenetic progress. In combination with vitamins E and C, β-carotene exhibits enhanced anti-cancer efficacy in several cancer cells. Lycopene exhibited potent anti-cancer effects against skin, breast, prostate, esophagus, stomach and colon cancers [32,[51], [52], [53]]. Glucosinolate derived from cruciferous vegetables has displayed chemo-preventive effect on liver, colon, mammary gland, lung, colorectal and pancreas cancer [54]. Di-indolylmethane have demonstrated exceptional anti-cancer effect against hormone-responsive breast, ovarian and prostate cancers (Table 2).
Table 2.
Mechanism of chemoprevention by dietary phytochemicals in different cancer subtypes.
| Sl. No | Cancer Subtype | Cell line | Compound name | Functional Involvement | Reference |
|---|---|---|---|---|---|
| 1. | Breast and ovarian cancer | MCF-7 | Fisetin | Mitochondrial apoptosis and autophagic cell death which is independent of apoptosis | [55] |
| 2. | MDA-MB-231 | Apigenin | Induction of autophagy via enhanced LC3 lipidation | [56] | |
| 3. | MCF-7 | Genistein | Bax/Bcl-2 modulation for subsequent onset of apoptosis | [57] | |
| 4. | A2780 | Genistein | Modulation the AKT signaling pathway for induction of autophagic cell death. Sustained onset of autophagy through modulation of PKC and ERK | [58] | |
| 5. | MDA-MB-231 and MCF-7 | Kaempferol | Inhibition of cellular proliferation via G2/M phase cycle arrest. Induction DNA fragmentation, caspase 3, 7 and 9, Bax, PARP, and p53 for onset of apoptotic cell death. | [59] | |
| 6. | MDA-MB-361 and MCF-7 | Epigallocatechin | ROS dependent onset of autophagy and apoptosis. Subsequent removal of dysfunctional mitochondria through mitophagy that mediate cell death | [47] | |
| 7. | MCF-7 | Resveratrol | Autophagy independent of Beclin1 and Vps34 signalling pathway | Li et al., 200 9 | |
| 8. | MCF-7 | Codeine | Induction of apoptosis | [60] | |
| 9. | Cervical and prostate cancer | HeLa | Fisetin | Modulation of ERK1/2 and caspase-8 and caspase-3 | [61] |
| 10. | PC-3 and DU-145 | Fisetin | Autophagic cell death though modulation of mTOR-AKT signaling pathway | [55] | |
| 11. | LNCaP | Genestein | Inhibition of invasion, migration and EMT | [58] | |
| 12. | PC-3 and DU145 | Genestein | Inhibition of AKT/mTOR/p70S6K leading to autophagic cell death | [62] | |
| 13. | PC-3 | β-carotene | Enhanced expression of cytochrome C and induction of caspase proteins | [63] | |
| 14. | Colon and gastric cancer | HCT-116 | Apigenin | Autophagic cell death via inhibition of the PI3K/Akt/mTOR signalling pathway | [64] |
| 15. | HT-29 and HCT-116 | Kaempferol | Induction of TRAIL mediated apoptosis | [65] | |
| 16. | MKN-28, SGC-7901 and BGC-823 cells | Curcumin | Induction of both autophagic and apoptotic cell death | [45] | |
| 17. | HCT-116 | Curcumin | Induction of autophagy associated senescence through enhanced LC3 lipidation | [66] | |
| 18. | SGC-7901 and MGC-803 | Caffeine | Induction of caspase-3 and -9 mediated apoptosis | [67] | |
| 19. | Liver and pancreatic cancer | MIA PaCa-2 | Genistein | Inhibition of the Bcl-2 expression for onset of apoptosis | [68] |
| 20. | Hep3B | Andrographolide | Induction of apoptosis through modulation of MAPK, pJNK, ERK1/2 signalling | [69] | |
| 21. | HepG2 | Oridonin | ROS mediated apoptosis for induction of apoptotic cell death through p53 and p38 expression, enhanced expression of caspase 3 and caspase-9 | [70] | |
| 22. | HepG2 | Cucurbitacin B | Regulation of the Bcl-2 expression, regulation of the cyclin D1 and cdc2 expression | [71] | |
| 23. | Glioma, melanoma and sarcoma | U87-MG and U373-MG | Curcumin | Induction of G2/M phase cycle arrest and AKT/mTOR/p70S6K mediated autophagy | [14] |
| 24. | A375 and C8161 | Curcumin | Autophagic cell death through regulation of AKT/mTOR/p70S6K signaling | [72] | |
| 25. | Head and neck carcinoma | Cal33 and FaDu | Gallic acid | Induction of apoptosis through upregulation of Bax, caspase-3 and downregulation of Bcl-2, NRF2, NQO1 and GCLC. Autophagic flux inhibition, enhanced LC3 lipidation. | [73,74] |
| 26. | Lung cancer | A549 | Curcumin | Regulation of AMPK, MAPK and ERK1/2 signalling | [45] |
| 27. | A549 and H1299 | Allicin | Inhibition of the cellular proliferation, invasion and metastasis via modulation of PI3K/AKT signaling | [75] | |
| 28. | Leukaemia | K562 | Curcumin | Induction of autophagy via enhanced expression of Beclin1 and downregulation of Bcl-2, inhibition of AKT/mTOR/p70S6K | [76] |
| 29. | CML | Resveratrol | Induction of autophagy through AMPK-mTOR signaling pathway | [14] | |
| 30. | CML | Quercetin | Induction of PKC mediated apoptosis | [77] | |
| 31. | HL-60 | Codeine | DNA damage and nuclear fragmentation, caspase 3 activation for induction of apoptosis | [78] |
Indoles and sulforaphane have been found to be more effective against breast cancer. Moreover, they have been influential in the protection of the skin against UV radiation damage. Sulforaphane has displayed very significant inhibitory effect on prostate tumorigenesis. Phenethyl isothiocyanate (PEITC) has been intensively studied for its chemo-preventive action against breast, lung, cervical, prostate and several myeloma cell lines. Effectively, PEITC has displayed very potent inhibitory activity against melanoma.
4. Chemo-preventive action of dietary phytochemicals
Carcinogenesis is documented as a complex, multistep process initiated with exposure to a carcinogenic agent [42]. In the cancer initiation stage, a normal cell gets transformed into a cancer cell. The cancer-initiating cell begins abnormal multiplication and gives rise to a heterozygous tumor cell population. In the tumor promotion stage, actively proliferating pre-neoplastic cells accumulate that lead to tumor progression which involves the growth of a tumor with potential invasion and metastatic potential. Chemoprevention is defined as the application of chemotherapeutic agents to inhibit, converse or hinder tumorigenesis/ carcinogenesis. Numerous dietary phytochemicals have been reported to act as chemo-preventive agents by interfering with a specific regulatory stage during the process of carcinogenic [79]. Overproduction of oxidants cause an oxidative imbalance that contributes towards oxidative damage of DNA, proteins and lipids to aid cancer pathophysiology [80]. In this context, to prevent oxidative stress dietary phytochemicals with potential antioxidant properties hold high importance as chemo-preventive agents [81].
Several phytochemicals seem to possess anti-inflammatory properties. The colossal interlink between cancer and inflammation is evident as several inflammatory conditions that influence the onset of cancer initiation [6,11,82,83]. Previous investigations have demonstrated that biological activity of these dietary phytochemicals are due to their potent free radicals scavenging and enhanced antioxidant activity that subsequently regulate the expression of oncogenes and tumor suppressor genes [5,6,10,11]. In addition to this, dietary phytochemicals regulate the cancer cell proliferation and differentiation, the arrest of cell cycle at different phases and onset of cell death pathways such as apoptosis and autophagy [11,84,85]. According to Lee Wattenberg’s conventional classification, the chemo-preventive agents are segmented into blocking and suppressing agents [86].
4.1. Blocking agents
As blocking agents, dietary phytochemicals prevent carcinogens to reach the target sites and subsequently inhibit the DNA damage [87]. These dietary phytochemicals neutralize the carcinogens by moderating the enzymatic systems responsible for them. These phyto-products either reduce their carcinogenic potential or increase their excretion [88,89]. Allyl sulfides present in garlic act as blocking agents by altering the host’s defense system against molecules that are responsible for DNA-damage. Similarly, tea polyphenols inhibit the binding of carcinogenic substances to genetic material preventing genetic mutations [90,91]. Quercetin, another prominent polyphenol increases the excretion of oxidative metabolites. Carotenoids react with the free radicals in a lipid-soluble environment to prevent oxidative stress. β-carotene supposed to inhibit cancer cell growth by enhancing cellular antioxidant propensity as well as improving immune response [92].
