Abstract
The RAS oncogene is among the most commonly mutated in cancer. RAS mutations are identified in about half of patients diagnosed with metastatic colorectal cancer (mCRC), conferring poor prognosis and lack of response to anti-epidermal growth factor receptor (EGFR) antibodies. In the last decades, several investigational attempts failed in directly targeting RAS mutations, thus RAS was historically regarded as ‘undruggable’. Recently, novel specific KRASG12C inhibitors showed promising results in different solid tumors, including mCRC, renewing interest in this biomarker as a target. In this review, we discuss different strategies of RAS targeting in mCRC, according to literature data in both clinical and preclinical settings. We recognized five main strategies focusing on those more promising: direct RAS targeting, targeting the mitogen-activated protein kinase (MAPK) pathway, harnessing RAS through immunotherapy combinations, RAS targeting through metabolic pathways, and finally other miscellaneous approaches. Direct KRASG12C inhibition is emerging as the most promising strategy in mCRC as well as in other solid malignancies. However, despite good disease control rates, tumor response and duration of response are still limited in mCRC. At this regard, combinational approaches with anti-epidermal growth factor receptor drugs or checkpoint inhibitors have been proposed to enhance treatment efficacy, based on encouraging results achieved in preclinical studies. Besides, concomitant therapies increasing metabolic stress are currently under evaluation and expected to also provide remarkable results in RAS codon mutations apart from KRASG12C. In conclusion, based on hereby reported efforts of translational research, RAS mutations should no longer be regarded as ‘undruggable’ and future avenues are now opening for translation in the clinic in mCRC.
Key words: RAS, KRAS, sotorasib, adagrasib, colorectal cancer
Highlights
-
•
RAS mutations were historically considered ‘undruggable’ despite their high prevalence in colorectal cancer.
-
•
Many strategies targeting RAS mutations were attempted in the past with poor results across all histologies.
-
•
Novel inhibitors of the KRASG12C mutation recently obtained encouraging results in early-phase clinical trials.
-
•
Combining KRASG12C inhibition with anti-EGFR drugs or immunotherapy may provide further opportunities in colorectal cancer.
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer-related death in the Western world.1 Five-year relative overall survival (OS) is around 14% for those patients diagnosed with metastatic CRC (mCRC).1 In this setting, molecular alterations occurring in Kirsten rat sarcoma virus (KRAS), neuroblastoma RAS viral oncogene homolog (NRAS), and B-Raf proto-oncogene (BRAF) significantly worsen disease prognosis.1 In particular, RAS and BRAF mutations confer more aggressive tumor biology, shorter OS in particular in microsatellite stable (MSS) mCRC, and are negative predictive factors for response to milestone anti-epidermal growth factor receptor (EGFR) therapy (cetuximab or panitumumab).2, 3, 4, 5, 6 Accordingly, current clinical guidelines for RAS mutant (MT) MSS mCRC recommend chemotherapy (FOLFOX or FOLFIRI or FOLFOXIRI) with the addition of anti-vascular endothelial growth factor agents (bevacizumab in first line, bevacizumab or aflibercept in second line) as mainstream for early lines of treatment.7 While new therapeutic strategies are emerging in other molecular subsets, like doublet or triplet combinations of anti-EGFR, anti-BRAF, and anti-MEK [mitogen-activated protein kinase 1 (MAPK1)] harnessing BRAFV600E mutations, and checkpoint inhibitor immunotherapy in microsatellite unstable (MSI) mCRC, KRAS/NRAS mutations still represent the main clinical unmet need in this disease.7, 8, 9, 10
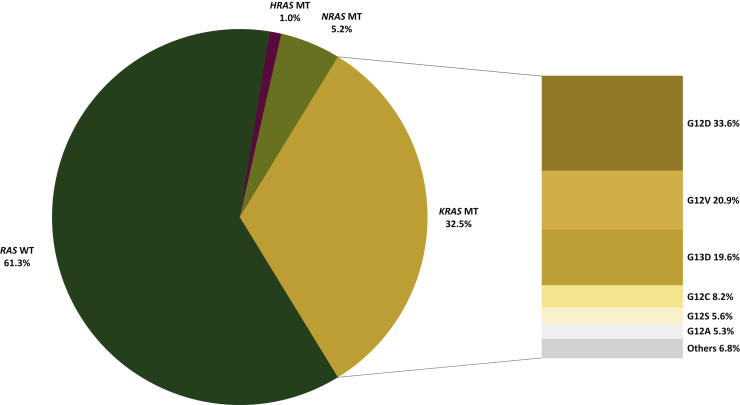
The RAS oncogene family consists of three oncogenes in humans, located in the short arm of chromosome 12, namely KRAS, NRAS, and Harvey rat sarcoma viral oncogene homolog (HRAS). The RAS family is one of the most frequently mutated across all malignancies, including CRC.11 Indeed, around 40% of CRC harbors KRAS mutations plus an additional 4% NRAS mutations (and a negligible <1% prevalence of HRAS mutations), with >95% of them occurring in KRAS G12, G13, or Q61 codons.4,11,12 G12 hotspot mutations account for around 68% of KRAS mutations in mCRC, most frequently G12D (~45%), G12V (~31%), and G12C (~11%) in MSS tumors, and predominantly G12D in MSI ones (Figure 1 and Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100156). Prevalence of KRAS mutations increases with age, except for MSI disease.13 In addition, acquired RAS mutations have been clearly demonstrated by our group and others to arise under the therapeutic pressure of EGFR blockade as secondary mechanisms of resistance.14,15
Figure 1.
Prevalence of RAS mutations in metastatic colorectal cancer.
Data were retrieved from the COSMIC database (https://cancer.sanger.ac.uk/cosmic) consulted on 27 February 2021. The percentage of mutated samples for point mutations regarding the ‘KRAS’, ‘NRAS’, and ‘HRAS’ genes was collected from COSMIC v92 using auto-filtering for ‘large intestinal’ tissue type, and ‘cecum’ or ‘left’ or ‘right’ or ‘colon’ or ‘rectum’ sub-site, and ‘carcinoma’ histology, and ‘adenocarcinoma’ sub-histology. For KRAS mutations, the most common codon variants were also collected. Raw data are available in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100156.
MT, mutant; WT, wild-type.
To assess the most efficient way of targeting the RAS oncogene, a comprehensive understanding of its biological role is needed. RAS proteins are members of a family of small guanosine triphosphate (GTP) phosphatases (GTPases) regulating many intracellular networks, which are fundamental in cell proliferation, migration, differentiation, senescence, and apoptosis.16 RAS is activated by ligand binding to membrane receptor tyrosine kinases (RTKs), including members of the human EGFR (HER) family. RAS proteins are turned off if guanosine diphosphate (GDP)-bound and turned on when GTP-bound. Despite RAS intrinsic capability of GTP hydrolysis and nucleotide exchange, this process is mainly regulated by extrinsic guanine nucleotide exchange factors (GEF) such as son of sevenless homologue 1 (SOS1) for GDP-to-GTP transition, and GTPase-activating proteins (GAP) such as neurofibromin for GTP hydrolysis.16,17 In its active GTP-bound state, RAS changes its conformation and activates several downstream effector pathways, including the MAPK pathway (RAS-RAF-MEK-MAPK or namely ERK) and phosphatidylinositol 3-kinase (PI3K) pathway (PI3K-AKT or protein kinase B-mTOR or mammalian target of rapamycin).17 To exert its function, RAS needs to be associated with the plasma membrane, through post-translational modifications of the cysteine-aliphatic-aliphatic-terminal amino acid (CAAX) motif within the hypervariable region (HVR) at the carboxyl terminus of the protein, mainly mediated by farnesyltransferase (FTase); phosphodiesterase-δ (PDEδ) chaperone protein then facilitates RAS localization to the plasma membrane.17 Oncogenic RAS mutations, altering its regulation and functioning, can lead to persistent MAPK pathway activation and unbalanced proliferative signaling.16
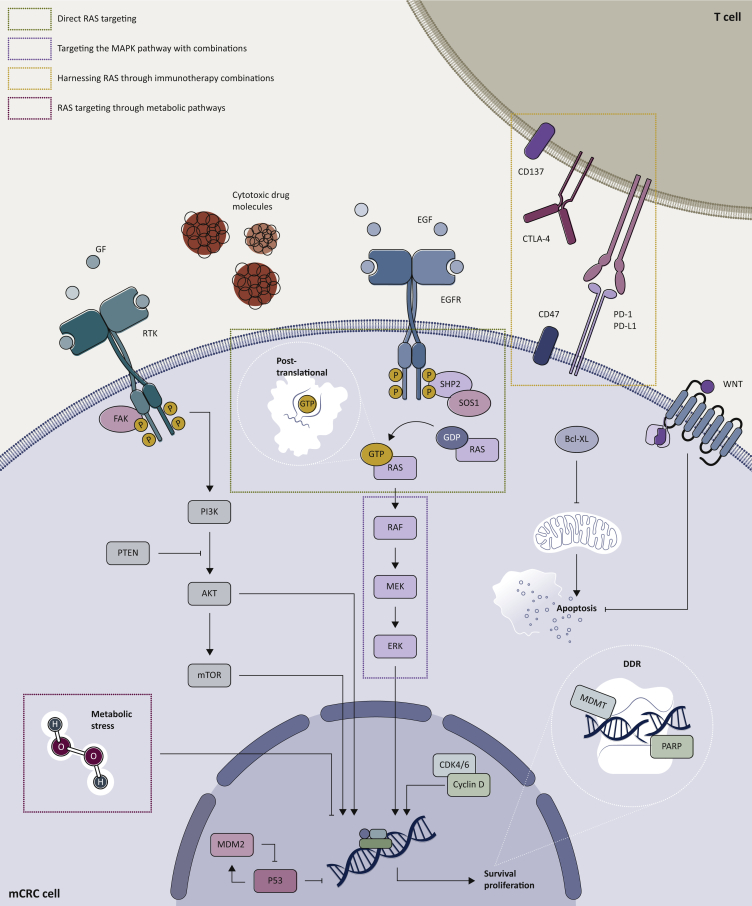
In this review, we discuss different therapeutic strategies tackling RAS mutations specifically in mCRC, focusing on those approaches which have already been tested in clinical trials (clinical trials with available results are reported in Table 1, whereas ongoing studies are listed in Table 2). Five main strategies were identified in this regard: direct RAS targeting, targeting the MAPK pathway, harnessing RAS through immunotherapy combinations, RAS targeting through metabolic pathways (all included in the visual summary Figure 2), and other miscellaneous approaches.
