Abstract
Background
Sub-Saharan Africa reported 550,000 new HIV infections among women in 2018. Pregnancy and the postpartum period are associated with an increased risk of HIV acquisition (adjusted risk ratio [RR]: 2.8 during pregnancy and 4.0 in postpartum period vs. non-pregnant or postpartum women, respectively). Acquisition of HIV during pregnancy and breastfeeding increases risk of mother to child transmission. We propose to test the impact of a peer-delivered oral storytelling intervention to increase retention in, and adherence to, pre-exposure prophylaxis (PrEP)/combination antiretroviral treatment (ART) among expectant couples.
Design
We propose a randomized controlled trial (RCT) (35 intervention and 35 control couples) at a health facility where 11% of expectant couples were in serodiscordant relationships in 2018. Couples randomized to the storytelling arm will be visited by a two community volunteers and who successfully adhered to PrEP/ART during a recent pregnancy. This expert couple will orate to participating couples three stories (at 1, 3 and 5 weeks after study enrollment) designed to empower, educate, and establish “ideal” interpersonal communication strategies within couples/families, and support adherence practices among participants. The primary outcome among HIV-uninfected women will be adherence to PrEP at 3 months.
Conclusions
PrEP among at-risk pregnant women must be implemented so that high levels of adherence and retention are achievable for them and their partners. We will test our storytelling intervention to identify an optimal strategy for PrEP education and family engagement in a region with high HIV prevalence. Our results will have an impact by effectively engaging serodiscordant couples in prevention/treatment during pregnancy and beyond.
Keywords: Mother-to-child transmission of HIV, Male-engagement, PrEP uptake and adherence, Antenatal care, Storytelling
1. Background
Acquisition of human immunodeficiency virus (HIV) during pregnancy increases mother-to-child-transmission (MTCT) risk [1,2]. The risk of HIV transmission among pregnant and post-partum women is significantly higher when compared to non-pregnant female counterparts; with adjusted risk ratios of 2.82 and 3.97, respectively [3,4]. Pregnant women in Mozambique seroconvert at a rate of 4.28/100 women-years [5], which is similar to published incidence data from Swaziland [6], Uganda [7], and South Africa [2,8]. Among pregnant women in known discordant relationships, HIV incidence is 12.5 per 100 women years [9,10]. In African cohorts, women with incident vs. chronic infection during the pregnancy/postpartum periods had a higher risk of MTCT (odds ratio [OR] 2.3, CI: 1.2–4.4)10 thus pre-exposure prophylaxis (PrEP) is essential to preventing incident mother and infant HIV infections.
PrEP offers a bridge to partner combination antiretroviral treatment (ART) initiation [11] and viral load suppression (VS) [12]. Among discordant couples, the risk of HIV transmission continues for up to six months post-ART initiation [9,13]. The World Health Organization (WHO) recommends concurrent usage of ART (for HIV-positive partner) and PrEP (for uninfected partner) for HIV-1 prevention [14]. Integration of PrEP delivery and ART initiation decreased HIV incidence in the negative partner to <0.5% per year [12]. Randomized controlled trials (RCT) have demonstrated that women on PrEP must maintain very high levels of adherence for the drug to be effective [[15], [16], [17]].
Family support can improve uptake of, retention in, and adherence to PrEP among pregnant/lactating women [12,18,19]. Families, including couples, are a proven, effective unit of intervention in the African HIV prevention and care context [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]]. While couple-centered behavioral interventions have been effective in reducing sexual and drug-risk behaviors, increasing access to HIV testing, and improving uptake of PrEP services [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]], there are few studies that have assessed the effect of family-centered interventions on adherence to maternal PrEP/paternal ART during pregnancy and lactation [12]. Families cannot provide substantive support to discordant couples unless they have adequate knowledge and skills to do so. Explaining how PrEP works and developing motivational messages that are effective in this population remains a challenge. Eligible patients are offered PrEP at the time of receiving discordant test results, yet discordant couples have little information about PrEP in Zambézia. (unpublished data from Zambézia, Audet et al., 2019). Development of couples-based education and messaging is essential to improving knowledge.