Kaempferol inhibits cellular proliferation through the induction of G2/M phase cycle arrest [59]. Moreover, it also regulates the expression of E-cadherin, N-cadherin, Slug and Snail for inhibition of EMT [59]. Genistein displays inhibition of cellular proliferation by arresting the cell cycle at the G2/M phase [93]. Moreover, inhibition of telomerase activity and angiogenic capacity support genistein-mediated chemo-prevention [58]. Moreover, apigenin-mediated inhibition of cellular proliferation is evident with G2/M phase cell cycle arrest [56]. Glucosinolates inhibit the enzyme activation to modify the steroid hormone metabolism and protect cells against oxidative damage, thus preventing tumor initiation [33]. They also expedite the cleansing of carcinogens by inducing Phase I and Phase II enzymes. Another antioxidant perillyl alcohol, protect cells from becoming cancerous, slow cancer cell growth and strengthen immune function to fight against cancer [33].
Allicin, another organic allyl sulfur compound exhibits inhibition of MMP-2 and MMP-9 expression for inhibition of cell proliferation [43]. It also regulates the STAT3 signalling pathway for inhibition of cell proliferation, migration and EMT [94]. Noscapine inhibits the cellular proliferation via binding to the tubulin microfilaments. In combination with docetaxel, noscapine binds to the tubulin for inhibition of cancer cell progression [95]. Noscapine-loaded nanocarriers in combination with doxorubicin enhanced the anti-cancer efficacy in several cancer cells [96]. Sulforaphane exhibits anti-cancer efficacy in several cancer cells through regulation of cell cycle progression, induction of apoptosis and autophagy, inhibition of angiogenesis and enhanced chemotherapeutic efficacy in combination with known anti-cancer drugs [63]. In colon and ovarian cancer cells, sulforaphane regulates the expression of HIF-1α and VEGF for inhibition of angiogenesis [97]. Gnidimacrin inhibits tumor progression through upregulation of p21WAF1/CIP1 signalling pathway [98]. In addition to this, gnidimacrin arrests the cell cycle at G2-phase and downregulates the expression of cdc2 [99].
Lycopene activates the phase II detoxification enzymes to reduce oxidative damage associated with cancer initiation [100,101]. Lycopene upregulates the cytochrome P450 expression to prevent carcinogenesis [102,103]. In several cancer cells, lycopene arrests the cell cycle at G0/G1 and S-phase [102,103]. Moreover, lycopene-mediated regulation of expression of MMP-2 and MMP-9 in several cancer cell lines inhibits the cancer growth and proliferation [[51], [52], [53]]. Lycopene exhibits anti-cancer efficacy through enhanced antioxidant, lipid peroxidation and ROS scavenging efficacy [32].
4.2. Suppressing agents
On the other hand, a certain class of dietary phytochemicals obstructs the malignant transformation of cancer-initiating cells by acting unswervingly on tumor cells and also by modifying their microenvironment through deploying hostile physiologic environments that are unfavorable for tumor growth and progression [104]. This group of phytochemicals inhibit tumor growth by induction of cell death pathways such as apoptosis [105]. In apoptosis deficient cancer cells, these phytochemicals deploy an alternative form of cell death known as autophagy [105]. Moreover, these dietary phytochemicals inhibit tumor angiogenesis [[106], [107], [108]]. Phenethyl isothiocyanate, curcumin and resveratrol have shown strong apoptosis and autophagy-inducing potential in several cancer cell lines [109]. These phytochemicals are known to regulate the pro-apoptotic and anti-apoptotic proteins that are responsible for the onset of apoptosis [110]. Moreover, they also regulate autophagy and autophagy-associated genes to regulate autophagic cell death [110].
Quercetin induces autophagy through induction of MAPK/PI3K/PKC/ERK signalling pathway [111,112]. Sulforaphane induces chemo-preventive action through the regulation of the NRF2 signaling pathway in several cancer cell lines [113,114]. Moreover, sulforaphane also regulates several epigenetic regulatory mechanisms for cancer prevention. Mechanistically, sulforaphane reverses the epigenetic alteration via DNA methyltransferases and histone deacetyltransferases and several non-coding RNAs [113]. Sulforaphane also alters the apoptotic signal through upregulation of Bax and downregulation of Bcl-2 expression [115]. Allicin activates the caspase cascades for induction of apoptosis. In addition to this, allicin upregulates the Bax and downregulates the Bcl-2 expression for induction of apoptosis [94]. Noscapine induces ROS for subsequent activation of apoptosis [116]. In several tumor cells, prolonged exposure to apigenin inhibits autophagy through inhibition of Beclin-1 expression that subsequently promotes caspase 3 and 9 dependent apoptosis [56]. In addition to this, apigenin induces apoptosis through downregulation of Bcl-2 and upregulation of caspase 3 expressions. Kaempferol mediated inhibition of PI3K/AKT/mTOR signaling, enhanced expression of Beclin-1, ATG-5, ATG-7, ATG-12 genes and enhanced LC3 lipidation for induction of autophagic cell death [117].
5. Chemotherapeutic efficacy of dietary phytochemicals in different cancer subtypes
5.1. Breast and ovarian cancer
In MCF-7 breast cancer cells, fisetin induces intrinsic apoptosis and autophagic cell death which is independent of apoptosis [55]. In MDA-MB-231 cells, apigenin induces autophagy via enhanced LC3 lipidation [56,64,118,119]. In MCF-7 cells, genistein enhances the Bax/Bcl-2 expression for subsequent onset of apoptosis [57]. In A2780 cells, genistein upregulates the AKT signaling pathway for induction of autophagic cell death. Moreover, genistein sustains the onset of autophagy through upregulation of PKC and ERK signaling [58]. In MDA-MB-231 and MCF-7 cells, kaempferol inhibits cellular proliferation via G2/M phase cell cycle arrest. Furthermore, it induces DNA fragmentation for the induction of apoptotic cell death [59]. Mechanistically, kaempferol regulates the expression of caspase 3, 7 and 9, Bax, PARP and p53 to induce apoptosis [59]. In addition to this, SOD and CAT regulation by kaempferol regulates the onset of apoptosis [59]. In MCF-7 and MDA-MB-361, EGCG induces ROS dependent onset of autophagy and apoptosis [47,48]. The mitochondrial dysfunction upon treatment of EGCG and their subsequent removal through mitophagy aid EGCG mediated autophagic cell death [47,48]. In the breast cancer-bearing xenograft mice model, EGCG inhibits lung metastasis by inducing apoptosis through DNA damage [47,120,121]. In MCF-7 cells, resveratrol induces autophagy independent of Beclin-1 and Vps34 signalling pathway [[122], [123], [124]]. In ovarian cancer cell lines, resveratrol induces autophagy through inhibition of PI3K/AKT/mTOR signalling pathway. In ovarian cancer cells, sulforaphane regulates the expression of HIF-1α and VEGF expression for inhibition of angiogenesis [97]. In MCF-7 cells, codeine induces apoptosis in a dose-dependent manner [60] (Fig. 3, Table 2).
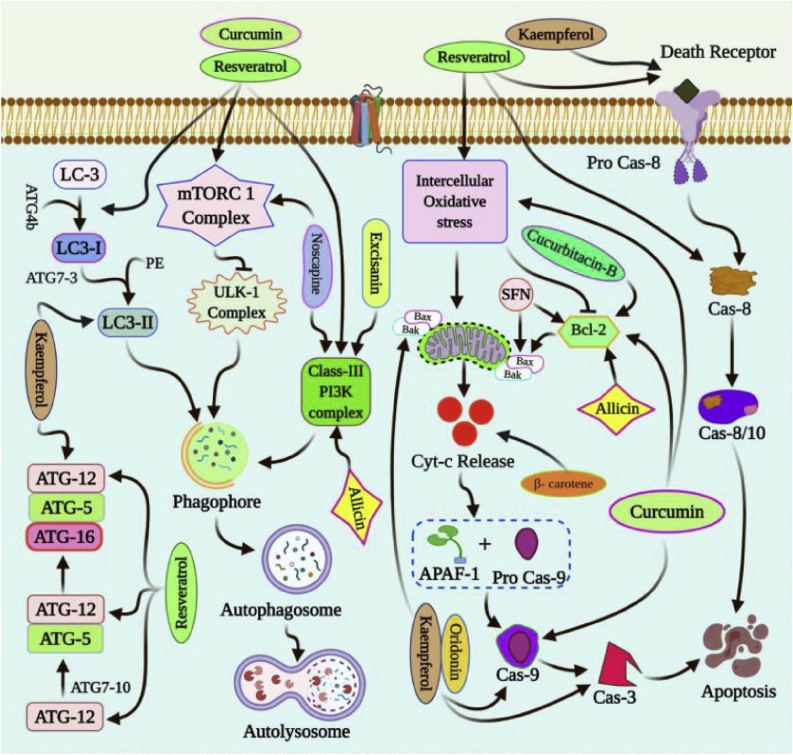
Fig. 3.
Molecular mechanisms of chemoprevention by dietary phytochemicals.
