Abstract
The Golgi complex plays a central role in protein secretion by regulating cargo sorting and trafficking. As these processes are of functional importance to cell polarity, motility, growth, and division, there is considerable interest in achieving a comprehensive understanding of Golgi complex biology. However, the unique stack structure of this organelle has been a major hurdle to our understanding of how proteins are secreted through the Golgi apparatus. Herein, we summarize available relevant research to gain an understanding of protein secretion via the Golgi complex. This includes the molecular mechanisms of intra-Golgi trafficking and cargo export in the trans-Golgi network. Moreover, we review recent insights on signaling pathways regulated by the Golgi complex and their physiological significance.
Keywords: Golgi complex, Golgi stress, Intracellular sorting, KDEL receptor, Protein secretion
INTRODUCTION
The intracellular transport system shuttles cargo (e.g., proteins and lipids) between organelles via transport carriers, including vesicles and tubules. Fundamentally, cargo is sorted into transport carriers generated from the membrane of donor organelles (1, 2). The cargo-containing carriers are then translocated to acceptor organelles along the microtubules (3). Upon synthesis, cargoes, which pass quality control in the endoplasmic reticulum (ER), are transported to the Golgi complex in COPII vesicles (4, 5). Thereafter, protein cargoes undergo post-translational modifications as they pass through the Golgi complex. Functional alterations in the Golgi complex have been described in different conditions, including various cancers, immunodeficiencies, and neurodegenerative diseases (6). Further, the question of how this organelle is regulated in pathological conditions has attracted considerable attention (7, 8). However, investigating the Golgi complex has been challenging due to technical limitations arising from the functional complexity and dynamic morphological changes of this organelle. In this short review, we summarize advances in the field of membrane biology from the past years and introduce the current issues in the field to obtain a better understanding of the role of the Golgi complex in the secretory pathway of cells.
COORDINATED REGULATION OF BIDIRECTIONAL TRANSPORT WITHIN THE GOLGI COMPLEX
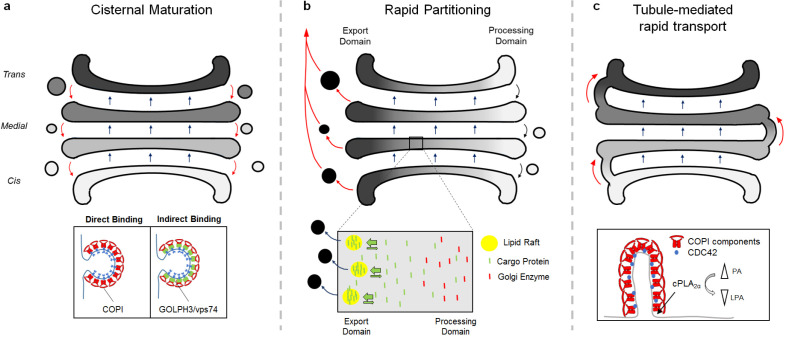
Secretory proteins undergo various post-translational modifications, including glycosylation, during their passage through the Golgi complex. How proteins are transported through this organelle has remained elusive for many years. Early studies identified the coat protein I (COPI) complex, which acts as a major coat component of vesicles formed in the Golgi (9). Extensive research pursued understanding the role of COPI vesicles in the Golgi complex. Considering the Golgi stacks as static, it was initially thought that COPI-coated vesicles are responsible for the anterograde and retrograde transport of cargoes within the organelle (10). However, this model could not explain the sorting of large cargoes such as procollagen into COPI vesicles with a small diameter of approximately 50 nm (11, 12). Subsequent studies revealed that procollagen is transported through the Golgi complex without leaving the cisternae (13, 14). Moreover, two independent research groups observed the change of cis-stack to trans-stack via live imaging in yeast (15, 16). These findings provided strong evidence for a possible mechanism through which stacks containing secretory cargoes move in the anterograde direction, referred to as the model of cisternal maturation (Fig. 1A). Given that COPI vesicles contain Golgi resident proteins, including mannosidase II and giantin (17), it was suggested that secretory cargo is transported through the Golgi complex in an anterograde direction via cisternal maturation, while the rearrangement of Golgi resident proteins is regulated by COPI vesicle-mediated retrograde transport.
Fig. 1.
