Highlights
-
•
Campylobacter fetus (C. fetus) is a rare cause of human disease but is responsible for over 50 % of invasive campylobacteriosis in humans.
-
•
C. fetus aortitis can rapidly progress to aneurysm and rupture, so urgent surgical intervention is required to prevent morbidity and mortality.
-
•
C. fetus has a tropism for endovascular surfaces and is able to evade host immune response which leads to recurrence and relapsing infections.
-
•
In the HIV-infected patient, C. fetus can persist due to defective cell mediated immunity and reduced production of C. fetus specific antibodies.
-
•
Carbapenems and aminoglycosides are bactericidal against C. fetus and are used to treat invasive C. fetus infections.
Keywords: Campylobacter fetusaortitis, Human immunodeficiency virus, HIV, Ertapenem
Abstract
A 36-year-old man with well controlled HIV developed Campylobacter fetus aortitis. To prevent aortic rupture, emergent surgical resection and neo-aortoiliac replacement with his left femoral vein was conducted. After surgical intervention, he was successfully treated with intravenous ertapenem for 6 weeks followed by oral amoxicillin for 3 months.
Introduction
Infective aortitis is a rare life-threatening condition that is usually secondary to microbial seeding of the aortic adventitia during bacteremia [1]. Symptoms are nonspecific including fever, abdominal pain, and back pain. Given the nonspecific clinical symptoms, aortitis can be difficult to diagnose thereby leading to complications including sepsis and aneurysm formation that can rapidly progress to aortic rupture [[1], [2], [3]]. Computerized topography (CT) is the diagnostic imaging of choice to aid in diagnosing this condition. During the pre-antimicrobial era, infective aortitis was largely a complication of endocarditis caused by Streptococcus and Haemophilus [1]. More recently, Staphylococcus has become the predominant pathogen in part due to intravascular procedures and injection drug use [1,4]. Enterobacteriaceae are occasional pathogens with Salmonella being the most prevalent gram-negative bacteria accounting for approximately 15 % of cases [4,5]. Other rare pathogens include Treponema, Listeria, and Aspergillus [[4], [5], [6]]. Treatment involves surgical resection of infected aortic tissue with aortic reconstruction in combination with aggressive antimicrobial therapy. We present a rare case of a patient with human immunodeficiency virus-1 (HIV) infection who developed Campylobacter fetus aortitis that was successfully treated with emergent surgical intervention and six weeks of intravenous ertapenem followed by three months of oral amoxicillin therapy.
Case report
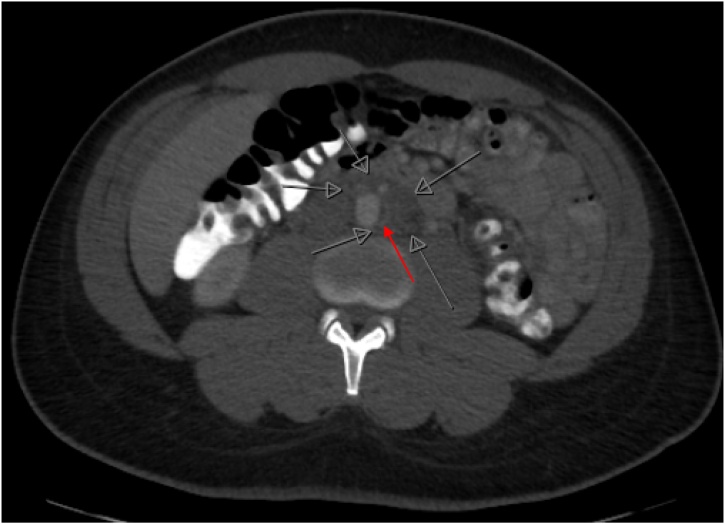
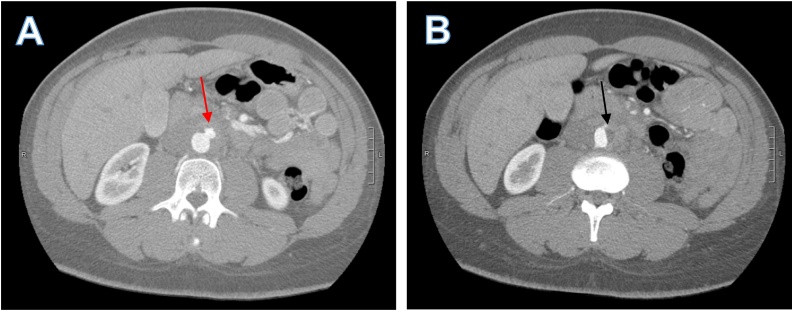
A 36-year-old man with well controlled HIV, on abacavir-lamivudine-dolutegravir (CD4 count of 931 cells/μL with undetectable viral load), presented with a one-week history of lower abdominal pain radiating to his back. He initially attributed the pain to constipation but when the pain progressed, he decided to seek medical advice at a local emergency room. CT scan of the abdomen and pelvis with oral and intravenous contrast showed an ill-defined infrarenal periaortic mass with an adjacent eccentric thrombus in the abdominal aorta and subtle enhancement of the vasa vasorum concerning for focal aortitis (Fig. 1). Initial concern was for a neoplastic process and outpatient CT guided biopsy was scheduled, but symptoms intensified over ten days prompting further medical evaluation. CT angiogram of the chest and abdomen showed a pseudoaneurysm of the aorta (Fig. 2A) and a focal dissection extending to the aortic bifurcation (Fig. 2B). Given these findings, the patient was transferred to our tertiary care center for further surgical and medical management.