Polyphenols like curcumin, resveratrol and noscapine regulate autophagic cell death via inhibition of mTORC1 signalling. Similarly, curcumin, resveratrol, noscapine, allicin, and excisanin activate the PI3K signalling for elongation of extending phagophore. Kaempferol, curcumin and resveratrol enhance the LC3 lipidation in the extending autophagosomes. Resveratrol enhances the expression of ATG-5 and ATG-12. Similarly, kaempferol and resveratrol also regulate the ATG-16 formation from ATG-5 and ATG-12. Curcumin, resveratrol and kaempferol enhance the Bax/Bcl-2 expression to regulate apoptosis. In addition to this, kaempferol, oridonin and curcumin upregulate the caspase cascades responsible for the onset of apoptosis. Oridonin regulates the expression of caspase 3 and caspase 9 while cucurbitacin B and allicin regulate the Bcl-2 expression for induction of apoptotic cell death. Sulforaphane also alters the apoptotic signal through upregulation of Bax and downregulation of Bcl-2. β-carotene enhances the release of cytochrome C to mediate mitochondrial apoptosis. Resveratrol enhances the expression of caspase 8 during the onset of extrinsic apoptosis while kaempferol elicits the death receptors that are responsible for extrinsic apoptosis.
5.2. Cervical and prostate cancer
Fisetin, a naturally occurring flavonoid induces apoptosis in the human cervical cancer cell line (HeLa) through upregulation of ERK1/2 and caspase 8 and caspase 3 [61]. In HeLa cells, quercetin induces autophagy and apoptosis for subsequent inhibition of cancer cell survival and proliferation [125,126]. In PC-3 and DU-145 cells, fisetin induces autophagic cell death through inhibition of mTOR-AKT signaling pathway [55]. In LNCaP cells, genestein treatment inhibits invasion, migration and EMT [58]. A similar regulation of inhibition of AKT/mTOR/p70S6K mechanistic involvement of signaling pathways is also responsible for the induction of autophagic cell death in PC-3 and DU-145 cells [62]. In PC-3 cells, β-carotene exhibits apoptosis through enhanced expression of cytochrome C and induction of caspase proteins. Sulforaphane has displayed very significant inhibitory effects on prostate tumorigenesis [63]. In human prostate cancer cells, paclitaxel in combination with noscapine exhibits enhanced anti-cancer activity [127,128] (Fig. 3).
5.3. Colon and gastric cancer
Apigenin induces autophagic cell death in human colon cancer (HCT-116) cells via inhibition of the PI3K/AKT/mTOR signalling pathway [56,64,118,119]. In HT-29 and HCT-116 cells, kaempferol induces TRAIL-mediated apoptosis [65]. In HT-29 and SSC-4 cells, EGCG elicits the onset of apoptosis for suppression of cell cellular proliferation and induction of cell death [47]. In MKN-28, SGC-7901 and BGC-823 cells, curcumin induces both autophagic and apoptotic cell death for cancer inhibition [45]. In HCT-116 cells, curcumin induces autophagy-associated senescence through enhanced LC3 lipidation [66]. In HT-29 cells, resveratrol induces ROS-dependent apoptosis [129]. In colon cancer cells, sulforaphane downregulates the expression of HIF-1α and VEGF expression for inhibition of angiogenesis [97]. In SGC-7901 and MGC-803 cells, caffeine induces caspase 3 and 9 mediated apoptosis [67]. In AGS cells, codeine induces apoptosis in a dose-dependent manner [60]. In human colon cancer cells, noscapine induces apoptosis via enhanced PTEN/PI3K/mTOR signaling [130] (Fig. 3, Table 2).
5.4. Liver and pancreatic cancer
In HepG2 cells, kaempferol induces apoptosis via regulation of ROS [59]. In MIA PaCa-2 cells, genistein downregulates the Bcl-2 expression for the onset of apoptosis. In combination with 5-FU, genistein exerts apoptosis in human pancreatic cells [68]. Andrographolide inhibits the growth of Hep3B cells by induction of apoptosis through the upregulation of MAPK, pJNK, ERK1/2 signalling pathways [69]. Excisanin A also decreased the cellular viability of Hep3B cells via induction of apoptosis through downregulation of AKT signaling [131]. Oridonin promotes the ROS-mediated apoptosis for the induction of apoptotic cell death in HepG2 cells [70]. Moreover, it also regulates p53 and p38 expression [132]. Oridonin regulates the expression of caspase 3 and caspase 9 for induction of apoptosis. Cucurbitacin B regulates the Bcl-2 expression in HepG2 cells for induction of apoptosis [133]. Moreover, it also regulates the cyclin D1 and cdc2 expression for the suppression of cellular proliferation [71] (Fig. 3, Table 2).
5.5. Glioma, melanoma and sarcoma
Curcumin induces G2/M phase cycle arrest and AKT/mTOR/p70S6K mediated autophagy in U87-MG and U373-MG cells [14,134,135]. In A375 and C8161 cells, curcumin induces autophagic cell death through the regulation of AKT/mTOR/p70S6K signaling [72,136]. In MG63 cells, quercetin in synergism with pharmacological and genetic inhibitors of autophagy induces apoptosis for inhibition of cell proliferation [125,126]. Noscapine also exhibits anti-cancer efficacy against glioblastoma by inhibiting the microtubules [137]. Caffeine induces selective cytotoxicity and DNA damage in human sarcoma cell lines [138]. Moreover, synergistic treatment of caffeine and conventional chemotherapeutic drugs exhibits enhanced chemo-sensitization through induction of apoptosis. In human osteosarcoma, caffeine induces G0/G1 phase cell cycle arrest and caspase 3/7 activation for the induction of apoptosis [139] (Fig. 3, Table 2).
5.6. Head and neck carcinoma
In oral cancer cells, curcumin induces autophagic cell death through bulk cellular vacuolation and enhanced conversion of LC3I to LC3II [45]. Similarly, in Cal33 oral cancer cells, gallic acid induces apoptosis through upregulation of Bax, caspase 3 and downregulation of Bcl-2 [74]. Moreover, it inhibits autophagy through enhanced expression of p62 to induce autophagy associated cell death. Gallic acid in combination with gamma irradiation inhibits lipophagy to promote lipophagy associated cell death [73]. A synergistic combination of sulforaphane and paclitaxel in Barrett esophageal adenocarcinoma, an enhanced antiproliferation is evident through the onset of apoptosis [140]. Moreover, it inhibits bronchial dysplasia and cellular proliferation which is evident through reduced Ki-67 expression (Fig. 3, Table 2).
5.7. Lung cancer
In A549 cells, curcumin regulates the AMPK, MAPK and ERK1/2 signaling pathway associated with cellular transformation, differentiation and proliferation [45]. In smoking-associated lung cancer patients, sulforaphane induces apoptosis through enhancement of caspases expression [63]. In A549 and H1299 cells, allicin inhibits the cellular proliferation, invasion and metastasis via regulation of PI3K/AKT signaling [75] (Fig. 3, Table 2).
5.8. Leukaemia
In K562 cells, curcumin induces autophagy via enhanced expression of Beclin-1 and downregulation of Bcl-2 [76]. Moreover, the inhibition of AKT/mTOR/p70S6K signaling is also responsible for the onset of autophagy [76]. Similarly, in myeloma and leukemia cells, resveratrol induces the Fas and its associated ligands as well as Bcl-2 and Bax signaling for the onset of both extrinsic and intrinsic apoptosis [141,142]. In chronic myeloid leukemia, resveratrol induces autophagy through the AMPK-mTOR signaling pathway [14,111,134]. In chronic lymphocytic leukemia, quercetin induces PKC-mediated apoptosis [77,143,144]. In HL-60 cells, codeine induces DNA damage and nuclear fragmentation for induction of apoptosis [145]. In HL-60 and HSC-2 cells, codeine activates the caspase 3 mediated apoptosis [78] (Fig. 3, Table 2).
6. Preclinical efficacy of dietary phytochemicals
In breast cancer (MCF-7) bearing mice xenograft model, curcumin exhibited anti-tumor efficacy in combination with paclitaxel and chemo-sensitize the tumor cells towards apoptosis [146]. In another breast cancer (MDA-MB-245) bearing mice xenograft model, curcumin and paclitaxel inhibited the NF-κβ and MMP signalling for inhibition of tumor metastasis [135]. Resveratrol regulated the breast cancer stem cells via regulating the Wnt/ β-catenin in breast cancer-bearing mice xenograft model [42]. In nude mice bearing MDA-MB-231 cells exhibit reduced angiogenesis and enhanced apoptosis post-treatment with resveratrol [42,135]. In addition to this, resveratrol mediated PI3K/AKT/mTOR signalling inhibition induced autophagic cell death. EGCG induced DNA damage as the precursor of apoptosis in nude mice model bearing breast tumor [121]. In addition to this, it also reduced the invasiveness of breast tumor thereby suppressing the lung metastasis. Fisetin inhibited the in vivo breast tumor growth via induction of apoptosis in a caspase-dependent manner [61]. Sulforaphane suppressed the tumor growth of triple-negative breast cancer cells, via inhibition of the Cripto/Alk4 protein complex formation [147].