Three models of protein transport within the Golgi complex. (A) Cisternal maturation model. Secretory cargo is transported in an anterograde direction along with cisternae maturation from cis-face to trans-face of the Golgi complex. Golgi resident proteins are transported in retrograde through COPI vesicles. (B) Rapid partitioning model. The Golgi stacks are distributed into two sub-domains. One is glycerophospholipids containing processing domain (white), while the other domain contains glycosphingolipids (black). Transmembrane secretory protein (green) are concentrated at export domain, while Golgi-resident proteins (red) are excluded from export domain and concentrated in processing domain. (C) Golgi tubule-mediated transport model. Each cisternae is connected by Golgi tubule which is regulated by the COPI complex. Golgi tubules are involve in the rapid bidirectional transport within the Golgi complex.
COPI is a large protein complex consisting of seven subunits (α, β, β’, γ, δ, ε, and ξ) (18, 19). It is structurally similar to clathrin and the AP2 complex, having an inner coat (β, γ, δ, ξ), which corresponds to the AP2 adaptor, and an outer coat (α, β’, ε) corresponding to the clathrin heavy chain (20). Coat proteins carry out two major roles, namely cargo sorting and vesicle formation. With regard to cargo sorting, the COPI complex contains several cargo binding sites (21, 22). Structural examination revealed that KKxx and KxKxx motifs in the cytoplasmic tail of retrograde cargo are inserted into the pores of the β’-COP subunit for binding (23). Cargo binding sites were additionally identified in the α- and γ-subunits, suggesting that cargoes bind to COPI subunits in different manners (24, 25). For instance, many Golgi resident proteins do not contain the canonical KKxx motif (26). In this case, cargoes are recognized by GOLPH3, which is a linker between Golgi-resident proteins and the COPI complex (27, 28). Indeed, various glycosyltransferases bind to GOLPH3 through a pentameric (F/L)-(L/I/V)-X-X-(R/K) motif for the COPI interaction (27, 28).
The molecular mechanisms underlying the regulation of COPI vesicle formation are unclear. It was initially suggested that small GTPases ARF1 and COPI are sufficient to form COPI vesicles based on the observation that vesicles could be reconstituted using an in vitro reconstitution assay wherein an intact Golgi membrane was incubated with purified COPI and recombinant ARF1 loaded with GTPγS (29). In this scenario, the COPI complex was recruited to the Golgi membrane upon activation of ARF1 (30). The GTPase-activating protein (GAP) for ARF1, ARFGAP1, then promoted the dissociation of COPI components from vesicles via GTP hydrolysis (29). However, given that the GAP acts as a coat component of vesicle formation in multiple transport pathways (31), various groups have questioned the role of ARFGAP1 in COPI vesicle formation (32, 33). By establishing a two-stage in vitro reconstitution system that separates the early (budding) and late (fission) stages of COPI vesicle formation, ARFGAP1 was found to be involved in the early stage, regulating cargo sorting (34). Additionally, ARFGAP1 bound to a highly curved membrane through the ALPS motif. Further, this binding induced the membrane curvature required for vesicle formation (32, 35, 36). Taken together, these results indicated that ARFGAP1 acts as a coat component in COPI vesicle formation (36, 37). Through cryo-EM, recent studies found irregular cage structures of COPI vesicles which were reconstituted using a coatomer and ARF1 (38). Considering that vesicles coated with COPII and clathrin have a regular cage structure, COPI vesicles of different structures may be observed through the addition of missing coat components.
