Abstract
Innate immunity plays a prominent role in the host defense against pathogens and must be precisely regulated. As vital orchestrators in cholesterol homeostasis, microRNA-33/33* have been widely investigated in cellular metabolism. However, their role in antiviral innate immunity is largely unknown. Here, we report that VSV stimulation decreased the expression of miR-33/33* through an IFNAR-dependent manner in macrophages. Overexpression of miR-33/33* resulted in impaired RIG-I signaling, enhancing viral load and lethality whereas attenuating type I interferon production both in vitro and in vivo. In addition, miR-33/33* specifically prevented the mitochondrial adaptor mitochondrial antiviral-signaling protein (MAVS) from forming activated aggregates by targeting adenosine monophosphate activated protein kinase (AMPK), subsequently impeding the mitophagy-mediated elimination of damaged mitochondria and disturbing mitochondrial homeostasis which is indispensable for efficient MAVS activation. Our findings establish miR-33/33* as negative modulators of the RNA virus-triggered innate immune response and identify a previously unknown regulatory mechanism linking mitochondrial homeostasis with antiviral signaling pathways.
Keywords: microRNA-33/33*, type I interferon, MAVS, AMPKα, mitophagy
Subject terms: RIG-I-like receptors, miRNA in immune cells
Introduction
Innate immunity constitutes the first line of host defense against pathogen invasion. Upon infection, viral nucleic acids can serve as pathogen-associated molecular patterns (PAMPs) and be detected by pattern recognition receptors (PRRs) in host cells.1–4 Retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) are major PRRs that mediate the RNA virus-triggered antiviral response. Activated by virus-derived double-stranded RNA (dsRNA), RIG-I and melanoma differentiation-associated gene 5 (MDA5) can recruit mitochondrial antiviral-signaling protein (MAVS) as an adaptor to initiate a series of signaling cascades, triggering the production of type I interferons (IFN-α and IFN-β) as well as proinflammatory cytokines to induce the expression of a diverse set of interferon-stimulated genes (ISGs) that eventually restrict virus replication.5–7 Antiviral immune responses are sophisticated processes, where, if not properly regulated, insufficient interferon production may result in chronic infection, whereas excessive interferon production possibly leads to autoimmune or inflammatory disease. Thus, the RLR signaling pathway needs to be precisely regulated at the cellular level to ensure the elimination of invading pathogens while avoiding harmful immunopathology.8,9
MicroRNAs (miRNAs) have emerged as important posttranscriptional ‘fine-tuners’ of gene expression in response to pathophysiological stimuli, which, by binding to the 3′-untranslated region (3′-UTR) of target mRNAs, can reduce protein expression by blocking mRNA translation and/or promoting mRNA degradation.10–13 Located within the gene encoding sterol-regulatory element-binding factor-2 (SREBF-2), a transcriptional regulator of cholesterol synthesis, miR-33 and its passenger strand miR-33* were initially identified as key regulators in cholesterol homeostasis and were reported to facilitate cellular cholesterol accumulation by targeting the lipid efflux transporters ATP-binding cassette A1 (ABCA1) and ABCG1.14–17 Cumulatively, subsequent studies have established that miR-33 and miR-33* function as crucial mediators to orchestrate cellular metabolism via suppression of critical genes involved in glucose metabolism (IRS2, PGC1α, G6PC),18,19 fatty-acid oxidation (CROT, CPT1α, HADHB),18,20 bile acid synthesis (CYP7A1),21 and mitochondrial homeostasis (AMPKα, PGC1α).22,23 However, little is known about whether they have a role in innate immunity, as it is becoming increasingly evident that the cellular metabolic status is closely associated with immune cell fate and function.24–27
Recent studies have indicated that the miR-33-dependent regulation of macrophage energy status directs macrophage polarization in atherosclerosis through AMPK silencing.20,28 In addition, we have previously reported that miR-33 reduction through the IFNβ-Ch25h-25HC axis downstream of TRIF regulates TLR-induced inflammatory cytokine production via ABCA1/ABCG1-mediated control of membrane microdomains.29,30 Given that miR-33/33* have a strong impact on the cellular metabolic status and are reduced by IFNβ, we surmised that they might be involved in antiviral innate immunity. In this study, we showed that vesicular stomatitis virus (VSV) infection could decrease the expression of the Srebf2-miR-33/33* axis via the IFNAR signaling pathway. MiR-33/33* suppressed the VSV-induced antiviral response, promoting virus susceptibility and attenuating the production of type I interferons. Moreover, miR-33/33* impeded the activation of MAVS by targeting the cellular energy sensor AMPK. Suppression of AMPK inhibited mitophagy, resulting in impaired elimination of damaged mitochondria and ultimately blocked MAVS activation. Taken together, our findings demonstrate that the type I IFN-induced decrease in miR-33/33* mediates a positive feedback on RIG-I signaling and highlight a previously unrecognized biological role for miR-33/33* as part of the antiviral innate immune response, in which they serve as a hub that links the induction of type I IFNs with mitochondrial homeostasis.
Results
Viral infection decreases the expression of miR-33/33* in an IFNAR-dependent manner in macrophages
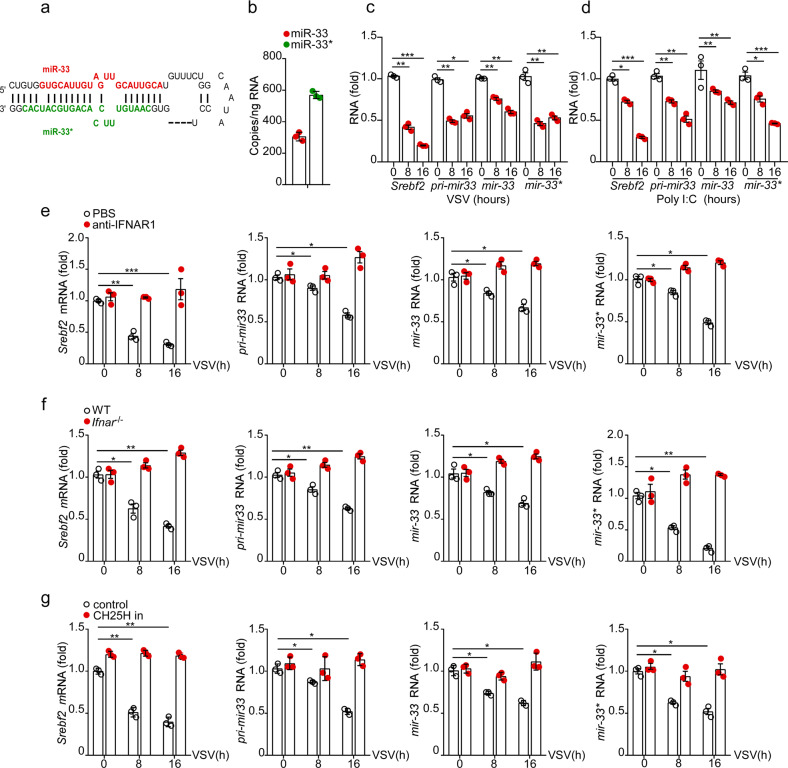
To explore the possible involvement of miR-33 and miR-33* (Fig. 1a) in host antiviral immunity, we first evaluated their expression in VSV-infected macrophages. Consistent with previous reports,31 copy numbers of miR-33* were found to be more abundant compared with miR-33 copy numbers in mouse peritoneal macrophages, suggesting that miR-33* might be equally important in this cell type (Fig. 1b). qRT-PCR results demonstrated that VSV challenge induced a progressive reduction in miR-33 and miR-33* expression. Moreover, expression of the host gene Srebf2 and the miR-33 precursor transcript (pri-mir-33), from which mature miR-33 and miR-33* arise, were also decreased following VSV infection, indicating a total repression of the Srebf2-miR-33 locus (Fig. 1c). A similar trend was also observed in macrophages stimulated with poly (I:C), a synthetic analog of viral RNA (Fig. 1d).
Fig. 1.
