Highlights
-
•
Prosthetic joint infection (PJI) due to Salmonella spp. is uncommon, with an estimated prevalence of <0.3 % of all PJI.
-
•
Salmonella enterica serovar Enteritidis and Salmonella enterica serovar Typhimurium are the most common isolates causing PJI.
-
•
Risk factors include malignancy, hemoglobinopathies, diabetes mellitus, HIV/AIDS, alcohol dependency and immunosuppressed state.
-
•
PJI due to Salmonella spp. can occur without significant preceding gastrointestinal symptoms of infection.
Keywords: Salmonella enterica serovars Enteritidis, Prosthetic joint infection
Abstract
Prosthetic joint infection (PJI) is a serious complication of prosthetic joint implantation with a prevalence of about 1–2 % of all prosthetic joint surgeries. While Staphylococcus spp. are the most common organisms isolated, Salmonella spp. are a rare cause of PJI (estimated prevalence < 0.3 %). We present a case of a 62-year-old patient with a history of previous joint trauma complicated by osteonecrosis, infection and chronic alcohol abuse with late hematogenous prosthetic hip infection due to Salmonella enterica serovar Enteritidis. PJI due to Salmonella spp. should be considered in the differential diagnosis when a patient has risk factors such as malignancy, hemoglobinopathies, diabetes mellitus, human immunodeficiency virus/acquired immunodeficiency syndrome, alcohol dependency or immunosuppressed state, even without significant preceding gastrointestinal symptoms. Our patient had a few of these risk factors and required surgical debridement in addition to antimicrobials for treatment of his PJI.
Introduction
Prosthetic joint infection (PJI) is a serious complication of prosthetic joint implantation. While Staphylococcus spp. are the most common organisms isolated, Salmonella spp. are a rare cause of PJI. We present a case of a 62-year-old patient who developed late hematogenous prosthetic hip infection due to Salmonella enterica serovar Enteritidis.
Case report
A 62-year-old African American male with a past medical history of severe osteoarthritis requiring bilateral hip arthroplasties, alcohol dependence, schizophrenia and chronic untreated hepatitis C presented with a six-day history of acute left hip pain. The patient’s history was significant for a fracture of the left hip prosthesis 3 years prior requiring open reduction and internal fixation (ORIF) with a course complicated by polymicrobial joint infection with methicillin resistant Staphylococcus aureus (MRSA), Enterobacter cloacae complex and Pseudomonas aeruginosa. He was treated with irrigation and debridement of the joint with retention of hardware followed by a six weeks course of intravenous antimicrobial therapy. He was not placed on any chronic antimicrobial suppression. He continued to do well until his current presentation to the hospital with acute left hip pain.
The patient denied any preceding trauma, fevers, chills, skin changes, vomiting or diarrhea but complained of abdominal pain and nausea. He reported drinking 6 alcoholic drinks per day, smoking 10 cigarettes a day with a 52-pack year history, and denied any intravenous drug use. He lived in the Midwest United States and denied any recent travel, sick contacts, pets, or contact with farm animals.
Vitals signs were notable for a blood pressure 94/68 mmHg, pulse 112 beats/min, respiratory rate 16 respirations/min and temperature 36.6 C. Physical examination noted a thin, cachectic male with tenderness to palpation and decreased passive and active range of motion of the left hip. There were no open wounds or drainage noted at the hip. Laboratory tests revealed a white blood cell count (WBC) 9300/μL (normal 3,700−10,500/μL), creatinine 1.1 mg/dL (normal 0.6–1.2 mg/dL), C-reactive protein 17.3 mg/dL (normal <0.5 mg/dL). Left hip X ray revealed sclerotic lesions concerning for osteonecrosis, as well as presence of orthopedic hardware (Fig. 1). Left hip arthrocentesis revealed synovial fluid with a glucose 2 mg/dL, total protein 5.1 mg/dL, and nucleated cell count 185,988 /μL with 94 % neutrophils. Gram stain demonstrated Gram-negative rods. The patient was started on piperacillin-tazobactam.
