Highlights
-
•
Microfibres from clothes laundering are the main source of primary microplastics in oceans.
-
•
Fibres are frequently the most common plastics found in aquatic animals.
-
•
The main plastic in polluting microplastics is PET.
-
•
Various toxic substances are incorporated in textile microfibres.
-
•
Fibres contribute up to 90% total plastic mass in wastewater treatment plants.
-
•
A Standard Test is required to allow the development of microfibre release control actions.
Keywords: Microplastics, Microfibres, PET, Laundry effluent, Waste treatment plants, Ecotoxicity
Microplastics; Microfibres; PET; Laundry effluent; Waste treatment plants; Ecotoxicity.
Abstract
Plastic microfibre pollution produced by domestic and commercial laundering of synthetic textiles has recently been incriminated in the press and the scientific literature as the main source (up to 90%) of primary microplastics in the oceans. Polyethylene terephthalate (PET) is the most common microfibre encountered. This review aims to provide updated information on worldwide plastic microfibre pollution caused by textile laundering and some possibilities for its control. Release of microfibres during domestic washing and tumble drying, their fate in wastewater treatment plants (WWTPs) and the oceans, and their environmental effects on the aquatic biota are discussed, as well as potential control methods at the levels of textile modification and laundry procedures. Environmental effects on aquatic biota are important; as a result of their small size and length-to-diameter ratio, microfibers are more effectively incorporated by organisms than other plastic particle groups. Simulation laundering studies may be useful in the development of a Standard Test Method and modification of WWTPs may reduce microfibre release into aquatic systems. However, improvements will be necessary in textile design and appliance design, and recommendations should be made to consumers about reducing their personal impact on the environment through their laundering choices, which can include appliances, fabric care products and washing conditions. Official regulation, such as that introduced recently by the French government, may be necessary to reduce plastic microfibre release from clothes’ laundering.
1. Introduction
Microplastic pollution caused by washing of synthetic textiles has recently been incriminated in the press (XerosTech, 2020; Vogue, 2020), as well as in the scientific literature (e.g., De Falco et al., 2019A; Suaria et al., 2020; Acharya et al., 2021; Liu et al., 2021), as the main source of primary microplastics in the oceans. Textiles manufactured from plastics can release microfibres throughout their lifecycle, adding immensely to the increasingly worrying world microplastics pollution levels. The floating plastics in our seas are dominated by microfibres (Green et al., 2018; Mishra et al., 2019; Xu et al., 2021a), which are defined as fibers that are 1 μm–5 mm in length, with a length to diameter ratio greater than 100 (Liu et al., 2019a, b). Such microfibres have a huge surface area:volume ratio, which increases their adverse environmental effects considerably (see later in this review, and Gaylarde et al., 2021; Liu et al., 2021; Rebelein et al., 2021).
Microplastics have been found in 90% of all surface waters worldwide, with microfibres making up 91% of this (Barrow et al., 2018; Napper et al., 2021). This is some orders of magnitude higher than the densities reported in even highly polluted waters (Ryan et al., 2019). McEachern et al. (2019), taking samples from Tampa Bay, Florida, USA, found that 76% of microplastic particles were microfibres in discrete samples taken with a Van Dorn sampler, but 88% in plankton tow samples. Hence the wide variation in reported figures is partially explained by sampling techniques. Heavy rainfall before the sampling also affects the results, with more microplastic particles normally being recorded at this time (Lima et al., 2014; Yonkos et al., 2014; McEachern et al., 2019; Xia et al., 2020).
At greater depths, microfibres have been found to be less abundant (McEachern et al., 2019); Sanchez-Vidal et al. (2018) analysed seafloor sediments in Southern European seas and determined that coastal seas retained 33% of seafloor microfibres, the rest being exported to the open sea, where they accumulated in sediments, around 20% of them at below 2000m deep. However, almost 80% of the microfibres were cellulose-based and only 12.9% contained the polyester polyethylene terephthalate (PET), generally regarded as indicative of textile plastic microfibres. Sub-surface waters along a latitudinal gradient in the Atlantic Ocean were found to contain 1.15 microplastic particles m−3, predominantly microfilaments and mainly polyethylene and polyamide (Kanhai et al., 2017). Ryan et al. (2019) suggest that microfibres should be treated as a separate category of pollutants, containing both natural and synthetic fibres and, in view of the relative difficulty in determining the exact nature of microfibres (Gong and Xie, 2020; Gaylarde et al., 2021; Ivar do Sul, 2021; Lu et al., 2021), this is a reasonable suggestion, although future improvements in analytical techniques (Castelvetro et al., 2021; Lin et al., 2021; Lu et al., 2021; Monteleone et al., 2021; Liu et al., 2020; Munno et al., 2020) may make it unnecessary. Synthetic textile fibres can be very varied and include the plastics polyamide, polyvinyl chloride, polyurethane/elastane, modacrylic, polyacrylonitrile, polyethylene terephthalate (PET), and other polyesters. Polyester is said to dominate the textile market at the moment (Schöpel and Stamminger, 2019); in 2015, 62900 kg of polyester fibres were produced throughout the world. In Finland alone, polyester emissions from household machine washing have been estimated to be 150,000 kg per year (Sillanpää and Sainio, 2017). Palacios-Mateo et al. (2021) wrote that PET is the main plastic used for polyester-based clothing and Richards (2015) had previously stated that PET is the predominant plastic worldwide.
The revelation that most microfiber pollution in water was due to effluent from clothes washing, often via wastewater treatment plants (WWTPs), was first made by Browne et al. (2011), who collected microplastics from sediments on beaches throughout the world, including Australia, Philippines, Portugal, S. America, USA and UK. A group of independent advisers to the UN (GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection) consolidated this information in 2015. In 2017, IUCN (the International Union for Conservation of Nature) considered that laundry effluent accounted for 35% of all microplastic contamination in the oceans (Boucher and Friot, 2017); this was without including textile microfibres deposited from the air, which can be considerable (De Falco et al., 2020; González-Pleiter et al., 2021; Yang et al., 2019). The actual proportion of marine microplastics pollution derived from textiles is thus probably even higher. Airbourne microplastics require considerably more research (Akanyange et al., 2021; Xu et al., 2020; Zhang et al, 2020); they are, for example, an important source of plastic microfibres in otherwise unpolluted areas such as the French Pyrenees, the Italian Alps and Arctic snow (Ambrosini et al., 2019; Bergmann et al., 2019). However, little is understood about the sources of atmospheric microfibers, their transport and deposition (Dris et al., 2016; Gasperi et al., 2018), although it is known that fibres may be released into indoor air during mechanical drying of textiles (Napper and Thompson, 2016; O'Brien et al., 2020).
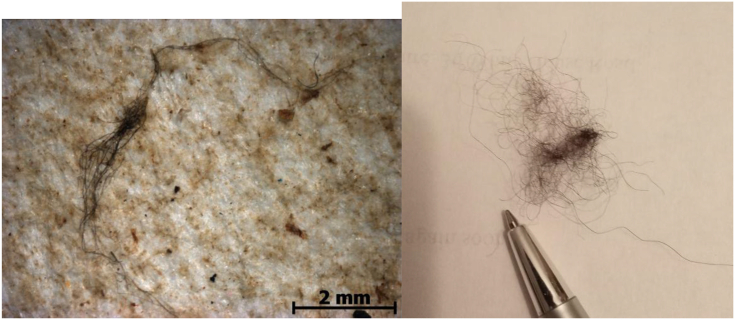
Relatively little laundry effluent will be liberated directly into the sea, mainly that in less industrialized countries, where there have been few measurements. Most effluents will be fed into wastewater treatment systems and thence into the rivers. Freshwater ecosystems, then, are particularly at risk, receiving not only domestic wastes, but also industrial wastewater from the fabric-producing factories, before liberating it into the oceans. Figure 1 shows microfibres taken directly from a domestic washing machine in the UK and some recovered from water in Guanabara Bay, Brazil.
Figure 1.
Plastic fibres retrieved from (left) Guanabara Bay, Brazil; (right) the drum of a washing machine in a UK household; the pen tip is around 0.2 mm across.
The recent recognition accorded to the polluting potential of laundry activities around the world means that several research projects have been initiated in the last few years, as indicated by the fact that the number of papers published in 2020 and 2021 that are cited herein comprise just over half of the total references. This review aims at gathering the current information on plastic microfibre pollution caused by textile laundering worldwide, together with potential control methods.
2. Release of microfibres during washing of textiles
For the purposes of this review, the release of plastic microfibres during the production of textiles will not be considered, although this is a significant polluting process (Sivakumar 2014; Chan et al., 2021; Dhir, 2021); the steps involved can be found in Palacios-Mateo et al. (2021).
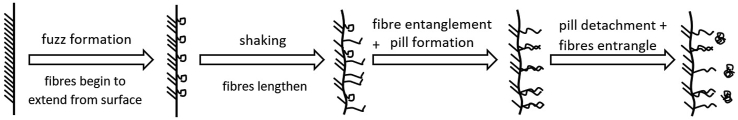
During use, clothes suffer a variety of onslaughts that result in “furring”, “felting”, or “pilling” of the exposed surfaces. This is basically the appearance of fibre ends on the outside of the fabric. The amount, strength and length of the extruded fibres depends on the type of textile. Properties such as chemical composition, tightness of the yarn and age of the fabric influence this process (Zambrano et al., 2019). Okubayashi et al. (2005) states the steps in the formation of microfibres from washing of cotton textiles. Adapting these for synthetics, we can list: 1. Formation of fuzz; 2. Fibrillation (shaking); 3. Pill formation (fibre entanglement); 4. Pill detachment (see Figure 2). The greater the number of exposed fibres on the surface, the more will be released into the environment, either broken or unbroken, during washing (Carney Almroth et al., 2018).
Figure 2.
The process of microfibre formation and release during washing of textiles.
Fibres may, of course, also be released without washing, simply during day-to-day wearing of the clothes, and it has been stated that the quantities released in this way, by rubbing, brushing, etc, are equal to those produced during laundering (Palacios-Mateo et al., 2021). The difference is that during the washing processes fibres are released into both air and water, unlike during dressing, wearing and undressing. However, as stated above, there has been only a little work done on release of plastic microfibres into the air; this will be discussed later in this article.