In prostate cancer-bearing mice model, curcumin inhibited the tumor growth and elicited induction of apoptosis via NF-κβ, MAPK and EGFR regulation. In TRAMP rat model, resveratrol reduced the growth of prostate cancer tumor via downregulation of ERK1/2 signalling and induction of apoptosis [135]. In PC-3 bearing mice xenograft model, lycopene reduced the angiogenesis via inhibition of VEGF and EGF expression [148]. In PC-3 cells bearing prostate cancer mice model, sulforaphane retarded the tumor growth via induction of apoptosis in a caspase-dependent manner [149]. In xenograft mice model bearing colorectal cancer, curcumin treatment sensitizes the tumor cells towards chemotherapy via inhibition of NF-κβ signalling. Curcumin in synergism with oxaliplatin or 5-fluorouracil inhibited tumor growth in human gastric cancer-bearing mice model [135]. Lycopene inhibited the growth of colorectal cancer via decreasing the PCNA, COX-2, MMP-9, ERK1/2 and induction of apoptosis via upregulation of caspase 3 and p21 [148]. In a xenograft tumor model in mice, noscapine induced apoptosis to restrain tumor growth via downregulation of Bcl-2 and upregulation of Bax, cytochrome C, caspase 3 and caspase 9 [150].
Curcumin in synergism with metformin decreased hepatocellular carcinoma tumor growth in xenograft mice model [151]. In SK-Hep-1 hepatoma cells bearing mice model, lycopene inhibited the tumor growth and metastasis via decreased expression of MMP-2 and MMP-9 [148]. In the human glioma cell bearing mice model, curcumin mediated inhibition of angiogenesis was by decreased MMP-9 expression [152]. In the A375 cell bearing melanoma mice model, curcumin inhibited the PI3K/AKT/mTOR signalling to induced autophagic cell death [153]. In DMBA induced skin tumor-bearing CD-1 mice model, resveratrol inhibited the tumor growth via downregulation of TGF-β and TNF-α and other inflammation-related key molecular proteins [154]. Apigenin induced apoptosis for reduction of chondrosarcoma tumor in mice model. Resveratrol reduced the tumor growth, angiogenesis and metastasis in Lewis Lung Carcinoma bearing C57BI/6 mice model [155]. Apigenin inhibited the tumor growth, metastasis and angiogenesis in the NSCLS xenograft mice model [42]. In the murine xenograft lung cancer model, noscapine in synergism with cisplatin reduced the tumor growth via induction of apoptosis with evident upregulation of caspase 3, caspase 8, PARP, p53, p21 and Bax [156].
7. Challenges in therapeutic intervention and clinical translation
The limited bioavailability, poor stability and permeability, reduced pharmacokinetics (reduced plasma, blood and tissue concentration) and pharmacodynamics, metabolism and absorption has been the major challenges for clinical translation of dietary phytochemicals. Then extensive digestion of curcumin is a major obstacle for maintaining higher plasma and tissue concentration [157]. In a phase II clinical trial (NCT00094445), even after 8 weeks of application of curcumin, the plasma concentration was found to be very low. Curcumin at 2.5−5 μg/mL concentration induced chromosomal alteration and DNA damage [158]. Curcumin at doses of 0.9–3.6 g/day for 14 days exhibited nausea and diarrhea [159]. Oleoresin has shown toxic and carcinogenic effect in in vivo rat model [157]. SRT501 in combination with bortezomib approved for multiple myeloma has noticed adverse renal toxicity, nausea, diarrhea, vomiting, fatigue and anemia [160,161]. In Male Sprague-Dawley rats, low bioavailability of kaemferol (nearly 2%) has reduced its use as chemopreventive in several cancer subtypes [162]. Similarly, the poor solubility, limited bioavailability, instability and poor permeability have also limited the use of quercetin as potent chemopreventive [163].
8. Conclusion and future perspective
Ever-increasing cancer incidences and associated mortalities despite advancement in prognosis, diagnosis and treatment have emerged as foremost challenge in cancer therapy. The cytotoxicity and non-target specificity have contributed to more problems with the application of chemotherapeutic drugs. To abide by the ill effects associated with chemotherapy, dietary phytochemicals have emerged as potent pharmacophores in cancer therapeutics. With mechanistic involvement of cell survival and cell death pathways (apoptosis and autophagy) associated with cancer-treating drugs, identification and evaluation of dietary phytochemical modulating such signaling pathways has appeared as possible pharmacophores for future generation cancer treatment. With apoptotic and autophagic target specificity, dietary phytochemicals will uncover several associated cellular networking that are potential targets for cancer chemoprevention. Moreover, as individual drug candidates and in combination with conventional cancer drugs, these phytochemicals will enhance chemo-sensitization for better chemotherapy. In addition to this, identifying and understanding the molecular key players involved, target-specific drug delivery through nano carriers, liposomes, polymers and micro emulsions will be formulated for enhanced bioactivity and bioavailability. Moreover, the identification of such key molecules will uncover novel targets for future generation personalized and precision medicine in cancer therapy.
Author contributions
SP, RN, SMP, BP. BS and CB have prepared the manuscript. MJ and SKB have done the conceptualization and proofreading of the manuscript.
Research involving human participants and/or animals
No Human participation and/or Animal have been used in this study
Informed consent
The corresponding author on behalf of all coauthors agree to accept the informed consent of compliance with ethical standard
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors are thankful to Berhampur University and National Institute of Technology Rourkela for providing the necessary facilities.
Contributor Information
Sujit Kumar Bhutia, Email: sujitb@nitrkl.ac.in.
Mrutyunjay Jena, Email: mrutyunjay.jena@gmail.com.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Nounou M.I., ElAmrawy F., Ahmed N., Abdelraouf K., Goda S., Syed-Sha-Qhattal H. Breast cancer: conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer Basic Clin. Res. 2015;9 doi: 10.4137/BCBCR.S29420. BCBCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatum J.L. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int. J. Radiat. Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 5.Law B.Y.K., Mok S.W.F., Wu A.G., Lam C.W.K., Yu M.X.Y., Wong V.K.W. New potential pharmacological functions of Chinese herbal medicines via regulation of autophagy. Molecules. 2016;21:359. doi: 10.3390/molecules21030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R.H. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J. Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 7.Si H., Liu D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014;25:581–591. doi: 10.1016/j.jnutbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surh Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 9.Russo G.L., Russo M., Spagnuolo C. The pleiotropic flavonoid quercetin: from its metabolism to the inhibition of protein kinases in chronic lymphocytic leukemia. Food Funct. 2014;5:2393–2401. doi: 10.1039/c4fo00413b. [DOI] [PubMed] [Google Scholar]
- 10.Tuorkey M.J. Cancer therapy with phytochemicals: present and future perspectives. Biomed. Environ. Sci. 2015;28:808–819. doi: 10.3967/bes2015.112. [DOI] [PubMed] [Google Scholar]
- 11.Grabacka M.M., Gawin M., Pierzchalska M. Phytochemical modulators of mitochondria: the search for chemopreventive agents and supportive therapeutics. Pharmaceuticals. 2014;7:913–942. doi: 10.3390/ph7090913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng S., Shanmugam M.K., Kumar A.P., Yap C.T., Sethi G., Bishayee A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 2019;125:1228–1246. doi: 10.1002/cncr.31978. [DOI] [PubMed] [Google Scholar]
- 13.Kiruthiga C., Devi K.P., Nabavi S.M., Bishayee A. Autophagy: a potential therapeutic target of polyphenols in hepatocellular carcinoma. Cancers. 2020;12:562. doi: 10.3390/cancers12030562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moosavi M.A., Haghi A., Rahmati M., Taniguchi H., Mocan A., Echeverría J., Gupta V.K., Tzvetkov N.T., Atanasov A.G. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018;424:46–69. doi: 10.1016/j.canlet.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 15.Sun C.-Y., Zhang Q.-Y., Zheng G.-J., Feng B. Autophagy and its potent modulators from phytochemicals in cancer treatment. Cancer Chemother. Pharmacol. 2019;83:17–26. doi: 10.1007/s00280-018-3707-4. [DOI] [PubMed] [Google Scholar]