People are seven times more likely to remember a story vs. facts alone [38]. Mozambique has a rich history of oral storytelling, a cultural tradition we will integrate into this intervention. The strength of family support provided to the discordant couple is correlated with adherence to medication and retention in care [39]. Developing and delivering engaging stories has proved essential via numerous RCTs for several reasons: (1) stories act as mnemonic devices for facts [40,41]; (2) stories engage our emotions, which results in increased empathy and adoption of messages [42]; and (3) stories that engage participants lead to story-consistent beliefs [43]. Narratives have been used to persuade patients to accurately assess their risk of a particular condition (e.g. lung cancer, cervical cancer, etc.), in efforts to change behavior (e.g. smoking, condom use, etc.) or uptake specific medical services (e.g. lung cancer screening, human papillomavirus (HPV) vaccination, etc.) [[44], [45], [46], [47], [48]]. Given the history of oral storytelling as an educational tool in Mozambique, there is evidence that this culturally-informed tradition can be used to reduce HIV stigma [49] and increase understanding of complex topics [50,51] like PrEP and ART use.
2. Objectives
The overall goal of this project is to develop and assess the effect of a partner-based PrEP/ART delivery intervention among expectant couples where the pregnant woman is HIV-negative, and her male partner is HIV-positive. Specifically, we will compare the effect of a storytelling intervention (vs. standard of care counseling and clinical services alone) on participants’ knowledge, motivation, and behavioral skills associated with PrEP retention and adherence.
2.1. Outcomes of interest
Our primary clinical outcome is PrEP/ART medication non-adherence at three months. Among women, PrEP adherence will be assessed using a urine assay for Tenofovir, with concentrations <100 ng/mL indicative of non-adherence. Additionally, for women lost to follow-up (LTFU), it will be assumed that Tenofovir concentrations are <100 ng/mL. Among men, ART adherence will be assessed using viral load, with viral load ≥200 copies/mL indicative of non-adherence. As for women, men LTFU will be assumed to be not virologically suppressed. Our secondary clinical outcome is not being retained in care at three months, defined as not completing a medical provider visit or prescription refill appointment 90 days (±7 days) after initiation of medication as documented in the ANC logbooks and Open MRS databases.
Other psychosocial outcomes of interest include the impact of the intervention on depression, HIV knowledge, partner support and empathy at 3 months post-enrollment.
3. Study procedures
The proposed study is a two sample, couple-randomized pilot to test the impact of an oral storytelling intervention (versus standard of care PrEP/ART delivery) to increase retention in, and adherence to, PrEP and ART among HIV-discordant expectant couples. The study protocol and informed consent documents have been approved by the Vanderbilt University Medical Center (VUMC) Institutional Review Board (IRB #191719, dated 30 December 2019) and the Comité Nacional de Bioética para a Saúde (Institutional Committee for Bioethics in Health in Mozambique, reference #01/CNBS/2020, dated 3 April 2020) in Mozambique. The trial is registered at ClinicalTrials.gov (NCT03149237).
3.1. Site selection
This pilot study will be conducted at Namacurra Sede health facility, a district-level hospital located in Zambézia Province, Mozambique. The site was selected due to the high number of discordant couples eligible for PrEP services.
3.2. Participant selection
Couples, comprised of one pregnant woman and her male partner, will be eligible to participate if: i) the pregnant woman is identified as HIV-negative at antenatal care (ANC)-based HIV testing; ii) the male partner is identified as HIV-positive at any ANC appointment, either through ANC-based HIV testing or having a known history of being HIV-positive (from clinical records or medication pick-up documents, or any other mechanism by which ANC staff identify him as HIV-positive); iii) both persons agree to take PrEP (pregnant woman)/ART (male partner) together and receive standard care in pre- and postnatal period, including care of the HIV-exposed infant at the Child at Risk Clinic (Consulta de Criança em Risco [CCR]); iv) the woman's due date is > 4 weeks from study enrollment; v) both persons are 18 years or older; vi) both persons are able to give informed consent; vii) both persons (parents) are willing to consent to an infant record search, and viii) both persons consent to be in the study.
Family members (as defined by relatives living in the participants’ household or relatives the participants identify as living in the study community) can be nominated to attend the storytelling sessions by the discordant couple as long as they are 18 years of age or older, are identified as a confidant, and are willing to attend at least one of the storytelling sessions. These family members will be eligible to participate in the qualitative interview about the storytelling session if i) they participated in at least one of the storytelling sessions; ii) are 18 years of age or older; iii) are able to give informed consent; and iv) are willing to participate in the interview.