The release of microfibres into washing water has been studied by a number of authors (e.g., Özkan and Gündoğdu, 2021; De Falco et al., 2019; Zambrano et al., 2019; Napper and Thompson, 2016), with varying results. This is due not only to the use of different types of material, but also to different methodologies used. Özkan and Gündoğdu (2021), for example, using the Gyrowash machine, found that recycled PET knitted fabrics released 4489.93 fibers L−1, or 368094.07 fibers kg−1, while virgin polyester fabrics released 2034.26 fibers L−1, or 167436.58 fibers kg−1. Both fabric types released fewer fibres with increasing washing cycles. Although Carney Almroth et al. (2018) used the same washing machine, their different fabrics produced different results, which were reported as different units (fibers/m⁻2/L⁻1). The use of different units by authors reporting microfibre loss does not aid comparisons. Table 1 lists some of these studies, showing the differences.
Table 1.
Some studies on microfibre release during laundering.
| Material(s) | Washing/drying method | Variables tested | Fibre release | Reference |
|---|---|---|---|---|
| Polyester (PE) | Domestic washing machine | Textile characteristics | 124–308 mg kg−1 (640 000–1500 000 fibres) | De Falco et al. (2019a) |
| PE, PE-cotton blend, acrylic | Domestic washing machine | Temperature and laundry aids | 0.27–0.46 mg kg−1 (app. 80 000 fibres) | Napper and Thompson (2016) |
| PE fleece, nylon shell | Domestic washing machine | Type and quantity of detergent, addition of surfactant | 25 and 100 mg kg−1 fibers (+and - surfactant) | O'Brien et al., 2020 |
| Fully synthetic | Domestic washing machine | Machine type, new or aged clothes | 0.3% of mass per wash (3300 mg kg−1) | Hartline et al. (2016) |
| PET fleece | Domestic washing machine and tumble dryer | Detergent and softener | 12 mg kg−1 (11 300 fibres) in wash cycles; approx 40 000 fibres in drying | Pirc et al. (2016) |
| Acetate | Domestic washing machines | Machine type, detergent | 74,816 ± 10,656 microfibers/m2 per wash (max.) | Yang et al. (2019) |
| PE | Laboratory machine (Launder-Ometer) | Temperature, detergent | 0.1–1 mg/g | Zambrano et al. (2019) |
| PE fleece | Domestic washing machine | Detergent | Cycle 1: 70 Cycle 4: 40 Cycle 8: 25 mg kg−1 |
Lant et al. (2020) |
| PE, PE fleece | Laboratory machine (Gyrowash) | Knit gauge, detergent | PE fleece: 7360 fibers/m⁻2/L⁻1 PE fabric: 87 fibers/m⁻2/L⁻1 |
Carney Almroth et al., 2018 |
In the first report of this phenomenon, that of Browne et al. (2011), 1900 microfibres/wash/garment was the estimated release; later studies have used more exact measurements, such as mg.kg−1, microfibres.m-2 (see Table 1). The effects of variables such as type of textile, detergent, temperature and washing action have been determined, some of them using special small simulation machines, in which parameters can be specified and held constant (e.g., Hernandez et al., 2017; Carney Almroth et al., 2018), whilst others (e.g., Lant et al., 2020; Dalla Fontana et al., 2020; Galvão et al., 2020; Kärkkäinen and Sillanpää 2021) have employed domestic washers of various types. Such studies can give useful insights into the effects of changes in laundry procedures and could be useful in the development of a standard test method.
Most published studies have employed commercial washing machines. The majority of microfibers are released into the washing water during the first 15 min, with older fabrics releasing more (Hernandez et al., 2017; Hartline et al., 2016). After the first wash, fibre release decreases until it reaches a plateau (De Falco et al., 2019; Kelly et al., 2019; Napper and Thompson, 2016). Apparently, the recently developed North American high-efficiency top-loaders produce considerably less microfibers than the old-fashioned type (Lant et al., 2020); it has been shown that the volume of water in relation to the quantity of textiles influences the amount of microfibre release (Kelly et al., 2019) and the lower amounts of water used in high-efficiency models could be the reason for reduced fibre production. In the study of Lant et al. (2020), the lower water to fabric ratio accompanied a 69.7% reduction in fibre release for polyester fleece (n = 32, p = 7.9 × 10-6) and 37.4% reduction for polyester T-shirt (n = 32, p = 0.0032).
Of course, it is not only the fabric:water ratio that influences microfibre release. Washing at higher temperatures also increases the output of microfibres (Yang et al., 2019; Cotton et al., 2020). These authors also found that adding detergent to the wash increased microfibre numbers, and this has been confirmed by some, but not all, other authors. O'Brien et al. (2020) stated that the presence of detergent increased microfibre release, but detergent composition and amount had little effect, a result that was echoed by Lant et al. (2020). The overall length of the released fibres was not affected by detergent according to O'Brien et al. (2020); Cesa et al. (2020) however, determined that detergent actually reduced the release of microfibres from synthetic garments during machine washing. The different results could depend, at least partially, on whether liquid or powder detergents were used (Xu et al., 2021a).
The many factors that can influence release of microfibres during laundering processes obviously lead to great variations in their environmental concentrations around the world and with season, a fact that has been reported by Stanton et al. (2019). This has led to suggestions that microfibres should be monitored regularly in environments of interest to gain a clearer picture of the extent of and changes in the problem (Palacios-Mateo et al., 2021; Henry et al., 2019).
3. Release of microfibres during tumble drying of textiles
In industrialized countries, the process of laundering clothes often involves the use of a tumble dryer, which may be integral or separate from the washing machine. During this drying process, any fibres that have been broken and moved to the fabric surface during washing will be liberated into the air or into the condensate and thence pass to wastewater systems. The use of automatic electric dryers is particularly prevalent in North America, where over 80% of households use this method (Kapp and Miller, 2020).
O'Brien et al. (2020) repeatedly washed and dried a blue polyester (PE) fleece blanket using the ‘Normal Dry’ program of a domestic dryer (56–59 °C for 20 min). Their well-controlled study showed that blue PE fibres in the surrounding air were increased on average from 7.6 to 56 per cubic metre of air, a highly significant increase in spite of the high standard deviations. These results were confirmed using greater numbers of garments by Kärkkäinen and Sillanpää (2021), who showed, additionally, that fibre emission decreased with increasing tumble drying cycles They found that PE fleeces released the most and, on average, the longest fibres, while PE or polyamide sports shirts released the least.
Apart from the influence of chemical and textural differences between the different types of fabric on microfibre release, adsorption or absorption of contaminants by the microfibres also varies (Turner, 2019). This obviously has an influence on the final ecological impact of the released microfibres; this is discussed in the later section on environmental effects of microfibres.
4. Wastewater systems
In industrialised countries, the microfibres released into machine effluents will eventually be transferred to wastewater treatment plants (WWTPs) that treat collected solid and liquid wastes, including microplastic fibers and microbeads. Modern WWTPs have been designed to retain up to 99% of the solid particles, concentrating them in activated sludge (Freeman et al., 2020; Koelmans et al., 2019). Even so, retained particles in sludge can enter the aquatic ecosystem via surface carriage (Corradini et al., 2019). It is not within the remit of this review to discuss the many different processes involved in the treatment of wastewaters around the world, or, equally, the ways in which wastes are transferred into the treatment plants. Certainly, however, WWTP effluents represent a critical path for microplastics to enter aquatic ecosystems (Magni et al., 2019; Iyare et al., 2020) and so their inclusion in this review is necessary.
Iyare et al. (2020), in a review of removal of microplastics from WWTPs, stated that average removal rates over 21 studies were 72% during preliminary and primary treatment steps, while secondary and tertiary WWTPs raised this figure to 88% and 94%, respectively. However, the majority of the studies focussed on plastic microbeads, and not fibres. Tian et al. (2021) indicated the importance of microfibres when they found that the PET microfibre mass in laundry wastewater contributed 50% to the total PET mass in WWTP influent, and fibres have been found to comprise over 50%, and up to 90%, of the total plastics by other authors (Prajapati et al., 2021; Magnusson and Norén, 2014; Mahon et al., 2017; Corradini et al., 2019).
De Falco et al. (2019a), using a household washing machine, found that the majority of the released microfibres was retained by the 60 μm pore-size machine filters; they had an average length of 360–660 μm and diameter of 12–16 μm. This size range would readily pass through the WWTP systems. Such microfibres have been shown to be the most abundant microplastics in the influent wastewater in WWTPs (Xu et al., 2019; Leslie et al., 2017), but their concentrations are, as stated above, reduced during the primary and secondary stages of waste treatment, after which they will become incorporated into the solid fraction that may be sent to landfill, incinerated, or spread on the land as fertilizer (Eerkes-Medrano et al., 2015). WWTPs were not, of course, originally designed to capture microplastics (Ben-David et al., 2021), and a certain amount will pass into the effluent. The average microplastics (MP) concentrations in wastewater and sludge released into the Persian Gulf were 70.66 (±14.12, SD) MP.35 L−1 and 6070 (±807.25) MP.kg−1, respectively. These consisted predominantly of PE and polypropylene (Naji et al., 2021). In other studies, 93.3% of the MP particles in effluent were found to be smaller than 300 μm (Mintenig et al., 2020) and Fortin et al. (2019), using Raman microspectroscopic analysis, found that the majority were 1–10 μm. Many studies report that microfibres are the main MP components in effluents (Mintenig et al., 2020; Talvitie et al., 2017a; Lares et al., 2018) and these are mostly polyethylene, especially PET (Mintenig et al., 2020; Wolff et al., 2018; Lares et al., 2018; Ziajahromi et al., 2017a; Talvitie et al., 2017a). The presence of even small quantities of microfibers in WWTP effluent means that a significant amount is discharged into the receiving waters, because of the large volumes of effluent discharged every day (Talvitie et al., 2017b). Even highly efficient WWTPs that approach 98% MP removal will still allow daily discharge of considerable MPs in treated sewage effluents (Conley et al., 2019; Hidayaturrahman and Lee, 2019). Such a high removal capacity is not the norm, however; Liu et al. (2019a, b) found only 64.4% removal in a WWTP in China. Interestingly, although they identified the major sources of MP as clothes’ washing and the polymer production industries, the main type of plastic identified in the wastewater by these authors was polyamide.