VSV stimulation in macrophages decreases miR-33 levels via the Ifn-Ch25h axis. a Stem loop structure of the miR-33 locus showing the guide strand miR-33 and passenger strand miR-33*. b Absolute quantitative PCR analysis of miR-33 and miR-33* copy numbers in peritoneal macrophages (MΦ). c–g qRT-PCR analysis of Srebf2 mRNA, pri-miR-33, miR-33, and miR-33* in c macrophages treated for various time with VSV (MOI = 1), d macrophages transfected for various time with the RIG-I agonist polyinosinic:polycytidylic acid (Poly (I:C), 500 ng/ml), e macrophages treated with VSV (MOI = 1) for various time in the presence of either vehicle or the anti-mouse IFNAR1 antibody (10 μg/ml), f WT or Ifnar−/− macrophages infected for various time with VSV (MOI = 1), g macrophages infected with VSV (MOI = 1) for various time in the presence of control or Ch25h siRNA (CH25H in) treatment. *p < 0.05; **p < 0.01; ***p < 0.001 (one-way analysis of variance (ANOVA) c, d or two-way ANOVA e–g). Data are presented as the mean ± s.e.m. and are representative of three independent experiments.
Srebf2 is the host gene of miR-33/33*, and the expression of it was previously demonstrated to be regulated by type I IFNs at multiple functional levels, particularly through the transcriptional suppression of Ch25h and posttranscriptional silencing of miR-342-5p.32 We preincubated macrophages with anti-IFNAR antibody prior to VSV infection and found that blocking type I IFN signaling completely rescued the VSV-induced downregulation of the miR-33 locus (Fig. 1e). In addition, VSV infection failed to decrease the expression of miR-33 and miR-33* in macrophages from Ifnar1−/− mice (Fig. 1f). qRT-PCR results revealed that the expression of both Ch25h and miR-342-5p was upregulated during VSV infection (Supplementary Fig. 1a and b). While the relief of Srebf2-miR-33 locus inhibition was observed by Ch25h knockdown using siRNA (Fig. 1g), when miR-342-5p was knocked down, the repressive effects on these transcripts were only reduced but not abrogated, suggesting that the Ch25h-dependent suppressing of Srebf2-miR-33/33* expression may predominate in our model (Supplementary Fig. 1c). Together, these results demonstrate that viral infection can reduce the expression of miR-33 and miR-33* mainly in an IFNAR-Ch25h-dependent manner in macrophages.
miR-33/33* downregulate RNA virus-triggered production of type I IFNs in macrophages
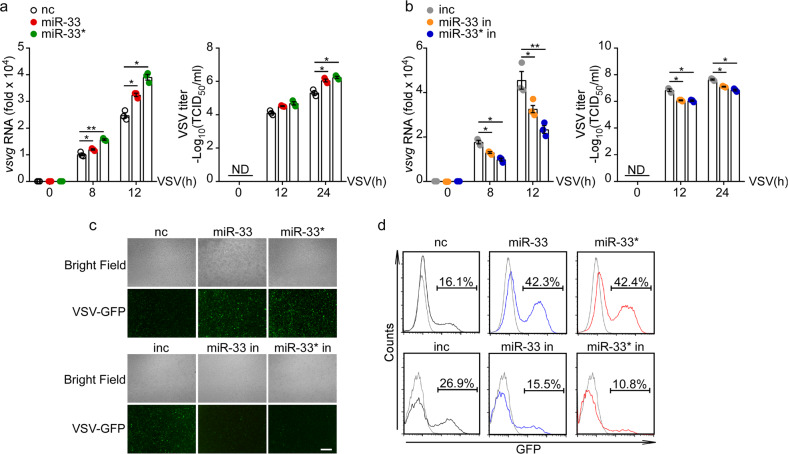
Next, we sought to determine whether changes in miR-33/33* expression influenced the host antiviral response in macrophages. We used miRNA mimics and inhibitors separately to overexpress and knockdown miR-33 and miR-33*, and then infected macrophages with VSV for different time. qRT-PCR and TCID50 results revealed that overexpression of miR-33/33* significantly enhanced the level of VSV-G transcript in macrophages as well as viral loads in supernatants post infection, whereas miR-33/33* inhibition had the opposite effect (Fig. 2a, b). By performing fluorescence microscopy and flow cytometry, we found that miR-33/33* overexpression promoted, whereas miR-33/33* inhibition reduced the replication of GFP-tagged VSV in macrophages (Fig. 2c, d), suggesting an enhanced viral susceptibility induced by miR-33/33*.
Fig. 2.
miR-33/33* promote VSV infection in macrophages. a and b qRT-PCR analysis of VSV transcripts and TCID50 determination of VSV loads in macrophages transfected with the negative control mimics (nc), miR-33 mimics (miR-33), miR-33* mimics (miR-33*) a, or negative control inhibitor (inc), miR-33 inhibitor (miR-33 in) or miR-33* inhibitor (miR-33* in) b followed by infection for the indicated time with VSV (MOI = 1). c Immunofluorescence assay of VSV-GFP in macrophages transfected with nc, miR-33, miR-33* or inc, miR-33 in, miR-33* in, followed by infection for 12 h with GFP-expressing VSV (MOI = 1 for nc/miR-33/33* mimics; MOI = 2 for inc/miR-33/33* inhibitors). Scale bars, 100 µm. d Flow cytometry analysis of GFP fluorescence intensity in macrophages treated as in c. ND, not detectable; vsvg, glycoprotein of VSV Indiana serotype. *p < 0.05; **p < 0.01 (one-way ANOVA a, b). Data are presented as the mean ± s.e.m. and are representative of three independent experiments.
Type I IFNs had a dominant effect in eliciting host defense against VSV infection, leading us to ask if the viral susceptibility conferred by miR-33/33* occurred through the impairment of the type I IFN response. In addition to a higher VSV load, miR-33/33* overexpression resulted in an impeded type I IFN response in which the transcription levels of Ifnα4 and Ifnβ, and IFN-β secretion were all decreased compared with those in control macrophages (Fig. 3a). In contrast, increased type I IFN transcription and secretion levels were observed by inhibiting miR-33/33* (Fig. 3b), indicating that miR-33/33* suppressed the type I IFN response in macrophages, which was further substantiated by qRT-PCR detection of the Isg15 (Fig. 3a, b). To ensure that those effect on interferon response was not countered by a viral protein, poly (I:C) transfection was used to mimic virus infection in macrophages, and similar results were also observed (Fig. 3c, d). On the other hand, miR-33/33* failed to affect the production of proinflammatory cytokines, e.g., Tnfα and Il6, in VSV-challenged macrophages (Supplementary Fig. 2a and b).
Fig. 3.
miR-33/33* attenuate the IFN response against VSV infection in macrophages. a and b qRT-PCR analysis of Ifnα4, Ifnβ, and Isg15 mRNA expression and ELISA of IFN-β production in macrophages transfected with nc, miR-33, or miR-33* a or inc, miR-33 in, or miR-33* in b, followed by infection for the indicated time with VSV (MOI = 1). c and d qRT-PCR analysis of Ifnα4, Ifnβ, and Isg15 mRNA expression and ELISA of IFN-β production in macrophages transfected with nc, miR-33, or miR-33* c or inc, miR-33 in, or miR-33* in d, followed by Poly (I:C) transfection (500 ng/ml) to mimic VSV infection. *p < 0.05; **p < 0.01; ***p < 0.001 (one-way ANOVA a–d). Data are presented as the mean ± s.e.m. and are representative of three independent experiments.
Unlike their modulatory effect on the RNA virus-triggered immune response, manipulation of miR-33/33* expression had negligible impact on either viral load or type I IFN production in macrophages challenged with HSV-1, a DNA virus (Supplementary Fig. 2c–e). Similar results were also observed in macrophages stimulated with poly (dA:dT), a synthetic analog of viral DNA (Supplementary Fig. 2 f and g).
Taken together, these data demonstrate that miR-33/33* suppress the type I IFN response in macrophages upon RNA virus infection.
miR-33/33* prevent MAVS from forming functional aggregates to blunt RIG-I signaling
Activation of the cytosolic dsRNA sensor RIG-I/MDA5 leads to a sophisticated network of downstream signaling cascades, among which TBK1/IKKε-induced IRF3 phosphorylation and subsequent dimerization and nucleus translocation serve as the most critical steps for type I IFN transcription.1,5,7 We therefore assessed the possible impact of miR-33/33* on these molecular events.