Although there are many advantages of the cisternal maturation model in explaining anterograde transport in the Golgi, it is still unclear why some cargoes are transported faster than others (39). As rapidly growing cells produce and secrete various molecules within a short period of time, understanding the molecular mechanisms underlying rapid secretion may be of great relevance to pathological conditions as well as normal physiology. Golgi stacks were found to be rapidly partitioned, providing different transport pathways for rapid secretion (Fig. 1B) (40). It was also suggested that certain cargoes use a special transport carrier for rapid secretion. Historically, rapidly secreted cargoes (e.g., VSV-G and proinsulin) were originally observed in COPI-coated transport carriers (10). However, this was not further investigated as the COPI complex was thought to be involved in retrograde transport. This issue was recently revisited using electron microscopy (EM) tomography, in which Z-stack images of the Golgi complex obtained via EM were applied for 3D-reconstruction. A Golgi structure in which stacks are connected by tubules was observed (41). These tubules were highly likely to be considered as vesicles in previous studies as horizontal images of the two were similar (41, 42). Subsequent studies revealed that the COPI complex promoted the formation of Golgi tubules (Fig. 1C). Mechanistically, after initial budding of the Golgi membrane induced by the COPI complex, the enzymatic activities of lysophosphatidic acid acyltransferase-γ (LPAATγ) and cytosolic phospholipase A2α (cPLA2α) determined the fate of these buds in becoming vesicles or tubules (43). In particular, cPLA2α induced the elongation of COPI-coated buds to form tubules by providing lysophosphatidic acids with an inverted cone shape, which inhibit COPI vesicle fission (43). In this regard, rapid protein secretion was abolished by disrupting cPLA2α, suggesting that the Golgi tubule is important for the rapid secretory pathway (43). Taken together, secretory proteins are transported through the Golgi complex by cisternal maturation (slow) and Golgi tubules (rapid) formed by the COPI complex, whereas rearrangement of Golgi resident proteins is regulated by retrograde transport via COPI vesicles.
How the same coat protein acts in the opposite direction during transport was recently revealed, as anterograde cargoes were found to bind to the COPI complex through a non-canonical basic sorting motif, distinct from retrograde cargo binding to the COPI subunit via the KKxx motif (44). This process is involved in the role of Rho GTPase CDC42 within the Golgi complex. Upon activation, CDC42 binds to the γ-subunit of the COPI complex through the KKxx motif at its C-terminus region, which causes the competitive inhibition of retrograde cargo binding to the COPI complex (25, 44). CDC42 also promotes anterograde transport through its ability to exert membrane curvature, which induces the formation of Golgi tubules (44). In this case, however, CDC42 did not affect anterograde cargo sorting into the tubules. These findings raised questions regarding how the role of CDC42 in Golgi transport is regulated. One possible mechanism is that GTPases are modulated by their respective GEF/GAP. Of note, various GEFs and GAPs of the Rho GTPase have been found in the Golgi complex (45, 46). In addition to the general hypothesis presented above, upstream kinases can target GEF/GAP to regulate GTPases as studies on Vav proteins, which are prototypic GEFs for Rho GTPases, have shown (47). The SRC kinase is a likely upstream regulator of CDC42 based on the observation that protein secretion through the Golgi complex is inhibited by SRC disruption (48). Taken together, these considerations suggest the high possibility that SRC targets the GEF/GAP for Golgi CDC42 to modulate the latter’s role in transport. Studies on the circuit of SRC-GEF/GAP-Rho GTPase in Golgi transport are necessary in order to achieve a complete understanding of bidirectional transport within the organelle. Moreover, investigating the involvement of other Rho GTPases, such as Rac1 and RhoA, in the secretory pathway is another intriguing topic the elucidation of which will contribute to a more complete understanding of secretion.
CARGO SORTING AND TRANSPORT IN THE TRANS-GOLGI NETWORK
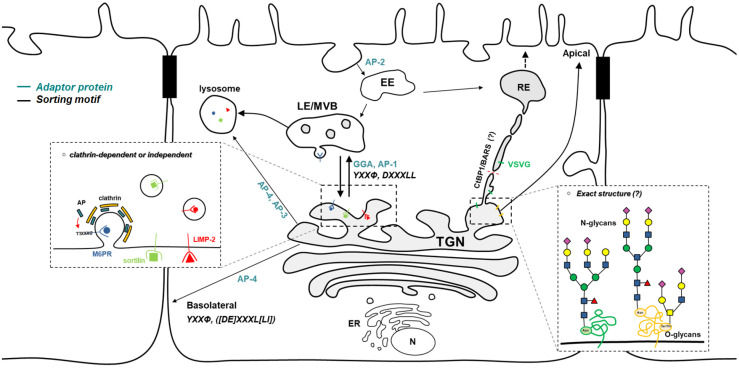