Table 1.
Clinical trials tackling RAS-mutant metastatic colorectal cancer with results available in the scientific literature
| Strategy | Drugs | Phase | RAS MT mCRC pts | ORR (%) | DCR (%) |
|---|---|---|---|---|---|
| Direct RAS targeting | |||||
| FTIs | Tipifarnib (R115777) | III | 235a | 1a (0.4) | 58a (24.7) |
| Lonafarnib (SCH 66336) | II | 21a | 0a (0.0) | 3a (14.3) | |
| BMS-214662 | I | 22a | 0a (0.0) | 0a (0.0) | |
| Statins | Simvastatin + irinotecan + cetuximab | II | 52 | 1 (1.9) | 34 (65.4) |
| KRASG12C inhibitors | Sotorasib (AMG 510) | I | 42 | 3 (7.1) | 31 (73.8) |
| Adagrasib (MRTX849) | II | 18 | 3 (16.7) | 17 (94.4) | |
| Multikinase inhibitors | Rigosertib | I | 10 | 0 (0.0) | 0 (0.0) |
| Targeting the MAPK pathway | |||||
| MEKi | Trametinib | I | 13 | 0 (0.0) | 4 (30.8) |
| Cobimetinib | I | 28 | 0 (0.0) | NA | |
| RO5126766b | I | 2 | 0 (0.0) | NA | |
| CDK4/6i | Palbociclib | II | 15 | 0 (0.0) | 5 (33.3) |
| mTORi | Temsirolimus | II | 64 | 0 (0.0) | 24 (37.5) |
| MEKi + anti-HER2 | Trametinib + lapatinib | I | 12 | 0 (0.0) | 10 (83.3) |
| MEKi + anti-EGFR | Cobimetinib + duligotuzumabc | Ib | 15 | 0 (0.0) | 5 (33.3) |
| Selumetinib + cetuximab | I | 14 | 0 (0.0) | 5 (35.7) | |
| MEKi + PI3K | Refametinib + copanlisib | Ib | 12 | 0 (0.0) | 5 (41.7) |
| Binimetinib + alpelisib | Ib | NA | 0 (0.0) | NA | |
| Cobimetinib + pictilisib | Ib | 47a | 0a (0.0) | NA | |
| Trametinib + buparlisib | Ib | 33 | 0 (0.0) | NA | |
| PD-0325901 + gedatolisibe | I | 21a | 0a (0.0) | NA | |
| Trametinib + omipalisibe | I | NA | 0 (0.0) | NA | |
| Pimasertib + voxtalisibe | I | 11 | 0 (0.0) | NA | |
| MEKi + AKT | Selumetinib + MK-2206 | I | 11 | 0 (0.0) | 1 (9.09) |
| Trametinib + afuresertib (GSK2110183) | I | 3a | 0 (0.0) | NA | |
| MEKi + ChT | Selumetinib + irinotecan | I/II | 31 | 3 (9.7) | 19 (61.2) |
| Pimasertib + FOLFIRI | I | 16 | 2 (12.5) | 11 (68.6) | |
| mTORi + ChT | Temsirolimus + irinotecand | II | 35 | 1 (2.9) | 30 (85.7) |
| MEKi + BCL-XLi | Trametinib + navitoclax | I/II | 9 | 0 (0.0) | 2 (22.2) |
| MEKi + cyclosporin A | Selumetinib + cyclosporin A | I/Ib | 14 | 1 (7.1) | 11 (78.6) |
| Harnessing RAS through immunotherapy combinations | |||||
| MEKi + anti-PD-L1 | Cobimetinib + atezolizumab | III | 183a | 1 (1.0) | 48a (26.2) |
| Anti-PD-L1 + anti-CTLA-4 + ChT | Durvalumab + tremelimumab + FOLFOX | I/II | 16 | 10 (62.5) | 14 (87.5) |
| Immunomodulator + anti-EGFR | Lenalidomide + cetuximab | II | 43 | 0 (0.0) | 9 (20.9) |
| Imprime PGG + cetuximab | II | 18 | 1 (5.6) | 10 (55.6) | |
| Magrolimab + cetuximab | Ib/II | 40 | 0 (0.0) | 18 (45.0) | |
| RAS MT vaccine | RAS MT vaccine + IL-2 and/or GM-CSF | II | 38 | 0 (0.0) | NA |
| RAS targeting through metabolic pathways | |||||
| High-dose AA + ChT | AA + FOLFOX6 or FOLFIRI | I | 10 | 3 (30.0) | 8 (80.0) |
| Other miscellaneous approaches | |||||
| Anti-PLK1 + ChT + anti-VEGF | Onvansertib + FOLFIRI + bevacizumab | I/II | 9 | 4 (44.4) | 8 (88.8) |
| Anti-DR5 + ChT | Conatumumab + FOLFIRI | II | 51 | 7 (13.7) | 35 (68.6) |
| Anti-EGFR | Cetuximab | II | 12f | 0 (0.0) | 3 (25.0) |
| Imgatuzumab | I/II | 25 | 0 (0.0) | 6 (24.0) | |
| Pan-HER inhibitors | Afatinib | II | 41 | 0 (0.0) | 5 (12.2) |
| Anti-EGFR + ChT | Imgatuzumab + FOLFIRI | II | NA | NA | NA |
AA, ascorbic acid; BCL-XL, B-cell lymphoma-extra large; CDK, cyclin-dependent kinase; ChT, cytotoxic chemotherapy; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DCR, disease control rate; EGFR, epidermal growth factor receptor; FTI, farnesyl transferase inhibitor; GM-CSF, granulocyte-monocyte colony-stimulating factor; HER2, human epidermal growth factor receptor 2; IL-2, interleukin-2; i, inhibitor; MAPK, mitogen-activated protein kinase; mCRC, metastatic colorectal cancer; MT, mutant; mTOR, mammalian target of rapamycin; ORR, overall response rate; NA, not available; PD-L1, programmed death-ligand 1; PI3K, phosphatidylinositol 3-kinase; PLK1, polo-like kinase 1; pts, patients; VEGF, vascular endothelial growth factor.
Data are reported on overall mCRC population as this trial did not provide adequate information regarding RAS mutational status.
RO5126766 is a RAF/MEK dual inhibitor.
Duligotuzumab is an EGFR/HER3 dual inhibitor.
Drug rechallenged in previously refractory patients.
PI3K/mTORi.
KRAS G13 MT mCRC.
Table 2.
Ongoing clinical trials tackling RAS-mutant metastatic colorectal cancer
| Strategy | NCT/trial name | Drugs | Phase |
|---|---|---|---|
| Direct RAS targeting | |||
| KRASG12C inhibitors | NCT03600883/CodeBreaK100 | Sotorasib (AMG 510) | II |
| NCT03785249/KRYSTAL-1 | Adagrasib (MRTX849) | I/II | |
| NCT04006301 | JNJ-74699157 | I | |
| NCT04165031b | LY3499446 | I/II | |
| KRASG12C inhibitor-based combinations | NCT04185883/CodeBreaK101 | AMG 510 with anti-PD-1, MEKi, SHP2 allosteric inhibitor, pan-ErbB inhibitor, anti-PD-L1, anti-EGFR, ChT, mTORi, or CDK4/6i | I |
| NCT03785249/KRYSTAL-1 | MRTX849 with pembrolizumab, cetuximab or afatininb | I/II | |
| NCT04330664/KRYSTAL-2 | MRTX849 + TNO155 (SHP2 inhibitor) | I/II | |
| NCT04793958/KRYSTAL-10 | MRTX849 + cetuximab versus ChT | III | |
| KRAS-derived mRNA binder | NCT03101839 | AZD4785 | I |
| SOS1 inhibitor | NCT04111458 | BI 1701963 ± trametinib (MEKi) | I |
| SHP2 inhibitors | NCT03634982 | RMC-4630 | I |
| NCT03518554 | JAB-3068 | I | |
| NCT03565003 | JAB-3068 | I/II | |
| SHP2 inhibitor-based combinations | NCT03989115 | RMC-4630 with osimertinib (anti-EGFR) or cobimetinib (MEKi) | I/II |
| Targeting the MAPK pathway | |||
| RAF/MEKi ± mTORi | NCT02407509 | RO5126766 ± everolimus | I |
| ERK inhibitors | NCT02857270 | LY3214996 ± midazolam, abemaciclib (CDK4/6i), nab-paclitaxel (ChT), gemcitabine (ChT), encorafenib (MEKi), or cetuximab (anti-EGFR) | I |
| NCT02313012 | CC-90003 | I | |
| Pan-ErbB inhibitor-based combinations | NCT03065387 | Neratinib with everolimus (mTORi), palbociclib (CDK4/6i), or trametinib (MEKi) | I |
| cMET inhibitor + MEKi | NCT02510001 | Crizotinib with PD-0325901 or binimetinib | I |
| EGFR inhibitor + MEKi | NCT03087071 | Panitumumab ± trametinib | II |
| NCT01927341 | Panitumumab + binimetininb | I/II | |
| MEKi + CDK4/6i | NCT02065063 | Trametinib + palbociclib | I |
| NCT03981614 | Binimetinib + palbociclib | II | |
| FAK inhibitor + RAF/MEKi | NCT03875820/FRAME trial | VS-6063 + RO5126766 | I |
| PI3K + MEKi | NCT01337765 | BEZ235c + binimetinib | I |
| NCT01859351 | WX-037 ± WX-554 | I | |
| MEKi + ChT | NCT02613650 | Binimetinib + mFOLFIRI | I |
| NCT03317119 | Trametinib + TAS-102 | I | |
| MEKi + MDM2 inhibitor | NCT03714958 | Trametinib + HDM201 | I |
| Harnessing RAS through immunotherapy combinations | |||
| Anti PD-1 + MEKi ± anti-CTLA-4 | NCT03271047 | Nivolumab + binimetinib ± ipilimumab | I/II |
| Anti-PD-L1 + MEKi + PARPi | NCT03637491 | Avelumab + binimetinib + talazoparib | I/II |
| Anti-CTLA-4 + anti-PD-L1 + ChT | NCT03202758/MEDETREME | Tremelimumab + durvalumab + FOLFOX | I/II |
| Anti PD-1 + ChT + anti-VEGF | NCT04194359 | Sintilimab + XELOX + bevacizumab | III |
| CD137 agonist + ChT + anti-EGFR | NCT03290937 | Utomilumab + irinotecan + cetuximab | I |
| Adoptive cell transfer | NCT03745326 | Anti-KRASG12D/G12V mTCR PBL | I/II |
| Sequential ChT and anti-PD-1 | NCT03519412/ARETHUSA | Temozolomide followed by pembrolizumab | II |
| RAS targeting through metabolic pathways | |||
| Glutaminase inhibitor + CDK4/6i | NCT03965845 | Telaglenastat (CB-839) + palbociclib | I/II |
| Fatty acid synthase inhibitor | NCT02980029 | TVB-2640 | I |
| Metabolic damaging | NCT03146962 | High-dose i.v. vitamin C | II |
| NCT02969681 | High-dose i.v. vitamin C + FOLFOX ± bevacizumab | III | |
| Other miscellaneous approaches | |||
| Selective WEE1 inhibitor + ChT | NCT02906059 | Adavosertib (AZD1775) + irinotecan | I |
| TRAIL receptor agonist | NCT03082209 | Eftozanermin (ABBV-621) ± FOLFIRI and bevacizumab | I |
| Anti-EGFR + ChT | ACTRN12612000901808a/ICECREAM | Cetuximab ± irinotecan | II |
| Pan-ErbB inhibitor + anticonvulsant | NCT03919292 | Neratinib + valproate | I/II |
CDK, cyclin-dependent kinase; ChT, cytotoxic chemotherapy; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGFR, epidermal growth factor receptor; ERK, extracellular-signal-regulated kinase; FAK, focal adhesion kinase; I, inhibitor; i.v., intravenous; MEK; mTCR, murine T-cell receptor; mTOR, mammalian target of rapamycin; NCT, unique identification code given to each clinical study upon registration at ClinicalTrials.gov; PARP, poly (ADP-ribose) polymerase; PBL, peripheral blood lymphocyte; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PI3K, phosphatidylinositol 3-kinase; SHP2, Src homology region 2 domain-containing phosphatase-2; SOS1, sevenless homologue 1; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor.