- 16.Vidoni C., Ferraresi A., Secomandi E., Vallino L., Dhanasekaran D.N., Isidoro C. Seminars in Cancer Biology. Elsevier; 2019. Epigenetic targeting of autophagy for cancer prevention and treatment by natural compounds. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Q., Peng C., Zheng C., He X.-H., Huang W., Han B. Recent advances in characterizing natural products that regulate autophagy. Anti-Cancer Agents Med. Chem. (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2019;19:2177–2196. doi: 10.2174/1871520619666191015104458. [DOI] [PubMed] [Google Scholar]
- 18.Kashyap D., Sharma A., Sak K., Tuli H.S., Buttar H.S., Bishayee A. Fisetin: a bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018;194:75–87. doi: 10.1016/j.lfs.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Patel S.M., Venkata K.C.N., Bhattacharyya P., Sethi G., Bishayee A. Seminars in Cancer Biology. Elsevier; 2016. Potential of neem (Azadirachta indica L.) for prevention and treatment of oncologic diseases; pp. 100–115. [DOI] [PubMed] [Google Scholar]
- 20.Singh A.K., Sharma N., Ghosh M., Park Y.H., Jeong D.K. Emerging importance of dietary phytochemicals in fight against cancer: role in targeting cancer stem cells. Crit. Rev. Food Sci. Nutr. 2017;57:3449–3463. doi: 10.1080/10408398.2015.1129310. [DOI] [PubMed] [Google Scholar]
- 21.Ames B.N., Wakimoto P. Are vitamin and mineral deficiencies a major cancer risk? Nat. Rev. Cancer. 2002;2:694–704. doi: 10.1038/nrc886. [DOI] [PubMed] [Google Scholar]
- 22.Amin A.R., Kucuk O., Khuri F.R., Shin D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009;27:2712. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awika J.M., Rooney L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry. 2004;65:1199–1221. doi: 10.1016/j.phytochem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Goel A., Jhurani S., Aggarwal B.B. Multi‐targeted therapy by curcumin: how spicy is it? Mol. Nutr. Food Res. 2008;52:1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- 25.Talero E., Avila-Roman J., Motilva V. Chemoprevention with phytonutrients and microalgae products in chronic inflammation and colon cancer. Curr. Pharm. Des. 2012;18:3939–3965. doi: 10.2174/138161212802083725. [DOI] [PubMed] [Google Scholar]
- 26.H Sarkar F., Li Y., Wang Z., Padhye S. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Curr. Pharm. Des. 2010;16:1801–1812. doi: 10.2174/138161210791208956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haghi A., Azimi H., Rahimi R. A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. J. Gastrointest. Cancer. 2017;48:314–320. doi: 10.1007/s12029-017-9997-7. [DOI] [PubMed] [Google Scholar]
- 28.Kantara C., O’Connell M., Sarkar S., Moya S., Ullrich R., Singh P. Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1-siRNA. Cancer Res. 2014;74:2487–2498. doi: 10.1158/0008-5472.CAN-13-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunnumakkara A.B., Bordoloi D., Harsha C., Banik K., Gupta S.C., Aggarwal B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 2017;131:1781–1799. doi: 10.1042/CS20160935. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi T., Song Y., He C., Wang D., Morita K., Tsukada J., Kanazawa T., Yoshida Y. Autophagy is associated with cucurbitacin D-induced apoptosis in human T cell leukemia cells. Med. Oncol. 2016;33:30. doi: 10.1007/s12032-016-0743-y. [DOI] [PubMed] [Google Scholar]
- 31.Toden S., Okugawa Y., Jascur T., Wodarz D., Komarova N.L., Buhrmann C., Shakibaei M., Boland C.R., Goel A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015;36:355–367. doi: 10.1093/carcin/bgv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Q., Yu H., Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010;75:R50–R57. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- 33.Moreno D.A., Carvajal M., López-Berenguer C., García-Viguera C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006;41:1508–1522. doi: 10.1016/j.jpba.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Rouzaud G., Young S.A., Duncan A.J. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol. Prev. Biomarkers. 2004;13:125–131. doi: 10.1158/1055-9965.epi-085-3. [DOI] [PubMed] [Google Scholar]
- 35.Rose P., Whiteman M., Moore P.K., Zhu Y.Z. Bioactive S-alk (en) yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005;22:351–368. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]
- 36.Arreola R., Quintero-Fabián S., López-Roa R.I., Flores-Gutiérrez E.O., Reyes-Grajeda J.P., Carrera-Quintanar L., Ortuño-Sahagún D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/401630. 401630-401630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salehi B., Zucca P., Orhan I.E., Azzini E., Adetunji C.O., Mohammed S.A., Banerjee S.K., Sharopov F., Rigano D., Sharifi-Rad J. Allicin and health: a comprehensive review. Trends Food Sci. Technol. 2019;86:502–516. [Google Scholar]
- 38.Kennedy D.O., Wightman E.L. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011;2:32–50. doi: 10.3945/an.110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matkowski A. Plant in vitro culture for the production of antioxidants—a review. Biotechnol. Adv. 2008;26:548–560. doi: 10.1016/j.biotechadv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Palevitch D., Craker L. Nutritional and medical importance of red pepper (Capsicum spp.) J. Herbs Spices Med. Plants. 1996;3:55–83. [Google Scholar]
- 41.Pathak S., Agarwal A.V., Agarwal P., Trivedi P.K. Springer; 2019. Secondary Metabolite Pathways in Medicinal Plants: Approaches in Reconstruction and Analysis, Molecular Approaches in Plant Biology and Environmental Challenges; pp. 339–364. [Google Scholar]
- 42.Choudhari A.S., Mandave P.C., Deshpande M., Ranjekar P., Prakash O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front. Pharmacol. 2020;10:1614. doi: 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patra S., Pradhan B., Nayak R., Behera C., Panda K.C., Das S., Jena M., Bhutia S.K. 2021. Apoptosis and Autophagy Modulating Dietary Phytochemicals in Cancer Therapeutics: Current Evidences and Future Perspectives. [DOI] [PubMed] [Google Scholar]
- 44.Aqil F., Munagala R., Jeyabalan J., Vadhanam M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013;334:133–141. doi: 10.1016/j.canlet.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu F., Gao S., Yang Y., Zhao X., Fan Y., Ma W., Yang D., Yang A., Yu Y. Curcumin induced autophagy anticancer effects on human lung adenocarcinoma cell line A549. Oncol. Lett. 2017;14:2775–2782. doi: 10.3892/ol.2017.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiselev V.I., Ashrafyan L.A., Muyzhnek E.L., Gerfanova E.V., Antonova I.B., Aleshikova O.I., Sarkar F.H. A new promising way of maintenance therapy in advanced ovarian cancer: a comparative clinical study. BMC Cancer. 2018;18:904. doi: 10.1186/s12885-018-4792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qazi A., Pal J., Maitah Mi., Fulciniti M., Pelluru D., Nanjappa P., Lee S., Batchu R.B., Prasad M., Bryant C.S. Anticancer activity of a broccoli derivative, sulforaphane, in barrett adenocarcinoma: potential use in chemoprevention and as adjuvant in chemotherapy. Transl. Oncol. 2010;3:389. doi: 10.1593/tlo.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thangapazham R.L., Singh A.K., Sharma A., Warren J., Gaddipati J.P., Maheshwari R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007;245:232–241. doi: 10.1016/j.canlet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 49.Mercader-Ros M., Lucas-Abellán C., Fortea M., Serrano-Martínez A., Gabaldón J., Núnez-Delicado E. Nova Science Publishers, Inc; New York, NY, USA: 2013. Biological Activities of Kaempferol: Effect of Cyclodextrins Complexation on the Properties of Kaempferol. Kaempferol: Chemistry, Natural Occurrences and Health Benefits; pp. 1–31. [Google Scholar]
- 50.Mahadevappa R., Fai Kwok H. Phytochemicals-A novel and prominent source of anti-cancer drugs against colorectal cancer. Comb. Chem. High Throughput Screen. 2017;20:376–394. doi: 10.2174/1386207320666170112141833. [DOI] [PubMed] [Google Scholar]