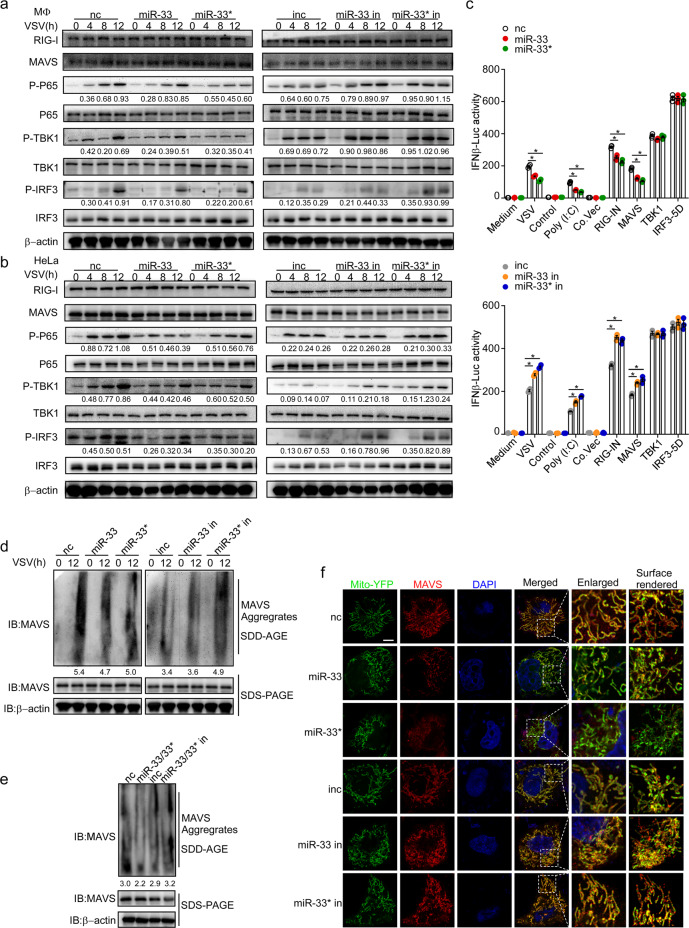
As detected by immunoblot assay, miR-33/33* overexpression led to reduced levels of phosphorylated TBK1, p65, and IRF3 in VSV-challenged macrophages, which were nevertheless enhanced by miR-33/33* inhibition (Fig. 4a). Similar results were also observed in HeLa cells infected with VSV (Fig. 4b), together indicating an impaired signal transduction mediated by miR-33/33* upon RIG-I activation in both mouse and human cells.
Fig. 4.
miR-33/33* inhibit the formation of functional MAVS aggregates to suppress RIG-I signaling. a and b Immunoblot analysis of phosphorylated (p-) or total proteins in lysates of macrophages (MΦ) a or HeLa cells b transfected with nc, miR-33, or miR-33* or inc, miR-33 in, or miR-33* in followed by infection for the indicated hours with VSV (MOI = 1). c IFN-β promoter luciferase reporter activity (IFN-β-Luc) in HEK293T cells co-transfected with nc, miR-33, or miR-33* or inc, miR-33 in, or miR-33* in, together with empty vector or an expression plasmid for the RIG-I amino-terminal ‘2CARD’ domain (RIG-IN), MAVS, TBK1, or IRF3-5D. d SDD-AGE analysis and SDS-PAGE analysis of the aggregation of MAVS in macrophages transfected as in a followed by infection for the indicated hours with VSV (MOI = 1). e SDD-AGE analysis and SDS-PAGE analysis of the aggregation of MAVS in HEK293T cells transfected with nc, miR-33 plus miR-33* (miR-33/33*), inc, or miR-33 in plus miR-33* in (miR-33/33* in) along with Flag-MAVS. f Immunofluorescence with confocal microscopy of HeLa cells transfected as in a along with the Mito-YFP plasmid and Flag-MAVS. Cellular nuclei are stained with the DNA-binding dye DAPI; Flag-MAVS is visualized using primary antibodies to MAVS followed by a secondary antibody expressing DyLight 594; enlarged region, enlargement of the area outlined on the left; surface rendered column, 3D surface rendering process of left column using Imaris 9.0. Scale bar, 2 µm. *p < 0.05 (one-way ANOVA c). Data are presented as the mean ± s.e.m. and are representative of three independent experiments.
To further investigate how miR-33/33* might interfere with RIG-I signaling, we transfected HEK293T cells with vectors expressing the RIG-I N terminus (RIG-IN), MAVS, TBK1, and a constitutively activated form of IRF3 (IRF3-5D) separately to initiate RIG-I signaling and then evaluated the effect of miR-33/33* on IFN-β induction. As shown in our data, the ability of miR-33/33* to decrease the luciferase activity of the IFNB promoter was observed in RIG-IN- and MAVS-overexpressing cells but not in TBK1- and IRF3-5D-overexpressing cells (Fig. 4c), suggesting that miR-33/33* might function downstream of RIG-I/MAVS and upstream of TBK1/IRF3.
MAVS is an indispensable signal transducer in the RIG-I pathway, where its conformational change leads to the prion-like formation of functional self-aggregates which is crucial for subsequent TBK1 and IRF3 activation.33–36 As the expression of MAVS was not affected by miR-33/33* overexpression or inhibition (Fig. 4a, b), we investigated whether miR-33/33* could interfere with certain steps involved in MAVS conformational activation. Herein, we employed semidenaturing detergent agarose gel electrophoresis (SDD-AGE), which has been previously used for the determination of prion-like structures and serves as the gold standard assay for detecting MAVS functional aggregates.34,35,37,38 Transfection of miR-33/33* mimics into macrophages led to remarkably reduced smears of SDS-resistant high-molecular weight MAVS aggregates upon VSV infection, whereas inhibiting miR-33/33* potentiated MAVS activation (Fig. 4d). Similar results were also observed in HEK293T cells co-transfected with MAVS and miR-33/33* mimics or inhibitors (Fig. 4e).
To further confirm our findings, we visualized MAVS protein in HeLa cells using confocal microscopy.34 HeLa cells were co-transfected with Mito-YFP, MAVS expression vector, and miR-33/33* mimics or inhibitors. As shown in Fig. 4f, overexpression of MAVS led to speckle-like MAVS clusters attached to mitochondria. Importantly, miR-33/33* overexpression remarkably impaired VSV-induced MAVS aggregation. Conversely, increased punctate structures were observed in cells transfected with miR-33/33* inhibitors.
Collectively, these results suggest that miR-33/33* inhibit the formation of MAVS functional self-aggregates to suppress RIG-I signaling.