When secretory proteins arrive at the trans-Golgi network (TGN), they must be properly sorted before being delivered to their final destinations (49). TGN export was previously considered a constitutive process (50). However, cumulative studies have revealed that this pathway is regulated (Fig. 2). In fact, polarized distribution of apical and basolateral proteins provides strong evidence for the regulation of transport in the TGN. Impaired sorting causes mislocalization of basolateral cargoes in the apical region (51). Basolateral proteins (e.g., Furin and LDLR) bind to the μ subunit of the AP complex through a tyrosine-based motif (YXXf) and a di-leucine motif ([DE]XXXL[LI]), promoting cargo export in the TGN (52). Impaired sorting of basolateral proteins by disruption of AP-4 μ subunit leads to mislocalization to the apical surface (49, 53). Cargo receptors also recognize ligands other than the peptide-based sorting motif. When apical-targeted proteins reach the TGN, the sugars in cargoes are recognized by lectins, which promotes rearrangement of apical cargoes on lipid rafts for the sorting process (54-56). The mannose-6-phosphate receptor (M6PR) also recognizes sugars on lysosomal enzymes (57). Upon cargo binding, the receptor binds to GGA and AP-1 to sort cargoes into clathrin-coated vesicles. Subsequently, low pH in the endosomal compartments induces the dissociation of cargoes from M6PR, which are then retrieved to the TGN (57). Notably, many proteins (e.g., insulin-like growth factor II (IGF II), retinoic acid, soluble enzymes, as well as lysosomal membrane proteins) are transported independently of M6PR (52). In this regard, an alternative mechanism regulated by sortilin and LIMP-2 was identified. Sortilin contains a VPS10 domain on the lumen side. The cleavage of the N-terminal propeptide on this VPS10 domain regulates the sorting of lysosome targeting proteins such as cathepsin D and cathepsin H. Further, the cytoplasmic tail of sortilin binds to the GGA and AP complex for TGN export (58, 59). LIMP-2 is a key regulator of β-glucocerebrosidase (βGC) transport in the TGN (60). A heavily glycosylated coiled-coil domain of LIMP-2 binds to βGC. Binding is disrupted at the acidic pH once cargo is delivered to the endosomal compartment. How soluble proteins such as cartilage oligomeric protein (COMP) and lysozyme C (LyzC) are sorted after exiting the TGN was recently elucidated. That is, ADF/Cofilin1 was shown to increase calcium concentration in the lumen of the TGN through a calcium pump, SPCA1 (61). Higher Ca2+ promoted the oligomerization of Cab45, inducing the binding to soluble cargoes for TGN export (62). Although extensive studies have uncovered regulatory factors of TGN export, the coat protein that regulates various transport pathways occurring in the TGN remains unclear.
Fig. 2.
Cargo sorting and export regulated in the trans-Golgi network. Transport of cargoes to various destinations is regulated in the TGN. Many key factors have been identified. TGN export to endolysosome is regulated through Clathrin dependent and independent machineries. Mannose-6-phosphasphate receptor (M6PR) and Sortilin act as a cargo receptor in this pathway. In polarized cells, transport of apical and basolateral cargoes is regulated by multiple mechanisms that involve not only proteins, but also sugars.
SIGNALING PATHWAYS REGULATED BY THE GOLGI COMPLEX FOR ORGANELLE HOMEOSTASIS
The Golgi complex is involved in the activation of signaling transduction by providing a platform for signaling molecules (Fig. 3). For example, the signaling of N-Ras in the Golgi complex increases T cell responses to antigen stimulation (63, 64). Ras signaling initiated from the Golgi complex triggers sustained T cell activity compared to that from the plasma membrane (65). These observations lead to the question of how signaling molecules are specifically recruited to the Golgi complex, but not to other organelles. The membrane association of small GTPases was described as regulated by lipid modi-fications, including farnesylation, prenylation, and palmitoylation (66). Further, the recruitment of Ras to the Golgi complex was determined by a balance between palmitoylation and depalmitoylation (67, 68). Given that many upstream regulators, such as GEF and GAP, exist in the Golgi complex (45, 46), it is possible that these are initially recruited to the apparatus to activate the Rho GTPase on its membrane. Notably, however, how GEF and GAP are recruited to specific organelles and what signaling activates Rho GTPase in TGN export are both poorly understood concepts.
Fig. 3.
Signaling transduction instigated by the Golgi complex. (Left) Palmitoylation of Ras obtained at the Golgi complex is required for membrane recruitment and activation of signaling pathways. Signaling pathway of N-Ras at the Golgi complex shows a sustained activity as compared to N-Ras signaling instigated from the plasma membrane. (Right) Retrograde COPI transport is regulated by KDELR-mediated PKA signaling pathway. Cargo export in TGN is regulated by SRC signaling pathway which is activated by KDELR.