Fig. 1.
Axial view of CT abdomen and pelvis with oral and intravenous contrast. Red arrow indicates thrombus and early dissection with focal aortitis. Open grey arrows show ill-defined soft tissue abnormality surrounding the infrarenal aorta, measuring 4.3cm × 4.2cm. (For interpretation of the references to colour in this Figure legend, the reader is referred to the web version of this article).
Fig. 2.
Axial views of CT angiogram of the abdomen. A) Pseudoaneurysm from the anterior left aspect of the aorta (red arrow) measuring about 1.2 cm. B) Flattening of left aortic border and non-enhancing short segment of the left side of the aorta representing a small focal dissection that extended to aortic bifurcation (Black arrow). (For interpretation of the references to colour in this Figure legend, the reader is referred to the web version of this article).
On arrival the patient was hemodynamically stable with no fever or chills. He did recall a brief transient febrile illness with malaise two months prior but denied any other symptoms at that time. The patient denied any history of vascular disease, endovascular infections, or injection drug use. He was in a monogamous relationship with his male sexual partner who also had well controlled HIV on antiretroviral therapy. However, his sexual partner did have a recurrent diarrheal illness over the past four months requiring repeated oral antimicrobial courses. Physical examination was unremarkable except for tenderness to direct palpation on all four abdominal quadrants. Laboratory data was remarkable for WBC of 23,200 cells/μL (normal range 4,500−11,000 cells/μL) with 85 % neutrophils, erythrocyte sedimentation rate of 67 mm/hour (normal range 0−15 mm/hour), and a C-reactive protein of 3.7 mg/dL (normal value <1 mg/dl). Blood cultures had no growth and rapid plasma reagin was nonreactive. Transthoracic echocardiogram did not have any abnormalities. Given the concern for infective aortitis, the patient was started on intravenous vancomycin 1.75 g every 12 h and piperacillin-tazobactam 4.5 g every six hours.
The patient underwent an emergent aortic repair and resection of the inferior abdominal aorta and neo-aortoiliac replacement with his left femoral vein. Intraoperatively, marked retroperitoneal edema, inflammation, and severe adhesions were observed around the distal aorta. Pathological evaluation of his resected abdominal aorta showed acute and chronic adventitial inflammation with pseudoaneurysm. Aortic tissue culture grew C. fetus and susceptibility testing was sent to a reference lab with results seen in Table 1. His postoperative course was unremarkable and he was discharged home on intravenous ertapenem 1 g daily. He completed a 6-week course of intravenous ertapenem therapy without any side effects and then was transitioned to oral amoxicillin 500 mg twice a day for three months. Following the completion of therapy, the patient has continued to do well and remains symptom free, off antimicrobial therapy.
Table 1.
C. fetus Susceptibility Results.
| Antimicrobial | MIC μg/mL* |
|---|---|
| Ampicillin | 1 |
| Ceftriaxone | 2 |
| Gentamicin | 0.5 |
| Meropenem | <0.06 |
MIC: minimum inhibitory concentration.
At the present time, there are no clinical and laboratory standards institute (CLSI) guidelines for the interpretation of these MICs of C. fetus susceptibility to these antimicrobials.