miR-33/33* impair the activation of MAVS by targeting AMPK for posttranscriptional silence
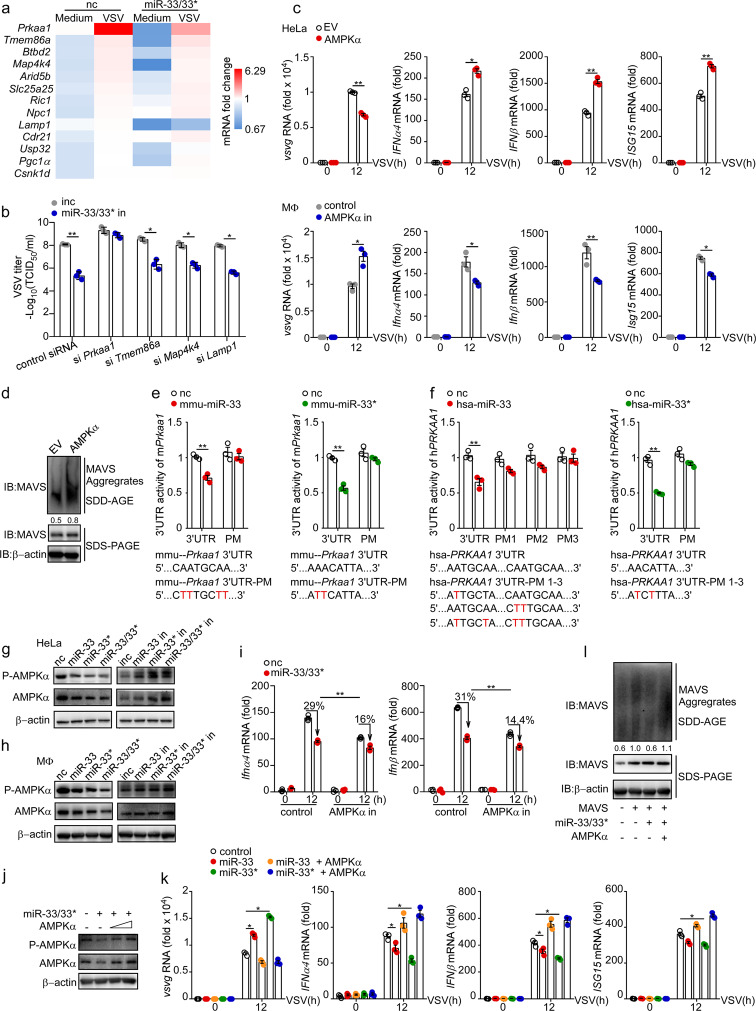
We then sought to investigate how miR-33/33* might modulate MAVS activation. Using algorithms for microRNA target prediction,39 we filtered a set of ~ 50 genes as potential targets of mouse miR-33 and miR-33* (Supplementary Table 1), among which 32 putative target genes expressed in macrophages were quantified by qRT-PCR for further validation (Supplementary Fig. 3). Four candidates, namely, Prkaa1, Tmem86a, Map4k4, and Lamp1, the expression levels of which were suppressed by miR-33/33* and increased during the antiviral response, were identified as the targets most likely to mediate the effects of miR-33/33* during VSV infection (Fig. 5a). We further evaluated the regulatory role of miR-33/33* in macrophages lacking these genes, and found that Prkaa1 inhibition largely abrogated the protective effect of miR-33/33* in during viral infection, whereas inhibition of the other candidates negligibly affected it, suggesting a special regulatory effect of miR-33/33* on Prkaa1 in this model (Fig. 5b). The gene Prkaa1 encodes the catalytic subunit of the cellular energy sensor AMPK (AMPKα) and has been reported as a target of both miR-33 and miR-33* in mouse and human cells.20,22,31 Previous studies have described a role for AMPK in antiviral immunity whereby it inhibits fatty-acid synthesis to restrict the nutrient supply necessary for virus replication.40 However, whether AMPK participates in the RIG-I signaling pathway, especially in MAVS activation, remains unclear, leading us to hypothesize that if miR-33/33* participate in type I IFN responses through interaction with AMPK.
Fig. 5.
miR-33/33* impair the activation of MAVS by targeting AMPK for posttranscriptional silence. a mRNA expression of indicated genes in peritoneal macrophages transfected with nc or miR-33/33* followed by VSV infection (MOI = 1) relative to that of uninfected macrophages transfected with nc. b TCID50 determination of VSV loads in macrophages transfected with negative control inhibitor (inc), miR-33 and miR-33* inhibitors (miR-33/33* in) along with siRNAs as indicated, followed by VSV infection for 12 h. c qRT-PCR analysis of VSV transcripts and Ifnα, Ifnβ, and Isg15 mRNA expression in HeLa cells transfected with empty vector (EV) or expression plasmid for AMPKα (encoded by PRKAA1) (top) or in macrophages transfected with control siRNA (control) or Prkaa1 siRNA (AMPK in) (bottom), followed by infection for the indicated time with VSV (MOI = 1). d SDD-AGE analysis and SDS-PAGE of the aggregation of MAVS in HeLa cells transfected with empty vector (EV) or expression plasmid for AMPKα followed by VSV infection for 12 h. e 3′-UTR luciferase reporter activity of mmu-Prkaa1 in HEK293T cells transfected with nc, mmu-miR-33 or mmu-miR-33*. The normal and mutated mmu-Prkaa1 3′-UTR sequences used are shown below. f 3′-UTR luciferase reporter activity of hsa-PRKAA1 in HEK293T cells transfected with nc, hsa-miR-33, or hsa-miR-33*. The normal and mutated hsa-PRKAA1 3′- sequences used are shown below. g and h Immunoblot analysis of phosphorylated (p-) or total AMPKα in HeLa cells g and macrophages h transfected with nc, miR-33, miR-33*, miR-33 along with miR-33* (miR-33/33*) or inc, miR-33 in, miR-33* in, miR-33 in along with miR-33* in (miR-33/33* in). i qRT-PCR analysis of Ifnα4 and Ifnβ mRNA expression in control and Prkaa1 siRNA (AMPK in)-treated macrophages, which were subsequently transfected with nc or miR-33/33*, followed by infection for the indicated hours with VSV (MOI = 1). j Immunoblot analysis of phosphorylated (p-) or total AMPKα in HeLa cells transfected with nc, miR-33/33*, EV and AMPKα (500–1000 ng/ml) as indicated. k qRT-PCR analysis of VSV transcripts and Ifnα, Ifnβ, Isg15 mRNA expression levels in HeLa cells transfected with empty vector (EV) or expression plasmid for AMPKα along with nc, miR-33, or miR-33* as indicated, followed by infection with VSV (MOI = 1) for the indicated hours relative to those of cells treated with nc plus EV (herein referred to as control). l SDD-AGE analysis and SDS-PAGE analysis of the aggregation of MAVS in HEK293T cells transfected with or without expression plasmid for MAVS, AMPKα along with nc or miR-33/33* as indicated in the figure. *p < 0.05; **p < 0.01 (Student’s t test (b, c, e), one-way ANOVA k or two-way ANOVA i). Data are presented as the mean ± s.e.m. and are representative of three independent experiments.
We first investigated the antiviral capability of AMPK in the RIG-I signaling pathway. Ectopically expressed AMPK inhibited viral infection, as evidenced by its ability to reduce the level of VSV-G transcript and to enhance the transcription of type I IFNs and ISG15 in VSV-challenged cells (Fig. 5c). The SDD-AGE results also demonstrated that AMPK overexpression boosted MAVS aggregation during VSV infection (Fig. 5d). Conversely, silencing endogenous AMPK resulted in impaired viral clearance and attenuated type I IFN response (Fig. 5c). These data suggest a protective role of AMPK in potentiating cellular antiviral responses.
We next verified the interactions between miR-33/33* and the Prkaa1 3′-UTR using the luciferase reporter assay system. Consistent with previous reports, miR-33 specifically reduced the 3'-UTR-luciferase activity of both the human and mouse PRKAA1 (Prkaa1) genes, which were also found to be significantly decreased in cells transfected with miR-33* mimics (Fig. 5e, f).31 In addition, the total and phosphorylated protein levels of both human and mouse AMPKα were also decreased by miR-33/33* overexpression but enhanced by miR-33/33* inhibition (Fig. 5g, h). These results indicated a role for miR-33/33* in the regulation of both Prkaa1 mRNA and protein expression.
To test whether miR-33/33* regulated the RIG-I signaling pathway through AMPK, we transfected miR-33/33* mimics into control and Prkaa1 siRNA-treated macrophages that were subsequently infected with VSV. miR-33/33* mimic treatment restricted Ifnα/β induction by ~ 30% in control macrophages but only by ~ 15% in AMPK-inhibited macrophages compared with control miRNA-treated cells, suggesting that miR-33/33* negatively regulated the type I IFN response, at least in part, through AMPK inhibition (Fig. 5i). Additionally, a transfection of 500 ng/ml AMPK plasmid could greatly abrogate the miR-33/33*-mediated AMPK downregulation (Fig. 5j), and replenishing AMPK (1 μg/ml) completely rescued the repressive effect of miR-33/33* on the type I IFN response, facilitating MAVS activation and Ifnα/β transcription, restricting VSV viability to an even lower viral load compared with cells transfected with control miRNA (Fig. 5k, l), hinting at a miR-33/33*-AMPK-MAVS regulatory axis in IFN production.
Taken together, these results suggest a possible regulatory network in which miR-33/33* impair the type I IFN response through AMPK inhibition.