Assuming a retention efficiency of 98.4% solids, a WWTP receiving influent from a population of only 100,000 inhabitants would discharge ~1 kg of microfibers into the environment every day (Hartline et al., 2016). Indeed, microfibers have been isolated from tap water all over the world, one global study measuring an average concentration of 4.34 particles/L and a maximum of 54 particles/L in 150 samples (Kosuth et al., 2017). The waste sludge itself may accumulate up to 98% of the MP (Gies et al., 2018), most of which is present as microfibres (Petroody et al., 2021), and this may be used as fertilizer if not incinerated or sent to landfill (Koyuncuoğlu and Erden, 2021; Edo et al., 2020), thus leading to potential contamination of agricultural land. Indeed, high levels of MP have been detected in land surrounding some WWTPs (e.g. Browne et al., 2011; Estahbanati and Fahrenfeld, 2016). It should certainly be an aim of WWTP managers to reduce their MP output.
Finally, Xu et al. (2021b) warn that the processes used in WWTPs could actually generate micro- and nano-plastics. Microbial enzymes may promote the hydrolysis of carrier materials, allowing MP to be released; secondary MP or nanoplastics may be produced during wastewater pumping, disinfection and thermal treatment of sludge, while processes such as thermal drying of sludge and lime stabilization have been found to lead to fragmentation of MPs. Considerably more testing and control is required on the microfibre situation in WWTPs.
5. Simulation studies
There is no standard method for measuring microfibre release during laundering, and this could explain the many different results found in the literature, some of which can be seen in Table 1. Both ASTM and ISO have published standards for pilling or linting in the dry state (ASTM pilling resistance tests D3511, D5512 and D4970; ISO 9073-10:2003 for lint and other particles generation in the dry state from non-woven fabrics) and these could be used as a basis, together, with ISO 12138:2017, which specifies domestic laundering procedures for fabrics prior to flammability testing, to add the details of the actual laundering process. Jönsson et al. (2018) developed a potential standard test consisting of six steps, all carefully specified: (1) Pre-Cleaning, (2) Cutting, (3) Welding, (4) Washing, using the GyroWash machine (5) Rinsing, (6) Analysis of filtered material by microscopy coupled to analytical software. Important points for a standard test, pointed out by Jönsson et al. (2018), are as follows: a) vacuum cleaning of the surfaces prior to test to ensure that superficial dust and already loose fibres are removed, b) cutting the sample by laser to ensure that there is no loss of fibres from the cut edges, c) the use of steel balls inside the bag, which aligns the test with the industry standard for testing colour fastness. Strict cleanliness in the environs of the test is also essential, in view of the omnipresence of microplastic fibres in today's environment (Hung et al., 2021; Brander et al., 2020).
The flammability test, ISO 12138:2017, specifies the type of domestic washing machine that should be used, but does not suggest the use of small laboratory models that were employed by Jönsson et al. (2018) and some other researchers. As suggested previously, it is unlikely that such small laboratory machines will produce results similar to those from the larger domestic or commercial models. Indeed, Lant et al. (2020) suggest that their results and those of Pirc et al. (2016) differ from those of Hernandez et al. (2017) and Carney Almroth et al. (2018) because of the use, by the latter two groups, of these small-scale simulation devices (Washtec P and GyroWash, respectively). The physical action of agitation, especially, differs between the machines and this must affect the fibrillation and breaking processes, with resulting differences in free microfibre production.
Cai et al. (2020a) used a modified ISO standard (105-C061:2010 with a modified washing solution), to investigate the differences between microplastic fibre release from 12 different textile samples that were prepared by cutting and sewing in different ways. They found that samples prepared by cutting with scissors released more fibres than those which were laser-cut and that fleece samples released more fibres than textiles with unprocessed surfaces. Their results fitted within the limits of those reported by some other workers who used either simulation machines or domestic washers. The same relative results were obtained using a specially developed, very small scale, laboratory sonication method for washing (Cai et al., 2020b). This suggests that, in some cases, the small scale simulation machines (which are utilized in a number of Standard Methods) can be appropriate. They have, at least, the environmental advantages of minimal use of materials and water. It is clear that the production of reliable, reproducible and quantitative test methods is extremely important for development of policies for studies on the impact of textiles on the environment (Rist et al., 2018).
6. Fate and environmental effects of microfibres from textiles
Environmental effects of microplastics from textiles are no different from those of other plastic microfibres, apart from the fact that their additives are relatively specific to their function. During manufacture, the fibres may incorporate or be coated with a variety of potentially harmful compounds, which are normally not removed during the laundry processes. These additives have the functions of protecting the fabric from environmental onslaughts such as microbial attack, or adding required properties to the finished article. Among the latter, we may cite softening agents, dyes, anti-wrinkle substances and water repellents. The latter may be toxic fluorinated compounds, which have been shown to resist washing and to remain on the released microfibres (Schellenberger et al., 2019). Pigments are incorporated into plastics to give the required colour. These may contain various toxic substances, such as heavy metals and azo, phthalocyanine and anthraquinone chromophores (Ismail et al., 2019). Plasticizers themselves can also be dangerous to aquatic life when leached from the material. For a general review of the ecotoxic effects of plastic additives, the reader is directed to the review of Campanale et al. (2020).
As well as toxic chemicals included in their formulation, microfibres may also carry adsorbed toxins on their surfaces. MPs have been shown to carry a wide variety of toxic metals and organic pollutants, such as PAHs, DDT, PCBs, and dioxins (Gaylarde et al., 2021, and references therein; Wang et al., 2018), hence increasing their danger to aquatic biota. The Earth's oceans are the final sink destination for microplastics that may have passed through various environmental departments (air, WWTPs, rivers, etc.) and marine organisms are constantly at risk from their toxic effects.
Synthetic microfibres have been shown to be ingested by many aquatic species, including zooplankton, polychaetes, bivalves and crustaceans (Alnajar et al., 2021; Chinfak et al., 2021; Mateos-Cárdenas et al., 2021; Bour et al., 2020; Avio et al., 2020; Jemec et al., 2016), deep-sea organisms (Pereira et al., 2020; Taylor et al., 2016; Murray and Cowie, 2011), marine, freshwater and farmed fish (Savoca et al., 2021; Avio et al., 2020; Halstead et al., 2018; Horton et al., 2018) and birds (Bourdages et al., 2021; Hamilton et al., 2021; Coughlan et al., 2020). Exposure to microfibers can affect growth, reproduction and survival of water fleas and amphipods (Ziajahromi et al., 2017b).
Microfibres are frequently the most common form of ingested MP detected in marine fish and invertebrates (Courtene-Jones et al., 2017; Taylor et al., 2016) and, as a result of their small size and length-to-diameter ratio, they are more effectively incorporated by organisms than are other plastic particle groups (Dris et al., 2018). Such MP are found throughout the world, in mountainous regions (Allen et al., 2019; Ambrosini et al., 2019) and rivers (Chinfak et al., 2021; Mani et al., 2015), in the deep oceans (Loughlin et al., 2021; Sanchez-Vidal et al., 2018; Courtene-Jones et al., 2017), in sediment samples from shorelines across a range of global locations (Adams et al., 2021; Browne et al., 2011), and in coastal waters (Kashiwabara et al., 2021; Green et al., 2018). They have been reported in the USA (Kashiwabara et al., 2021; Said and Heard, 2020), in Europe (Fischer et al., 2016; Leslie et al., 2017), in Asia (Jiang et al., 2019; Naji et al., 2021) and in the Arctic (Bergmann et al., 2019; González-Pleiter et al., 2020). Since they are so prevalent on our planet, it is not surprising that they have been detected in many organisms (Rebelein et al., 2021; Guzzetti et al., 2018; Egbeocha et al., 2018), including humans, in whom it is estimated that over 50% of human annual consumption of plastics is as microfibres (Cox et al., 2019). The actual consumption of microplastics by humankind is not known, but it has been estimated as 0.5–1 g/week (Senathirajah et al., 2021). Microfibres have been found in agricultural food crops (Oliveri Conti et al., 2020) and in mussels from the Belgian and Dutch coasts (Leslie et al., 2017; De Witte et al., 2014). The impact of MP on human health has recently been emphasized by Vijayaraman et al. (2020). Ingestion of microplastics has also been recorded in many crustaceans, seabirds, sea snakes, sea turtles, penguins, seals, sea lions, manatees, sea otters, fish, and half of all marine mammals (Taylor et al., 2016, and references therein), Ingested microfibres can lead to various toxic effects in aquatic animals. Zhao et al. (2021) exposed zebrafish (both adults and larvae) to MP fibres of different lengths and showed that both 50 (±26) μm and 200 (±90) μm long fibres adversely affected both life stages of the fish. They caused length-dependent histopathological changes and biomarker responses in the intestines, with long fibers inducing more serious effects. Food intake was decreased by 54 %–67 % more than with short fibers. The authors also used the new investigative technique of metabolomics and found that 124 and 123 metabolites were significantly changed by short and long fibers, respectively. MP fibers were shown to up-regulate glycerophospholipid metabolism, exacerbating oxidative damage and inflammation, and to down-regulate fatty acyl metabolism, inducing nutritional deficiency.
McCormick et al. (2020) showed by controlled experiments that damselfish that had been fed MP as part of the standard diet developed more risky behaviour; they suggested that this was caused by the need for more food forays because their stomachs were partially filled with the undigested plastics. This can obviously be a harmful effect in any animal that ingests microfibres.
Bour et al. (2020) compared the toxic effects of high concentrations of polyester microfibres (500 μm long) and polyethylene microbeads (27–32 μm) in brine shrimp (Artemia sp.) and fish (Gasterosteus aculeatus), invertebrate and vertebrate aquatic species, respectively. The shrimp ingested high amounts of microbeads, but not microfibres, when no other food was available. Ingestion of MP by fish was relatively unimportant, but they were found to accumulate in the gills, when added to the freshwater or seawater. Damaged gill epithelium has also been observed in Japanese medaka exposed long-term to microfibers (Hu et al., 2020). The sea anemone, Aiptasia pallida, has been shown to ingest microfibres of various polymer types, especially when offered with a food source of brine shrimp homogenate (Romanó de Orte et al., 2019). The authors considered that the shrimp was acting as a chemical source of prey, stimulating ingestion. In spite of the results of Bour et al. (2020), which suggested that microbeads might be more damaging than microfibres, more studies suggest that microfibres have a negative impact on organisms than those that show little impact (Kapp and Miller, 2020, and references therein; Bucci et al., 2019). Examples in which plastic microfibres have been shown to have greater impact than microbeads include studies on the amphipod Hyalella azteca (Au et al., 2015) and the waterflea Ceriodaphnia dubia (Ziajahromi et al., 2017b).