Discussion
Campylobacteriosis is a bacterial gastrointestinal illness causing an estimated 1.3 million diarrheal illnesses annually [7]. Campylobacter spp. are microaerophilic, nonfermenting gram-negative bacteria. There are over 20 species of Campylobacter but C. jejuni, C. coli and C. fetus are the predominant species implicated in human infections. C. fetus is primarily a zoonotic pathogen that rarely causes human infections, accounting for less than 3% of all campylobacter diarrheal illnesses but over 50 % of invasive campylobacteriosis, with a mortality rate up to 14 % [8,9]. Humans usually contract C. fetus through the fecal-oral route, with some association to consumption of raw beef, raw liver, and cheese [8]. Immunosuppression and older age are known risk factors for invasive C. fetus infections [8]. However, there is a paucity of data on invasive C. fetus in HIV patients and to our knowledge this is the first reported case of C. fetus aortitis in a patient with HIV.
This patient did not present with the characteristic gastrointestinal symptoms that are typical with campylobacteriosis, but his transient febrile illness two months prior to presentation may have represented C. fetus bacteremia and subsequent seeding of his abdominal aorta. He likely acquired his infection fecal-orally given that his male sexual partner had recurrent enteritis that may have been due to C. fetus as well. This is not surprising because C. fetus clusters have been identified among men who have sex with men [10,11].
C. fetus aortitis characteristically has rapid progression to aneurysm formation and subsequently, to aortic rupture. Overall mortality of C. fetus aortitis is 20–30 % but mortality approaches 100 % if rupture occurs before surgical intervention can be conducted [[10], [11], [12]]. Our case illustrates the rapid progression of C. fetus from focal aortitis (Fig. 1) to the development of pseudoaneurysm over ten days (Fig. 2). Once the pseudoaneurysm was identified, emergent open resection and neo-aortoiliac replacement with his left femoral vein was conducted. This case reinforces the need for a high index of suspicion to identify and surgically treat C. fetus aortitis, thereby preventing progression to aortic rupture and reducing mortality.
While surgical resection is paramount, persistence and aptness to evade the immune system by C. fetus predisposes patients to a high risk for recurrence even after aggressive surgical and medical management. This can be attributed to C. fetus’ paracrystalline surface layer comprised of high molecular weight surface layer proteins (SLPs) which allows the organism to evade compliment binding and subsequent compliment-mediated destruction in non-immune serum [13]. Although antibodies are active against the SLPs, the SLPs can develop high frequency antigenic variations that can evade opsonization and antibody-dependent phagocytosis [14]. By so doing, C. fetus can evade both innate and adaptive immunity leading to persistent and relapsing infections. In addition, C. fetus has a tropism for endovascular surfaces due to high affinity binding of its surface receptor to the vascular endothelium and the release of pro-coagulants that promote intravascular thrombosis [8]. This is supported by case series reporting high rates of aortic aneurysms in patients with C. fetus bacteremia [10,11].
The ability of C. fetus to evade the immune system and its predilection for recurrence complicates treatment which leads to a wide variety of treatment durations ranging from six weeks to life-long oral antibacterial suppression after intravenous antibiotics [10,12]. While macrolides remain the drug of choice for noninvasive C. fetus infections, the optimal therapy for invasive disease has not been established [15]. Third generation cephalosporins are the agents of choice against invasive salmonellosis, but they are not bactericidal against C. fetus and have high rates of resistance [16]. Therefore, carbapenems and aminoglycosides are commonly used for invasive C. fetus disease given that these agents are bactericidal, have low MICs, and have had successful clinical outcomes as documented in different case series and reports [[9], [10], [11]]. Furthermore, fluoroquinolones should be used with caution given the increased rates of resistance, but amoxicillin and ampicillin have documented bactericidal activity especially at high concentrations [9,16]. Based on this data, we treated our patient with six weeks of a carbapenem, using intravenous ertapenem for the convenience of once daily administration as has been used by others [17,18]. However, the duration of oral antimicrobial therapy after initial intravenous carbapenem therapy rests on individualized treatment decisions that consider the severity of underlying immunodeficiencies and other host factors rather than standardized protocols for all. Therefore given his underlying HIV which was well controlled, we elected to treat with oral amoxicillin for three months similar to other documented regimens in patients with underlying conditions [12].