Adenylyl cyclase, which converts cellular ATP to cyclic AMP (cAMP), regulates PKA signaling upon activation of G-protein coupled receptors (GPCRs) (69, 70). PKA promotes the recruitment of ARF1 to the Golgi complex, resulting in membrane trafficking (71). Since GPCR signaling is mostly regulated at the plasma membrane, it is surprising that the Golgi complex is involved. In this regard, recent studies have uncovered a new role of the KDEL receptor (KDELR), a GPCR, in signaling transduction (72, 73). KDELR was originally known as a cargo receptor for COPI transport. Under ER stress conditions, ER resident proteins often leak from the ER and are captured by the KDELR located at the Golgi complex, which binds to the “KDEL” tetrapeptide sequence of ER resident proteins. This promotes the retrieval of proteins back to the ER through COPI vesicles (74, 75). Studies investigating its physiological role revealed that different signaling pathways activated by KDELR regulate distinct transport pathways, including Gq/SRC for cargo export at the TGN as well as Gs/PKA for COPI transport (72, 73). This raises the key question regarding how various signaling pathways are regulated by this single receptor. Among the three types of KDELR (KDELR1/2/3) in mammalian cells, only KDELR1-mediated signaling has been studied. We suspect that the multitude of different G-proteins coupled to KDELRs are likely to activate different downstream effector proteins. A proteomic approach using engineered ascorbic acid peroxidase (APEX) was recently utilized to determine the spatiotemporal protein networks of GPCRs in living cells (76, 77). This could be an appropriate technical approach for identifying new pathways regulated by KDELR in the Golgi complex.
Intracellular compartments maintain organelle homeostasis in response to environmental stress through a number of mechanisms. Over the past decades, the endoplasmic reticulum (ER) has been extensively studied with regard to the maintenance of homeostasis under stress. Protein synthesis is considerably enhanced under various physiological and pathological conditions. Such increased rates of protein synthesis overwhelm the protein folding capacity of the ER, leading to the accumulation of misfolded protein (78). Multiple stress response mechanisms are activated to maintain organelle homeostasis and are cumulatively known as the unfolded protein response (UPR). As proteins are secreted through the Golgi, how it maintains homeostasis in the context of increased transport flux is another topic of considerable interest. However, a limited number of studies have the Golgi complex stress response. In this regard, proteins such as TFE3, proteoglycans, CREB3, and HSP47 have been identified as key factors in the maintenance Golgi homeostasis (79, 80). However, the mechanisms through which these factors regulate the Golgi stress response remain unclear. Further, since the Golgi is involved in various signaling pathways, it is likely that KDELR-associated signaling pathways promote transport through the organelle, reducing protein amounts within. Given the complex dynamics of the Golgi complex in diverse conditions, further research is needed to elucidate aspects such as the Golgi stress response and related signaling pathways.
CONCLUSION
The Golgi complex plays an important role in various cellular processes. Extensive studies have been conducted in order to understand the organelle from a membrane trafficking perspective. However, Golgi regulation under different conditions remains unclear. In addition to studies on membrane trafficking and related signaling, there are various fundamental questions that need to be addressed regarding the Golgi complex, such as the identification of signaling cues, understanding how organelle homeostasis is maintained, and elucidating the significance of glycosylation in the context of membrane trafficking. Moreover, a new form of intracellular communication regulated by direct organelle contact was recently discovered. This raises the question of whether the Golgi complex also communicates with other organelles through membrane contact and, if so, what the physiological significance of Golgi contact is.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Research Foundation (NRF-2020R1C1C1008823, NRF-2017R1A5A1015 366), the Korea Health Industry Development Institute (KHIDI-HR20C0025), POSCO Science Fellowship, and the BK21 Plus and BK21 FOUR Research Fellowship.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Sato K, Nakano A. Mechanisms of COPII vesicle formation and protein sorting. FEBS Lett. 2007;581:2076–2082. doi: 10.1016/j.febslet.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 2.Hsu VW, Lee SY, Yang JS. The evolving understanding of COPI vesicle formation. Nat Rev Mol Cell Biol. 2009;10:360–364. doi: 10.1038/nrm2663. [DOI] [PubMed] [Google Scholar]