7. Degradation of microfibres in the aqueous environment
It is an accepted fact that plastics are very slowly degraded, especially by the biological components of the natural environment (Ali et al., 2021). One of the major problems with microplastics pollution of aquatic systems is the prolonged time periods for which it persists. Despite all the available information in the literature about these contaminants, few studies assess the degradation process of textile fibres in aquatic environments (Zambrano et al., 2019). Well-controlled aquatic biodegradation experiments have shown that cotton and rayon microfibres should degrade in natural aquatic aerobic environments whereas polyester microfibres will persist for long periods of time (Palacios-Mateo et al., 2021). Degradation of plastics in the environment is influenced by both intrinsic properties such as polymer type, density, molecular size, any added chemicals, as well as by extrinsic parameters such as agitation, UV exposure and, potentially, biological activity.
7.1. Non-biological degradation
Plastic particles or fibres are subjected to several abiotic degradative reactions in aquatic ecosystems, including photodegradation (UV catalysed oxidation), hydrolysis and mechanical degradation (Gewert et al., 2018; Zambrano et al, 2019, 2020). Although available data concerning the environmental degradation of plastic microfibres is scarce, it is expected that the degradation dynamics of these pollutants is analogous to other plastic particles. Thus solar energy causes discoloration and surface cracking (Da Costa et al., 2018; Gewert et al., 2018), resulting in polymer surface increase and subsequent fragmentation by additional mechanical forces (e.g. water currents, waves, etc). Sait et al. (2021) investigated the photodegradation of microfibres composed of PET, polyamide (PA) and polyacrylonitrile (PAN). They were exposed to UV for 10 months in freshwater and seawater. PET and PA fibres became fragmented and showed surface changes, while PA also showed chemical changes. PAN showed no significant photodegradation. UltraHPLC identified bisphenol A, bisphenol S and benzophenone-3 in all samples at concentrations between 4.3 and 501 ng/g. In a less extensive study, Sørensen et al. (2021) showed that 56 days of artificial sunlight exposure in seawater led to surface changes in PA, but little fragmentation, while PET showed both changes in surface morphology and fragmentation. Other groups have shown that PE is rather resistant to UV degradation (Da Costa et al., 2018; Lin et al., 2020).
7.2. Biodegradation
Although MP particles in the marine environment have been shown to carry their own, rather specific, biofilm (called the “plastisphere” by Zettler et al., 2013), it is doubtful whether these microorganisms could be involved in degradation of the plastic material. Bryant et al. (2016) noted that the plastisphere contains a core of microbial clades that are functionally different from those in the water column around them. They considered that the plastisphere microorganisms were utilizing adsorbed pollutants or carbon sources produced by marine eukaryotes, rather than the plastic itself.
Zhao et al. (2021) discuss the possibilities for biodegradation of MP, the challenges and potential of various developments. Gong et al. (2018) produced an engineered and alkali-tolerant strain of Comamonas testosterone F4, denoted F6, that was able to degrade PET. The average PET particle size was reduced from 7.3 to 1.58 and 2.63 μm after up to 50h of incubation. The marine fungus, Zalerion maritimum, has been shown to participate in degradation of PE (Paço et al., 2017), as have other fungi (Sánchez, 2020). However, there is still no well documented report of rapid plastics biodegradation by environmental microorganisms. Kumar et al. (2020) consider that enhancing biodegradation of MP in the environment will be an important part of their control. However, it is probably easier to reduce the release of microplastic fibres from laundering activities than to augment their biodegradation.
8. Potential methods for controlling microfibre production and release
Proposed methods for the reduction of microfibre release from laundry activities fall into 3 camps, changes to washing machines or washing procedures, changes in the textiles themselves and improvement of wastewater treatment processes.
8.1. Modification of washing machines
The initial choice of washing machine can influence microfibre release, with front-loading machines generating fewer microfibres than top loaders (Henry et al., 2019). Addition of filters to washing machines has been proposed to mitigate the issue; the Lint LUV-R system that fits onto the machine outlet shows higher efficiency than the in-drum fibre-trapping ‘Cora ball’ device (McIlwraith et al., 2019). However, such domestic user additions can never be a satisfactory solution. Modern washing machines need to be fitted at the factory with suitable filters to avoid microfibre release to the waste pipe. Browne et al. (2020) compared the ability of various pore sized filters to reduce microfibre release. Micrometer-sized filters did effectively reduce released PE fibre mass by over 65%, but filtered effluent still contained up to one third of the original fibre mass.
The results of Lant et al. (2020), who measured microfibre release under both European and North American laundry conditions, suggest that high water to fabric ratio increases microfibre loss, thus encouraging the current environmental aims of reducing water usage. This provides an additional reason why the appliance industry should continue to develop approaches to reduce water consumption during laundering.
8.2. Modification of washing procedures
The increase of public preoccupation with microfibre release during clothes washing has led to the development of devices that can be inserted into washing machines by their users to trap and retain the fibres released during laundering. The Guppyfriend, for example, is a fine mesh bag in which clothes can be placed for washing, while the Cora Ball traps the fibres that are released during washing. Kärkkäinen and Sillanpää (2021) found that these two devices reduced microfibres in the outlet of the washing machines by only 39% and 10%, respectively, while Napper et al. (2020) measured a 54% reduction with the Guppyfriend bag. The latter authors concluded that the most effective device was the XFiltra, which, when fitted to the effluent water, reduced fibre release by 78%. Using fleece blankets as the test textile, however, McIlwraith et al. (2019) achieved a capture of 87% of the total number of microfibres using the Lint LUV-R, compared to 26% with the Cora Ball. Obviously, the efficiency of these devices depends on the types of fabrics being washed and the actual conditions and mechanics of the washing process.
8.3. Modification of textiles
Avoiding loose knits and fleeces can reduce microfibre release during laundering (Carney Almroth et al., 2018); other textile characteristics that affect fibre release are the origin and type of polymer, fabric construction (yarn length, width and twist), and the finishing and cutting of the fabric. Water repellent and high abrasion resistant finishes reduce microfibre shedding (Zambrano et al., 2021a), as does the extra cross-linking that accompanies the durable-press finish, the latter, however, also reducing biodegradability (Zambrano et al., 2021b). The Textile Industry is concerned to improve their environmental footprint and there is research into new finishing treatments to reduce microfibre release. These include the electrofluidodynamic (EFD) method (De Falco et al., 2019b), in which homogeneous coatings are produced on polyamide fabrics, and finishes based on glycidyl methacrylate-modified pectin (De Falco et al., 2018). The dip-coating of a polyester fabric produced a textile that resisted 200 washing cycles, as well as treatment with 1M acid or alkali (Tian et al., 2020). Although developed specifically as a scald-prevention fabric, it could offer insights into new low-microfibre-release textile developments.
8.4. Improvement of WWTP technology
Removal of microfibres at wastewater treatment plants is being considered, although this is challenging as the microfibres would need to be eliminated from both effluent and sludge to prevent release into the environment (Talvitie et al., 2017a, b; Michielssen et al., 2016). The removal process of microfibres mainly occurs in the initial phase of sedimentation in a typical WWTP, where multiple treatment steps based on physicochemical and biological treatments are used (Xu et al., 2019). Some tertiary treatments may be used to further clean the aqueous effluent. Membrane reactors, sand filters, disc filters and biofilters have been shown as effective approaches (Gatidou et al., 2019; Simon et al., 2019; Liu et al., 2020), although these methods are not completely efficient, since small sized particles can still be released into the receiving environment. Studies suggest that advanced technologies can improve the removal of MP from treatment systems (Carr et al., 2016; Talvitie et al., 2017b; Ziajahromi et al., 2017; Lares et al., 2018). Both the treatment process and the physicochemical properties of the polymer influence removal efficiency (Bond et al., 2018) and it is the MP in the smaller size range, which are difficult for the wastewater treatment plants to capture, that are of the greatest concern for the environment (Zambrano et al., 2019; De Falco et al., 2019a; Hou et al., 2020). Lares et al. (2018), for example, found that most MP were removed before the activated sludge process; the overall retention capacity was 98.3%, and this could be improved by incorporating a pilot-scale membrane bioreactor. Membrane bioreactors have been found to increase the efficiency of MP removal from wastewater. In a study of a full-scale WWTP in E. China, Lv et al. (2019) found that the membrane bioreactor removed 99.5% of the MP mass, compared to 97% in the oxidation ditch system. The plastics still, however, accumulated in the sludge. They suggested that the best way to reduce WWTP effluents was to control the source, especially eliminating fibres from laundry facilities. Indeed, this would appear to be the optimum answer, requiring concentrated research in the areas of non-fibre-release fabrics and improved air and water filters in laundry facilities.
9. Recent initiatives by official bodies
An important decision of the French government is the recently approved law aimed at reducing the release of microplastics from all washing machines by 2025 (“Loi no 2020-105 du 10 février 2020 relative à la lutte contre le gaspillage et à l'économie circulaire”, available at www.legifrance.gouv.fr). Doubtless such an initiative will be copied by other countries. The choice of policy implements to reduce microplastics release that could be put in place by the Finnish government is discussed by Virta and Räisänen (2021). At the waste treatment level, which is sometimes administered by local government, the use of tertiary granular sand filtration and membrane filtration in WWTPs will assist removal of MP filaments (Michielssen et al., 2016).
Appropriate standard testing methods will be needed if further improvements are to be made in textile design, appliance design, or recommendations to consumers about reducing their personal impact on the environment through their laundering choices. e.g. fabric care products and washing conditions. Bodies such as ASTM and ISO should be developing such tests. Current reproducible results suggest that liquid detergents and fabric softeners can continue to be used without negative effects and that washing in colder water with shorter cycles will allow a reduction in domestic microfibre production. The new High-Efficiency washing machines, with reduced water use, may also assist (Kelly et al., 2019).