- 51.Paur I., Lilleby W., Bøhn S.K., Hulander E., Klein W., Vlatkovic L., Axcrona K., Bolstad N., Bjøro T., Laake P., Taskén K.A., Svindland A., Eri L.M., Brennhovd B., Carlsen M.H., Fosså S.D., Smeland S.S., Karlsen A.S., Blomhoff R. Tomato-based randomized controlled trial in prostate cancer patients: effect on PSA. Clin. Nutr. (Edinburgh, Scotland) 2017;36:672–679. doi: 10.1016/j.clnu.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Ranjan A., Ramachandran S., Gupta N., Kaushik I., Wright S., Srivastava S., Das H. Role of Phytochemicals in Cancer Prevention . 2019:20. doi: 10.3390/ijms20204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shanbhag V.K. Lycopene in cancer therapy. J. Pharm. Bioallied Sci. 2016;8:170–171. doi: 10.4103/0975-7406.171740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayes J.D., Kelleher M.O., Eggleston I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008;47:73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- 55.Suh Y., Afaq F., Khan N., Johnson J.J., Khusro F.H., Mukhtar H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis. 2010;31:1424–1433. doi: 10.1093/carcin/bgq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao X., Liu B., Cao W., Zhang W., Zhang F., Zhao H., Meng R., Zhang L., Niu R., Hao X. Autophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cells. Chinese J. Cancer Res. 2013;25:212. doi: 10.3978/j.issn.1000-9604.2013.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hung J.-Y., Hsu Y.-L., Li C.-T., Ko Y.-C., Ni W.-C., Huang M.-S., Kuo P.-L. 6-Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells. J. Agric. Food Chem. 2009;57:9809–9816. doi: 10.1021/jf902315e. [DOI] [PubMed] [Google Scholar]
- 58.Gossner G., Choi M., Tan L., Fogoros S., Griffith K.A., Kuenker M., Liu J.R. Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol. Oncol. 2007;105:23–30. doi: 10.1016/j.ygyno.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Choi E.J., Ahn W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008;2:322–325. doi: 10.4162/nrp.2008.2.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uddin S.J. Griffith University; 2011. Cytotoxicity Screening of Bangladeshi Medicinal Plants and Isolation and Structural Elucidation of Novel Anti-cancer Compounds from Acrostichum aureum. [Google Scholar]
- 61.Yang P.-M., Tseng H.-H., Peng C.-W., Chen W.-S., Chiu S.-J. Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int. J. Oncol. 2012;40:469–478. doi: 10.3892/ijo.2011.1203. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Q., Peng C., Zheng C., He X.H., Huang W., Han B. Recent advances in characterizing natural products that regulate autophagy. Anticancer Agents Med. Chem. 2019;19:2177–2196. doi: 10.2174/1871520619666191015104458. [DOI] [PubMed] [Google Scholar]
- 63.Choudhari A.S., Mandave P.C., Deshpande M., Ranjekar P., Prakash O. Phytochemicals in Cancer treatment: from preclinical studies to clinical practice. Front. Pharmacol. 2019;10:1614. doi: 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kashyap D., Sharma A., Tuli H.S., Sak K., Garg V.K., Buttar H.S., Setzer W.N., Sethi G. Apigenin: a natural bioactive flavone-type molecule with promising therapeutic function. J. Funct. Foods. 2018;48:457–471. [Google Scholar]
- 65.Imran M., Salehi B., Sharifi-Rad J., Aslam Gondal T., Saeed F., Imran A., Shahbaz M., Tsouh Fokou P.V., Umair Arshad M., Khan H. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24:2277. doi: 10.3390/molecules24122277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mosieniak G., Adamowicz M., Alster O., Jaskowiak H., Szczepankiewicz A.A., Wilczynski G.M., Ciechomska I.A., Sikora E. Curcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagy. Mech. Ageing Dev. 2012;133:444–455. doi: 10.1016/j.mad.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 67.Liu H., Zhou Y., Tang L. Caffeine induces sustained apoptosis of human gastric cancer cells by activating the caspase‑9/caspase‑3 signalling pathway. Mol. Med. Rep. 2017;16:2445–2454. doi: 10.3892/mmr.2017.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki R., Kang Ya., Li X., Roife D., Zhang R., Fleming J.B. Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res. 2014;34:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 69.Ji L., Liu T., Liu J., Chen Y., Wang Z. Andrographolide inhibits human hepatoma-derived Hep3B cell growth through the activation of c-Jun N-terminal kinase. Planta Med. 2007;73:1397–1401. doi: 10.1055/s-2007-990230. [DOI] [PubMed] [Google Scholar]
- 70.Liu X., Kang J., Wang H., Huang T. Mitochondrial ROS contribute to oridonin-induced HepG2 apoptosis through PARP activation. Oncol. Lett. 2018;15:2881–2888. doi: 10.3892/ol.2017.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan K.T., Meng F.Y., Li Q., Ho C.Y., Lam T.S., To Y., Lee W.H., Li M., Chu K.H., Toh M. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010;294:118–124. doi: 10.1016/j.canlet.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 72.Lee Y.J., Kim N.-Y., Suh Y.-A., Lee C. Involvement of ROS in curcumin-induced autophagic cell death. Korean J. Physiol. Pharmacol. 2011;15:1–7. doi: 10.4196/kjpp.2011.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patra S., Bhol C.S., Panigrahi D.P., Praharaj P.P., Pradhan B., Jena M., Bhutia S.K. Gamma irradiation promotes chemo-sensitization potential of gallic acid through attenuation of autophagic flux to trigger apoptosis in an NRF2 inactivation signalling pathway. Free Radic. Biol. Med. 2020;160:111–124. doi: 10.1016/j.freeradbiomed.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 74.Patra S., Panda P.K., Naik P.P., Panigrahi D.P., Praharaj P.P., Bhol C.S., Mahapatra K.K., Padhi P., Jena M., Patil S. Terminalia bellirica extract induces anticancer activity through modulation of apoptosis and autophagy in oral squamous cell carcinoma. Food Chem. Toxicol. 2020;136 doi: 10.1016/j.fct.2019.111073. [DOI] [PubMed] [Google Scholar]
- 75.Huang L., Song Y., Lian J., Wang Z. Allicin inhibits the invasion of lung adenocarcinoma cells by altering tissue inhibitor of metalloproteinase/matrix metalloproteinase balance via reducing the activity of phosphoinositide 3-kinase/AKT signaling. Oncol. Lett. 2017;14:468–474. doi: 10.3892/ol.2017.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ozpolat B., Dalby K., Lopez-Berestein G. Springer; 2012. Induction of Autophagy by Polyphenolic Compounds in Cancer: A Novel Strategy to Induce Cell Death and to Treat Cancer, Natural Compounds as Inducers of Cell Death; pp. 237–261. [Google Scholar]
- 77.Reyes-Farias M., Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019;20:3177. doi: 10.3390/ijms20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takeuchi R., Hoshijima H., Nagasaka H., Chowdhury S.A., Kikuchi H., Kanda Y., Kunii S., Kawase M., Sakagami H. Induction of non-apoptotic cell death by morphinone in human promyelocytic leukemia HL-60 cells. Anticancer Res. 2006;26:3343–3348. [PubMed] [Google Scholar]
- 79.Naithani R., Huma L.C., Moriarty R.M., McCormick D.L., Mehta R.G. Comprehensive review of cancer chemopreventive agents evaluated in experimental carcinogenesis models and clinical trials. Curr. Med. Chem. 2008;15:1044–1071. doi: 10.2174/092986708784221403. [DOI] [PubMed] [Google Scholar]
- 80.Franceschi C., Capri M., Monti D., Giunta S., Olivieri F., Sevini F., Panourgia M.P., Invidia L., Celani L., Scurti M. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 81.Palmieri B., Sblendorio V. Oxidative stress tests: overview on reliability and use. Eur. Rev. Med. Pharmacol. Sci. 2007;11:383–399. [PubMed] [Google Scholar]
- 82.Moosavi M.A., Sharifi M., Ghafary S.M., Mohammadalipour Z., Khataee A., Rahmati M., Hajjaran S., Łos M.J., Klonisch T., Ghavami S. Photodynamic N-TiO 2 nanoparticle treatment induces controlled ROS-mediated autophagy and terminal differentiation of leukemia cells. Sci. Rep. 2016;6:1–16. doi: 10.1038/srep34413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J., Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol. Ther. 2008;7:1875–1884. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- 84.Chen S., Rehman S.K., Zhang W., Wen A., Yao L., Zhang J. Autophagy is a therapeutic target in anticancer drug resistance. Biochimica et Biophysica Acta (BBA)-Rev. Cancer. 2010;1806:220–229. doi: 10.1016/j.bbcan.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Marquez R.T., Tsao B.W., Faust N.F., Xu L. Drug resistance and molecular cancer therapy: apoptosis versus autophagy. Apoptosis. 2013:155–177. [Google Scholar]
- 86.Farombi E.O. Diet-related cancer and prevention using anticarcinogens. Afr. J. Biotechnol. 2004;3:651–661. [Google Scholar]
- 87.Surh Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 88.Khambete N., Kumar R. Carcinogens and cancer preventors in diet. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014;4:4. [Google Scholar]
- 89.Surh Y.-J. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. Mol. Mech. Mutagen. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 90.Bartoszek A. Chemical and Functional Properties of Food Components. 2002. Mutagenic, carcinogenic, and chemo-preventive compounds in foods; pp. 307–336. [Google Scholar]
- 91.Ng C.Y., Yen H., Hsiao H.-Y., Su S.-C. Phytochemicals in skin cancer prevention and treatment: an updated review. Int. J. Mol. Sci. 2018;19:941. doi: 10.3390/ijms19040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gliszczynska-Swigło A., Oszmianski J. Food Oxidants and Antioxidants: Chemical, Biological, and Functional Properties. 2013. 14 antioxidant and prooxidant activity of food components; p. 375. [Google Scholar]