- 3.Fourriere L, Jimenez AJ, Perez F, Boncompain G. The role of microtubules in secretory protein transport. J Cell Sci. 2020;133:jcs237016. doi: 10.1242/jcs.237016. [DOI] [PubMed] [Google Scholar]
- 4.Barlowe C, Orci L, Yeung T, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2011;14:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 6.Makhoul C, Gosavi P, Gleeson PA. Golgi dynamics: the morphology of the mammalian Golgi apparatus in health and disease. Front Cell Dev Biol. 2019;7:112. doi: 10.3389/fcell.2019.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee TH, Linstedt AD. Osmotically induced cell volume changes alter anterograde and retrograde transport, Golgi structure, and COPI dissociation. Mol Biol Cell. 1999;10:1445–1462. doi: 10.1091/mbc.10.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellokumpu S, Sormunen R, Kellokumpu I. Abnormal glycosylation and altered Golgi structure in colorectal cancer: dependence on intra-Golgi pH. FEBS Lett. 2002;516:217–224. doi: 10.1016/S0014-5793(02)02535-8. [DOI] [PubMed] [Google Scholar]
- 9.Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 10.Orci L, Stamnes M, Ravazzola M, et al. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/S0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 11.Becker B, Melkonian M. The secretory pathway of protists: spatial and functional organization and evolution. Microbiol Rev. 1996;60:697. doi: 10.1128/MR.60.4.697-721.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonfanti L, Mironov AA, Jr, Martínez-Menárguez JA, et al. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/S0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- 13.Mironov AA, Beznoussenko GV, Nicoziani P, et al. Small cargo proteins and large aggregates can traverse the Golgi by a common mechanism without leaving the lumen of cisternae. J Cell Biol. 2001;155:1225–1238. doi: 10.1083/jcb.200108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donohoe BS, Kang B-H, Staehelin LA. Identification and characterization of COPIa- and COPIb-type vesicle classes associated with plant and algal Golgi. Proc Natl Acad Sci U S A. 2007;104:163. doi: 10.1073/pnas.0609818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- 16.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Menárguez JA, Prekeris R, Oorschot VMJ, et al. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J Cell Biol. 2001;155:1213–1224. doi: 10.1083/jcb.200108029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe M, Kreis TE. Regulation of membrane traffic in animal cells by COPI. Biochim Biophys Acta. 1998;1404:53–66. doi: 10.1016/S0167-4889(98)00046-9. [DOI] [PubMed] [Google Scholar]
- 19.Nickel W, Brügger B, Wieland FT. Vesicular transport: the core machinery of COPI recruitment and budding. J Cell Sci. 2002;115:3235. doi: 10.1242/jcs.115.16.3235. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman GR, Rahl PB, Collins RN, Cerione RA. Conserved structural motifs in intracellular trafficking pathways: structure of the γCOP appendage domain. Mole Cell. 2003;12:615–625. doi: 10.1016/j.molcel.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosson P, Letourneur F. Coatomer interaction with dilysine endoplasmic reticulum retention motifs. Science. 1994;263:1629. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- 23.Jackson Lauren P, Lewis M, Kent Helen M, et al. Molecular basis for recognition of dilysine trafficking motifs by COPI. Dev Cell. 2012;23:1255–1262. doi: 10.1016/j.devcel.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma W, Goldberg J. Rules for the recognition of dilysine retrieval motifs by coatomer. EMBO J. 2013;32:926–937. doi: 10.1038/emboj.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu WJ, Erickson JW, Lin R, Cerione RA. The γ-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature. 2000;405:800–804. doi: 10.1038/35015585. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz KR, Liu J, Li S, et al. Golgi localization of glycosyltransferases requires a Vps74p oligomer. Dev Cell. 2008;14:523–534. doi: 10.1016/j.devcel.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu L, Tai WCS, Chen L, Banfield DK. Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science. 2008;321:404. doi: 10.1126/science.1159411. [DOI] [PubMed] [Google Scholar]