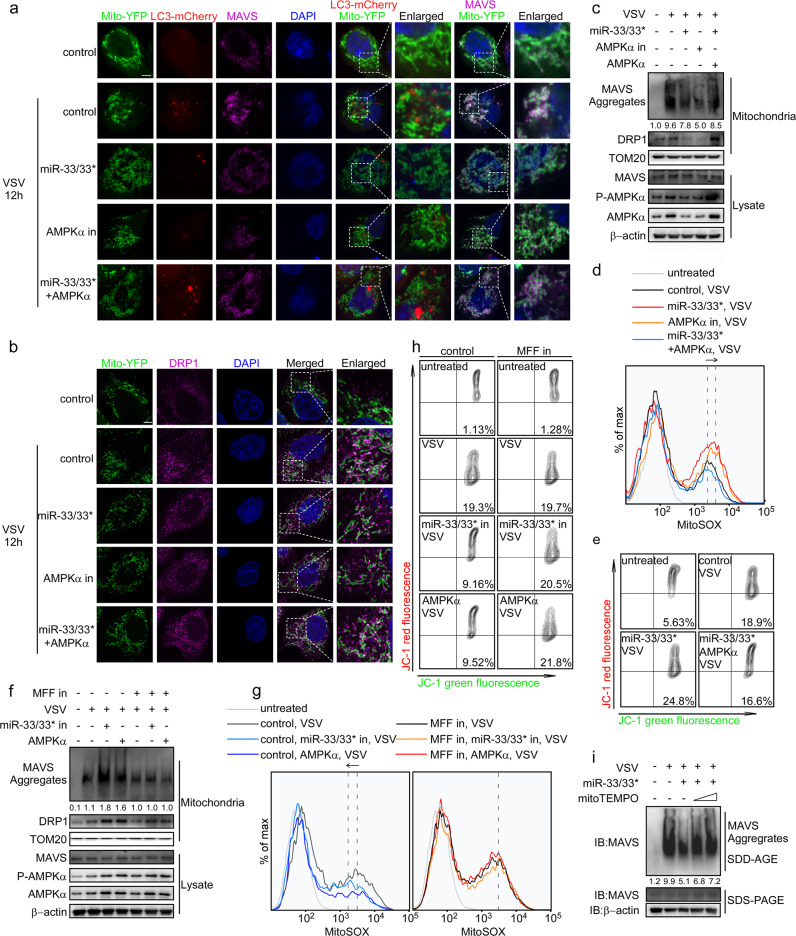
The miR-33/33*-AMPK axis modulates MAVS activation by interfering with mitophagy
AMPK has recently been revealed to participate in various aspects of mitochondrial homeostasis, including mitophagy. It has been reported that toxin-induced AMPK activation can facilitate mitochondrial fission and selectively eliminate damaged mitochondria through mitophagy, thus acting as a quality-control process upon mitochondrial stress.41 Because efficient MAVS activation requires a highly dynamic rearrangement of mitochondria that can be associated with increased mitochondrial stress, low-level mitophagy could remove those damaged mitochondria timely and ensure a robust mitochondrial platform healthy enough for further MAVS activation. We surmised that this could be the possible mechanism by which the miR-33/33*-AMPK axis engaged MAVS activation.
To test our hypothesis, we performed a set of assays detecting mitochondrial homeostasis during VSV infection. First, mitophagy and MAVS activation were visualized using confocal microscopy. As the results show, with the formation of functional MAVS clusters, increased LC3B aggregates were observed colocalized with mitochondria, implying the induction of mitophagy during VSV infection. miR-33/33* overexpression and AMPK inhibition reduced the colocalization of LC3B aggregates with mitochondria, which was accompanied by impaired MAVS activation. Accordingly, co-transfection of AMPK not only greatly rescued the miR-33/33*-induced reduction of MAVS aggregates but also enhanced mitophagy (Fig. 6a). AMPK-mediated mitophagy has been reported to depend on the phosphorylation and activation of mitochondrial fission factor (MFF), a core component of the mitochondrial fission pathway, which can subsequently increase the localization of mitochondrial fission protein DRP1 at mitochondria, thus promoting mitochondrial fragmentation and mitophagy.42,43 With this in mind, the DRP1 protein was also visualized during VSV infection. As shown in Fig. 6b, both miR-33/33* overexpression and AMPK inhibition impaired DRP1 accumulation in mitochondria, which could be rescued by replenishing AMPK. These results were further confirmed by immunoblot assay (Fig. 6c), in which reduced DRP1 levels in mitochondria were observed along with MAVS repression in cells with miR-33/33* overexpression or AMPK inhibition. Together, these results suggested the possible induction of DRP1-mediated mitophagy under the regulation of miR-33/33*-AMPK axis during VSV infection.
Fig. 6.
The miR-33/33*-AMPK axis modulates MAVS activation by interfering with mitophagy. a and b Confocal fluorescence microscopy of HeLa cells transfected with miRNAs, siRNAs or plasmids as indicated, along with Mito-YFP (1 μg/ml) and LC3-mCherry (1 μg/ml) plasmids, followed by infection for 12 h with VSV (MOI = 1). Enlarged area, enlargement of the area outlined on the left; scale bar, 2 µm. c Immunoblot analysis of the indicated proteins in HeLa cells transfected with miRNAs, siRNAs, or plasmids as indicated followed by VSV stimulation for 12 h. d Flow cytometry analysis of HeLa cells transfected with miRNAs, siRNAs, or plasmids as indicated and stained with the mitochondrial superoxide–specific stain MitoSOX after VSV infection for 12 h. e Flow cytometry analysis of HeLa cells transfected as in d and stained with JC-1 during VSV infection for 12 h. f Immunoblot analysis of the indicated proteins of HeLa cells transfected with control miRNAs, siRNAs or vectors, miR-33/33* in AMPKα expressing vectors and MFF siRNA (MFF in) as indicated followed by VSV stimulation for 12 h. g Flow cytometry analysis of HeLa cells transfected as in f stained with the mitochondrial superoxide–specific stain MitoSOX after VSV infection for 12 h. h Flow cytometry analysis of HeLa cells transfected as in f and stained with JC-1 after VSV infection for 12 h. i Macrophages transfected with nc or miR-33/33* were pretreated with MitoTEMPO (0.5–1 μm) as indicated, followed by VSV infection for 8 h (MOI = 1), and MAVS aggregation was analyzed by SDD-AGE and SDS-PAGE. Cells transfected with control miRNAs, siRNAs plus EV, are referred to as control in this figure. Data are representative of three independent experiments.
Insufficient mitophagy has been linked to the accumulation of impaired mitochondria where the increase in mitochondrial reactive oxygen species (mtROS) and disturbance of the mitochondrial membrane potential (ΔΨm) could ultimately lead to severe mitochondrial damage. Thus, we hypothesized that defective mitophagy induced by the miR-33/33*-AMPK axis during VSV infection could destabilize ΔΨm because of the accumulation of mtROS. To investigate this possibility, we examined mitochondrial superoxide production and ΔΨm, and the results showed that VSV infection increased the production of mitochondrial superoxide and the ΔΨm-sensitive color shift detected by MitoProbe JC-1 in HeLa cells with miR-33/33* overexpression or AMPK inhibition to a greater extent compared with the control cells, whereas replenishing AMPK reduced the ROS levels and restored ΔΨm (Fig. 6d, e), indicating a potential mechanism of mitophagy the miR-33/33*-AMPK axis may engage to modulate MAVS activation.
To confirm whether mitophagy was responsible for miR-33/33*-AMPK axis-mediated MAVS repression, we knocked down MFF to remove the effect of AMPKα on mitophagy and then examined the effect of the miR-33/33*-AMPK axis on MAVS activation during VSV infection. Although miR-33/33* inhibition or AMPK overexpression greatly increased MAVS aggregation after infection, this effect was abolished in cells lacking MFF (Fig. 6f). Moreover, miR-33/33* inhibition or AMPK overexpression failed to reduce mtROS release and maintain ΔΨm when mitophagy was impaired, as there was no significant difference in mtROS production and ΔΨm-sensitive color shift between controls and cells with miR-33/33* inhibition or AMPK overexpression in the MFFsi group after VSV infection (Fig. 6g, h). On the other hand, mitochondrial ROS quenching by the mitochondria-targeted antioxidant MitoTEMPO could greatly reverse miR-33/33*-induced MAVS suppression (Fig. 6i).
Therefore, we confirmed that the inhibitory effect on type I IFNs by miR-33/33* requires AMPK-mediated mitophagy during VSV infection.