Referring to Australian New Zealand Clinical Trials Registry (ANZCTR).
Early termination due to unexpected toxicity.
BEZ235 is MEK/mTORi.
Figure 2.
Main therapeutic strategies targeting RAS-mutant metastatic colorectal cancer.
Five therapeutic strategies targeting RAS-mutant metastatic colorectal cancer were identified and categorized by different pharmacodynamic interferences with the RAS signal: direct RAS targeting, targeting the MAPK pathway, harnessing RAS through immunotherapy combinations, RAS targeting through metabolic pathways, and other miscellaneous approaches. The main molecular targets are shown and grouped according to the most suitable anti-RAS therapeutic strategy. Created with BioRended.com.
Bcl-XL, B-cell lymphoma-extra large; CDK, cyclin-dependent kinase; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DDR, DNA damage response and repair; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; GF, growth factor; GDP, guanosine diphosphate; GTP, guanosine triphosphate; MAPK, mitogen-activated protein kinase; mCRC, metastatic colorectal cancer; MDM2, murine double minute 2; MGMT, O6-methylguanine-DNA methyl-transferase; mTOR, mammalian target of rapamycin; PARP, poly (ADP-ribose) polymerase; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog; RTK, receptor tyrosine kinase; SHP2, Src homology region 2 domain-containing phosphatase-2; SOS1, sevenless homologue 1.
Direct RAS targeting
Post-translational inhibitors: the ancestry of RAS-targeting
Targeting post-translational RAS modifications was one of the first attempted strategies to reduce the expression of the MAPK pathway, with the aim of preventing the RAS protein interaction with the plasma membrane, and thus the subsequent activation of its downstream signaling. Owing to the central role of farnesylation in this process and to the accessibility of HVR and CAAX motifs, FTase became a promising target.17 Despite inhibiting tumor growth in preclinical models of HRAS-driven cancers, however, FTase inhibitors (FTI) tipifarnib, lonafarnib, and BMS-214662 showed no clinical efficacy in patients with advanced RAS MT mCRC.18, 19, 20 These results have been attributed to RAS isoform-specific differences in the post-translational processing, as FTase can be superseded by alternative prenylation by geranylgeranyltransferase (GGTase) in NRAS- and KRAS-driven tumors, but not HRAS-driven ones.17 Dual targeting of FTase and GGTase has been proposed as a circumventing strategy, as well as targeting downstream RAS processing proteins like PDEδ (with deltarasin or novel PDEδ inhibitor deltazinone 1). However, these strategies have never been tested in clinical trials, since increased toxicity is expected from shared activity of these enzymes on both normal and transformed cells.21 Novel lipophilic bisphosphonate BPH1222 also showed preclinical inhibition of the post-translational processing of RAS prenylation and may be considered for future clinical trials in mCRC.22 Besides, lipid-lowering statins were proven to interfere with the post-translational modification of the RAS protein, through blockade of the mevalonate pathway by means of β-hydroxy-β-methylglutaryl-CoA reductase inhibition. The mevalonate pathway is a metabolic cascade resulting in cholesterol synthesis, together with various end-products including farnesyl and geranylgeranyl moieties, which are both critical for the post-translational prenylation of RAS. By impeding farnesylation and geranylgeranylation, statins interfere with RAS binding to the plasma membrane, and thereby its activation. In spite of this, the addition of simvastatin to anti-EGFR drugs in order to restore sensitivity reported lack of efficacy in clinical trials.23,24 Conflicting data emerged from the addition of statins to chemotherapy regimens, with negative results from a retrospective analysis of the CAIRO2 trial (with chemotherapy + bevacizumab + cetuximab).25 However, a phase II trial showed an encouraging 65.4% disease control rate (DCR) and 7.6 months progression-free survival (PFS) for irinotecan-refractory KRAS MT mCRC patients treated with irinotecan, cetuximab, and simvastatin.26
RAS direct inhibitors: new perspectives limited to the KRASG12C mutation
Differently from FTI, direct inhibition of specific RAS isoforms and codon mutations accounts for a more encouraging approach. Among the most common RAS mutations, KRASG12C has been recently demonstrated druggable.27,28 KRASG12C is identified in approximately 4% of CRCs.12 Its oncogenic activity is linked to impaired GAP-mediated hydrolysis, resulting in marked predominance of the GTP-bound active state. However, KRASG12C preserves peculiar near-wild-type (WT) intrinsic GTPase activity and thus slight GTP-to-GDP cycling ability, differently from other KRAS codon mutations.29 This diverseness enables direct inhibitors to halt the KRASG12C protein in its inactive GDP-bound conformation, by means of covalent binding to a reactive thiol group in the cysteine 12 residue.30 The discovery of this allosteric nucleotide-binding pocket (called ‘switch-II pocket’) was pioneered by Shokat and colleagues,31 and led to the development of chemical compounds irreversibly targeting KRASG12C, and translating into decreased viability and increased apoptosis in cell lines. Thenceforward, several novel inhibitors were developed. Sotorasib (AMG 510 by Amgen, Inc., Thousand Oaks, CA) has been the first small molecule to be tested in clinical trials. A phase I study with sotorasib monotherapy evaluated 42 heavily pretreated patients (median of three prior lines) with refractory KRASG12C mCRC. Overall response rate (ORR) and DCR were 7.1% (3/42) and 73.8% (31/42), respectively. Of interest, these increased to 12.0% (3/25) and 80.0% (20/25), respectively, in patients receiving the expansion phase dosage (960 mg daily). Among all dose levels, median duration of stable disease (SD) was 5.4 months and median PFS was 4.0 months. Sotorasib was well tolerated with no dose-limiting toxicities or adverse events causing treatment discontinuation.27,32 The phase II monotherapy trial is ongoing (CodeBreaK100/NCT03600883). Adagrasib (MRTX849 by Mirati Therapeutics, Inc., San Diego, CA) is another KRASG12C inhibitor which is currently being tested in a phase Ib/II clinical trial (KRYSTAL-1/NCT03785249). Of 24 patients treated with the recommended phase II dose (600 mg twice), results are available for 18 with 16.7% ORR (3/18) and 94.4% DCR (17/18). Treatment duration was ≥4 months for 55% of patients (10/18).28,33 Further phase I/II trials exploiting different KRASG12C inhibitors such as JNJ-74699157 (NCT04006301) and LY3499446 (NCT04165031) are ongoing. Given its good toxicity profile and in order to increase its efficacy, sotorasib has been combined with MEK inhibitors (MEKi), immune checkpoint inhibitors (ICIs), cytotoxic agents and anti-EGFR drugs, significantly improving inhibition of tumor growth in vivo.34, 35, 36 Several phase I/II trials combining an anti-KRASG12C together with ICIs (extensive topic addressed in the following chapter Harnessing RAS through immunotherapy combinations), MEKi, anti-AKT drugs, Src homology region 2 domain-containing phosphatase-2 (SHP2) allosteric inhibitors (TNO155), pan-HER RTK inhibitors, EGFR inhibitors, and cytotoxic agents are ongoing (CodeBreaK101/NCT04185883, KRYSTAL-1/NCT03785249, KRYSTAL 2/NCT04330664).34,37 Besides, a phase III study is now randomizing patients to receive adagrasib and cetuximab versus chemotherapy as the second-line treatment of KRASG12C MT mCRC (KRYSTAL-10/NCT04793958).