3.3. Control arm
The participants randomized to the control arm will receive standard of care (SOC) services that include: (1) male engagement (male invitation to ANC services and couples HIV testing); (2) opt-out rapid HIV testing of all pregnant women attending ANC, to ensure patient status [Determine HIV-1/HIV-2®, (Abbott Laboratories, Abbott Park, Illinois, USA) followed by Uni-Gold HIV-1/HIV-2® (Trinity Biotech Plc, Bray, Co. Wicklow, Ireland)]; (3) universal ART services for HIV-positive male partner per national guidelines [52]; (4) counseling for the HIV-positive partner as per national guidelines; (5) PrEP medications, condoms and psychosocial support/counseling (i.e., PrEP services package as per national guidelines) free of charge; and (6) follow-up HIV testing at time of PrEP medication pick-up (per national guidelines). In addition to SOC services, will offer monthly measurement of PrEP (tenofovir) drug level via urine assay testing [53].
At six-months post-ART initiation, eligible male partners will be referred to one of the differentiated models of care if their viral load (VL) is suppressed (at which point the PrEP regimen for female partner may also be suspended with clinician's approval). If the male partner abandons HIV care and treatment, the woman will continue to be eligible for PrEP. Postpartum women on PrEP will receive follow-up for PrEP services at the adult ART clinic; her infant is followed at the Healthy Child Clinic (Consulta de Criança Sadia [CCS]) for clinical follow-up and vaccinations; if the woman seroconverts, she will start ART for life while infants will have access to HIV testing by dried blood spot (DBS) polymerase chain reaction (PCR) testing as early as four weeks after birth at the CCR. If the infant seroconverts, s/he is provided ART for life. In the event of seroconversion for mother, follow-up for mother and infant continues at the CCR until the child is 18 months of age or until two months after the mother ceases breastfeeding (with final diagnosis for infant's HIV status post-exposure). Relevant medications are also offered free of charge in the adult HIV clinic to male partners. If the woman seroconverts at any point during the study, HIV-specific counseling and support, provision of cotrimoxazole prophylaxis, isoniazid preventive therapy (IPT), and universal ART are provided free of charge [54]. Viral load testing is routinely available for all ART-treated children and adults, implemented according to national guidelines.
3.4. Intervention arm
The participants randomized to the intervention arm will receive all of the SOC services and PrEP drug level testing (for females) as described above (i.e., will receive the same SOC clinical services as those couples randomized to the control arm). In addition, the couples randomized to intervention arm will be invited to participate together in three storytelling sessions with eligible family members of their choosing.
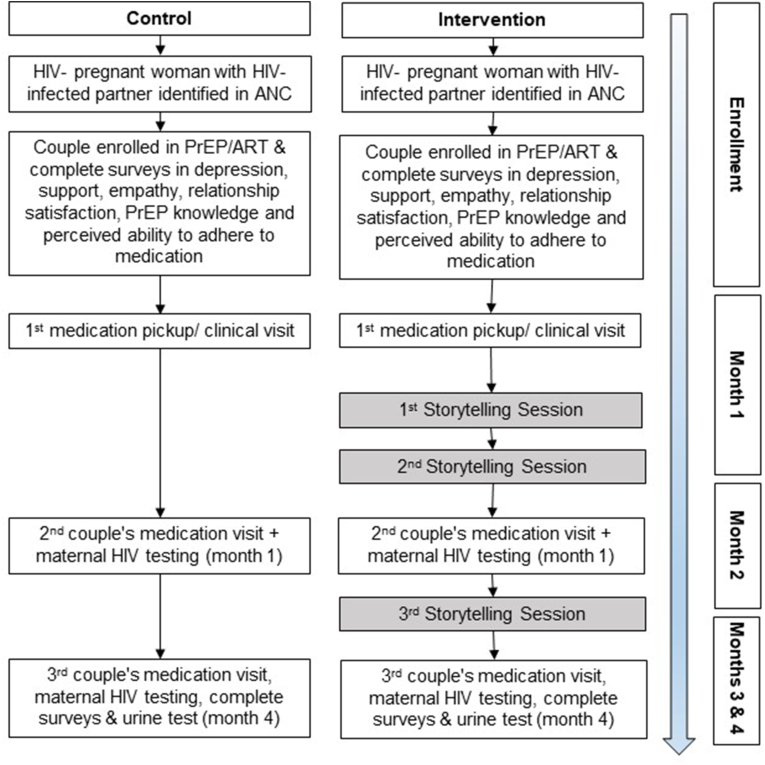
The stories were developed based on the experiences of 20 individuals taking PrEP at the study site. This group provided their experiences initiating PrEP, disclosing their discordant relationship status with family and friends, and the challenges to remaining adherent to medication. Positive examples, including those who overcome challenges to remain on PrEP, were extracted and used to create three coherent narratives. The stories will be told by volunteer community members who have successfully completed adhered to PrEP/ART. This “expert” peer couple will deliver the narratives in the first person, each person playing/voicing an assigned character, at the participant couple's home (or in a preferred location chosen by the participants). Each narrative story will last 12–15 min. At the conclusion of the narrative, the trained peer couple will facilitate a discussion with those listening to the story. They will engage the participants and any attending family members in a discussion, asking them about the storyline they just heard. They will ask questions to specifically probe for reactions to events and difficult characters/interactions. They will lead the discussion about ways the main characters in the story (a fictional discordant couple) overcome challenges in adherence to ART/PrEP medication; seeking to learn from the participants if there are ways the stories' messages can be related to their own lives. We will conduct the first two storytelling sessions in the first month of study enrollment to try and influence participants' behavior/thinking prior to their first follow-up visit for medication pick-up (in an effort to assess storytelling's impact on attendance at scheduled appointments, as a proxy for adherence) (Fig. 1).