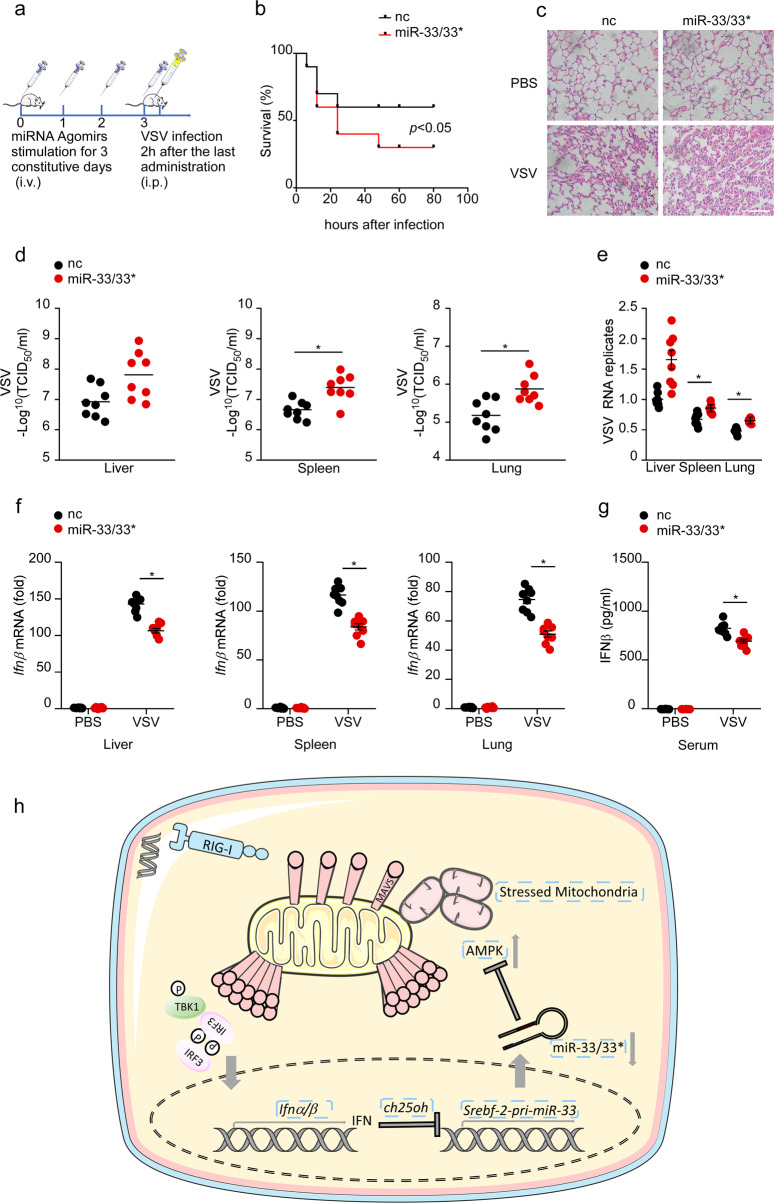
miR-33/33* agomirs predispose mice to viral infection in vivo
Finally, we investigated the physiological function of miR-33/33* in host antiviral responses in vivo. To this end, mice were primed with miR-33/33* agomir oligonucleotides through tail vein injection followed by VSV challenge (Fig. 7a). Compared with mice that received control-agomir, miR-33/33* agomir-primed mice were more susceptible to VSV infection-induced lethality and lung injury (Fig. 7b, c). As expected, mice that received miR-33/33* agomirs had significantly higher VSV titers and VSV-G transcript levels in the liver, spleen, and lung (Fig. 7d, e). Furthermore, the level of IFN-β was significantly lower in liver, spleen, lung, and serum of miR-33/33* agomir-primed mice than control mice upon VSV infection (Fig. 7f, g). These data suggest that miR-33/33* negatively regulates the VSV-induced antiviral immune response in vivo, thus rendering mice more susceptible to VSV infection.
Fig. 7.
miR-33/33* agomirs predispose mice to viral infection in vivo. a Schematic model of the in vivo miR-33/33* experiment. Mice were primed with nc or miR-33/33* agomirs through tail vein injection for 3 constitutive days followed by VSV infection (i.p.) (108 pfu per mouse, n = 8 per group). b Survival curve of 8-week-old, nc-, or miR-33/33* agomir-primed mice given intraperitoneal injection of VSV (108 pfu/g) (n = 8 per group); *p < 0.05 (log-rank test). c Hematoxylin and eosin staining of lung sections from mice treated as in a 24 h i.p. Scale bar, 100 mm. d Determination of VSV loads in organs by TCID50 assay from mice treated as in a 24 h i.p. e qRT-PCR analysis of VSV expression in organs from mice treated as in a 24 h i.p. f qRT-PCR analysis of Ifnβ mRNA expression in organs from mice treated as in a 24 h i.p. g ELISA of IFN-β production in serum from mice treated as in a 24 h i.p. h Schematic model of the regulatory role of miR-33/33* in the RIG-I signaling pathway wherein decreased Srebf2-miR-33/33* expression induced by type I IFNs produced in response to virus infection mediates positive feedback on type I IFN signaling to potentiate the antiviral state. *p < 0.05 (Student’s t test). Data are presented as the mean ± s.e.m. and are representative of three independent experiments.
Discussion
In this study, we demonstrate that miR-33/33* target the cellular energy sensor AMPK to disturb mitochondrial homeostasis, which prevents MAVS activation and finally blunts RIG-I signaling (Fig. 7h), demonstrating a new regulatory circuit that serves as an important component of the host defense against virus infection.
One striking implication of our data is that miR-33/33* blunt MAVS activation by suppressing the mitophagy process via an AMPK-dependent mechanism. Mitochondria are highly mobile organelles that constantly undergo fusion and division events to generate a dynamic and interconnected network. Mitochondria act as platforms where MAVS is embedded, and the morphology of them has been closely related to MAVS stability and activation.44–47 Studies to date support the notion that activation of the RIG-I signaling pathway triggers a fusion process of mitochondria, wherein elongated mitochondria serve to (1) facilitate MAVS redistribution, increasing the local accumulation of MAVS on a mitochondrion48; (2) promote mitochondrion-endoplasmic reticulum interactions, enhancing the association of MAVS with STING to augment RIG-I signaling49; and (3) maintain a robust ΔΨm which may be a necessary component of MAVS signaling.50 Here, we speculate that in the beginning of viral infection, when there is a high demand for RIG-I signaling to be activated, mitochondria quickly elongate in order to support efficient MAVS activation. However, with continued signaling and mitochondria continually being exposed to a high fusion activity, the concomitant mitochondrial stress must be resolved properly to avoid mitochondrial dysfunction and thus enable further MAVS activation. Previous studies have documented AMPK as an important component of mitochondrial homeostasis through activation of MFF in the mitochondrial fission pathway.42 In our present study, we confirmed that miR-33/33* post transcriptionally silenced AMPKα expression and its catalytic activity to exert their repressive effect on IFN production upon VSV infection. In addition, we showed that impaired MAVS activation induced through the miR-33/33*-AMPK axis was accompanied by a defective mitophagy process, as evidenced by decreased colocalization of DRP1 with mitochondria, increased accumulation of mtROS, and dissipation of the membrane potential (Fig. 6a–e). Moreover, blocking MFF largely abrogated the protective function of miR-33/33* inhibitor and AMPK overexpression on MAVS activation (Fig. 6f–h), thus confirming AMPK-mediated mitophagy through MFF as an indispensable node downstream of miR-33/33* in the regulation of the RIG-I signaling pathway. Therefore, we propose a model in which decreased miR-33/33* expression results in an increase in AMPKα, which promotes mitophagy to ensure a timely elimination of damaged mitochondria, and provides MAVS with a suitable platform for its further activation.
However, mitochondrial fusion and fission are balanced processes. A continuous mitophagy would lead to decreased mitochondrial mass, which perturbs MAVS expression on mitochondria and finally its activation. In our results, although a mitophagy process mediated by the miR-33/33*-AMPKα axis was emphasized, neither mitochondrial mass nor MAVS expression seems to be affected, as the protein level of TOM20, which reflects mitochondrial quantities, as well as total MAVS expression, were not changed by miR-33/33* manipulation (Figs. 4a, b, 6c, f). Thus, it remains possible that there also exist other mechanisms (e.g., mitochondrial biogenesis) controlled by miR-33/33* orchestrating together with AMPKα to regulate mitochondrial homeostasis and induce MAVS activation. To address this, we screened our predicted targets for genes related to mito-biogenesis and found that genes including Pgc1-α (a gene that has a central role in regulating cellular energy homeostasis and mitochondrial metabolism), Nrf-1 (an activator of mitochondrial biogenesis), and Slc25a25 (encodes a mitochondrial solute carrier protein) were suppressed by miR-33/33* at the mRNA level, though at a relatively small extent compared with Prkaa1 suppression. Thus, we considered the possibility that miR-33/33* manipulation may affect a network of genes including Prkaa1, Pgc1-α, Nrf-1, and Slc25a25, which cooperate to eliminate damaged mitochondria and take over with healthy ones. It will be important in future studies to mechanistically address these questions.