Collaboration with the textile industry will be essential in this task. Yan et al. (2020) interviewed 10 experts from the textile industry and found that microfibre pollution does not have a clear-cut definition. They concluded that the problem and challenges need to be identified by multiple and integrated stakeholders, overseeing the entire product life cycle. Such collaboration, together with government directives such as that put in place by the French, will help to reduce a great part of the plastics pollution on the planet.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Acharya S., Rumi S.S., Hu Y. Microfibers from synthetic textiles as a major source of microplastics in the environment: a review. Textil. Res. J. 2021 [Google Scholar]
- Adams J.K., Dean B.Y., Athey S.N. Anthropogenic particles (including microfibers and microplastics) in marine sediments of the Canadian Arctic. Sci. Total Environ. 2021;784:147155. doi: 10.1016/j.scitotenv.2021.147155. [DOI] [PubMed] [Google Scholar]
- Akanyange S.N., Lyu X., Zhao X. Does microplastic really represent a threat? A review of the atmospheric contamination sources and potential impacts. Sci. Total Environ. 2021;777:146020. doi: 10.1016/j.scitotenv.2021.146020. [DOI] [PubMed] [Google Scholar]
- Ali S.S., Elsamahy T., Koutra E. Degradation of conventional plastic wastes in the environment: a review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021;771:144719. doi: 10.1016/j.scitotenv.2020.144719. [DOI] [PubMed] [Google Scholar]
- Allen S., Allen D., Phoenix V.R. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019;12:339–344. [Google Scholar]
- Alnajar N., Jha A.N., Turner A. Impacts of microplastic fibres on the marine mussel, Mytilus galloprovinciallis. Chemosphere. 2021;262:128290. doi: 10.1016/j.chemosphere.2020.128290. [DOI] [PubMed] [Google Scholar]
- Ambrosini R., Azzoni R.S., Pittino F. First evidence of microplastic contamination in the supraglacial debris of an alpine glacier. Environ. Pollut. 2019;253:297–301. doi: 10.1016/j.envpol.2019.07.005. Pmid:31323612. [DOI] [PubMed] [Google Scholar]
- Au S.Y., Bruce T.F., Bridges W.C., Klaine S.J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015;34:2564–2572. doi: 10.1002/etc.3093. pmid:26042578. [DOI] [PubMed] [Google Scholar]
- Avio C.G., Pittura L., d’Errico G. Distribution and characterization of microplastic particles and textile microfibers in Adriatic food webs: general insights for biomonitoring strategies. Environ. Pollut. 2020;258:113766. doi: 10.1016/j.envpol.2019.113766. [DOI] [PubMed] [Google Scholar]
- Barrows A.P.W., Cathey S.E., Petersen C.W. Marine environment microfiber contamination: global patterns and the diversity of microparticle origins. Environ. Pollut. 2018;237:275–284. doi: 10.1016/j.envpol.2018.02.062. [DOI] [PubMed] [Google Scholar]
- Ben-David E.A., Habibi M., Haddad E. Microplastic distributions in a domestic wastewater treatment plant: removal efficiency, seasonal variation and influence of sampling technique. Sci. Total Environ. 2021;752:141880. doi: 10.1016/j.scitotenv.2020.141880. [DOI] [PubMed] [Google Scholar]
- Bergmann M., Mützel S., Primpke S. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax1157. eaax1157. pmid:31453336; PMCID: PMC6693909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J., Friot D. IUCN; Gland, Switzerland: 2017. Primary Microplastics in the Oceans: a Global Evaluation of Sources. [Google Scholar]
- Bond T., Ferrandiz-Mas V., Felipe-Sotelo M., van Sebille E. The occurrence and degradation of aquatic plastic litter based on polymer physicochemical properties: A review. Crit. Revs. Environ. Sci. Technol. 2018;48(7-9):685–722. [Google Scholar]
- Bour A., Hossain S., Taylor M. Synthetic microfiber and microbead exposure and retention time in model aquatic species under different exposure scenarios. Front Environ. Sci. 2020;8:83. [Google Scholar]
- Bourdages M.P.T., Provencher J.F., Baak J.E. Breeding seabirds as vectors of microplastics from sea to land: evidence from colonies in Arctic Canada. Sci. Total Environ. 2021;764 doi: 10.1016/j.scitotenv.2020.142808. [DOI] [PubMed] [Google Scholar]
- Brander S.M., Renick V.C., Foley M.M. EXPRESS: sampling and QA/QC: a guide for scientists investigating the occurrence of microplastics across matrices. Appl. Spectrosc. 2020;74:1099–1125. doi: 10.1177/0003702820945713. [DOI] [PubMed] [Google Scholar]
- Browne M.A., Crump P., Niven S.J. Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ. Sci. Technol. 2011;45:9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- Browne M.A., Ros M., Johnston E.L. Pore-size and polymer affect the ability of filters for washing-machines to reduce domestic emissions of fibres to sewage. PloS One. 2020;15 doi: 10.1371/journal.pone.0234248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J.A., Clemente T.M., Viviani D.A. Diversity and activity of communities inhabiting plastic debris in the North Pacific Gyre. mSystems. 2016;1 doi: 10.1128/mSystems.00024-16. e00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci K., Tulio M., Rochman C.M. What is known and unknown about the effects of plastic pollution: a meta-analysis and systematic review. Ecol. Appl. 2019;30(2) doi: 10.1002/eap.2044. pmid:31758826. [DOI] [PubMed] [Google Scholar]
- Cai Y., Mitrano D.M., Heuberger M. The origin of microplastic fiber in polyester textiles: the textile production process matters. J. Clean. Prod. 2020;267:121970. [Google Scholar]
- Cai Y., Yang T., Mitrano D.M. Systematic study of microplastic fiber release from 12 different polyester textiles during washing. Environ. Sci. Technol. 2020;54:4847–4855. doi: 10.1021/acs.est.9b07395. [DOI] [PubMed] [Google Scholar]
- Campanale C., Massarelli C., Savino I. A detailed review study on potential effects of microplastics and additives of concern on human health. MDPI Int. J. Environ. Res. Pub. Health. 2020:1–26. doi: 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney Almroth B.M., Astrom L., Roslund S. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018;25:1191–1199. doi: 10.1007/s11356-017-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr S.A., Liu J., Tesoro A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016;91:174–182. doi: 10.1016/j.watres.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Castelvetro V., Corti A., Biale G. New methodologies for the detection, identification, and quantification of microplastics and their environmental degradation by-products. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-021-12466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesa F.S., Turra A., Checon H.H. Laundering and textile parameters influence fibers release in household washings. Environ. Pollut. 2020;257:113553. doi: 10.1016/j.envpol.2019.113553. [DOI] [PubMed] [Google Scholar]
- Chan C.K.M., Curie P., Ming C.K. Microplastic fibre releases from industrial wastewater effluent: a textile wet-processing mill in China. Environ. Chem. 2021 [Google Scholar]
- Chinfak N., Sompongchaiyakul P., Charoenpong C. Abundance, composition, and fate of microplastics in water, sediment, and shellfish in the Tapi-Phumduang River system and Bandon Bay, Thailand. Sci. Total Environ. 2021;781:146700. doi: 10.1016/j.scitotenv.2021.146700. [DOI] [PubMed] [Google Scholar]
- Conley K., Clum A., Deepe J. Wastewater treatment plants as a source of microplastics to an urban estuary: removal efficiencies and loading per capita over one year. Water Res. X. 2019;3:100030. doi: 10.1016/j.wroa.2019.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini F., Meza P., Eguiluz R. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019;671:411e420. doi: 10.1016/j.scitotenv.2019.03.368. [DOI] [PubMed] [Google Scholar]
- Cotton L., Hayward A.S., Lant N.J., Blackburn R.S. Improved garment longevity and reduced microfibre release are important sustainability benefits of laundering in colder and quicker washing machine cycles. Dyes Pigments. 2020;177:108120. [Google Scholar]
- Coughlan N.E., Doyle S., Baker-Arney C. Ingestion of anthropogenic debris by migratory barnacle geese Branta leucopsis on a remote north-eastern Atlantic island. Mar. Pollut. Bull. 2020;160:111588. doi: 10.1016/j.marpolbul.2020.111588. [DOI] [PubMed] [Google Scholar]
- Courtene-Jones W., Quinn B., Gary S.F. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017;231:271–280. doi: 10.1016/j.envpol.2017.08.026. [DOI] [PubMed] [Google Scholar]
- Cox K.D., Covernton G.A., Davies H.L. Consumption of microplastics. Environ. Sci. Technol. 2019;53:7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- Da Costa J.P., Nunes A.R., Santos P.S.M. Degradation of polyethylene microplastics in seawater: insights into the environmental degradation of polymers. J. Environ. Sci. Health Part A. 2018;53:866–875. doi: 10.1080/10934529.2018.1455381. [DOI] [PubMed] [Google Scholar]
- Dalla Fontana G., Mossotti R., Montarsolo A. Assessment of microplastics release from polyester fabrics: the impact of different washing conditions. Environ. Pollut. 2020;264:113960. doi: 10.1016/j.envpol.2020.113960. [DOI] [PubMed] [Google Scholar]
- De Falco F., Gentile G., Avolio R. Pectin based finishing to mitigate the impact of microplastics released by polyamide fabrics. Carbohydr. Polym. 2018;198:175–180. doi: 10.1016/j.carbpol.2018.06.062. [DOI] [PubMed] [Google Scholar]
- De Falco F., Di Pace E., Cocca M.C., Avella M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019;9:6633. doi: 10.1038/s41598-019-43023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Falco F., Cocca M., Guarino V. Novel finishing treatments of polyamide fabrics by electrofluidodynamic process to reduce microplastic release during washings. Polym. Degrad. Stabil. 2019;165:110–116. [Google Scholar]
- De Falco F., Cocca M., Avella M., Thompson R.C. Microfibre release to water, via laundering, and to air, via everyday use: a comparison between polyester clothing with differing textile parameters. Environ. Sci. Technol. 2020;54:3288–3296. doi: 10.1021/acs.est.9b06892. [DOI] [PubMed] [Google Scholar]