Fig. 1.
Timeline of Study Activities.
3.5. Participant assessments
Surveys: Among discordant couples, we will use an interviewer assisted survey consisting of the following measures: (1) depression screening [55]; (2) partner and family support [39,56]; (3) relationship empathy [57]; (4) relationship satisfaction [58]; (5) PrEP stigma [59]; and (6) motivation/perceived ability to adhere to medication [60] at baseline and three months.
Clinical Assessments: For female participants in both study groups, we will measure their PrEP adherence using a point of care (POC) urine test at each medication pick-up for the first three months on the PrEP regimen.
Interviews: We will complete post-intervention qualitative interviews with a smaller sample of participants to gain a better understanding of the perceived positive and negative aspects of the intervention and the overall project. Interviewers will follow a semi-structured interview guide.
4. Statistical analysis
Primary analyses for PrEP/ART adherence biomarker will follow an intent-to-treat protocol. We operationalize the outcome in the negative (e.g., not adherent, such that the odds ratios (OR) for the intervention effect will be < 1.0 if effective. Given the small sample size of n = 140 (70 women and 70 men), we will estimate the intervention effect on the dichotomous outcome of non-adherence with a logistic regression model using generalized estimating equations (GEE) and an exchangeable working correlation structure to account for clustering within couples and an interaction term between intervention arm and sex to identify sex-specific intervention effects. The regression model will be further adjusted for education level (none, some primary, completed primary or higher), perceived community HIV stigma (continuous variable), and affective empathy (continuous variable) as these have remained imbalanced at baseline in an earlier couple's intervention study. because the outcome is relatively common (>30%), the magnitude of the OR may exaggerate the magnitude of the relative risk reduction. In addition to reporting crude and adjusted ORs and 95% confidence intervals from the logistic regression model, we will also report regression-based standardized risk ratios and 95% confidence intervals [61]. Analysis of our secondary clinical outcome of retention in care at three months will follow the same analysis plan as described above for non-adherence.
For the other outcomes of interest that are psychosocial measures obtained at three months, we will follow a similar analysis plan as described for the clinical outcomes. Because the psychosocial measures are based on scales or indices, outcomes will be continuous and analyzed with linear regression models using GEE and an exchangeable working correlation structure to account for clustering within couples and an interaction term between intervention arm and sex to identify sex-specific intervention effects. The regression model for each psychosocial outcome will be further adjusted for education level and the baseline value of the psychosocial measure of interest (e.g., for analyses of the intervention effect on depression, the model will include the baseline measure of depression).
Prior work on interventions with couples receiving ART indicates that missing data is rare for socio-demographic data. However, for psychosocial measures such as depression or stigma, missingness may reach 10–30%. Given our small sample size, we will utilize multiple imputation methods to retain as much of the sample as possible despite missing data. Because missingness is likely to occur in the psychosocial measures, which are continuous measures and often normally distributed, we will implement joint model imputation (or multivariate normal imputation) on any continuous variables with missing data. Joint model multiple imputation has been shown to be valid for sample sizes as small as 100, even when 30% of data are missing [62].