- 28.Dippold HC, Ng MM, Farber-Katz SE, et al. GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337–351. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1371. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci U S A. 1992;89:6408. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kung LF, Pagant S, Futai E, et al. Sec24p and Sec16p cooperate to regulate the GTP cycle of the COPII coat. EMBO J. 2012;31:1014–1027. doi: 10.1038/emboj.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bigay J, Gounon P, Robineau S, Antonny B. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature. 2003;426:563–566. doi: 10.1038/nature02108. [DOI] [PubMed] [Google Scholar]
- 33.Reinhard C, Schweikert M, Wieland FT, Nickel W. Functional reconstitution of COPI coat assembly and disassembly using chemically defined components. Proc Natl Acad Sci U S A. 2003;100:8253. doi: 10.1073/pnas.1432391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanoix J, Ouwendijk J, Stark A, et al. Sorting of Golgi resident proteins into different subpopulations of COPI vesicles : a role for ArfGAP1. J Cell Biol. 2001;155:1199–1212. doi: 10.1083/jcb.200108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bigay J, Casella J-F, Drin G, Mesmin B, Antonny B. ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J. 2005;24:2244–2253. doi: 10.1038/sj.emboj.7600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S-Y, Yang J-S, Li Z, et al. The late stage of COPI vesicle fission requires shorter forms of phosphatidic acid and diacylglycerol. Nat Commun. 2019;10:3409. doi: 10.1038/s41467-019-11324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J-S, Lee SY, Gao M, et al. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159:69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dodonova SO, Diestelkoetter-Bachert P, von Appen A, et al. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Science. 2015;349:195. doi: 10.1126/science.aab1121. [DOI] [PubMed] [Google Scholar]
- 39.Glick BS, Nakano A. Membrane traffic within the Golgi apparatus. Ann Rev Cell Dev Biol. 2009;25:113–132. doi: 10.1146/annurev.cellbio.24.110707.175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson GH, Hirschberg K, Polishchuk RS, Gerlich D, Phair RD, Lippincott-Schwartz J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trucco A, Polishchuk RS, Martella O, et al. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–1081. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- 42.Pietro ES, Capestrano M, Polishchuk EV, et al. Group IV phospholipase A2α controls the formation of inter-cisternal continuities involved in intra-Golgi transport. PLOS Biology. 2009;7:e1000194. doi: 10.1371/journal.pbio.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J-S, Valente C, Polishchuk RS, et al. COPI acts in both vesicular and tubular transport. Nat Cell Biol. 2011;13:996–1003. doi: 10.1038/ncb2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park S-Y, Yang J-S, Schmider AB, Soberman RJ, Hsu VW. Coordinated regulation of bidirectional COPI transport at the Golgi by CDC42. Nature. 2015;521:529. doi: 10.1038/nature14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farhan H, Hsu VW. Cdc42 and cellular polarity: emerging roles at the Golgi. Trends Cell Biol. 2016;26:241–248. doi: 10.1016/j.tcb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baschieri F, Confalonieri S, Bertalot G, et al. Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis. Nat Commun. 2014;5:4839. doi: 10.1038/ncomms5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bustelo XR. Vav proteins, adaptors and cell signaling. Oncogene. 2001;20:6372–6381. doi: 10.1038/sj.onc.1204780. [DOI] [PubMed] [Google Scholar]
- 48.Pulvirenti T, Giannotta M, Capestrano M, et al. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat Cell Biol. 2008;10:912–922. doi: 10.1038/ncb1751. [DOI] [PubMed] [Google Scholar]
- 49.Guo Y, Sirkis DW, Schekman R. Protein sorting at the trans-Golgi network. Ann Rev Cell Dev Biol. 2014;30:169–206. doi: 10.1146/annurev-cellbio-100913-013012. [DOI] [PubMed] [Google Scholar]
- 50.Polishchuk EV, Di Pentima A, Luini A, Polishchuk RS. Mechanism of constitutive export from the Golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-Golgi network tubular domains. Mol Biol Cell. 2003;14:4470–4485. doi: 10.1091/mbc.e03-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 52.Dittmer F, Ulbrich EJ, Hafner A, et al. Alternative mechanisms for trafficking of lysosomal enzymes in mannose 6-phosphate receptor-deficient mice are cell type-specific. J Cell Sci. 1999;112:1591. doi: 10.1242/jcs.112.10.1591. [DOI] [PubMed] [Google Scholar]