- De Witte B., Devriese L., Bekaert K. Quality assessment of the blue mussel (Mytilus edulis): comparison between commercial and wild types. Mar. Pollut. Bull. 2014;85:146–155. doi: 10.1016/j.marpolbul.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Dhir Y.J. 2 - hazards of fashion and textile waste: approaches for effective waste management. In: Nayak R., Patnaik A., editors. The Textile Institute Book Series, Waste Management in the Fashion and Textile Industries. Woodhead Publishing; 2021. pp. 31–58. 2021. [Google Scholar]
- Dris R., Gasperi J., Saad M. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar. Pollut. Bull. 2016;104:290–293. doi: 10.1016/j.marpolbul.2016.01.006. Pmid:26787549. [DOI] [PubMed] [Google Scholar]
- Dris R., Gasperi J., Rocher V., Tassin B. Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris Megacity: sampling methodological aspects and flux estimations. Sci. Total Environ. 2018;618:157–164. doi: 10.1016/j.scitotenv.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Edo C., González-Pleiter M., Leganés F. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020;259:113837. doi: 10.1016/j.envpol.2019.113837. [DOI] [PubMed] [Google Scholar]
- Egbeocha C.O., Malek S., Emenike C.U., Milow P. Feasting on microplastics : ingestion by and effects on marine organisms. Aquat. Biol. 2018;27:93–106. [Google Scholar]
- Eerkes-Medrano D., Thompson R.C., Aldridge D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015;75:63–82. doi: 10.1016/j.watres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Fischer E.K., Paglialonga L., Czech E., Tamminga M. Microplastic pollution in lakes and lake shoreline sediments—a case study on Lake Bolsena and Lake Chiusi (central Italy) Environ. Pollut. 2016;213:648–657. doi: 10.1016/j.envpol.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Fortin S., Song B., Burbage C. Quantifying and identifying microplastics in the effluent of advanced wastewater treatment systems using Raman microspectroscopy. Mar. Pollut. Bull. 2019;149:110579. doi: 10.1016/j.marpolbul.2019.110579. [DOI] [PubMed] [Google Scholar]
- Freeman S., Booth A.M., Sabbah I. Between source and sea: The role of wastewater treatment in reducing marine microplastics. J. Environ. Manage. 2020;266:110642. doi: 10.1016/j.jenvman.2020.110642. [DOI] [PubMed] [Google Scholar]
- Galvão A., Aleixo M., De Pablo H. Microplastics in wastewater: microfiber emissions from common household laundry. Environ. Sci. Pollut. Res. 2020;27(2020):26643–26649. doi: 10.1007/s11356-020-08765-6. [DOI] [PubMed] [Google Scholar]
- Gasperi J., Wright S.L., Dris R. Microplastics in air: are we breathing it in? Curr. Opin. Environ. Sci. Health. 2018;1:1–5. [Google Scholar]
- Gatidou G., Arvaniti O.S., Stasinakis A.S. Review on the occurrence and fate of microplastics in Sewage Treatment Plants. J. Hazard. Mats. 2019;367:504–512. doi: 10.1016/j.jhazmat.2018.12.081. [DOI] [PubMed] [Google Scholar]
- Gaylarde C.C., Baptista Neto J.A., da Fonseca E.M. Nanoplastics in aquatic systems - are they more hazardous than microplastics? Environ. Pollut. 2021;272:115950. doi: 10.1016/j.envpol.2020.115950. [DOI] [PubMed] [Google Scholar]
- GESAMP . IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection; 2015. “Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment”, GESAMP Reports and Studies. [Google Scholar]
- Gewert B., Plassmann M., Sandblom O., MacLeod M. Identification of chain scission products released to water by plastic exposed to ultraviolet light. Environ. Sci. Technol. Lett. 2018;5:272–276. [Google Scholar]
- Gies E.A., LeNoble J.L., Noël M. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018;133:553–561. doi: 10.1016/j.marpolbul.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Gong J., Xie P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere. 2020;254:126790. doi: 10.1016/j.chemosphere.2020.126790. [DOI] [PubMed] [Google Scholar]
- Gong J., Kong T., Li Y. Biodegradation of microplastic derived from poly(ethylene terephthalate) with bacterial whole-cell biocatalysts. Polymers. 2018;10:1326. doi: 10.3390/polym10121326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Pleiter M., Velazquez D., Edo C. Fibers spreading worldwide: microplastics and other anthropogenic litter in an Arctic freshwater lake. Sci. Total Environ. 2020;722:137904. doi: 10.1016/j.scitotenv.2020.137904. [DOI] [PubMed] [Google Scholar]
- González-Pleiter M., Edo C., Aguilera A. Occurrence and transport of microplastics sampled within and above the planetary boundary layer. Sci. Total Environ. 2021;761:143213. doi: 10.1016/j.scitotenv.2020.143213. [DOI] [PubMed] [Google Scholar]
- Green D.S., Kregting L., Boots B. A comparison of sampling methods for seawater microplastics and a first report of the microplastic litter in coastal waters of Ascension and Falkland Islands. Mar. Pollut. Bull. 2018;137:695–701. doi: 10.1016/j.marpolbul.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Guzzetti E., Sureda A., Tejada S., Faggio C. Microplastic in marine organisms: environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018;64:164–171. doi: 10.1016/j.etap.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Hamilton B.M., Bourdages M.P.T., Geoffroy C. Microplastics around an Arctic seabird colony: particle community composition varies across environmental matrices. Sci. Total Environ. 2021;773:145536. doi: 10.1016/j.scitotenv.2021.145536. [DOI] [PubMed] [Google Scholar]
- Hartline N.L., Bruce N.J., Karba S.N. Microfiber masses recovered from conventional machine washing of new or aged garments. Environ. Sci. Technol. 2016;50:11532–11538. doi: 10.1021/acs.est.6b03045. Epub 2016 Oct 13. PMID: 27689236. [DOI] [PubMed] [Google Scholar]
- Halstead J.E., Smith J.A., Carter E.A., Lay P.A., Johnston E.L. Assessment tools for microplastics and natural fibres ingested by fish in an urbanised estuary. Environ. Pollut. 2018;234:552–561. doi: 10.1016/j.envpol.2017.11.085. [DOI] [PubMed] [Google Scholar]
- Henry B., Laitala K., Klepp I.G. Microfibres from apparel and home textiles: prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 2019;652:483–494. doi: 10.1016/j.scitotenv.2018.10.166. [DOI] [PubMed] [Google Scholar]
- Hernandez E., Nowack B., Mitrano D.M. Polyester textiles as a source of microplastics from households: a mechanistic study to understand microfiber release during washing. Environ. Sci. Technol. 2017;51:7036–7046. doi: 10.1021/acs.est.7b01750. [DOI] [PubMed] [Google Scholar]
- Hidayaturrahman H., Lee T.-G. A study on characteristics of microplastic in wastewater of South Korea: identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull. 2019;146:696–702. doi: 10.1016/j.marpolbul.2019.06.071. [DOI] [PubMed] [Google Scholar]
- Horton A.A., Jürgens M.D., Lahive E. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018;236:188–194. doi: 10.1016/j.envpol.2018.01.044. [DOI] [PubMed] [Google Scholar]
- Hou L., Kumar D., Yoo C.G. Conversion and removal strategies for microplastics in wastewater treatment plants and landfills. Chem. Eng. J. 2020;406:126715. [Google Scholar]
- Hu L., Chernick M., Lewis A.M. Chronic microfiber exposure in adult Japanese medaka (Oryzias latipes) PloS One. 2020;15 doi: 10.1371/journal.pone.0229962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C., Klasios N., Zhu X. Methods matter: methods for sampling microplastic and other anthropogenic particles and their implications for monitoring and ecological risk assessment. Integrated Environ. Assess. Manag. 2021;17:282–291. doi: 10.1002/ieam.4325. [DOI] [PubMed] [Google Scholar]
- Ismail M., Akhtar K., Khan M. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharmaceut. Des. 2019;25:3645–3663. doi: 10.2174/1381612825666191021142026. [DOI] [PubMed] [Google Scholar]
- Ivar do Sul J.A. Why it is important to analyze the chemical composition of microplastics in environmental samples. Mar. Pollut. Bull. 2021;165:112086. doi: 10.1016/j.marpolbul.2021.112086. [DOI] [PubMed] [Google Scholar]
- Iyare P.U., Ouki S.K., Bond T. Microplastics removal in wastewater treatment plants: a critical review. Environ. Sci.: Water Res. Technol. 2020;6:2664. [Google Scholar]
- Jemec A., Horvat P., Kunej U. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016;219:201–209. doi: 10.1016/j.envpol.2016.10.037. [DOI] [PubMed] [Google Scholar]
- Jiang C., Yin L., Li Z. Microplastic pollution in the rivers of the tibet plateau. Environ. Pollut. 2019;249:91–98. doi: 10.1016/j.envpol.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Jönsson C., Levenstam Arturin O., Hanning A.-C. Microplastics shedding from textiles—developing analytical method for measurement of shed material representing release during domestic washing. Sustainability. 2018;10:2457. [Google Scholar]
- Kanhai D.K., Officer R., Lyashevska O. Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean. Mar. Pollut. Bull. 2017;115:307–314. doi: 10.1016/j.marpolbul.2016.12.025. [DOI] [PubMed] [Google Scholar]
- Kapp K.J., Miller R.Z. Electric clothes dryers: an underestimated source of microfiber pollution. PloS One. 2020;15(10) doi: 10.1371/journal.pone.0239165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärkkäinen N., Sillanpää M. Quantification of different microplastic fibres discharged from textiles in machine wash and tumble drying. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-020-11988-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwabara L.M., Kahane-Rapport S.R., King C. Microplastics and microfibers in surface waters of monterey Bay National marine sanctuary, California. Mar. Pollut. Bull. 2021;165:112148. doi: 10.1016/j.marpolbul.2021.112148. [DOI] [PubMed] [Google Scholar]
- Kelly M.R., Lant N.J., Kurr M., Burgess J.G. Importance of water-volume on the release of microplastic fibers from laundry. Environ. Sci. Technol. 2019;53:11735–11744. doi: 10.1021/acs.est.9b03022. [DOI] [PubMed] [Google Scholar]
- Koelmans A.A., Mohamed Nor N.H., Hermsen E. Vol. 155. 2019. Microplastics in freshwaters and drinking water: critical review and assessment of data quality Water Res; pp. 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuth M., Mason S., Wattenberg E. Synthetic polymer contamination of global drinking water. PloS One. 2017;13 [Google Scholar]
- Koyuncuoğlu P., Erden G. Sampling, pre-treatment, and identification methods of microplastics in sewage sludge and their effects in agricultural soils: a review. Environ. Monit. Assess. 2021;193:175. doi: 10.1007/s10661-021-08943-0. [DOI] [PubMed] [Google Scholar]