Qualitative Analyses: Audio files will be transcribed verbatim by a research assistant and two researchers will independently code and analyze the interviews using MAXQDA® software. We will employ a thematic approach to identify and analyze themes in the data [63]. We will focus on two key reactions to the storytelling: (1) the experience of listening to the stories, with a focus on the effectiveness of the narrators' ability to transport the listener, the level of identification and perceived similarity with characters; and (2) the impact of the storytelling intervention on information related to PrEP/ART adherence, with a focus on motivation to adhere to medication due to self-efficacy, family support and feelings of depression/hopelessness, and behavioral skills learned. We will use a combination of deductive codes based on findings from previous adherence research and inductive codes to categorize newly identified factors associated with uptake, adherence, and retention in PrEP/ART services (e.g., self-efficacy, partner support, information etc.). Two researchers will read through the transcripts several times and highlight relevant examples of storytelling impact on information, behavioral skills and motivation. Researchers will generate codes and highlight themes by collating codes across the dataset and review themes to develop a thematic map (e.g., the influence of a character's successful use of a strategy on a listener's self-efficacy to employ the same tactic). The final coding framework will have at least 85% agreement between the two coders. This exercise will allow us to modify our behavioral model if necessary.
4.1. Power and sample size determination
Our pilot will involve 70 couples (70 HIV-negative pregnant women and their HIV-positive male partners; 35 couples in each arm) from a single clinic. Limited data on PrEP in Mozambique suggest 55% of pregnant women were not retained at three months after PrEP initiation (Friends in Global Health [FGH], unpublished routine program data). Given our sample size of 70 women and a 5% type I error, we will have 82% power to detect a RR of 0.40 for not being retained. Should there be evidence the intervention effect is similar for men and women with regards to retention, the two groups can be analyzed together to further improve power to detect RRs closer to 0.50. We do not have estimates of tenofovir levels among PrEP users in Mozambique, but other studies indicate as many as 70% may have no active drug detected [64]. Given our sample of 70 women and a 5% type I error, we will have 85% power to detect a RR of 0.50 for no detectable drug level.
4.2. Limitations
Our sample size of 35 couples in each arm is small, which limits our ability to detect a small change in behavior among couples. However, we believe this pilot will allow us to detect signals of the intervention's success, which we can subsequently use to fund a larger trial.
5. Conclusions/summary
We propose to test the effect of a peer-delivered oral storytelling intervention to increase retention in and adherence to PrEP/ART among serodiscordant expectant couples. We will contrast this model to standard of care services and counseling. This innovative intervention tests a culturally relevant approach (e.g., couples/family engagement via oral storytelling) to ART/PrEP delivery and retention support.
Disclosures
CMA has received compensation from ViiV Healthcare to develop an implementation science curriculum.
Funding
This work is supported by the National Institute of Mental Health (NIMH) grants R01MH113478-01. CMA was also supported by K01MH107255. The authors are solely responsible for the design and conduct of this study, study analyses, and the drafting of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Birkhead G.S., Pulver W.P., Warren B.L., Hackel S., Rodriguez D., Smith L. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002-2006. Obstet. Gynecol. 2010;115(6):1247–1255. doi: 10.1097/AOG.0b013e3181e00955. [DOI] [PubMed] [Google Scholar]
- 2.Moodley D., Esterhuizen T., Reddy L. Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J. Infect. Dis. 2011;203(9):1231–1234. doi: 10.1093/infdis/jir017. [DOI] [PubMed] [Google Scholar]
- 3.Thomson K., Hughes J.P., Baeten J.M. CROI; Boston, MA: 2018. Female HIV Acquisition Per Sex Act Is Elevated in Late Pregnancy and Postpartum. March 4-7, 2018. [Google Scholar]