Immunocompromised patients are theorized to be more susceptible to invasive C. fetus infections secondary to impaired cell-mediated and humoral immunity compared to immunocompetent hosts. In one cohort, HIV infection was associated with up to a 40-fold increase in the incidence of severe, chronic and recurrent campylobacteriosis compared to non-HIV individuals [19]. This is likely secondary to the HIV, which impairs not only cell mediated immunity, but also creates a dysregulated humoral response, with reduced ability to produce effective pathogen-specific antibodies, especially to encapsulated pathogens [20,21]. In addition, HIV has also been shown to cause a depressed production of mucosal secretory IgA and abnormally low campylobacter-specific IgA, IgM, and IgG antibodies in HIV patients with recurrent, invasive infections compared to those patients who did not experience relapse [22,23]. Therefore, in the HIV-infected patient, C. fetus appears to be a very elusive pathogen that has the ability to evade the host’s innate and adaptive immunity with its SLPs acting as a proteinaceous capsule [14]. In our patient, he had well controlled HIV with CD4 count of 931 cells/μL and HIV viral load that was undetectable, but given the concern for recurrence, we treated him with oral amoxicillin for three months after he had received six weeks of intravenous ertapenem. He will continue to follow with infectious diseases to ensure that there is no clinical recurrence and that he continues to be compliant with antiretroviral therapy for his underlying HIV infection.
Conclusion
To our knowledge, this is the first case report of C. fetus aortitis in a HIV-infected patient. He was successfully treated with surgical resection of the pseudoaneurysm and a six-week course of intravenous ertapenem followed by three months of oral amoxicillin therapy given his underlying immunosuppression. This case reinforces the need for prompt surgical and medical treatment of this rare infection to prevent devastating aortic rupture. Further studies are needed to clarify the pathophysiology of the deficient innate and acquired immune responses that are associated with C. fetus invasive infections. This may help determine the proper duration of antimicrobial therapy. However, given the rarity and heterogeneity with respect to the immune statuses of infected hosts, the duration of antimicrobial therapy will likely have to be individualized rather than standardized.
Conflicts of interest
The authors have nothing to disclose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
Ethical approval
Not applicable because it is a case report.
Author contribution
Uzoamaka A. Eke: Conceptualization, Writing-original draft preparation, reviewing and editing. James B Doub: Writing-reviewing and editing. Joel V. Chua-Writing-reviewing, editing and supervision.
References
- 1.Foote E.A., Postier R.G., Greenfield R.A., Bronze M.S. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89–97. doi: 10.1007/s11936-005-0010-6. [DOI] [PubMed] [Google Scholar]
- 2.Oderich G.S., Panneton J.M., Bower T.C. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg. 2001;34(5):900–908. doi: 10.1067/mva.2001.118084. [DOI] [PubMed] [Google Scholar]
- 3.Deipolyi A.R., Czaplicki C.D., Oklu R. Inflammatory and infectious aortic diseases. Cardiovasc Diagn Ther. 2018;8(Suppl 1):S61–s70. doi: 10.21037/cdt.2017.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown S.L., Busuttil R.W., Baker J.D., Machleder H.I., Moore W.S., Barker W.F. Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg. 1984;1(4):541–547. [PubMed] [Google Scholar]
- 5.Müller B.T., Wegener O.R., Grabitz K., Pillny M., Thomas L., Sandmann W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001;33(1):106–113. doi: 10.1067/mva.2001.110356. [DOI] [PubMed] [Google Scholar]
- 6.Maeda H., Umezawa H., Goshima M. Primary infected abdominal aortic aneurysm: surgical procedures, early mortality rates, and a survey of the prevalence of infectious organisms over a 30-year period. Surg Today. 2011;41(3):346–351. doi: 10.1007/s00595-010-4279-z. [DOI] [PubMed] [Google Scholar]