- Kumar A.G., Anjana K., Hinduja M. Review on plastic wastes in marine environment – biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020;150:110733. doi: 10.1016/j.marpolbul.2019.110733. [DOI] [PubMed] [Google Scholar]
- Lant N.J., Hayward A.S., Peththawadu M.M.D. Microfiber release from real soiled consumer laundry and the impact of fabric care products and washing conditions. PloS One. 2020;15 doi: 10.1371/journal.pone.0233332. PMID: 32502152; PMCID: PMC7274375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lares M., Ncibi M.C., Sillanpää M., Sillanpää M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018;133:236–246. doi: 10.1016/j.watres.2018.01.049. [DOI] [PubMed] [Google Scholar]
- Leslie H.A., Brandsma S.H., van Velzen M.J.M., Vethaak A.D. Microplastics en route: field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017;101:133–142. doi: 10.1016/j.envint.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Lima A.R.A., Costa M.F., Barletta M. Distribution patterns of microplastics within the plankton of a tropical estuary. Environ. Res. 2014;132:146–155. doi: 10.1016/j.envres.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Lin J., Yan D., Fu J. Ultraviolet-C and vacuum ultraviolet inducing surface degradation of microplastics. Water Res. 2020;186:116360. doi: 10.1016/j.watres.2020.116360. [DOI] [PubMed] [Google Scholar]
- Lin J., Xu X.-P., Yue B.-Y. A novel thermoanalytical method for quantifying microplastics in marine sediments. Sci. Total Environ. 2021;760:144316. doi: 10.1016/j.scitotenv.2020.144316. [DOI] [PubMed] [Google Scholar]
- Liu J., Yang Y., Ding J. Microfibers: a preliminary discussion on their definition and sources. Environ. Sci. Pollut. Res. 2019;26:29497–29501. doi: 10.1007/s11356-019-06265-w. [DOI] [PubMed] [Google Scholar]
- Liu X., Yuan W., Di M. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019;362:176–182. [Google Scholar]
- Liu M., Lu S., Chen Y. Analytical methods for microplastics in environments: current advances and challenges. In: He D., Luo Y., editors. Microplastics in Terrestrial Environments. The Handbook of Environmental Chemistry. Vol. 95. Springer; Cham: 2020. [Google Scholar]
- Liu J., Liang J., Ding J. Microfiber pollution: an ongoing major environmental issue related to the sustainable development of textile and clothing industry. Environ. Dev. Sustain. 2021 [Google Scholar]
- Loughlin C., Mendes A.R.M., Morrison L., Morley A. The role of oceanographic processes and sedimentological settings on the deposition of microplastics in marine sediment: Icelandic waters. Mar. Pollut. Bull. 2021;164:111976. doi: 10.1016/j.marpolbul.2021.111976. [DOI] [PubMed] [Google Scholar]
- Lu H.-C., Ziajahromi S., Neale P.A., Leusch F.D.L. A systematic review of freshwater microplastics in water and sediments: recommendations for harmonisation to enhance future study comparisons. Sci. Total Environ. 2021;781:146693. [Google Scholar]
- Lv X., Dong Q., Zuo Z. Microplastics in a municipal wastewater treatment plant: fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 2019;225:579–586. [Google Scholar]
- Magnusson K., Norén F. 2014. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant.http://urn.kb.se/resolve?urn=urn:nbn:se:naturvardsverket:diva-2226 Accessed on March 5th, 2021. [Google Scholar]
- Mahon A.M., O'Connell B., Healy M.G. Microplastics in sewage sludge: effects of treatment. Environ. Sci. Technol. 2017;51:810–818. doi: 10.1021/acs.est.6b04048. [DOI] [PubMed] [Google Scholar]
- Mani T., Hauk A., Walter U., Burkhardt-Holm P. Microplastics profile along the Rhine river. Sci. Rep. 2015;5:17988. doi: 10.1038/srep17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni S., Binelli A., Pittura L. The fate of microplastics in an Italian wastewater treatment plant. Sci. Total Environ. 2019;652:602–610. doi: 10.1016/j.scitotenv.2018.10.269. [DOI] [PubMed] [Google Scholar]
- Mateos-Cárdenas A., O'Halloran J., van Pelt F.N.A.M., Jansen M.A.K. Beyond plastic microbeads – short-term feeding of cellulose and polyester microfibers to the freshwater amphipod Gammarus duebeni. Sci. Total Environ. 2021;753:141859. doi: 10.1016/j.scitotenv.2020.141859. [DOI] [PubMed] [Google Scholar]
- McCormick M.I., Chivers D.P., Ferrari M.C.O. Microplastic exposure interacts with habitat degradation to affect behaviour and survival of juvenile fish in the field. Proc. R. Soc. B: Biol. Sci. 2020 doi: 10.1098/rspb.2020.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern K., Alegria H., Kalagher A.L. Microplastics in Tampa Bay, Florida: abundance and variability in estuarine waters and sediments. Mar. Pollut. Bull. 2019;48:97–106. doi: 10.1016/j.marpolbul.2019.07.068. [DOI] [PubMed] [Google Scholar]
- McIlwraith H.K., Lin J., Erdle L.M. Capturing microfibers - marketed technologies reduce microfiber emissions from washing machines. Mar. Pollut. Bull. 2019;139:40–45. doi: 10.1016/j.marpolbul.2018.12.012. PMID: 30686443. [DOI] [PubMed] [Google Scholar]
- Michielssen M.R., Michielssen E.R., Ni J., Duhaime M.B. Fate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed. Environ. Sci. Water Res. Technol. 2016;2:1064–1073. [Google Scholar]
- Mintenig S.M., Kooi M., Erich M.W. A systems approach to understand microplastic occurrence and variability in Dutch riverine surface waters. Water Res. 2020;176:115723. doi: 10.1016/j.watres.2020.115723. [DOI] [PubMed] [Google Scholar]
- Mishra S., Rath C.C., Das A.P. Marine microfiber pollution: a review on present status and future challenges. Mar. Pollut. Bull. 2019;140:188–197. doi: 10.1016/j.marpolbul.2019.01.039. [DOI] [PubMed] [Google Scholar]
- Monteleone A., Wenzel F., Langhals H., Dietrich D. New application for the identification and differentiation of microplastics based on fluorescence lifetime imaging microscopy (FLIM) J. Environ. Chem. Eng. 2021;9:104769. doi: 10.1016/j.cbi.2021.109466. [DOI] [PubMed] [Google Scholar]
- Munno K., De Frond H., O’Donnell B., Rochman C.M. Increasing the accessibility for characterizing microplastics: introducing new application-based and spectral libraries of plastic particles (SLoPP and SLoPP-E) Anal. Chem. 2020;92:2443–2451. doi: 10.1021/acs.analchem.9b03626. [DOI] [PubMed] [Google Scholar]
- Murray F., Cowie P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758) Mar. Pollut. Bull. 2011;62:1207–1217. doi: 10.1016/j.marpolbul.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Naji A., Azadkhah S., Farahani H. Microplastics in wastewater outlets of Bandar Abbas city (Iran): a potential point source of microplastics into the Persian Gulf. Chemosphere. 2021;262:128039. doi: 10.1016/j.chemosphere.2020.128039. [DOI] [PubMed] [Google Scholar]
- Napper I.E., Thompson R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016;112:39–45. doi: 10.1016/j.marpolbul.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Napper I.E., Barrett A.C., Thompson R.C. The efficiency of devices intended to reduce microfibre release during clothes washing. Sci. Total Environ. 2020;738:140412. doi: 10.1016/j.scitotenv.2020.140412. [DOI] [PubMed] [Google Scholar]
- Napper I.E., Baroth A., Barrett A.C. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 2021;274:116348. doi: 10.1016/j.envpol.2020.116348. [DOI] [PubMed] [Google Scholar]
- O'Brien S., Okoffo E.D., O'Brien J.W. Airborne emissions of microplastic fibres from domestic laundry dryers. Sci. Total Environ. 2020;747:141175. doi: 10.1016/j.scitotenv.2020.141175. [DOI] [PubMed] [Google Scholar]
- Okubayashi S., Campos R., Rohrer C., Bechtold T. A pilling mechanism for cellulosic knit fabrics – effects of wet processing. J. Text. Inst. 2005;96:37–41. [Google Scholar]
- Oliveri Conti G., Ferrante M., Banni M. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020;187:109677. doi: 10.1016/j.envres.2020.109677. [DOI] [PubMed] [Google Scholar]
- Özkan I., Gündoğdu S. Investigation on the microfiber release under controlled washings from the knitted fabrics produced by recycled and virgin polyester yarns. J. Textil. Inst. 2021;112:264–272. [Google Scholar]
- Palacios-Mateo C., van der Meer Y., Seide G. Analysis of the polyester clothing value chain to identify key intervention points for sustainability. Environ. Sci. Eur. 2021;33:2. doi: 10.1186/s12302-020-00447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paço A., Duarte K., da Costa J.P. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017;586:10–15. doi: 10.1016/j.scitotenv.2017.02.017. [DOI] [PubMed] [Google Scholar]
- Pereira J.M., Rodríguez Y., Blasco-Monleon S. Microplastic in the stomachs of open-ocean and deep-sea fishes of the North-East Atlantic. Environ. Pollut. 2020;265:115060. doi: 10.1016/j.envpol.2020.115060. [DOI] [PubMed] [Google Scholar]
- Petroody S.S.A., Hashemi S.H., van Gestel C.A.M. Transport and accumulation of microplastics through wastewater treatment sludge processes. Chemosphere. 2021;278:130471. doi: 10.1016/j.chemosphere.2021.130471. [DOI] [PubMed] [Google Scholar]
- Pirc U., Vidmar M., Mozer A., Kržan A. Emissions of microplastic fibres from microfiber fleece during domestic washing. Environ. Sci. Pollut. Res. 2016;23:22206–22211. doi: 10.1007/s11356-016-7703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati S., Beal M., Maley J. Qualitative and quantitative analysis of microplastics and microfiber contamination in effluents of the City of Saskatoon wastewater treatment plant. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-021-12898-7. [DOI] [PubMed] [Google Scholar]
- Rebelein A., Int-Veen I., Kammann U., Scharsack J.P. Microplastic fibers — underestimated threat to aquatic organisms? Sci. Total Environ. 2021;777:146045. doi: 10.1016/j.scitotenv.2021.146045. [DOI] [PubMed] [Google Scholar]
- Richards P.R. Chapter 19–fabric finishing: dyeing and colouring. In: Sinclair R., editor. Textiles and Fashion. Woodhead Publishing; Sawston: 2015. pp. 475–505. [Google Scholar]
- Rist S., Carney B., Hartmann N.B., Karlsson T.M. Critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018;626:720–726. doi: 10.1016/j.scitotenv.2018.01.092. [DOI] [PubMed] [Google Scholar]
- Romanó de Orte M., Clowez S., Caldeira K. Response of bleached and symbiotic sea anemones to plastic microfiber exposure. Environ. Pollut. 2019;249:512–517. doi: 10.1016/j.envpol.2019.02.100. [DOI] [PubMed] [Google Scholar]