- 4.Thomson K.A., Hughes J., Baeten J.M. Increased risk of female HIV-1 acquisition throughout pregnancy and postpartum: a prospective per-coital act analysis among women with HIV-1 infected partners. J. Infect. Dis. 2018;218(1):16–25. doi: 10.1093/infdis/jiy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Schacht C., Hoffman H.J., Mabunda N. High rates of HIV seroconversion in pregnant women and low reported levels of HIV testing among male partners in Southern Mozambique: results from a mixed methods study. PloS One. 2014;9(12) doi: 10.1371/journal.pone.0115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kieffer M.P., Nhlabatsi B., Mahdi M., Hoffman H.J., Kudiabor K., Wilfert C.M. Improved detection of incident HIV infection and uptake of PMTCT services in labor and delivery in a high HIV prevalence setting. J. Acquir. Immune Defic. Syndr. 1999;57(4):e85–91. doi: 10.1097/QAI.0b013e31821acc6e. [DOI] [PubMed] [Google Scholar]
- 7.Gray R.H., Li X., Kigozi G. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 8.Kateja N., Agarwal H., Saraswat A., Bhat M., Rathore A.S. Continuous precipitation of process related impurities from clarified cell culture supernatant using a novel coiled flow inversion reactor (CFIR) Biotechnol. J. 2016;11(10):1320–1331. doi: 10.1002/biot.201600271. [DOI] [PubMed] [Google Scholar]
- 9.Mujugira A., Celum C., Coombs R.W. HIV transmission risk persists during the first 6 Months of antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 1999;72(5):579–584. doi: 10.1097/QAI.0000000000001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake A.L., Wagner A., Richardson B., John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med. 2014;11(2) doi: 10.1371/journal.pmed.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngure K., Heffron R., Curran K. I knew I would Be safer. Experiences of Kenyan HIV serodiscordant couples soon after pre-exposure prophylaxis (PrEP) initiation. AIDS Patient Care STDS. 2016;30(2):78–83. doi: 10.1089/apc.2015.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeten J.M., Heffron R., Kidoguchi L. Integrated delivery of antiretroviral treatment and pre-exposure prophylaxis to HIV-1-Serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLoS Med. 2016;13(8) doi: 10.1371/journal.pmed.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoenigl M., Chaillon A., Moore D.J. Rapid HIV viral load suppression in those initiating antiretroviral therapy at first visit after HIV diagnosis. 2016;6:32947. doi: 10.1038/srep32947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . 2012. Guidance on Couples HIV Testing and Counseling and Antiretroviral Therapy for Treatment and Prevention in Serodiscordant Couples: Recommendations for a Public Health Approach. Geneva. [PubMed] [Google Scholar]
- 15.Mascolini M. 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy; May 19-21, 2014. 2014. Estimated time to protection and duration of protection with daily TDF/FCT PreP. (Washington, D.C) [Google Scholar]
- 16.Kashuba A. Does pharmacology support on demand PrEP? Int. AIDS. Soc. 2017 July 24, 2017. (Paris, France) [Google Scholar]
- 17.Janes H., Corey L., Ramjee G. Weighing the evidence of efficacy of oral PrEP for HIV prevention in women in southern Africa. AIDS Res. Hum. Retrovir. 2018;34(8):645–656. doi: 10.1089/aid.2018.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amico K.R., Wallace M., Bekker L.G. Experiences with HPTN 067/ADAPT study-provided open-label PrEP among women in cape town: facilitators and barriers within a mutuality framework. AIDS Behav. 2017;21(5):1361–1375. doi: 10.1007/s10461-016-1458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Defechereux P.A., Mehrotra M., Liu A.Y. Depression and oral FTC/TDF pre-exposure prophylaxis (PrEP) among men and transgender women who have sex with men (MSM/TGW) AIDS Behav. 2016;20(7):1478–1488. doi: 10.1007/s10461-015-1082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguiar C., Jennings L. Impact of male partner antenatal accompaniment on perinatal health outcomes in developing countries: a systematic literature review. Matern. Child Health J. 2015;19(9):2012–2019. doi: 10.1007/s10995-015-1713-2. [DOI] [PubMed] [Google Scholar]
- 21.Aluisio A., Richardson B.A., Bosire R., John-Stewart G., Mbori-Ngacha D., Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J. Acquir. Immune Defic. Syndr. 1999;56(1):76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Audet C.M., Blevins M., Chire Y.M. Engagement of men in antenatal care services: increased HIV testing and treatment uptake in a community participatory action program in Mozambique. AIDS Behav. 2016;20(9):2090–2100. doi: 10.1007/s10461-016-1341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auvinen J., Kylma J., Suominen T. Male involvement and prevention of mother-to-child transmission of HIV in Sub-Saharan Africa: an integrative review. Curr. HIV Res. 2013;11(2):169–177. doi: 10.2174/1570162x11311020009. [DOI] [PubMed] [Google Scholar]