- 93.Li H.-Q., Luo Y., Qiao C.-H. The mechanisms of anticancer agents by genistein and synthetic derivatives of isoflavone. Mini Rev. Med. Chem. 2012;12:350–362. doi: 10.2174/138955712799829258. [DOI] [PubMed] [Google Scholar]
- 94.Chen H., Zhu B., Zhao L., Liu Y., Zhao F., Feng J., Jin Y., Sun J., Geng R., Wei Y. Allicin inhibits proliferation and invasion in vitro and in vivo via SHP-1-Mediated STAT3 signaling in Cholangiocarcinoma. Cell. Physiol. Biochem. 2018;47:641–653. doi: 10.1159/000490019. [DOI] [PubMed] [Google Scholar]
- 95.Dash S.G., Suri C., Nagireddy P.K.R., Kantevari S., Naik P.K. Rational design of 9-vinyl-phenyl noscapine as potent tubulin binding anticancer agent and evaluation of the effects of its combination on Docetaxel. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1785945. [DOI] [PubMed] [Google Scholar]
- 96.Esnaashari S.S., Muhammadnejad S., Amanpour S., Amani A. A combinational approach towards treatment of breast Cancer: an analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech. 2020;21:166. doi: 10.1208/s12249-020-01710-3. [DOI] [PubMed] [Google Scholar]
- 97.Luo H., Rankin G.O., Liu L., Daddysman M.K., Jiang B.H., Chen Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer. 2009;61:554–563. doi: 10.1080/01635580802666281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thoppil R.J., Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011;3:228. doi: 10.4254/wjh.v3.i9.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoshida M., Matsui Y., Iizuka A., Ikarashi Y. G2-phase arrest through p21(WAF1 / Cip1) induction and cdc2 repression by gnidimacrin in human hepatoma HLE cells. Anticancer Res. 2009;29:1349–1354. [PubMed] [Google Scholar]
- 100.Lian F., Wang X.D. Enzymatic metabolites of lycopene induce Nrf2‐mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int. J. Cancer. 2008;123:1262–1268. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trejo-Solís C., Pedraza-Chaverrí J., Torres-Ramos M., Jiménez-Farfán D., Cruz Salgado A., Serrano-García N., Osorio-Rico L., Sotelo J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. Based Comp. Altern. Med. 2013;2013 doi: 10.1155/2013/705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aggarwal B.B., Ichikawa H., Garodia P., Weerasinghe P., Sethi G., Bhatt I.D., Pandey M.K., Shishodia S., Nair M.G. From traditional Ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin. Ther. Targets. 2006;10:87–118. doi: 10.1517/14728222.10.1.87. [DOI] [PubMed] [Google Scholar]
- 103.Ridley A.J., Whiteside J.R., McMillan T.J., Allinson S.L. Cellular and sub-cellular responses to UVA in relation to carcinogenesis. Int. J. Radiat. Biol. 2009;85:177–195. doi: 10.1080/09553000902740150. [DOI] [PubMed] [Google Scholar]
- 104.Béliveau R., Gingras D. Dorling Kindersley Ltd; 2017. Foods to Fight Cancer: What to Eat to Reduce Your Risk. [Google Scholar]
- 105.Delmas D., Solary E., Latruffe N. Resveratrol, a phytochemical inducer of multiple cell death pathways: apoptosis, autophagy and mitotic catastrophe. Curr. Med. Chem. 2011;18:1100–1121. doi: 10.2174/092986711795029708. [DOI] [PubMed] [Google Scholar]
- 106.Bhat T.A., Singh R.P. Tumor angiogenesis–a potential target in cancer chemoprevention. Food Chem. Toxicol. 2008;46:1334–1345. doi: 10.1016/j.fct.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 107.Singh R.P., Agarwal R. Tumor angiogenesis: a potential target in cancer control by phytochemicals. Curr. Cancer Drug Targets. 2003;3:205–217. doi: 10.2174/1568009033481985. [DOI] [PubMed] [Google Scholar]
- 108.Zhou J.-R., Gugger E.T., Tanaka T., Guo Y., Blackburn G.L., Clinton S.K. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J. Nutr. 1999;129:1628–1635. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 109.Sayeed M.A., Bracci M., Lucarini G., Lazzarini R., Di Primio R., Santarelli L. Regulation of microRNA using promising dietary phytochemicals: possible preventive and treatment option of malignant mesothelioma. Biomed. Pharmacother. 2017;94:1197–1224. doi: 10.1016/j.biopha.2017.07.075. [DOI] [PubMed] [Google Scholar]
- 110.Ouhtit A., Gaur R.L., Abdraboh M., Ireland S.K., Rao P.N., Raj S.G., Al-Riyami H., Shanmuganathan S., Gupta I., Murthy S.N. Simultaneous inhibition of cell-cycle, proliferation, survival, metastatic pathways and induction of apoptosis in breast cancer cells by a phytochemical super-cocktail: genes that underpin its mode of action. J. Cancer. 2013;4:703. doi: 10.7150/jca.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hasima N., Ozpolat B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.467. e1509-e1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim H., Moon J.Y., Ahn K.S., Cho S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/596496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Su X., Jiang X., Meng L., Dong X., Shen Y., Xin Y. Anticancer activity of sulforaphane: the epigenetic mechanisms and the Nrf2 signaling pathway. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/5438179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou J.W., Wang M., Sun N.X., Qing Y., Yin T.F., Li C., Wu D. Sulforaphane-induced epigenetic regulation of Nrf2 expression by DNA methyltransferase in human Caco-2 cells. Oncol. Lett. 2019;18:2639–2647. doi: 10.3892/ol.2019.10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choi S., Singh S.V. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res. 2005;65:2035–2043. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- 116.Li S., He J., Li S., Cao G., Tang S., Tong Q., Joshi H.C. Noscapine induced apoptosis via downregulation of survivin in human neuroblastoma cells having wild type or null p53. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kannan M., Jayamohan S., Mohanakumar A.K., Moorthy R.K., Purushothama K.M., Arockiam A.J.V. Bcl-2/BCL2L12 mediated apoptosis and cell cycle arrest induced by Kaempferol through the suppression of PI3K/AKT signaling pathway in Hepatocellular carcinoma. J. Adv. Appl. Sci. Res. 2019;2 [Google Scholar]
- 118.Lee Y., Sung B., Kang Y.J., Kim D.H., Jang J.-Y., Hwang S.Y., Kim M., Lim H.S., Yoon J.-H., Chung H.Y. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014;44:1599–1606. doi: 10.3892/ijo.2014.2339. [DOI] [PubMed] [Google Scholar]
- 119.Sameiyan E., Hayes A.W., Karimi G. The effect of medicinal plants on multiple drug resistance through autophagy: a review of in vitro studies. Eur. J. Pharmacol. 2019;852:244–253. doi: 10.1016/j.ejphar.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 120.Budisan L., Gulei D., Zanoaga O.M., Irimie A.I., Sergiu C., Braicu C., Gherman C.D., Berindan-Neagoe I. Dietary intervention by phytochemicals and their role in modulating coding and non-coding genes in cancer. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsai C.-Y., Chen C.-Y., Chiou Y.-H., Shyu H.-W., Lin K.-H., Chou M.-C., Huang M.-H., Wang Y.-F. Epigallocatechin-3-gallate suppresses human herpesvirus 8 replication and induces ROS leading to apoptosis and autophagy in primary effusion lymphoma cells. Int. J. Mol. Sci. 2018;19:16. doi: 10.3390/ijms19010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li J., Qin Z., Liang Z. The prosurvival role of autophagy in Resveratrol-induced cytotoxicity in human U251 glioma cells. BMC Cancer. 2009;9:215. doi: 10.1186/1471-2407-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scarlatti F., Maffei R., Beau I., Codogno P., Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–1329. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 124.Scarlatti F., Maffei R., Beau I., Ghidoni R., Codogno P. Non-canonical autophagy: an exception or an underestimated form of autophagy? Autophagy. 2008;4:1083–1085. doi: 10.4161/auto.7068. [DOI] [PubMed] [Google Scholar]
- 125.Bądziul D., Jakubowicz-Gil J., Paduch R., Głowniak K., Gawron A. Combined treatment with quercetin and imperatorin as a potent strategy for killing HeLa and Hep-2 cells. Mol. Cell. Biochem. 2014;392:213–227. doi: 10.1007/s11010-014-2032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaminskyy V.O., Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Signal. 2014;21:86–102. doi: 10.1089/ars.2013.5746. [DOI] [PubMed] [Google Scholar]