- 53.Simmen T, Höning S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 54.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Str Biol. 2002;12:616–623. doi: 10.1016/S0959-440X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 55.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheiffele P, Peränen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- 57.Kornfeld S, Mellman I. The Biogenesis of lysosomes. Ann Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- 58.Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J. 2003;22:6430–6437. doi: 10.1093/emboj/cdg629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ni X, Morales CR. The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic. 2006;7:889–902. doi: 10.1111/j.1600-0854.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 60.Reczek D, Schwake M, Schröder J, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-Independent targeting of β-glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 61.von Blume J, Alleaume A-M, Cantero-Recasens G, et al. ADF/Cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev Cell. 2011;20:652–662. doi: 10.1016/j.devcel.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 62.von Blume J, Alleaume A-M, Kienzle C, Carreras-Sureda A, Valverde M, Malhotra V. Cab45 is required for Ca2+-dependent secretory cargo sorting at the trans-Golgi network. J Cell Biol. 2012;199:1057–1066. doi: 10.1083/jcb.201207180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bivona TG, Quatela S, Philips MR. Analysis of Ras activation in living cells with GFP-RBD. Methods Enzymol. 2006;407:128–143. doi: 10.1016/S0076-6879(05)07012-6. [DOI] [PubMed] [Google Scholar]
- 64.Ibiza S, Perez-Rodriguez A, Ortega A, et al. Endothelial nitric oxide synthase regulates N-Ras activation on the Golgi complex of antigen-stimulated T cells. Proc Natl Acad Sci U S A. 2008;105:10507–10512. doi: 10.1073/pnas.0711062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasuda T, Kurosaki T. Regulation of lymphocyte fate by Ras/ERK signals. Cell Cycle. 2008;7:3634–3640. doi: 10.4161/cc.7.23.7103. [DOI] [PubMed] [Google Scholar]
- 66.Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. Regulating the regulator: post-translational modification of RAS. Nat Rev Mol Cell Biol. 2011;13:39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodwin JS, Drake KR, Rogers C, et al. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J Cell Biol. 2005;170:261–272. doi: 10.1083/jcb.200502063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rocks O, Peyker A, Kahms M, et al. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 69.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 70.Clark RB, Knoll BJ, Barber R. Partial agonists and G protein-coupled receptor desensitization. Trends Pharm Sci. 1999;20:279–286. doi: 10.1016/S0165-6147(99)01351-6. [DOI] [PubMed] [Google Scholar]
- 71.Muniz M, Martin ME, Hidalgo J, Velasco A. Protein kinase A activity is required for the budding of constitutive transport vesicles from the trans-Golgi network. Proc Natl Acad Sci U S A. 1997;94:14461–14466. doi: 10.1073/pnas.94.26.14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cancino J, Capalbo A, Di Campli A, et al. Control systems of membrane transport at the interface between the endoplasmic reticulum and the Golgi. Dev Cell. 2014;30:280–294. doi: 10.1016/j.devcel.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 73.Giannotta M, Ruggiero C, Grossi M, et al. The KDEL receptor couples to Gαq&11 to activate Src kinases and regulate transport through the Golgi. EMBO J. 2012;31:2869. doi: 10.1038/emboj.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Semenza JC, Hardwick KG, Dean N, Pelham HRB. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990;61:1349–1357. doi: 10.1016/0092-8674(90)90698-E. [DOI] [PubMed] [Google Scholar]
- 75.Lewis MJ, Pelham HRB. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 1992;68:353–364. doi: 10.1016/0092-8674(92)90476-S. [DOI] [PubMed] [Google Scholar]
- 76.Paek J, Kalocsay M, Staus DP, et al. Multidimensional tracking of GPCR signaling via peroxidase-catalyzed proximity labeling. Cell. 2017;169:338–349.e311. doi: 10.1016/j.cell.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lobingier BT, Hüttenhain R, Eichel K, et al. An approach to spatiotemporally resolve protein interaction networks in living cells. Cell. 2017;169:350–360.e312. doi: 10.1016/j.cell.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Ann Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 79.Sun Z, Brodsky JL. Protein quality control in the secretory pathway. J Cell Biol. 2019;218:3171–3187. doi: 10.1083/jcb.201906047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sasaki K, Yoshida H. Golgi stress response and organelle zones. FEBS Lett. 2019;593:2330–2340. doi: 10.1002/1873-3468.13554. [DOI] [PubMed] [Google Scholar]