Beyond KRASG12C: future options for RAS inhibition
KRASnon-G12C mutations translate into biologically distinct proteins from KRASG12C, lacking cysteinic substrate for covalent inhibition and expressing a lower intrinsic GTP hydrolysis rate.29 Thus, novel compounds are being studied in preclinical models such as the RAS(ON) platform by Revolution Medicines (Redwood City, CA).21 Dealing with the diversity of RAS codon mutants also led to the conceiving of pan-RAS inhibitors (binding both WT and MT KRAS beyond G12C) and protein-interaction disrupters, despite concerns for their tolerability.16 Besides, the RAS protein-interaction disrupter rigosertib did not show any benefit in a clinical trial.38 Novel compounds demonstrated in vitro and in vivo activity against KRASG12D, KRASQ61H, and KRASG12V codon mutations in CRC and other histologies.39, 40, 41, 42, 43 As the KRASG12D mutation is the most common in mCRC, the development of a specific effective inhibitor would be relevant in clinical practice.11 A genetic depletion strategy by novel antisense oligonucleotide AZD4785 (binding RAS-derived mRNA) is also under clinical investigation (NCT03101839).44 Finally, GEF inhibition was identified as a potential target, through binding of effectors such as SOS1 and SHP2, the latter being a scaffold protein that increases SOS1 nucleotide exchange activity by tethering SOS1 together with growth factor receptor-bound protein 2 (GRB2).45 A currently ongoing phase I clinical trial is testing the SOS1 inhibitor BI-170196 (NCT04111458). Inhibitors of SHP2, like RMC-4630 and JAB-3068, are also in phase I/II clinical trials, alone or combined with MEKi (NCT03634982, NCT03518554, NCT03565003, NCT04111458, NCT03989115). Strategies impeding RAS oligomerization have not reached clinical trials yet.21
Targeting the MAPK pathway
Single-agent MAPK blockade
The inhibition of MAPK effectors other than RAS represents a further strategy targeting RAS MT mCRC, mainly in the form of combination therapies targeting multiple downstream kinases or upstream membrane RTK.46 According to current evidence, MEKi were established as the cornerstone for drug association in this setting, favored over BRAF inhibitors in RAS MT cancers. Indeed, clinically approved BRAFV600E inhibitors, such as vemurafenib, dabrafenib, and encorafenib, are only effective with RAF monomers like BRAFV600, and not BRAF and CRAF dimers, and can lead to paradoxical activation of the EGFR/MAPK pathway through ERK-mediated regulatory feedback.47 Conversely, MEKi such as trametinib, binimetinib, and cobimetinib, prevent MEK phosphorylating ERK1/2, thus avoiding its dimerization and nuclear translocation.48 However, trametinib, cobimetinib, and also RO5126766 (a potent RAF/MEKi; NCT02407509 still recruiting) alone were not proven active in this subset of patients.49, 50, 51 Again, this can be explained by redundant signaling through upstream RTK and activation of parallel signal transduction cascades bypassing MEK inhibition and reactivating ERK signaling.52 Likewise, preliminary results of ERK inhibitor monotherapy with LY3214996 or CC-90003 did not show marked activity (NCT02857270, NCT02313012).53,54 Given the poor outcome with monotherapies, combinations exploiting vertical and/or horizontal (on parallel pathways) blockade have been assessed.
MEK and RTKs blockade
Several recent early studies focused on concurrent blockade of MEK and upstream RTK. For instance, MEKi were combined with anti-EGFR drugs, with the aim of overcoming primary resistance to the latter in RAS MT mCRC.14,55 The benefit of combining cetuximab, lapatinib (anti-HER2), or the EGFR/HER3 dual inhibitor duligotuzumab with MEKi produced, at most, disease stabilization with no objective responses.56, 57, 58, 59 Two phase I trials are evaluating the combination of MEKi with other RTK inhibitors, neratinib or crizotinib (NCT03065387, NCT02510001). Other trials combining MEKi and panitumumab are ongoing (NCT03087071, NCT01927341).
MEK inhibition and cell cycle regulation
MAPK pathway activation might lead to cell cycle dysregulation through the cyclin-dependent kinase (CDK) pathway, contributing to the G1-S phase progression through retinoblastoma protein phosphorylation, the latter seldom inactivated in CRC.60 Palbociclib alone showed limited activity in a phase II trial (0% ORR, 33% DCR).61 Given the limited toxicity of CDK4/6 inhibitors (CDK4/6i), trametinib was added to palbociclib which was demonstrated to be effective in KRAS MT CRC patient-derived xenograft (PDX) models. This combination has been tested in a phase Ib study (NCT02065063), the results of which have not been published yet. Up to now, the only available outcome data come from a case report of an NRAS MT mCRC patient achieving a prolonged partial response (PR) of 10.8 months with this combination.62,63 Finally, a phase II trial will compare binimetinib plus palbociclib versus trifluridine/tipiracil (TAS-102) in refractory RAS MT mCRC (NCT03981614). Focal adhesion kinase (FAK) is a major focal adhesion-associated protein kinase involved in cellular proliferation. It acts through elicitation of intracellular signal transduction pathways such as PI3K-AKT-mTOR, and inhibition of apoptosis in several types of cancer, including CRC. The FAK inhibitor VS-6063 in combination with RO5126766 (dual RAF/MEKi) is currently under investigation (NCT03875820).64
MEK and mTOR pathway inhibition
PIK3CA mutations or up-regulation of the PI3K-AKT-mTOR signaling pathway through ERBB3 gene amplification can preclude responsiveness to MEKi. Precisely, PIK3CA mutations can restore G1-S cell cycle progression making cancer cells independent from MAPK signaling.65 Available data on PI3K-AKT-mTOR pathway inhibition suggest minimal antitumor activity of mTOR inhibitors alone in KRAS MT mCRC. Indeed, temsirolimus led to 38% SD in pretreated patients.66 Combinations of MEKi with PI3K inhibitors, AKT inhibitors, or mTOR inhibitors are in clinical trials, despite several combinations having been proved ineffective in refractory mCRC. Indeed, 0% ORR was observed with combinations of MEKi plus PI3K inhibitors (copanlisib, alpelisib, pictilisib, or buparlisib), PI3K/mTOR dual inhibitors (gedatolisib, voxtalisib, or omipalisib), and AKT inhibitors (MK-2206, ipatasertib, or afuresertib).67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 Results from three similar studies are pending (NCT01337765, NCT01859351, NCT02407509).
MEKi and cytotoxic agents
MEKi can alter the expression of the B-cell lymphoma 2 (Bcl-2) family protein favoring cell apoptosis. Thus, synergistic activity of MEKi combination with cytotoxic agents was investigated.79 In a phase I/II study with selumetinib and irinotecan as second-line therapy, 9.7% ORR and 61.3% DCR were achieved.80 Likewise, temsirolimus achieved 63% SD when associated with irinotecan.66 Since chemotherapy doublets became the standard of care for RAS MT mCRC in the second-line setting, FOLFIRI plus pimasertib was evaluated in a phase I study, but early stopped due to toxicity concerns.81 However, further phase I trials with binimetinib and FOLFIRI, or trametinib plus TAS-102, are ongoing (NCT02613650, NCT03317119).
RAS MT TP53 WT CRC: prospect of further MEK-based therapy
The protein p53 is the main determinant of cell cycle arrest and a pivotal tumor suppressor encoded by the TP53 gene, even if other regulatory proteins can interfere with cell cycle functioning such as murine double minute 2 protein (MDM2). Cell cycle regulation in TP53 WT cells may be harnessed by MDM2 gene amplification or, likewise, by CDKN2A loss, which encodes the MDM2 antagonist p14ARF. Disruption of the interaction between p53 and MDM2, with subsequent reactivation of p53, represents a potential strategy in TP53 WT cancer cells.82 In RAS MT TP53 WT mCRC patients, a phase I study is evaluating the combination of trametinib and HDM201 (MDM2 inhibitor) (NCT03714958). Besides, further novel molecules are being proposed to restore TP53 activity (i.e. inhibitors of the oncogenic KRAS-induced p53-binding ‘Snail’) and may soon enter clinical trials.83
Other combinations exploiting MEK inhibition
Bcl-XL (Bcl-2-like protein 1 or Bcl-extra large) is an anti-apoptotic Bcl-2 family protein and a key suppressor of the apoptotic response to MEKi, since it binds and inhibits pro-apoptotic proteins induced by MEKi such as Bim (Bcl-2 interacting mediator of cell death). After proof of tumor regression in mouse models of RAS MT cancers, combined Bcl-XL/MEK inhibition entered clinical trials.84 In a phase Ib/II trial, navitoclax (Bcl-XL inhibitor) was given together with trametinib in subjects with RAS MT advanced solid tumors. Differently from other histologies, no sign of activity was noted in mCRC.85 Likewise, preclinical studies attributed MEKi resistance to Wnt pathway overexpression in KRAS MT cells. Thus, selumetinib combined with cyclosporin A (a non-canonical Wnt pathway modulator) achieved 5% ORR (2/38) and 47% DCR (18/38).86
Harnessing RAS through immunotherapy combinations
Immunotherapy is emerging as a new standard treatment in the upfront setting for MSI mCRC patients. However, in the RAS MT subgroup pembrolizumab was not superior to cytotoxic regimens.10 Indeed, KRAS mutations might facilitate cancer immunoescape mechanisms. In preclinical models, KRAS mutations modulate tumor microenvironment (TME), inducing immunosuppressive chemokines like interleukin (IL) 6 and IL-10, transforming growth factor-β, and granulocyte-macrophage colony-stimulating factor. This leads to M2 macrophages, myeloid-derived suppressor cells, and CD4+ regulatory T cell (Treg) recruitment, and CD8+ T cell depletion.87 Moreover, KRAS mutations were associated with programmed cell death protein 1 (PD-1) and reduction of expression of programmed death-ligand 1 (PD-L1) in MSI CRC.88
Sensitizing TME to immunotherapy by blocking the RAS pathway
MAPK pathway inhibition may revert immunosuppressive TME, thus enhancing the activity of ICIs in KRAS MT mCRC. In preclinical models, the combination of sotorasib (AMG 510) with ICIs increased intratumoral CD8+ T cells, interferon-γ (IFN-γ) pathway activation, and boosted secretion of chemokines and cytokines. In vivo, this led to sustained complete tumor regression in 9 out of 10 PDX (90%) derived from human KRASG12C MT CRC cells.34 Similar results were obtained when combining adagrasib (MRTX849) with anti-PD-1 agents.89 Thus, two clinical trials are evaluating sotorasib and adagrasib combined with anti-PD-1 (CodeBreaK101/NCT04185883, KRYSTAL-1/NCT03785249). Based on similar preclinical evidence, a MEKi and anti-PD-L1 combination has been investigated. However, in a phase III trial (IMblaze 370) atezolizumab and cobimetinib demonstrated no OS and PFS improvement compared with regorafenib in a molecular-unselected population of mCRC.90 A phase Ib/II trial is currently evaluating nivolumab and binimetinib with or without ipilimumab (anti-cytotoxic T-lymphocyte-associated protein 4, namely anti-CTLA-4) in patients with KRAS MT MSS mCRC (NCT03271047). In vitro, poly (ADP-ribose) polymerase inhibitors (PARPi) increased the formation of neoantigens triggering IFN release, potentially sensitizing immunotherapy, even if the role of PARPi in CRC remains to be established.91 In addition, MEKi decreased the expression of multiple homologous recombination (HR) components, thus sensitizing cancer cells to PARPi.92 Accordingly, a phase Ib/II study is currently investigating a triple combination of avelumab (anti-PD-L1), binimetinib, and talazoparib (PARPi) in patients with KRAS MT solid tumors (NCT03637491).