- 7.Tack D.M., Ray L., Griffin P.M. Preliminary incidence and trends of infections with pathogens transmitted commonly through food - Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016-2019. MMWR Morb Mortal Wkly Rep. 2020;69(17):509–514. doi: 10.15585/mmwr.mm6917a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagenaar J.A., van Bergen M.A., Blaser M.J., Tauxe R.V., Newell D.G., van Putten J.P. Campylobacter fetus infections in humans: exposure and disease. Clin Infect Dis. 2014;58(11):1579–1586. doi: 10.1093/cid/ciu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremblay C., Gaudreau C., Lorange M. Epidemiology and antimicrobial susceptibilities of 111 Campylobacter fetus subsp. Fetus strains isolated in Québec, Canada, from 1983 to 2000. J Clin Microbiol. 2003;41(1):463–466. doi: 10.1128/JCM.41.1.463-466.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cochennec F., Gazaigne L., Lesprit P., Desgranges P., Allaire E., Becquemin J.P. Aortoiliac aneurysms infected by Campylobacter fetus. J Vasc Surg. 2008;48(4):815–820. doi: 10.1016/j.jvs.2008.05.076. [DOI] [PubMed] [Google Scholar]
- 11.Gazaigne L., Legrand P., Renaud B. Campylobacter fetus bloodstream infection: risk factors and clinical features. Eur J Clin Microbiol Infect Dis. 2008;27(3):185–189. doi: 10.1007/s10096-007-0415-0. [DOI] [PubMed] [Google Scholar]
- 12.Rutherford E.J., Eakins J.W., Maxwell J.G., Tackett A.D. Abdominal aortic aneurysm infected with Campylobacter fetus subspecies fetus. J Vasc Surg. 1989;10(2):193–197. [PubMed] [Google Scholar]
- 13.Thompson S.A., Shedd O.L., Ray K.C., Beins M.H., Jorgensen J.P., Blaser M.J. Campylobacter fetus surface layer proteins are transported by a type I secretion system. J Bacteriol. 1998;180(24):6450–6458. doi: 10.1128/jb.180.24.6450-6458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaser M.J., Smith P.F., Hopkins J.A., Heinzer I., Bryner J.H., Wang W.L. Pathogenesis of Campylobacter fetus infections: serum resistance associated with high-molecular-weight surface proteins. J Infect Dis. 1987;155(4):696–706. doi: 10.1093/infdis/155.4.696. [DOI] [PubMed] [Google Scholar]
- 15.Geissler A.L., Bustos Carrillo F., Swanson K. Increasing campylobacter infections, outbreaks, and antimicrobial resistance in the United States, 2004-2012. Clin Infect Dis. 2017;65(10):1624–1631. doi: 10.1093/cid/cix624. [DOI] [PubMed] [Google Scholar]
- 16.Wieczorek K., Osek J. Antimicrobial resistance mechanisms among Campylobacter. Biomed Res Int. 2013;2013:340605. doi: 10.1155/2013/340605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghimire R., Urban C., Lee A., Pokhrel A., Wehbeh W., Turett G. Campylobacter fetus infection of the aorta: a case report and review of literature. Am J Infect Dis. 2015;11(2):26–32. [Google Scholar]
- 18.Melendez B.A., Hollis H.W., Jr., Rehring T.F. Mycotic popliteal aneurysm rupture secondary to Campylobacter fetus. Ann Vasc Surg. 2015;29(1) doi: 10.1016/j.avsg.2014.05.021. 122.e9-11. [DOI] [PubMed] [Google Scholar]
- 19.Sorvillo F.J., Lieb L.E., Waterman S.H. Incidence of campylobacteriosis among patients with AIDS in Los Angeles County. J Acquir Immune Defic Syndr (1988) 1991;4(6):598–602. [PubMed] [Google Scholar]
- 20.Müller F., Frøland S.S., Hvatum M., Radl J., Brandtzaeg P. Both IgA subclasses are reduced in parotid saliva from patients with AIDS. Clin Exp Immunol. 1991;83(2):203–209. doi: 10.1111/j.1365-2249.1991.tb05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carson P.J., Schut R.L., Simpson M.L., O’Brien J., Janoff E.N. Antibody class and subclass responses to pneumococcal polysaccharides following immunization of human immunodeficiency virus-infected patients. J Infect Dis. 1995;172(2):340–345. doi: 10.1093/infdis/172.2.340. [DOI] [PubMed] [Google Scholar]
- 22.Bernard E., Roger P.M., Carles D., Bonaldi V., Fournier J.P., Dellamonica P. Diarrhea and Campylobacter infections in patients infected with the human immunodeficiency virus. J Infect Dis. 1989;159(1):143–144. doi: 10.1093/infdis/159.1.143. [DOI] [PubMed] [Google Scholar]
- 23.Perlman D.M., Ampel N.M., Schifman R.B. Persistent Campylobacter jejuni infections in patients infected with the human immunodeficiency virus (HIV) Ann Intern Med. 1988;108(4):540–546. doi: 10.7326/0003-4819-108-4-540. [DOI] [PubMed] [Google Scholar]