Finally, our observation that IFN-mediated repression of Srebf2-miR-33/33* induces MAVS activation raises the intriguing possibility that interference with cholesterol metabolic pathways constitutes one of the core properties of IFN-mediated antiviral responses. Recently, increasing evidence has indicated the protective roles of type I IFN-mediated cholesterol reprogramming as predominant effectors and regulators in defending virus infection. Previous studies have identified the sterol pathway as an important regulator involved in the protection against virus infection through the mevalonate isoprenoid branch.51 Studies by Blanc et al.52 and Liu et al.53 showed Ch25h as an ISG that mediates the production of the antiviral oxysterol 25HC, which alters cell membrane permissiveness to prevent the entry of enveloped viruses. In addition, interferon production can induce antiviral miRNAs such as miR-342-5p, which restricts viral replication by targeting multiple genes in the host cholesterol synthetic pathway, including Srebf2, for posttranscriptional silence.32 It has also been suggested that a SREBP2-mediated decrease in flux through the cholesterol biosynthetic pathway supports an increased responsiveness of the STING ligand and primes cells for heightened antiviral immunity.54,55 Here, our data imply that type I IFN-mediated regulation of the Srebf2-miR-33/33* locus ensures a timely elimination of damaged mitochondria, keeping them healthy enough to support further MAVS activation. Additional experiments will be required to fully elucidate the regulatory role of metabolic reprogramming during antiviral responses in future investigations.
In conclusion, the studies presented herein provide mechanistic insights into a previously undiscovered regulatory circuit wherein the reduction in miR-33/33* by type I IFNs reciprocally induces type I IFN production and primes cells for heightened antiviral immunity. These findings support a model in which metabolic reprogramming may serve to regulate the antiviral pathway and advance our understanding of the cross-talk between cellular metabolism and host defense.
Materials and methods
Reagents
For a list of reagents used, see Supplementary Table 2.
Mouse models
C57BL/6 J mice (6–8 weeks old) were purchased from SIPPR-BK Experimental Animal Ltd. Co. (Shanghai, China). Ifnar−/− mice were kindly provided by professor Xuetao Cao (Second Military Medical University, Shanghai, China). All mice were housed in the University Laboratory Animal Center in a specific-pathogen-free environment. All animal experiments were performed according to the protocol approved by the Animal Ethics Committee of Zhejiang University and were in compliance with institutional guidelines.
Primary cell culture
Mouse peritoneal macrophages were harvested by peritoneal lavage with 10 mL DMEM from mice 4 days after intraperitoneal thioglycolate (BD, Sparks, MD) injection. Cells were centrifuged and resuspended in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (P–S) under 5% CO2, atmospheric oxygen, at 37 °C in a humidified incubator. The cells were seeded at 1 × 106 cells/well in a 12-well plate or 5 × 106 cells/well in a 6 cm dish.
Cell lines
HEK293T and HeLa cells were obtained from American Type Culture Collection (ATCC) and maintained in DMEM with 10% FBS, 4 mm l-glutamine, 100 U/mL penicillin and streptomycin (all from Gibco) under 5% CO2, atmospheric oxygen, at 37 °C in a humidified incubator.
Plasmid construction
The recombinant vector encoding human PRKAA1 (NM_206907.3) was created by PCR-based amplification of HeLa cDNA, followed by subcloning into the pcDNA3.1 eukaryotic expression vector (Invitrogen). Expression plasmids for RIG-IN, IRF3-5D, MAVS, TBK1, and plasmids for the IFNB-Luc transcriptional reporters and were kindly provided by professor Zongping Xia (Zhejiang University, Hangzhou, China). The microRNA expression reporter vector pMIR-report (Ambion) was used to validate the wild-type and mutant mouse Prkaa1 and human PRKAA1 3′-UTRs for microRNA binding sites. All constructs were confirmed by DNA sequencing.
Transfection of cells with siRNA, miRNA, and plasmids
siRNA and miRNA transfection into cells was performed using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol. In brief, cells were transfected with siRNA (40 nm), miRNA mimics (80 nm), or miRNA inhibitors (120 nm) in the presence of Lipofectamine RNAiMAX in OPTI-MEM media for 8 h and then replaced with complete medium containing 10% FBS. Cells were analyzed at 48 h post transfection. The plasmids were transfected into HEK293T cells or HeLa cells with JetPrime (Polyplus) according to the standard protocol.
Mouse miR-33/33* agomir nucleotides treatment
A total of 40 μg/g/day miR-33/33* agomirs or control-agomir was dissolved in a total volume of 200 µl PBS and administered for 3 consecutive days through daily tail vein injections at the same time each day. Mice were infected with virus 24 h after the last tail vein injection.
Virus infection
VSV, HSV-1, and VSV-GFP were gifts kindly provided by professor Xiaojian Wang (Zhejiang University, Hangzhou, China). Cells were infected with VSV (MOI = 1), VSV-GFP (MOI = 1), or HSV-1 (MOI = 10) for the indicated hours. For in vivo studies, mice were intraperitoneally infected with VSV (108 pfu per mouse) and killed 24 h after infection. For the in vivo lethality test, mice were intraperitoneally infected with VSV (108 pfu/g).
3′-UTR luciferase reporter assay
The miRNA 3′-UTR luciferase reporter assay was performed as described previously.56 In brief, HEK293T cells described above were co-transfected with 80 ng of luciferase reporter plasmid, 40 ng of thymidine kinase promoter-Renilla luciferase reporter plasmid (pRL-tk), and the indicated miRNA mimics or controls (final concentration, 30 nm). Twenty-four hours after transfection, firefly, and Renilla luciferase activities were measured consecutively using the Dual-Luciferase Assay System (Promega) according to the manufacturer’s instructions.
Quantitative reverse transcriptase PCR
Total RNA was isolated from cells using TRIzol reagent (Takara) according to the manufacturer’s instructions. First strand cDNA was generated from total RNA using reverse transcriptase (Toyobo). The SYBR Green Master Rox (Roche) was used for quantitative real-time RT-PCR analysis according to the manufacturer’s protocol. The primer sequences are shown in Supplementary Table 1. miRNA reverse transcription and PCR analysis were performed as described previously.56 The absolute quantification of miR-33/33* was performed using the Hairpin-it miRNA and U6 snRNA Normalization RT-PCR Quantitation Kit (GenePharma) according to the standard protocol. Primers used in qRT-PCR are shown in Supplementary Table 3. m, Mus musculus. h, Homo sapiens.
VSV TCID50 assay
This experiment was performed as we described previously.56
ELISA
IFN-β levels were detected with ELISA kits (InvivoGen) according to the manufacturer’s protocols.
Immunoblot analysis
For immunoblot analysis, cells were lysed with cell lysis buffer (Cell Signaling Technology, 9803) supplemented with a protease inhibitor ‘cocktail’ (Sigma, P8340). Protein concentrations in the extracts were measured by BCA assay (Pierce, 23235). Equal amounts of extracts from the samples were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The resolved proteins were then electrically transferred to a polyvinylidene difluoride membrane (Bio-Rad). Immunoblots were probed with the indicated antibodies. The protein bands were visualized by using a Pierce chemiluminescence ECL kit (Thermo).
Mitochondrial homeostasis determination
MitoProbe JC-1 is a cationic dye that indicates mitochondrial depolarization through a reduction in its red–green fluorescence ratio. JC-1 dye exhibits potential-dependent accumulation in mitochondria, indicated by a fluorescence emission shift from green (~ 529 nm) to red (~ 590 nm). Consequently, mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio. For this assay, HeLa cells treated as indicated in the figure legends were stained for 15 min in fresh complete RPMI-1640 medium containing 2.5 µm JC-1 MitoProbe. For MitoSOX determination, HeLa cells treated as described in the figure legends were stained for 30 min in fresh complete RPMI-1640 medium containing 5 µm MitoSOX indicator. Cells were then washed with HBSS, resuspended in flow cytometry buffer (DPBS with 1% FBS and 0.04% NaN3) and analyzed immediately by flow cytometry.