- 127.Aneja R., Miyagi T., Karna P., Ezell T., Shukla D., Gupta M.V., Yates C., Chinni S.R., Zhau H., Chung L.W. A novel microtubule-modulating agent induces mitochondrially driven caspase-dependent apoptosis via mitotic checkpoint activation in human prostate cancer cells. Eur. J. Cancer. 2010;46:1668–1678. doi: 10.1016/j.ejca.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chougule M.B., Patel A.R., Jackson T., Singh M. Antitumor activity of Noscapine in combination with Doxorubicin in triple negative breast cancer. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miki H., Uehara N., Kimura A., Sasaki T., Yuri T., Yoshizawa K., Tsubura A. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int. J. Oncol. 2012;40:1020–1028. doi: 10.3892/ijo.2012.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tian X., Liu M., Huang X., Zhu Q., Liu W., Chen W., Zou Y., Cai Y., Huang S., Chen A. Noscapine induces apoptosis in human Colon Cancer cells by regulating mitochondrial damage and warburg effect via PTEN/PI3K/mTOR signaling pathway. Oncol. Ther. 2020;13:5419. doi: 10.2147/OTT.S232137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deng R., Tang J., Xia L.P., Li D.D., Zhou W.J., Wang L.L., Feng G.K., Zeng Y.X., Gao Y.H., Zhu X.F. ExcisaninA, a diterpenoid compound purified from Isodon MacrocalyxinD, induces tumor cells apoptosis and suppresses tumor growth through inhibition of PKB/AKT kinase activity and blockade of its signal pathway. Mol. Cancer Ther. 2009;8:873–882. doi: 10.1158/1535-7163.MCT-08-1080. [DOI] [PubMed] [Google Scholar]
- 132.Huang J., Wu L., Tashiro S., Onodera S., Ikejima T. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J. Pharmacol. Sci. 2008;107:370–379. doi: 10.1254/jphs.08044fp. [DOI] [PubMed] [Google Scholar]
- 133.Zhang M., Zhang H., Sun C., Shan X., Yang X., Li-Ling J., Deng Y. Targeted constitutive activation of signal transducer and activator of transcription 3 in human hepatocellular carcinoma cells by cucurbitacin B. Cancer Chemother. Pharmacol. 2009;63:635–642. doi: 10.1007/s00280-008-0780-0. [DOI] [PubMed] [Google Scholar]
- 134.Choi K.S. Autophagy and cancer. Exp. Mol. Med. 2012;44:109–120. doi: 10.3858/emm.2012.44.2.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patra S., Pradhan B., Nayak R., Behera C., Rout L., Jena M., Efferth T., Bhutia S.K. Seminars in Cancer Biology. 2020. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. [DOI] [PubMed] [Google Scholar]
- 136.Singletary K., Milner J. Diet, autophagy, and cancer: a review. Cancer Epidemiol. Prev. Biomarkers. 2008;17:1596–1610. doi: 10.1158/1055-9965.EPI-07-2917. [DOI] [PubMed] [Google Scholar]
- 137.Qi Q., Liu X., Li S., Joshi H.C., Ye K. Synergistic suppression of noscapine and conventional chemotherapeutics on human glioblastoma cell growth. Acta Pharmacol. Sin. 2013;34:930–938. doi: 10.1038/aps.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cui W.-Q., Wang S.-T., Pan D., Chang B., Sang L.-X. Caffeine and its main targets of colorectal cancer. World J. Gastrointest. Oncol. 2020;12:149. doi: 10.4251/wjgo.v12.i2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luo Y., Leverson J.D. New opportunities in chemosensitization and radiosensitization: modulating the DNA-damage response. Expert Rev. Anticancer Ther. 2005;5:333–342. doi: 10.1586/14737140.5.2.333. [DOI] [PubMed] [Google Scholar]
- 140.Qazi A., Pal J., Maitah M., Fulciniti M., Pelluru D., Nanjappa P., Lee S., Batchu R.B., Prasad M., Bryant C.S., Rajput S., Gryaznov S., Beer D.G., Weaver D.W., Munshi N.C., Goyal R.K., Shammas M.A. Anticancer activity of a broccoli derivative, sulforaphane, in barrett adenocarcinoma: potential use in chemoprevention and as adjuvant in chemotherapy. Transl. Oncol. 2010;3:389–399. doi: 10.1593/tlo.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Delmas D., Solary E., Latruffe N. Resveratrol, a phytochemical inducer of multiple cell death pathways: apoptosis, autophagy and mitotic catastrophe. Curr. Med. Chem. 2011;18:1100–1121. doi: 10.2174/092986711795029708. [DOI] [PubMed] [Google Scholar]
- 142.Reis-Sobreiro M., Gajate C., Mollinedo F. Involvement of mitochondria and recruitment of Fas/CD95 signaling in lipid rafts in resveratrol-mediated antimyeloma and antileukemia actions. Oncogene. 2009;28:3221–3234. doi: 10.1038/onc.2009.183. [DOI] [PubMed] [Google Scholar]
- 143.Granato M., Montani M.S.G., Romeo M.A., Santarelli R., Gonnella R., D’Orazi G., Faggioni A., Cirone M. Metformin triggers apoptosis in PEL cells and alters bortezomib-induced unfolded protein response increasing its cytotoxicity and inhibiting KSHV lytic cycle activation. Cell. Signal. 2017;40:239–247. doi: 10.1016/j.cellsig.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 144.Wang K., Liu R., Li J., Mao J., Lei Y., Wu J., Zeng J., Zhang T., Wu H., Chen L. Quercetin induces protective autophagy in gastric cancer cells: involvement of Akt-mTOR-and hypoxia-induced factor 1α-mediated signaling. Autophagy. 2011;7:966–978. doi: 10.4161/auto.7.9.15863. [DOI] [PubMed] [Google Scholar]
- 145.Sirdaarta J.P. Thesis PhD Doctorate School of Natural Sciences Griffith University; Brisbane: 2016. Phytochemical Study and Anticancer Potential of High Antioxidant Australian Native Plants. [Google Scholar]
- 146.Calaf G.M., Ponce-Cusi R., Carrión F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 2018;40:2381–2388. doi: 10.3892/or.2018.6603. [DOI] [PubMed] [Google Scholar]
- 147.Castro N.P., Rangel M.C. 2019. Sulforaphane Suppresses the Growth of Triple-negative Breast Cancer Stem-like Cells in Vitro and in Vivo; pp. 147–158. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Trejo-Solís C., Pedraza-Chaverrí J., Torres-Ramos M., Jiménez-Farfán D., Cruz Salgado A., Serrano-García N., Osorio-Rico L., Sotelo J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. Comp. Altern. Med. 2013;2013 doi: 10.1155/2013/705121. 705121-705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Singh A.V., Xiao D., Lew K.L., Dhir R., Singh S.V. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 150.Yang Z.R., Liu M., Peng X.L., Lei X.F., Zhang J.X., Dong W.G. Noscapine induces mitochondria-mediated apoptosis in human colon cancer cells in vivo and in vitro. Biochem. Biophys. Res. Commun. 2012;421:627–633. doi: 10.1016/j.bbrc.2012.04.079. [DOI] [PubMed] [Google Scholar]
- 151.Zhang H.H., Zhang Y., Cheng Y.N., Gong F.L., Cao Z.Q., Yu L.G., Guo X.L. 2018. Metformin Incombination With Curcumin Inhibits the Growth, Metastasis, and Angiogenesis of Hepatocellular Carcinoma in Vitro and in Vivo; pp. 44–56. 57. [DOI] [PubMed] [Google Scholar]
- 152.Lee J.E., Yoon S.S., Lee J.W., Moon E.Y. Curcumin-induced cell death depends on the level of autophagic flux in A172 and U87MG human glioblastoma cells. Chin. J. Nat. Med. 2020;18:114–122. doi: 10.1016/S1875-5364(20)30012-1. [DOI] [PubMed] [Google Scholar]
- 153.Zhao G., Han X., Zheng S., Li Z., Sha Y., Ni J., Sun Z., Qiao S., Song Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016;35:1065–1074. doi: 10.3892/or.2015.4413. [DOI] [PubMed] [Google Scholar]
- 154.Jang M., Pezzuto J.M. Effects of resveratrol on 12-O-tetradecanoylphorbol-13-acetate-induced oxidative events and gene expression in mouse skin. Cancer Lett. 1998;134:81–89. doi: 10.1016/s0304-3835(98)00250-x. [DOI] [PubMed] [Google Scholar]
- 155.Ko J.H., Sethi G., Um J.Y., Shanmugam M.K., Arfuso F., Kumar A.P., Bishayee A., Ahn K.S. 2017. The Role of Resveratrol in Cancer Therapy; p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chougule M., Patel A.R., Sachdeva P., Jackson T., Singh M. Anticancer activity of Noscapine, an opioid alkaloid in combination with Cisplatin in human non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 2011;71:271–282. doi: 10.1016/j.lungcan.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Burgos-Morón E., Calderón-Montaño J.M., Salvador J., Robles A., López-Lázaro M. The dark side of curcumin. Int. J. Cancer. 2010;126:1771–1775. doi: 10.1002/ijc.24967. [DOI] [PubMed] [Google Scholar]
- 158.Goodpasture C.E., Arrighi F.E. Effects of food seasonings on the cell cycle and chromosome morphology of mammalian cells in vitro with special reference to turmeric. Food Cosmet. Toxicol. 1976;14:9–14. doi: 10.1016/s0015-6264(76)80356-2. [DOI] [PubMed] [Google Scholar]
- 159.Sharma R.A., Euden S.A., Platton S.L., Cooke D.N., Shafayat A., Hewitt H.R., Marczylo T.H., Morgan B., Hemingway D., Plummer S.M., Pirmohamed M., Gescher A.J., Steward W.P. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 160.Popat R., Plesner T., Davies F., Cook G., Cook M., Elliott P., Jacobson E., Gumbleton T., Oakervee H., Cavenagh J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013;160:714–717. doi: 10.1111/bjh.12154. [DOI] [PubMed] [Google Scholar]
- 161.Singh C.K., Ndiaye M.A., Ahmad N. Resveratrol and cancer: challenges for clinical translation. Biochim. Biophys. Acta. 2015;1852:1178–1185. doi: 10.1016/j.bbadis.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barve A., Chen C., Hebbar V., Desiderio J., Saw C.L.-L., Kong A.-N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2009;30:356–365. doi: 10.1002/bdd.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cai X., Fang Z., Dou J., Yu A., Zhai G. Bioavailability of quercetin: problems and promises. Curr. Med. Chem. 2013;20:2572–2582. doi: 10.2174/09298673113209990120. [DOI] [PubMed] [Google Scholar]