- 24.Brusamento S., Ghanotakis E., Tudor Car L., van-Velthoven M.H., Majeed A., Car J. Male involvement for increasing the effectiveness of prevention of mother-to-child HIV transmission (PMTCT) programmes. Cochrane Database Syst. Rev. 2012;10:CD009468. doi: 10.1002/14651858.CD009468.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byamugisha R., Astrom A.N., Ndeezi G., Karamagi C.A., Tylleskar T., Tumwine J.K. Male partner antenatal attendance and HIV testing in eastern Uganda: a randomized facility-based intervention trial. J. Int. AIDS Soc. 2011;14:43. doi: 10.1186/1758-2652-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conkling M., Shutes E.L., Karita E. Couples' voluntary counselling and testing and nevirapine use in antenatal clinics in two African capitals: a prospective cohort study. J. Int. AIDS Soc. 2010;13:10. doi: 10.1186/1758-2652-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farquhar C., Kiarie J.N., Richardson B.A. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J. Acquir. Immune Defic. Syndr. 1999;37(5):1620–1626. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas A.D., Tenthani L., Msukwa M.T. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi's option B+ programme: an observational cohort study. Lancet HIV. 2016;3(4):e175–182. doi: 10.1016/S2352-3018(16)00008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remien R.H., Stirratt M.J., Dolezal C. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19(8):807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 30.Allen S., Karita E., Chomba E. Promotion of couples' voluntary counselling and testing for HIV through influential networks in two African capital cities. BMC Publ. Health. 2007;7:349. doi: 10.1186/1471-2458-7-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cremin I., Morales F., Jewell B.L., O'Reilly K.R., Hallett T.B. Seasonal PrEP for partners of migrant miners in southern Mozambique: a highly focused PrEP intervention. J. Int. AIDS Soc. 2015;18(4 Suppl 3):19946. doi: 10.7448/IAS.18.4.19946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murnane P.M., Celum C., Mugo N. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27(13):2155–2160. doi: 10.1097/QAD.0b013e3283629037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ndase P., Celum C., Campbell J. Successful discontinuation of the placebo arm and provision of an effective HIV prevention product after a positive interim efficacy result: the partners PrEP study experience. J. Acquir. Immune Defic. Syndr. 1999;66(2):206–212. doi: 10.1097/QAI.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 34.Wall K.M., Kilembe W., Nizam A. Promotion of couples' voluntary HIV counselling and testing in Lusaka, Zambia by influence network leaders and agents. BMJ open. 2012;2(5) doi: 10.1136/bmjopen-2012-001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman R.M., Jaycocks A., Vardavas R. Benefits of PrEP as an adjunctive method of HIV prevention during attempted conception between HIV-uninfected women and HIV-infected male partners. J. Infect. Dis. 2015;212(10):1534–1543. doi: 10.1093/infdis/jiv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiwatram-Negrón T., El-Bassel N. Systematic review of couple-based HIV intervention and prevention studies: advantages, gaps, and future directions. AIDS Behav. 2014;18(10):1864–1887. doi: 10.1007/s10461-014-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crepaz N., Tungol-Ashmon M.V., Vosburgh H.W., Baack B.N., Mullins M.M. Are couple-based interventions more effective than interventions delivered to individuals in promoting HIV protective behaviors? A meta-analysis. AIDS Care. 2015;27(11):1361–1366. doi: 10.1080/09540121.2015.1112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bower G.H., Clark M.C. Narrative stories as mediators for serial learning. Psychonomic Sci. 1969;14(4):181–182. [Google Scholar]
- 39.Poudel K.C., Buchanan D.R., Amiya R.M., Poudel-Tandukar K. Perceived family support and antiretroviral adherence in HIV-positive individuals: results from a community-based positive living with HIV study. Int. Q Community Health Educ. 2015;36(1):71–91. doi: 10.1177/0272684X15614220. [DOI] [PubMed] [Google Scholar]
- 40.Gucciardi E., Jean-Pierre N., Karam G., Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv. Res. 2016;16:249. doi: 10.1186/s12913-016-1474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCabe J. OTRP Online: Office of Teaching Resources in Psychology; 2011. Integrating Mnemonics into Psychology Instruction. [Google Scholar]