Immunotherapy and cytotoxic agents
In preclinical studies, several chemotherapy regimens showed the capability to stimulate antitumor immunity through different pathways, especially by increasing PD-L1 expression and CD8+ T-cell recruitment. In addition, PD-1/PD-L1 blockade enhanced cancer vulnerability to oxaliplatin by reducing the expression of ERK or p38 MAPK, a potential mechanism involved in secondary resistance to platinum.93 The phase I/II MEDETREME trial (NCT03202758) is evaluating tremelimumab (anti-CTLA-4) and durvalumab (anti-PD-L1) on top of FOLFOX in the upfront setting for KRAS MT mCRC. An interim analysis supported its efficacy with a 6-month PFS of 62.5% (10 of 16 patients), of which 5 were complete response (CR) and 5 PR.94 Similarly, a phase III trial is assessing the efficacy of sintilimab (anti-PD-1) in association with XELOX and bevacizumab in the same setting (NCT04194359).
Other approaches to elicit immune response
Lenalidomide is an immunomodulatory agent enhancing inflammatory response through T-cell proliferation, IL-2, IL-12, and IFN-γ up-regulation and Treg inhibition. A lenalidomide and cetuximab combination failed to achieve meaningful activity in a clinical trial of KRAS MT mCRC patients.95 Similarly, Imprime PGG (a novel innate immune cell modulator) was adopted in combination with cetuximab with poor results in RAS MT CRC.96 Based on the inhibition of phagocytosis by tumor protein CD47, magrolimab (anti-CD47 antibody, Hu5F9-G4) proved antitumoral activity in a phase I basket trial, thus a phase Ib/II trial combined with cetuximab demonstrated at most 45% SD in KRAS MT mCRC patients.97 Cancer vaccines with mutant RAS peptides were also investigated in CRC patients, demonstrating the induction of specific immune responses in 54% of patients. The adjuvant use of granulocyte-macrophage colony-stimulating factor increased the immune response up to 92%, despite no patient showing a clinical response, likely due to Treg up-regulation.98 Moreover, triggering CD137 (4-1BB), a costimulatory receptor expressed on T lymphocytes and natural killer (NK) cells, increased antibody-dependent cellular toxicity (ADCC) by NK cells in vivo. Its potential synergistic activity with cetuximab led to an ongoing phase I trial of utomilumab (CD137 agonist) plus cetuximab and irinotecan in mCRC patients including those with KRAS MT disease (NCT03290937).99 In recent times, adoptive cell transfer (ACT) entered clinical research in solid tumors. ACT exploits patients' peripheral blood lymphocytes, which are transfected with a retroviral vector encoding a murine T-cell receptor (mTCR) directed against a specific cancer antigen. After in vitro expansion, the lymphocytes are reinfused in the same patient to boost the immune response. A phase I/II trial is evaluating the administration of lymphocytes loaded with anti-KRASG12D or anti-KRASG12V mTCR in patients with KRASG12D/G12V MT cancers (NCT03745326). Finally, the usefulness of alkylating drugs has also been assessed as a bridge loophole taking advantage of tumor resilience, with the aim of providing immunotherapy to previously insensitive tumors. Some 55% of KRAS MT mCRC cells present O6-methylguanine-DNA methyltransferase methylation (dMGMT), thus implying an increased vulnerability to temozolomide and dacarbazine.100 Besides, in proficient mismatch repair (pMMR)/MSS CRC cells, temozolomide increased tumor mutational burden (TMB) and infiltrating lymphocytes with IFN-γ release.101 The phase II ARETHUSA trial (NCT03519412) is currently evaluating the effect of temozolomide priming in chemorefractory KRAS MT dMGMT pMMR/MSS mCRC patients, followed by pembrolizumab in case of TMB elevation.102
RAS targeting through metabolic pathways
KRAS mutations can induce metabolic reprogramming through enhanced glucose uptake and increased expression of glutamine metabolic proteins.103 In this regard, a phase Ib/II trial is evaluating the association of the glutaminase inhibitor telaglenastat (CB-839) plus palbociclib in pretreated KRAS MT mCRC patients (NCT03965845). Fatty acid synthase (FASN) is an enzyme involved in lipid synthesis and frequently up-regulated in KRAS MT cells. A phase I trial is testing the effect of preoperative doses of TVB-2640 (FASN inhibitor) in resectable solid tumors, including KRAS MT CRC (NCT02980029).104 In addition, it has been reported that, when exposed to high-dose ascorbic acid (AA, vitamin C), KRAS MT cells are driven to energetic crisis and cell death. Indeed, increased dehydroascorbate uptake (oxidized form of AA) requires glutathione to reduce dehydroascorbate to AA, and its depletion induces oxidative stress. Oxygen radicals are then responsible for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inactivation, which is pivotal for the high glycolytic metabolic profile of KRAS MT mCRC cells (to a greater extent than RAS WT cells).105 Besides, MAPK pathway is selectively inhibited by AA in KRAS MT CRC cells.106 Phase I clinical trials found that high dose (1.5 g/kg or 90 g/m2) intravenous AA can be safely given with chemotherapy regimens FOLFOX and FOLFIRI.107,108 A phase II trial is studying high-dose AA monotherapy in KRAS MT mCRC in a cohort of pretreated patients and in the perioperative treatment before and after Y90 radioembolization in a cohort of patients with resectable hepatic metastases (NCT03146962). Finally, a phase III randomized trial is combining high-dose AA and FOLFOX plus bevacizumab in a first-line setting (NCT02969681).
Other miscellaneous approaches
Targeting cell cycle effectors and DNA damage response system
WEE1 is an oncogenic nuclear protein kinase that operates at the G2/M checkpoint through the inactivation of CDK1 in response to DNA damage. Selective inhibition of WEE1 (i.e. by adavosertib) favors DNA damage buildup and thus promotes mitotic catastrophe.109 On this basis, a phase I trial tested adavosertib (AZD1775) and irinotecan as second-line treatment of KRAS or BRAF MT mCRC and the results are awaited (NCT02906059). Similarly, polo-like kinase 1 (PLK1) is a serine/threonine nuclear kinase regulating mitotic checkpoints and cell division, often overexpressed in CRC. PLK1 was identified as a synthetic lethal target in KRAS MT CRC, since its inhibition by onvansertib (PCM-075) induced apoptosis. Based on preclinical evidence, onvansertib plus FOLFIRI and bevacizumab in second-line treatment of KRAS MT mCRC patients achieved 44% PR and 44% SD.110 As previously discussed, the therapeutic application of PARPi in CRC was recently addressed.111 In a preclinical study of RAS or BRAF MT MSS CRC cells, around 13% of CRC lines (13/99) were highly sensitive to olaparib and displayed cross-sensitivity to oxaliplatin, potentially underpinning a defect in the HR repair pathway.112 Another potential tumor target is the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Due to its in vivo capacity to induce selective apoptosis in tumor cells, TRAIL receptor agonists like eftozanermin (ABBV-621) were developed and tested in clinical trials. To activate the apoptotic cascade, these drugs need to bind to TRAIL membrane-death receptors (DR) TRAIL-R1 (DR4) and TRAIL-R2 (DR5), the expression of which was found higher in KRAS MT tumors.113 A phase I trial is now recruiting RAS MT mCRC patients to receive eftozanermin monotherapy or combined with FOLFIRI and bevacizumab (NCT03082209). Activation of DR leading to apoptosis was also investigated in a randomized phase II trial using an agonistic monoclonal antibody (moAb) against DR5 (conatumumab) combined with FOLFIRI versus FOLFIRI in the second-line setting and the experimental arm showed a trend toward an improved PFS with a better ORR.114
Anti-EGFR-based treatments for RAS MT mCRC
It is knowledge that RAS mutations cause primary and secondary resistance to anti-EGFR moABs in mCRC.4,14,55 However, different RAS codon mutations have been hypothesized to underlie discrepancies in the anti-EGFR response. Conflicting results from retrospective analyses raised doubts whether patients with KRASG13D MT mCRC could benefit from anti-EGFR drugs similarly to WT ones.115 In a phase II trial, 0% ORR and 25% DCR were observed in 12 KRASG13D MT mCRC patients treated with cetuximab monotherapy.116 A cohort of the phase II ICECREAM trial will answer this question by assessing the efficacy of cetuximab alone or in combination with irinotecan in patients with KRASG13D MT mCRC (ACTRN12612000901808).117 Pan-HER inhibitors like afatinib and neratinib were also tested in clinical trials for RAS MT mCRC patients, with the aim of expanding RTK blockade and in virtue of preclinical models indicating tumor growth inhibition in RAS MT mCRC. However, DCR with afatinib was modest (12%).118 Efficacy of neratinib in combination with divalproex sodium (histone deacetylase inhibitor) is under evaluation in a phase I/II clinical trial (NCT03919292). Finally, a novel humanized engineered anti-EGFR moAb (imgatuzumab) designed to enhance ADCC, showed poor ORR as monotherapy in EGFR-positive KRAS MT mCRC, and achieved no benefit in a phase II trial when added to FOLFIRI when compared with FOLFIRI plus cetuximab in a second-line setting, in both RAS WT and RAS MT mCRC patients.119
Discussion
RAS mutations in mCRC are moving from being only an unfavorable prognostic and predictive biomarker into an integral part of the evolving engine of dynamic preclinical and clinical research. Despite the failure of most of the previous wide-ranging approaches, novel RAS-directed drugs and therapeutic strategies have been developing, driven by the high unmet clinical need.11,16 After being considered ‘undruggable’ for decades, the RAS oncogene was finally proven actionable in clinical trials with the advent of several novel inhibitors directed against the KRASG12C subtype. These agents are certainly the most promising therapeutic discovery in this setting, even if differences in ORR emerged for mCRC as compared with non-small-cell lung cancer (NSCLC).27,28 This discrepancy might be attributed to a likely higher molecular heterogeneity and thus lower KRAS oncogene addiction in mCRC rather than NSCLC. Also, KRASG12C CRC cells deeply rely on RTK activation for their proliferation, as proven by higher detection of basal phosphorylated RTKs (including EGFR) in CRC as compared with NSCLC, and thus stronger residual ERK signaling despite KRAS inhibition in preclinical models.36,120 Nevertheless, given the good tolerability of KRASG12C inhibitors, further implementation through combinational strategies with other anticancer drugs has been proposed.27 Noteworthy, KRASG12C inhibitors combined with anti-EGFR agents might revert resistance to KRASG12C blockade in mCRC patients, as this approach was proven highly effective in CRC cells, patient-derived organoids and PDX, and is now in clinical trials.36 These studies might underlie the prospect of a new combinational strategy in the future of RAS MT mCRC, where the use of EGFR drugs has been inconceivable up to now.4 Similarly, combining the KRASG12C blockade with inhibitors of MAPK effectors, or parallel pathways such as PI3K-AKT-mTOR, was proposed in order to achieve maximal signal suppression.35 Promising proof of activity came from the combination of KRASG12C inhibitors with ICI in vivo, as the anti-inflammatory TME associated with RAS mutations was significantly reverted by these new agents in preclinical models, to a higher extent than that previously achieved by MEKi.34 These combinational strategies are now in clinical trials. Finally, an unexpected contribution might come from the supplementation of high-dose vitamin C to cytotoxic agents, which is expected to induce metabolic stress with limited additional toxicity in RAS MT mCRC cells.105,106 In conclusion, further translation research studies and clinical investigations are warranted to improve and broaden the initial promising results of RAS targeting in mCRC, learning from the limitations of previous therapeutic approaches and taking into account the peculiar histology-dependent bio-molecular features of RAS MT mCRC cells.