- Ryan P.G., Suaria G., Perold V. Sampling microfibres at the sea surface: the effects of mesh size, sample volume and water depth. Environ. Pollut. 2019 doi: 10.1016/j.envpol.2019.113413. [DOI] [PubMed] [Google Scholar]
- Said L., Heard M.J. Variation in the presence and abundance of anthropogenic microfibers in the Cumberland River in Nashville, TN, USA. Environ. Sci. Pollut. Res. 2020;27:10135–10139. doi: 10.1007/s11356-020-08091-x. [DOI] [PubMed] [Google Scholar]
- Sait S.T.L., Sørensen L., Kubowicz S. Microplastic fibres from synthetic textiles: environmental degradation and additive chemical content. Environ. Pollut. 2021;268:115745. doi: 10.1016/j.envpol.2020.115745. Part B. [DOI] [PubMed] [Google Scholar]
- Sánchez C. Fungal potential for the degradation of petroleum-based polymers: an overview of macro- and microplastics biodegradation. Biotechnol. Adv. 2020;40:107501. doi: 10.1016/j.biotechadv.2019.107501. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vidal A., Thompson R.C., Canals M., De Haan W.P. The imprint of microfibres in Southern European deep seas. PloS One. 2018;13:1–12. doi: 10.1371/journal.pone.0207033. Pmid:30395638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoca S., Matanović K., D'Angelo G. Ingestion of plastic and non-plastic microfibers by farmed gilthead sea bream (Sparus aurata) and common carp (Cyprinus carpio) at different life stages. Sci. Total Environ. 2021;782:146851. [Google Scholar]
- Schellenberger S., Jonsson C., Mellin P. Release of side-chain fluorinated polymer-containing microplastic fibers from functional textiles during washing and first estimates of perfluoroalkyl acid emissions. Environ. Sci. Technol. 2019 doi: 10.1021/acs.est.9b04165. [DOI] [PubMed] [Google Scholar]
- Schöpel B., Stamminger R. A comprehensive literature study on microfibres from washing machines. Tenside Surfactants Deterg. 2019;56:94–104. [Google Scholar]
- Senathirajah K., Attwood S., Bhagwat G. Estimation of the mass of microplastics ingested – a pivotal first step towards human health risk assessment. J. Hazard Mats. 2021;404:124004. doi: 10.1016/j.jhazmat.2020.124004. Part B. [DOI] [PubMed] [Google Scholar]
- Sillanpää M., Sainio P. Release of polyester and cotton fibres from textiles in machine washing. Environ. Sci. Pollut. Res. 2017;24:19313–19321. doi: 10.1007/s11356-017-9621-1. [DOI] [PubMed] [Google Scholar]
- Simon M., Vianello A., Vollertsen J. Removal of >10 µm microplastic particles from treated wastewater by a disc filter. Water. 2019;11:1935. [Google Scholar]
- Sivakumar D. Role of Lemna minor Lin in treating the textile industry wastewater. Int. J. Environ. Ecol. Geol. Min. Eng. 2014;8:203–207. [Google Scholar]
- Sørensen L., Synnøve Groven A.S., Hovsbakken I.A. UV degradation of natural and synthetic microfibers causes fragmentation and release of polymer degradation products and chemical additives. Sci. Total Environ. 2021;755:143170. doi: 10.1016/j.scitotenv.2020.143170. [DOI] [PubMed] [Google Scholar]
- Stanton T., Johnson M., Nathanail P. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 2019;666:377–389. doi: 10.1016/j.scitotenv.2019.02.278. [DOI] [PubMed] [Google Scholar]
- Suaria G., Achtypi A., Perold V. Microfibers in oceanic surface waters: a global characterization. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aay8493. pmid:32548254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvitie J., Mikola A., Setälä O. How well is microlitter purified from wastewater?–A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017;109:164–172. doi: 10.1016/j.watres.2016.11.046. Pmid:27883921. [DOI] [PubMed] [Google Scholar]
- Talvitie J., Mikola A., Koistinen A.P., Setala O. Solutions to microplastic pollution—removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017;123:407. doi: 10.1016/j.watres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Taylor M., Gwinnett C., Robinson L. Plastic microfibre ingestion by deep-sea organisms. Sci. Rep. 2016;6:33997. doi: 10.1038/srep33997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N., Wei J., Li Y. Efficient scald-preventing enabled by robust polyester fabrics with hot water repellency and water impalement resistance. J. Colloid Interface Sci. 2020;566:69–78. doi: 10.1016/j.jcis.2020.01.067. [DOI] [PubMed] [Google Scholar]
- Tian Y., Chen Z., Zhang J. An innovative evaluation method based on polymer mass detection to evaluate the contribution of microfibers from laundry process to municipal wastewater. J. Hazard Mats. 2021;407:124861. doi: 10.1016/j.jhazmat.2020.124861. [DOI] [PubMed] [Google Scholar]
- Turner A. Trace elements in laundry dryer lint: a proxy for household contamination and discharges to waste water. Sci. Total Environ. 2019;665:568–573. doi: 10.1016/j.scitotenv.2019.02.025. [DOI] [PubMed] [Google Scholar]
- Vijayaraman S., Mondal P., Nandan A., Siddiqui N.A. Presence of microplastic in water bodies and its impact on human health. In: Siddiqui N., Tauseef S., Abbasi S., Khan F., editors. Advances in Air Pollution Profiling and Control. Springer; 2020. (Springer Transact Civil Environ Eng, Singapore). [Google Scholar]
- Virta L., Räisänen R. Three futures scenarios of policy instruments for sustainable textile production and consumption as portrayed in the Finnish news media. Sustainability. 2021;13:594. [Google Scholar]
- Vogue magazine. 2020. https://www.vogue.co.uk/arts-and-lifestyle/article/microplastics-laundry [Google Scholar]
- Wang F., Wang F., Zeng E.Y. Chapter 7 - sorption of toxic chemicals on microplastics. In: Zeng E.Y., editor. Microplastic Contamination in Aquatic Environments. Elsevier; 2018. pp. 225–247. [Google Scholar]
- Wolff S., Kerpen J., Prediger J. Determination of the microplastics emission in the effluent of a municipal waste water treatment plant using Raman microspectroscopy. Water Res. X. 2018;2:100014. doi: 10.1016/j.wroa.2018.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XerosTech. 2020. https://www.xerostech.com/updates/how-to-stop-the-microplastics-in-your-clothes-polluting-the-ocean [Google Scholar]
- Xia W., Rao Q., Deng X. Rainfall is a significant environmental factor of microplastic pollution in inland waters. Sci. Total Environ. 2020;732:139065. doi: 10.1016/j.scitotenv.2020.139065. [DOI] [PubMed] [Google Scholar]
- Xu C., Zhang B., Gu C. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Haz. Mats. 2020;400:123228. doi: 10.1016/j.jhazmat.2020.123228. [DOI] [PubMed] [Google Scholar]
- Xu Y., Chan F.K.S., Stanton T. Synthesis of dominant plastic microfibre prevalence and pollution control feasibility in Chinese freshwater environments. Sci. Total Environ. 2021;783:146863. doi: 10.1016/j.scitotenv.2021.146863. [DOI] [PubMed] [Google Scholar]
- Xu Z., Bai X., Ye Z. Removal and generation of microplastics in wastewater treatment plants: a review. J. Clean. Prod. 2021;291:125982. [Google Scholar]
- Yan S., Jones C., Henninger C.E., McCormick H. Textile industry insights towards impact of regenerated cellulosic and synthetic fibres on microfibre pollution. In: Muthu S., Gardetti M., editors. Sustainability in the Textile and Apparel Industries. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. Springer; Cham: 2020. [Google Scholar]
- Yang L., Qiao F., Lei K. Microfiber release from different fabrics during washing. Environ. Pollut. 2019;249:136–143. doi: 10.1016/j.envpol.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Yonkos L.T., Friedel E.A., Perez-Reyes A.C. Microplastics in four estuarine rivers in the Chesapeake Bay, USA. Environ. Sci. Technol. 2014;48:14195–14202. doi: 10.1021/es5036317. [DOI] [PubMed] [Google Scholar]
- Zambrano M.C., Pawlak J., Daystar J. Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar. Pollut. Bull. 2019;142:394–407. doi: 10.1016/j.marpolbul.2019.02.062. [DOI] [PubMed] [Google Scholar]
- Zambrano M.C., Pawlak J.J., Daystar J. Venditti Aerobic biodegradation in freshwater and marine environments of textile microfibers generated in clothes laundering: effects of cellulose and polyester-based microfibers on the microbiome. Mar. Pollut. Bull. 2020;151:110826. doi: 10.1016/j.marpolbul.2019.110826. [DOI] [PubMed] [Google Scholar]
- Zambrano M.C., Pawlak J.J., Daystar J. Impact of dyes and finishes on the microfibers released on the laundering of cotton knitted fabrics. Environ. Pollut. 2021;272:115998. doi: 10.1016/j.envpol.2020.115998. [DOI] [PubMed] [Google Scholar]
- Zambrano M.C., Pawlak J.J., Daystar J. Impact of dyes and finishes on the aquatic biodegradability of cotton textile fibers and microfibers released on laundering clothes: correlations between enzyme adsorption and activity and biodegradation rates. Mar. Pollut. Bull. 2021;165:112030. doi: 10.1016/j.marpolbul.2021.112030. [DOI] [PubMed] [Google Scholar]
- Zettler E., Mincer T., Amaral-Zettler L. Life in the “plastisphere”: microbial communities on plastic marine debris. Environ. Sci. Technol. 2013;47:7137–7146. doi: 10.1021/es401288x. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kang S., Allen S. Atmospheric microplastics: a review on current status and perspectives. Earth Sci. Rev. 2020;203:103118. [Google Scholar]
- Zhao Y., Qiao R., Zhang S., Wang G. Metabolomic profiling reveals the intestinal toxicity of different length of microplastic fibers on zebrafish (Danio rerio) J. Hazard Mater. 2021;403:123663. doi: 10.1016/j.jhazmat.2020.123663. [DOI] [PubMed] [Google Scholar]
- Ziajahromi S., Neale P.A., Rintoul L., Leusch F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017;112:93–99. doi: 10.1016/j.watres.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Ziajahromi S., Neale P.A., Rintoul L., Leusch F.D.L. Wastewater treatment plants as a pathway for microplastics: development of a new approach to sample wastewater-based microplastics. Water Res. 2017;112:93–99. doi: 10.1016/j.watres.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Ziajahromi S., Kumar A., Neal P.A., Leusch F.D.L. Impact of microplastic beads and fibers on waterflea (Ceriodaphnia dubia) survival, growth, and reproduction: implications of single and mixture exposures. Environ. Sci. Technol. 2017;51:13397–13406. doi: 10.1021/acs.est.7b03574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.