- 42.Johnson D.R., Huffman B.L., Jasper D.M. Changing race boundary perception by reading narrative fiction. Basic Appl. Soc. Psychol. 2014;36(1):83–90. [Google Scholar]
- 43.Green M.C., Brock T.C. The role of transportation in the persuasiveness of public narratives. J. Pers. Soc. Psychol. 2000;79(5):701–721. doi: 10.1037//0022-3514.79.5.701. [DOI] [PubMed] [Google Scholar]
- 44.Kim H.S., Bigman C.A., Leader A.E., Lerman C., Cappella J.N. Narrative health communication and behavior change: the influence of exemplars in the news on intention to quit smoking. J. Commun. 2012;62(3):473–492. doi: 10.1111/j.1460-2466.2012.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunlop S.M., Wakefield M., Kashima Y. The contribution of antismoking advertising to quitting: intra- and interpersonal processes. J. Health Commun. 2008;13(3):250–266. doi: 10.1080/10810730801985301. [DOI] [PubMed] [Google Scholar]
- 46.Hinyard L.J., Kreuter M.W. Using narrative communication as a tool for health behavior change: a conceptual, theoretical, and empirical overview. Health Educ. Behav. 2007;34(5):777–792. doi: 10.1177/1090198106291963. the official publication of the Society for Public Health Education. [DOI] [PubMed] [Google Scholar]
- 47.Moran M.B., Murphy S.T., Frank L., Baezconde-Garbanati L. The ability of narrative communication to address health-related social norms. Int. Rev. Soc. Res. 2013;3(2):131–149. doi: 10.1515/irsr-2013-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moyer-Gusé E. Toward a theory of entertainment persuasion: explaining the persuasive effects of entertainment-education messages. Commun. Theor. 2008;18(3):407–425. [Google Scholar]
- 49.Jacques Z., Hieke W., Marga V., Gideon dJ. Beyond silence and rumor: storytelling as an educational tool to reduce the stigma around HIV/AIDS in South Africa. Health Educ. 2010;110(5):382–398. [Google Scholar]
- 50.Tp K.J., Retha S. Once upon a time in Africa: a case study of storytelling for knowledge sharing. ASLIB Proc. 2008;60(2):130–142. [Google Scholar]
- 51.Janelle F.P., Benissa S., Felicia Schanche H., Cyndi R.A., Ann A., Teodocia Maria H.-B. Storytelling: a qualitative tool to promote health among vulnerable populations. J. Transcult. Nurs. 2014;26(4):346–353. doi: 10.1177/1043659614524253. [DOI] [PubMed] [Google Scholar]
- 52.Ministry of Health & National Directorate for Medical Assistance Republic of Mozambique . Maputo, Mozambique. Ministry of Health; 2014. Guia de Tratamento Antiretroviral e Infecções Oportunistas no Adulto, Criança, Adolescente e Grávida. [Google Scholar]
- 53.Spinelli M.A., Glidden D.V., Rodrigues W.C. Low tenofovir level in urine by a novel immunoassay is associated with seroconversion in a preexposure prophylaxis demonstration project. AIDS. 2019;33(5):867–872. doi: 10.1097/QAD.0000000000002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Interagency Task Team. UNICEF, World Health Organization . 2013. Toolkit, Expanding and Simplifying Treatment Foe Pregnant Women Living with HIV: Managing the Transition to Option B/B+http://www.who.int/hiv/pub/mtct/iatt_optionBplus_toolkit/en/ [Google Scholar]
- 55.Kroenke K., Strine T.W., Spitzer R.L., Williams J.B., Berry J.T., Mokdad A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 56.Schwarzer R., Schulz U. 2013. Berlin Social Support Scales (BSSS)http://www.midss.org/content/berlin-social-support-scales-bsss 2016. [Google Scholar]
- 57.Schmidt C.D., Gelhert N.C. Couples therapy and empathy. Fam. J. 2016;25(1):23–30. [Google Scholar]
- 58.Hendrick S.S. A generic measure of relationship satisfaction. J. Marriage Fam. 1988;50(1):93–98. [Google Scholar]
- 59.Siegler A.J., Wiatrek S., Mouhanna F. Validation of the HIV pre-exposure prophylaxis stigma scale: performance of likert and semantic differential scale versions. AIDS Behav. 2020;24(9):2637–2649. doi: 10.1007/s10461-020-02820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erlen J.A., Cha E., Kim K.H., Caruthers D., Sereika S.M. The HIV medication taking self-efficacy scale: psychometric evaluation. J. Adv. Nurs. 2010;66(11):2560–2572. doi: 10.1111/j.1365-2648.2010.05400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cummings P. Methods for estimating adjusted risk ratios. STATA J. 2009;9(2):175–196. [Google Scholar]
- 62.McNeish D. Missing data methods for arbitrary missingness with small samples. J. Appl. Stat. 2017;44(1):24–39. [Google Scholar]
- 63.Braun V., Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3(2):77–101. [Google Scholar]
- 64.Marrazzo J.M., Ramjee G., Richardson B.A. Tenofovir-based preexposure prophylaxis for HIV infection among african women. N. Engl. J. Med. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]