Acknowledgements
We thank Fondazione Oncologia Niguarda Onlus for the support and the inspiration during the writing of this article.
Funding
This work was supported by Fondazione Oncologia Niguarda Onlus.
Disclosure
SS is an advisory board member for Amgen, Bayer, Bristol-Myers Squibb, CheckmAb, Clovis, Daiichi-Sankyo, Merck, Roche-Genentech, and Seattle Genetics. ASB is an advisory board member for Amgen, Bayer, Sanofi, and Servier. FS reports grants from Pfizer, lecture fees from Novartis and Merck Sharp & Dohme, and has served on advisory board for GlaxoSmithKline, outside the submitted work. The other authors declare no conflicts of interest.
Supplementary data
References
- 1.Siegel R.L., Miller K.D., Sauer A.G. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Benvenuti S., Sartore-Bianchi A., Di Nicolantonio F. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 3.Lièvre A., Bachet J.-B., Boige V. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 4.Douillard J.-Y., Oliner K.S., Siena S. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 5.Stintzing S., Miller-Phillips L., Modest D.P. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer. 2017;79:50–60. doi: 10.1016/j.ejca.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Taieb J., Le Malicot K., Shi Q. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst. 2017;109:djw272. doi: 10.1093/jnci/djw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Cutsem E., Cervantes A., Adam R. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 8.Russo M., Di Nicolantonio F., Bardelli A. Climbing RAS, the Everest of oncogenes. Cancer Discov. 2014;4:19–21. doi: 10.1158/2159-8290.CD-13-0906. [DOI] [PubMed] [Google Scholar]
- 9.Mauri G., Bonazzina E., Amatu A. The evolutionary landscape of treatment for BRAFV600E mutant metastatic colorectal cancer. Cancers (Basel) 2021;13:137. doi: 10.3390/cancers13010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.André T., Shiu K.-K., Kim T.W. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 11.Prior I.A., Lewis P.D., Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dienstmann R., Connor K., Byrne A.T. Precision therapy in RAS mutant colorectal cancer. Gastroenterology. 2020;158:806–811. doi: 10.1053/j.gastro.2019.12.051. [DOI] [PubMed] [Google Scholar]
- 13.Serebriiskii I.G., Connelly C., Frampton G. Comprehensive characterization of RAS mutations in colon and rectal cancers in old and young patients. Nat Commun. 2019;10:3722. doi: 10.1038/s41467-019-11530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misale S., Yaeger R., Hobor S. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siravegna G., Mussolin B., Buscarino M. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:827. doi: 10.1038/nm0715-827b. [DOI] [PubMed] [Google Scholar]
- 16.Ryan M.B., Corcoran R.B. Therapeutic strategies to target RAS-mutant cancers. Nat Rev Clin Oncol. 2018;15:709–720. doi: 10.1038/s41571-018-0105-0. [DOI] [PubMed] [Google Scholar]
- 17.Simanshu D.K., Nissley D.V., McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao S., Cunningham D., de Gramont A. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol. 2004;22:3950–3957. doi: 10.1200/JCO.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S., Kemeny N., Kelsen D.P. A phase II trial of farnesyl protein transferase inhibitor SCH 66336, given by twice-daily oral administration, in patients with metastatic colorectal cancer refractory to 5-fluorouracil and irinotecan. Ann Oncol. 2002;13:1067–1071. doi: 10.1093/annonc/mdf173. [DOI] [PubMed] [Google Scholar]
- 20.Tabernero J., Rojo F., Marimón I. Phase I pharmacokinetic and pharmacodynamic study of weekly 1-hour and 24-hour infusion BMS-214662, a farnesyltransferase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2005;23:2521–2533. doi: 10.1200/JCO.2005.00.398. [DOI] [PubMed] [Google Scholar]
- 21.Moore A.R., Rosenberg S.C., McCormick F., Malek S. RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19:533–552. doi: 10.1038/s41573-020-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baranyi M., Rittler D., Molnár E. Next generation lipophilic bisphosphonate shows antitumor effect in colorectal cancer in vitro and in vivo. Pathol Oncol Res. 2020;26:1957–1969. doi: 10.1007/s12253-019-00789-9. [DOI] [PubMed] [Google Scholar]
- 23.Baas J.M., Krens L.L., ten Tije A.J. Safety and efficacy of the addition of simvastatin to cetuximab in previously treated KRAS mutant metastatic colorectal cancer patients. Invest New Drugs. 2015;33:1242–1247. doi: 10.1007/s10637-015-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baas J.M., Krens L.L., Bos M.M. Safety and efficacy of the addition of simvastatin to panitumumab in previously treated KRAS mutant metastatic colorectal cancer patients. Anticancer Drugs. 2015;26:872–877. doi: 10.1097/CAD.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 25.Krens L.L., Simkens L.H.J., Baas J.M. Statin use is not associated with improved progression free survival in cetuximab treated KRAS mutant metastatic colorectal cancer patients: results from the CAIRO2 study. PLoS One. 2014;9:e112201. doi: 10.1371/journal.pone.0112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J., Hong Y.S., Hong J.Y. Effect of simvastatin plus cetuximab/irinotecan for KRAS mutant colorectal cancer and predictive value of the RAS signature for treatment response to cetuximab. Invest New Drugs. 2014;32:535–541. doi: 10.1007/s10637-014-0065-x. [DOI] [PubMed] [Google Scholar]
- 27.Hong D.S., Fakih M.G., Strickler J.H. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson M.L., Ou S.H.I., Barve M. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with colorectal cancer (CRC) and other solid tumors harboring a KRAS G12C mutation. Eur J Cancer. 2020;138:S2. [Google Scholar]
- 29.Hunter J.C., Manandhar A., Carrasco M.A. Biochemical and structural analysis of common cancer-associated KRAS mutations. Mol Cancer Res. 2015;13:1325–1335. doi: 10.1158/1541-7786.MCR-15-0203. [DOI] [PubMed] [Google Scholar]
- 30.Visscher M., Arkin M.R., Dansen T.B. Covalent targeting of acquired cysteines in cancer. Curr Opin Chem Biol. 2016;30:61–67. doi: 10.1016/j.cbpa.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostrem J.M., Peters U., Sos M.L., Wells J.A., Shokat K.M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fakih M., O'Neil B., Price T.J. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. J Clin Oncol. 2019;37:3003. [Google Scholar]
- 33.Hallin J., Engstrom L.D., Hargis L. The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canon J., Rex K., Saiki A.Y. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 35.Jiao D., Yang S. Overcoming resistance to drugs targeting KRASG12C mutation. Innovation. 2020;1:100035. doi: 10.1016/j.xinn.2020.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amodio V., Yaeger R., Arcella P. EGFR blockade reverts resistance to KRASG12C inhibition in colorectal cancer. Cancer Discov. 2020;10:1129–1139. doi: 10.1158/2159-8290.CD-20-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bar-Sagi D., Knelson E.H., Sequist L.V. A bright future for KRAS inhibitors. Nat Cancer. 2020;1:25–27. doi: 10.1038/s43018-019-0016-8. [DOI] [PubMed] [Google Scholar]
- 38.Bowles D.W., Diamond J.R., Lam E.T. Phase I study of oral rigosertib (ON 01910.Na), a dual inhibitor of the PI3K and Plk1 pathways, in adult patients with advanced solid malignancies. Clin Cancer Res. 2014;20:1656–1665. doi: 10.1158/1078-0432.CCR-13-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamoto K., Masutani T., Hirokawa T. Generation of KS-58 as the first K-Ras(G12D)-inhibitory peptide presenting anti-cancer activity in vivo. Sci Rep. 2020;10:21671. doi: 10.1038/s41598-020-78712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy M.J., Pagba C.V., Prakash P. Discovery of high-affinity noncovalent allosteric KRAS inhibitors that disrupt effector binding. ACS Omega. 2019;4:2921–2930. doi: 10.1021/acsomega.8b03308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurer T., Garrenton L.S., Oh A. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci U S A. 2012;109:5299–5304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Q., Burke J.P., Phan J. Discovery of small molecules that bind to K-ras and inhibit sos-mediated activation. Angew Chem Int Ed Engl. 2012;51:6140–6143. doi: 10.1002/anie.201201358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zorde Khvalevsky E., Gabai R., Rachmut I.H. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20723–20728. doi: 10.1073/pnas.1314307110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross S.J., Revenko A.S., Hanson L.L. Targeting KRAS-dependent tumors with AZD4785, a high-affinity therapeutic antisense oligonucleotide inhibitor of KRAS. Sci Transl Med. 2017;9:eaal5253. doi: 10.1126/scitranslmed.aal5253. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed T.A., Adamopoulos C., Karoulia Z. SHP2 Drives adaptive resistance to ERK signaling inhibition in molecularly defined subsets of ERK-dependent tumors. Cell Rep. 2019;26:65–78.e5. doi: 10.1016/j.celrep.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tolcher A.W., Peng W., Calvo E. Rational approaches for combination therapy strategies targeting the MAP kinase pathway in solid tumors. Mol Cancer Ther. 2018;17:3–16. doi: 10.1158/1535-7163.MCT-17-0349. [DOI] [PubMed] [Google Scholar]