Lung histology
Lungs from control or virus-infected mice were dissected, fixed in 10% phosphate-buffered formalin, embedded into paraffin, sectioned, stained with hematoxylin and eosin solution, and examined by light microscopy for histological changes. Immunohistochemical staining was performed using standard procedures.
SDD-AGE
SSDD-AGE was performed according to a published protocol with minor modifications.34 In brief, crude mitochondria (P5) were resuspended in 1 × sample buffer (0.5 × TBE, 10% glycerol, 2% SDS, and 0.0025% bromophenol blue) and loaded onto a vertical 1.5% agarose gel (Bio-Rad). After electrophoresis in running buffer (1 × TBE and 0.1% SDS) for 35 min with a constant voltage of 100 V at 4 °C, the proteins were transferred to an Immobilon membrane (Millipore) for immunoblotting.
Immunofluorescence confocal microscopy
HeLa cells plated on glass coverslips in six-well plates were infected or not with VSV at indicated time. Cells were fixed with 4% paraformaldehyde for 30 min, permeabilized using 0.1% Triton X-100, blocked with 1% BSA in PBS for 1 h and stained with the indicated primary antibodies followed by incubation with fluorescent-dye-conjugated secondary antibodies. The nuclei were stained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich). The colocalization was finally detected using the ZEISS LSM 880 with fast Airy Scan microscope under a × 63 oil objective.
Statistical analysis
Statistical analysis was carried out with Prism 8. All data are shown as the mean ± s.e.m. The mean values for biochemical data from each group were compared by Student’s t test, one-way ANOVA or two-way ANOVA. For the mouse survival study, Kaplan–Meier survival curves were generated and analyzed for statistical significance. P values < 0.05 were considered statistically significant.
Supplementary information
Acknowledgements
We thank Professor Xuetao Cao (Second Military Medical University, Shanghai, China) for the Ifnar−/− mice; professor Wei Liu (Zhejiang University, Hangzhou, China) and Wei Chen (Zhejiang University, Hangzhou, China) for the Mito-YFP and LC3-mCherry plasmids; Guifeng Xiao (Core Facilities, Zhejiang University School of Medicine) and Shuangshuang Liu (Core Facilities, Zhejiang University School of Medicine) for their excellent technical assistance with confocal microscopy; Shasha Chen (Zhejiang University) for help with SDD-AGE; Yuchuan Zhang (Zhejiang University) for help with flow cytometry; and Lijia Zhong (Zhejiang University) and Xinfang He (Zhejiang University) for help with in vivo experiments. This study was supported by the National Natural Science Foundation of China (81401283, 81771699), Zhejiang Provincial Natural Science Foundation of China (LZ19H100001, LY18H100004, and LY15C080001), and Fundamental Research Funds for the Central Universities (2018QNA7008).
Author contributions
DL, QT, and JZ performed the experiments and data analysis. DL and LL designed the experiments and wrote the manuscript. QW and YL helped with manuscript editing. YZ provided reagents and mice. YX and YS helped with infections of mice and related analysis.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Danhui Liu, Qinchun Tan, Jie Zhu
Contributor Information
Qingqing Wang, Email: wqq@zju.edu.cn.
Lihua Lai, Email: lailihua@zju.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-019-0326-x) contains supplementary material.
References
- 1.Goubau D, Deddouche S, Reis e Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38:870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 2008;8:911–22.. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev. Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loo YM, Gale M., Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692.. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki T, Kawai T, Akira S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol. Rev. 2011;243:61–73. doi: 10.1111/j.1600-065X.2011.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016;16:35–50. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 10.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev. Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat. Immunol. 2008;9:839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 12.Nilsen TW. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet. 2007;23:243–249. doi: 10.1016/j.tig.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 14.Horie T, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl. Acad. Sci. USA. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najafi-Shoushtari SH, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. USA. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayner KJ, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davalos A, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez CM, et al. MicroRNA 33 regulates glucose metabolism. Mol. Cell Biol. 2013;33:2891–2902. doi: 10.1128/MCB.00016-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouimet M, et al. MicroRNA-33–dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J. Clin. Investig. 2015;125:4334–4348. doi: 10.1172/JCI81676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Francl JM, Boehme S, Chiang JY. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7alpha-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology. 2013;58:1111–1121. doi: 10.1002/hep.26427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karunakaran D, et al. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by Anti-miR33 in atherosclerosis. Circ. Res. 2015;117:266–278. doi: 10.1161/CIRCRESAHA.117.305624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price NL, Fernandez-Hernando C. Novel role of miR-33 in regulating of mitochondrial function. Circ. Res. 2015;117:225–228. doi: 10.1161/CIRCRESAHA.117.306949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron AM, Lawless SJ, Pearce EJ. Metabolism and acetylation in innate immune cell function and fate. Semin. Immunol. 2016;28:408–416. doi: 10.1016/j.smim.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce E. J., Pearce E. L. Immunometabolism in 2017: driving immunity: all roads lead to metabolism. Nat. Rev. Immunol.18, 81–82 (2017). [DOI] [PMC free article] [PubMed]
- 27.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacy-Hulbert A, Moore KJ. Designer macrophages: oxidative metabolism fuels inflammation repair. Cell Metab. 2006;4:7–8. doi: 10.1016/j.cmet.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Lai L, et al. MicroRNA-33 regulates the innate immune response via ATP binding cassette transporter-mediated remodeling of membrane microdomains. J. Biol. Chem. 2016;291:19651–19660. doi: 10.1074/jbc.M116.723056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao GJ, et al. NF-kappaB suppresses the expression of ATP-binding cassette transporter A1/G1 by regulating SREBP-2 and miR-33a in mice. Int J. Cardiol. 2014;171:e93–e95. doi: 10.1016/j.ijcard.2013.11.093. [DOI] [PubMed] [Google Scholar]
- 31.Ouimet M, et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol. 2016;17:677–686. doi: 10.1038/ni.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson KA, et al. An interferon regulated MicroRNA provides broad cell-intrinsic antiviral immunity through multihit host-directed targeting of the sterol pathway. PLoS Biol. 2016;14:e1002364. doi: 10.1371/journal.pbio.1002364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai X, Chen ZJ. Prion-like polymerization as a signaling mechanism. Trends Immunol. 2014;35:622–630. doi: 10.1016/j.it.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou F, et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 37.Qi N, et al. Multiple truncated isoforms of MAVS prevent its spontaneous aggregation in antiviral innate immune signalling. Nat. Commun. 2017;8:15676. doi: 10.1038/ncomms15676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu B, et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor MAVS through Lys63-linked polyubiquitination. Nat. Immunol. 2017;18:214–224. doi: 10.1038/ni.3641. [DOI] [PubMed] [Google Scholar]
- 39.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moser TS, Schieffer D, Cherry S. AMP-activated kinase restricts Rift Valley fever virus infection by inhibiting fatty acid synthesis. PLoS Pathog. 2012;8:e1002661. doi: 10.1371/journal.ppat.1002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toyama EQ, et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351:275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egan DF, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–261. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr. Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 45.McWhirter SM, Tenoever BR, Maniatis T. Connecting mitochondria and innate immunity. Cell. 2005;122:645–647. doi: 10.1016/j.cell.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 46.Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onoguchi K, et al. Virus-infection or 5’ppp-RNA activates antiviral signal through redistribution of IPS-1 mediated by MFN1. PLoS Pathog. 2010;6:e1001012. doi: 10.1371/journal.ppat.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010;11:133–8. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci. Signal. 2011;4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- 51.Blanc M, et al. Host defense against viral infection involves interferon mediated down-regulation of sterol biosynthesis. PLoS Biol. 2011;9:e1000598. doi: 10.1371/journal.pbio.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanc M, et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity. 2013;38:106–118. doi: 10.1016/j.immuni.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilkins C, Gale M., Jr. Sterol-izing innate immunity. Immunity. 2013;38:3–5. doi: 10.1016/j.immuni.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 54.York Autumn G, et al. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell. 2015;163:1716–1729. doi: 10.1016/j.cell.2015.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Neill LA. How low cholesterol is good for anti-viral immunity. Cell. 2015;163:1572–1574. doi: 10.1016/j.cell.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Chen L, et al. MicroRNA-223 promotes type I interferon production in antiviral innate immunity by targeting forkhead box protein O3 (FOXO3) J. Biol. Chem. 2016;291:14706–16. doi: 10.1074/jbc.M115.700252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.