- 47.Prahallad A., Sun C., Huang S. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 48.Khokhlatchev A.V., Canagarajah B., Wilsbacher J. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 49.Infante J.R., Fecher L.A., Falchook G.S. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 50.Rosen L.S., LoRusso P., Ma W.W. A first-in-human phase I study to evaluate the MEK1/2 inhibitor, cobimetinib, administered daily in patients with advanced solid tumors. Invest New Drugs. 2016;34:604–613. doi: 10.1007/s10637-016-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chenard-Poirier M., Kaiser M., Boyd K. Results from the biomarker-driven basket trial of RO5126766 (CH5127566), a potent RAF/MEK inhibitor, in RAS- or RAF-mutated malignancies including multiple myeloma. J Clin Oncol. 2017;35:2506. [Google Scholar]
- 52.Migliardi G., Sassi F., Torti D. Inhibition of MEK and PI3K/mTOR suppresses tumor growth but does not cause tumor regression in patient-derived xenografts of RAS-mutant colorectal carcinomas. Clin Cancer Res. 2012;18:2515–2525. doi: 10.1158/1078-0432.CCR-11-2683. [DOI] [PubMed] [Google Scholar]
- 53.Pant S., Bendell J.C., Sullivan R.J. A phase I dose escalation (DE) study of ERK inhibitor, LY3214996, in advanced (adv) cancer (CA) patients (pts) J Clin Oncol. 2019;37:3001. [Google Scholar]
- 54.Mita M.M., LoRusso P., McArthur G.A. A phase Ia study of CC-90003, a selective extracellular signal-regulated kinase (ERK) inhibitor, in patients with relapsed or refractory BRAF or RAS-mutant tumors. J Clin Oncol. 2017;35:2577. [Google Scholar]
- 55.Bardelli A., Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 56.Huijberts S.C.F.A., van Geel R.M.J.M., van Brummelen E.M.J. Phase I study of lapatinib plus trametinib in patients with KRAS-mutant colorectal, non-small cell lung, and pancreatic cancer. Cancer Chemother Pharmacol. 2020;85:917–930. doi: 10.1007/s00280-020-04066-4. [DOI] [PubMed] [Google Scholar]
- 57.Lieu C.H., Hidalgo M., Berlin J.D. A phase Ib dose-escalation study of the safety, tolerability, and pharmacokinetics of cobimetinib and duligotuzumab in patients with previously treated locally advanced or metastatic cancers with mutant KRAS. Oncologist. 2017;22:e1024–e1089. doi: 10.1634/theoncologist.2017-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ledys F., Derangère V., Réda M. Anti-MEK and Anti-EGFR mAbs in RAS-mutant metastatic colorectal cancer: case series and rationale. Adv Ther. 2019;36:1480–1484. doi: 10.1007/s12325-019-00949-y. [DOI] [PubMed] [Google Scholar]
- 59.Deming D.A., Cavalcante L.L., Lubner S.J. A phase I study of selumetinib (AZD6244/ARRY-142866), a MEK1/2 inhibitor, in combination with cetuximab in refractory solid tumors and KRAS mutant colorectal cancer. Invest New Drugs. 2016;34:168–175. doi: 10.1007/s10637-015-0314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee M.S., Helms T.L., Feng N. Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models. Oncotarget. 2016;7:39595–39608. doi: 10.18632/oncotarget.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Hara M.H., Edmonds C., Farwell M. Phase II pharmacodynamic trial of palbociclib in patients with KRAS mutant colorectal cancer. J Clin Oncol. 2015;33:626. [Google Scholar]
- 62.Ziemke E.K., Dosch J.S., Maust J.D. Sensitivity of KRAS-mutant colorectal cancers to combination therapy that co-targets MEK and CDK4/6. Clin Cancer Res. 2016;22:405–414. doi: 10.1158/1078-0432.CCR-15-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan R.J., Amaria R.N., Lawrence D.P. Abstract PR06: phase 1b dose-escalation study of trametinib (MEKi) plus palbociclib (CDK4/6i) in patients with advanced solid tumors. Mol Cancer Ther. 2015;14:PR06. [Google Scholar]
- 64.Jeong K.-Y. Inhibiting focal adhesion kinase: a potential target for enhancing therapeutic efficacy in colorectal cancer therapy. World J Gastrointest Oncol. 2018;10:290–292. doi: 10.4251/wjgo.v10.i10.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarke P.A., Roe T., Swabey K. Dissecting mechanisms of resistance to targeted drug combination therapy in human colorectal cancer. Oncogene. 2019;38:5076–5090. doi: 10.1038/s41388-019-0780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spindler K.-L.G., Sorensen M.M., Pallisgaard N. Phase II trial of temsirolimus alone and in combination with irinotecan for KRAS mutant metastatic colorectal cancer: outcome and results of KRAS mutational analysis in plasma. Acta Oncol. 2013;52:963–970. doi: 10.3109/0284186X.2013.776175. [DOI] [PubMed] [Google Scholar]
- 67.Ramanathan R.K., Von Hoff D.D., Eskens F. Phase Ib trial of the PI3K inhibitor copanlisib combined with the allosteric MEK inhibitor refametinib in patients with advanced cancer. Target Oncol. 2020;15:163–174. doi: 10.1007/s11523-020-00714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juric D., Soria J.-C., Sharma S. A phase 1b dose-escalation study of BYL719 plus binimetinib (MEK162) in patients with selected advanced solid tumors. J Clin Oncol. 2014;32:9051. [Google Scholar]
- 69.Shapiro G.I., LoRusso P., Kwak E. Phase Ib study of the MEK inhibitor cobimetinib (GDC-0973) in combination with the PI3K inhibitor pictilisib (GDC-0941) in patients with advanced solid tumors. Invest New Drugs. 2020;38:419–432. doi: 10.1007/s10637-019-00776-6. [DOI] [PubMed] [Google Scholar]
- 70.Bedard P.L., Tabernero J., Janku F. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res. 2015;21:730–738. doi: 10.1158/1078-0432.CCR-14-1814. [DOI] [PubMed] [Google Scholar]
- 71.Bardia A., Gounder M., Rodon J. Phase Ib study of combination therapy with MEK inhibitor binimetinib and phosphatidylinositol 3-kinase inhibitor buparlisib in patients with advanced solid tumors with RAS/RAF alterations. Oncologist. 2020;25:e160–e169. doi: 10.1634/theoncologist.2019-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wainberg Z.A., Alsina M., Soares H.P. A multi-arm phase I study of the PI3K/mTOR inhibitors PF-04691502 and gedatolisib (PF-05212384) plus irinotecan or the MEK inhibitor PD-0325901 in advanced cancer. Target Oncol. 2017;12:775–785. doi: 10.1007/s11523-017-0530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schram A.M., Gandhi L., Mita M.M., Damstrup L., Campana F., Hidalgo M. A phase Ib dose-escalation and expansion study of the oral MEK inhibitor pimasertib and PI3K/MTOR inhibitor voxtalisib in patients with advanced solid tumours. Br J Cancer. 2018;119:1471–1476. doi: 10.1038/s41416-018-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grilley-Olson J.E., Bedard P.L., Fasolo A. A phase Ib dose-escalation study of the MEK inhibitor trametinib in combination with the PI3K/mTOR inhibitor GSK2126458 in patients with advanced solid tumors. Invest New Drugs. 2016;34:740–749. doi: 10.1007/s10637-016-0377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tolcher A.W., Khan K., Ong M. Antitumor activity in RAS-driven tumors by blocking AKT and MEK. Clin Cancer Res. 2015;21:739–748. doi: 10.1158/1078-0432.CCR-14-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Do K., Speranza G., Bishop R. Biomarker-driven phase 2 study of MK-2206 and selumetinib (AZD6244, ARRY-142886) in patients with colorectal cancer. Invest New Drugs. 2015;33:720–728. doi: 10.1007/s10637-015-0212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bendell J.C., LoRusso P., Cho D.C. Abstract CT328: clinical results of a phase Ib dose-escalation study of the Mek inhibitor cobimetinib (GDC-0973) and the Akt inhibitor ipatasertib (GDC-0068) in patients (pts) with solid tumors. Cancer Res. 2014;74:CT328. [Google Scholar]
- 78.Tolcher A.W., Patnaik A., Papadopoulos K.P. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother Pharmacol. 2015;75:183–189. doi: 10.1007/s00280-014-2615-5. [DOI] [PubMed] [Google Scholar]
- 79.Okumura S., Jänne P.A. Molecular pathways: the basis for rational combination using MEK inhibitors in KRAS-mutant cancers. Clin Cancer Res. 2014;20:4193–4199. doi: 10.1158/1078-0432.CCR-13-2365. [DOI] [PubMed] [Google Scholar]
- 80.Hochster H.S., Uboha N., Messersmith W. Phase II study of selumetinib (AZD6244, ARRY-142886) plus irinotecan as second-line therapy in patients with K-RAS mutated colorectal cancer. Cancer Chemother Pharmacol. 2015;75:17–23. doi: 10.1007/s00280-014-2609-3. [DOI] [PubMed] [Google Scholar]
- 81.Macarulla T., Cervantes A., Tabernero J. Phase I study of FOLFIRI plus pimasertib as second-line treatment for KRAS-mutated metastatic colorectal cancer. Br J Cancer. 2015;112:1874–1881. doi: 10.1038/bjc.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hata A.N., Rowley S., Archibald H.L. Synergistic activity and heterogeneous acquired resistance of combined MDM2 and MEK inhibition in KRAS mutant cancers. Oncogene. 2017;36:6581–6591. doi: 10.1038/onc.2017.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S.-H., Shen G.-N., Jung Y.S. Antitumor effect of novel small chemical inhibitors of Snail-p53 binding in K-Ras-mutated cancer cells. Oncogene. 2010;29:4576–4587. doi: 10.1038/onc.2010.208. [DOI] [PubMed] [Google Scholar]
- 84.Corcoran R.B., Cheng K.A., Hata A.N. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell. 2013;23:121–128. doi: 10.1016/j.ccr.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corcoran R.B., Do K.T., Cleary J.M. Phase I/II study of combined BCL-XL and MEK inhibition with navitoclax (N) and trametinib (T) in KRAS or NRAS mutant advanced solid tumours. Ann Oncol. 2019;30:v164. doi: 10.1158/1078-0432.CCR-23-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krishnamurthy A., Dasari A., Noonan A.M. Phase Ib results of the rational combination of selumetinib and cyclosporin A in advanced solid tumors with an expansion cohort in metastatic colorectal cancer. Cancer Res. 2018;78:5398–5407. doi: 10.1158/0008-5472.CAN-18-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dias Carvalho P., Machado A.L., Martins F., Seruca R., Velho S. Targeting the tumor microenvironment: an unexplored strategy for mutant KRAS tumors. Cancers (Basel) 2019;11:2010. doi: 10.3390/cancers11122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marco M., Chen C.-T., Choi S.-H., Pelossof R., Shia J., Garcia-Aguilar J. A KRAS mutation is associated with an immunosuppressive tumor microenvironment in mismatch-repair proficient colorectal cancer. J Clin Oncol. 2019;37:609. [Google Scholar]
- 89.Briere D.M., Calinisan A., Aranda R. Abstract LB-C09: the KRASG12C inhibitor MRTX849 reconditions the tumor immune microenvironment and leads to durable complete responses in combination with anti-PD-1 therapy in a syngeneic mouse model. Mol Cancer Ther. 2019;18 doi: 10.1158/1535-7163.MCT-20-0462. LB-LB-C09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eng C., Kim T.W., Bendell J. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20:849–861. doi: 10.1016/S1470-2045(19)30027-0. [DOI] [PubMed] [Google Scholar]
- 91.Vikas P., Borcherding N., Chennamadhavuni A., Garje R. Therapeutic potential of combining PARP inhibitor and immunotherapy in solid tumors. Front Oncol. 2020;10:570. doi: 10.3389/fonc.2020.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun C., Fang Y., Labrie M., Li X., Mills G.B. Systems approach to rational combination therapy: PARP inhibitors. Biochem Soc Trans. 2020;48:1101–1108. doi: 10.1042/BST20191092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bailly C., Thuru X., Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer. 2020;2:zcaa002. doi: 10.1093/narcan/zcaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghiringhelli F., Chibaudel B., Taieb J. Durvalumab and tremelimumab in combination with FOLFOX in patients with RAS-mutated, microsatellite-stable, previously untreated metastatic colorectal cancer (MCRC): results of the first intermediate analysis of the phase Ib/II MEDETREME trial. J Clin Oncol. 2020;38:3006. [Google Scholar]
- 95.Siena S., Van Cutsem E., Li M. Phase II open-label study to assess efficacy and safety of lenalidomide in combination with cetuximab in KRAS-mutant metastatic colorectal cancer. PLoS One. 2013;8:e62264. doi: 10.1371/journal.pone.0062264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Segal N.H., Gada P., Senzer N., Gargano M.A., Patchen M.L., Saltz L.B. A phase II efficacy and safety, open-label, multicenter study of imprime PGG injection in combination with cetuximab in patients with stage IV KRAS-mutant colorectal cancer. Clin Colorectal Cancer. 2016;15:222–227. doi: 10.1016/j.clcc.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fisher G.A., Lakhani N.J., Eng C. A phase Ib/II study of the anti-CD47 antibody magrolimab with cetuximab in solid tumor and colorectal cancer patients. J Clin Oncol. 2020;38:114. [Google Scholar]
- 98.Rahma O.E., Hamilton J.M., Wojtowicz M. The immunological and clinical effects of mutated ras peptide vaccine in combination with IL-2, GM-CSF, or both in patients with solid tumors. J Transl Med. 2014;12:55. doi: 10.1186/1479-5876-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chester C., Sanmamed M.F., Wang J., Melero I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 100.Amatu A., Sartore-Bianchi A., Moutinho C. Promoter CpG island hypermethylation of the DNA repair enzyme MGMT predicts clinical response to dacarbazine in a phase II study for metastatic colorectal cancer. Clin Cancer Res. 2013;19:2265–2272. doi: 10.1158/1078-0432.CCR-12-3518. [DOI] [PubMed] [Google Scholar]
- 101.Germano G., Lamba S., Rospo G. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552:116–120. doi: 10.1038/nature24673. [DOI] [PubMed] [Google Scholar]
- 102.Siena S., Sartore-Bianchi A., Personeni N. Pembrolizumab in MMR-proficient metastatic colorectal cancer pharmacologically primed to trigger dynamic hypermutation status: the ARETHUSA trial. J Clin Oncol. 2019;37:TPS2659. [Google Scholar]
- 103.Hutton J.E., Wang X., Zimmerman L.J. Oncogenic KRAS and BRAF drive metabolic reprogramming in colorectal cancer. Mol Cell Proteomics. 2016;15:2924–2938. doi: 10.1074/mcp.M116.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gouw A.M., Eberlin L.S., Margulis K. Oncogene KRAS activates fatty acid synthase, resulting in specific ERK and lipid signatures associated with lung adenocarcinoma. Proc Natl Acad Sci U S A. 2017;114:4300–4305. doi: 10.1073/pnas.1617709114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yun J., Mullarky E., Lu C. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aguilera O., Muñoz-Sagastibelza M., Torrejón B. Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer. Oncotarget. 2016;7:47954–47965. doi: 10.18632/oncotarget.10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stephenson C.M., Levin R.D., Spector T., Lis C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;72:139–146. doi: 10.1007/s00280-013-2179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang F., He M.-M., Wang Z.-X. Phase I study of high-dose ascorbic acid with mFOLFOX6 or FOLFIRI in patients with metastatic colorectal cancer or gastric cancer. BMC Cancer. 2019;19:460. doi: 10.1186/s12885-019-5696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Webster P.J., Littlejohns A.T., Gaunt H.J., Prasad K.R., Beech D.J., Burke D.A. AZD1775 induces toxicity through double-stranded DNA breaks independently of chemotherapeutic agents in p53-mutated colorectal cancer cells. Cell Cycle. 2017;16:2176–2182. doi: 10.1080/15384101.2017.1301329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ahn D.H., Erlander M., Ridinger M. 436P - Phase Ib/II study of the polo-like kinase 1 (PLK1) inhibitor, onvansertib, in combination with FOLFIRI and bevacizumab for second line treatment of KRAS-mutated metastatic colorectal cancer. Ann Oncol. 2020;31:S427. [Google Scholar]
- 111.Mauri G., Arena S., Siena S., Bardelli A., Sartore-Bianchi A. The DNA damage response pathway as a land of therapeutic opportunities for colorectal cancer. Ann Oncol. 2020;31:1135–1147. doi: 10.1016/j.annonc.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 112.Arena S., Corti G., Durinikova E. A subset of colorectal cancers with cross-sensitivity to olaparib and oxaliplatin. Clin Cancer Res. 2020;26:1372–1384. doi: 10.1158/1078-0432.CCR-19-2409. [DOI] [PubMed] [Google Scholar]
- 113.Kretz A.-L., Trauzold A., Hillenbrand A. TRAILblazing strategies for cancer treatment. Cancers (Basel) 2019;11:456. doi: 10.3390/cancers11040456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cohn A.L., Tabernero J., Maurel J. A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann Oncol. 2013;24:1777–1785. doi: 10.1093/annonc/mdt057. [DOI] [PubMed] [Google Scholar]
- 115.Peeters M., Douillard J.-Y., Van Cutsem E. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol. 2013;31:759–765. doi: 10.1200/JCO.2012.45.1492. [DOI] [PubMed] [Google Scholar]
- 116.Schirripa M., Loupakis F., Lonardi S. Phase II study of single-agent cetuximab in KRAS G13D mutant metastatic colorectal cancer. Ann Oncol. 2015;26:2503. doi: 10.1093/annonc/mdv385. [DOI] [PubMed] [Google Scholar]
- 117.Segelov E., Waring P., Desai J. ICECREAM: randomised phase II study of cetuximab alone or in combination with irinotecan in patients with metastatic colorectal cancer with either KRAS, NRAS, BRAF and PI3KCA wild type, or G13D mutated tumours. BMC Cancer. 2016;16:339. doi: 10.1186/s12885-016-2389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hickish T., Cassidy J., Propper D. A randomised, open-label phase II trial of afatinib versus cetuximab in patients with metastatic colorectal cancer. Eur J Cancer. 2014;50:3136–3144. doi: 10.1016/j.ejca.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 119.Bridgewater J.A., Cervantes A., Markman B. GAIN-(C): efficacy and safety analysis of imgatuzumab (GA201), a novel dual-acting monoclonal antibody (mAb) designed to enhance antibody-dependent cellular cytotoxicity (ADCC), in combination with FOLFIRI compared to cetuximab plus FOLFIRI in second-line KRAS exon 2 wild type (e2WT) or with FOLFIRI alone in mutated (e2MT) metastatic colorectal cancer (mCRC) J Clin Oncol. 2015;33:669. [Google Scholar]
- 120.Lazzari L., Corti G., Picco G. Patient-derived xenografts and matched cell lines identify pharmacogenomic vulnerabilities in colorectal cancer. Clin Cancer Res. 2019;25:6243–6259. doi: 10.1158/1078-0432.CCR-18-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.