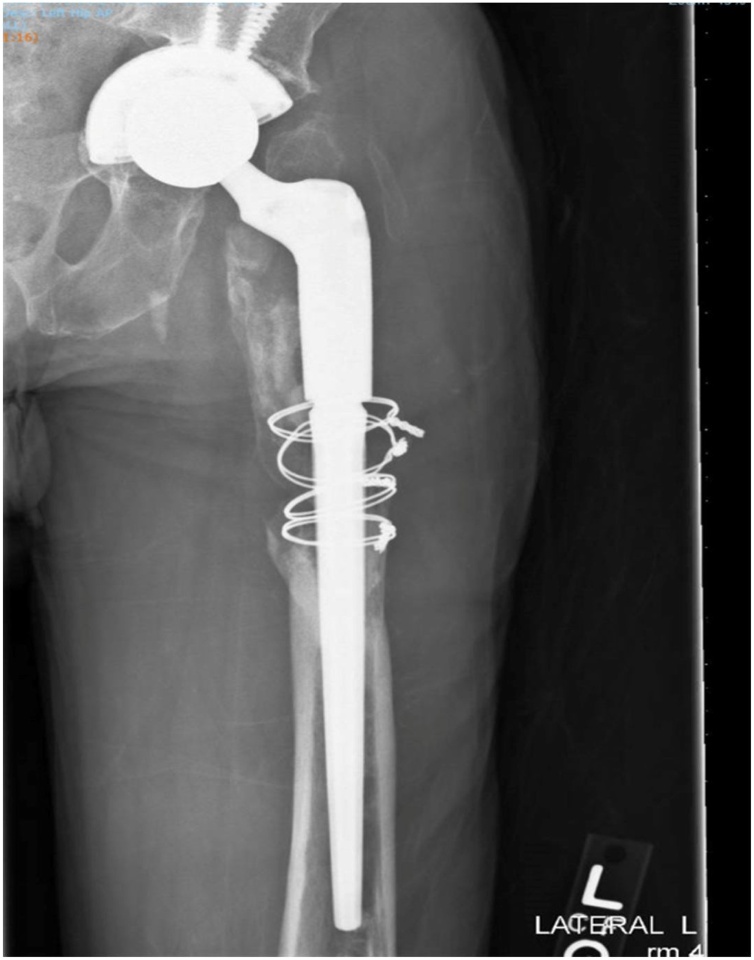
Fig. 1.
X-ray of the left hip showing arthroplasty with long stem implant and cerclage wires with cortical gap and no significant bone bridging in the proximal shaft. There were erosive lesions in the proximal shaft/metaphysis of the femur with prominent heterotopic ossification. Sclerotic lesions in the distal shaft of the femur is concerning for osteonecrosis.
Both blood cultures and synovial fluid grew Salmonella spp., which was eventually identified as S. enterica serovar Enteritidis, and he was diagnosed with late onset hematogenous PJI due to S. enterica serovar Enteritidis. Repeated blood culture remained negative. His antimicrobial was changed to ceftriaxone, based on susceptibility results (Table 1). As additional work up, he underwent computed tomographic angiography of the chest, abdomen and pelvis, which revealed no sign of enteritis, abscess or endovascular disease. Approximately a week after his admission, right upper quadrant abdominal pain developed, and evaluation revealed acute cholecystitis, necessitating cholecystectomy. Orthopedic surgeons deferred surgical intervention in the setting of recent cholecystectomy as well as his multiple comorbidities. His left hip pain significantly improved with ceftriaxone and he was discharged to a rehabilitation facility with a plan to continue a course of 6 weeks of ceftriaxone.
Table 1.
Salmonella enterica serovar Enteritidis susceptibility.
| Salmonella enterica serovar Enteritidis | |
|---|---|
| Antimicrobial | MIC/Interpretation |
| Ampicillin | 2.0 μg/mL/Susceptible |
| Ceftriaxone | 0.12 μg/mL/Susceptible |
| Ciprofloxacin | 0.03 μg/mL/Susceptible |
| TMP-SMX | 0.12 μg/mL/Susceptible |
At his follow up, he was transitioned to oral trimethoprim-sulfamethoxazole (TMP-SMX) as a chronic suppressive therapy, as he had not had any debridement surgery and had a retained prosthesis. He had adverse events associated with TMP-SMX (rash and hyperkalemia) necessitating transition to amoxicillin. Approximately 10 months after his initial first presentation, he presented with progressive worsening of left hip pain with decreased weight bearing capacity. A repeat left hip arthrocentesis revealed nucleated cell count 18,570/μL with 90 % neutrophils and cultures showing S. enterica serovar Enteritidis. Left hip X-ray showed orthopedic hardware with loosening and fractured screw at the acetabular component of his left total hip arthroplasty (Fig. 2). During this second admission, he underwent resection of the acetabular component of the hip with retention of the well-fixed femoral stem. The patient was treated with 6 weeks of intravenous ceftriaxone following his surgery. At follow up, the patient’s pain was significantly better and given the retained femoral stem the decision was made to transition him to amoxicillin indefinitely for suppression.
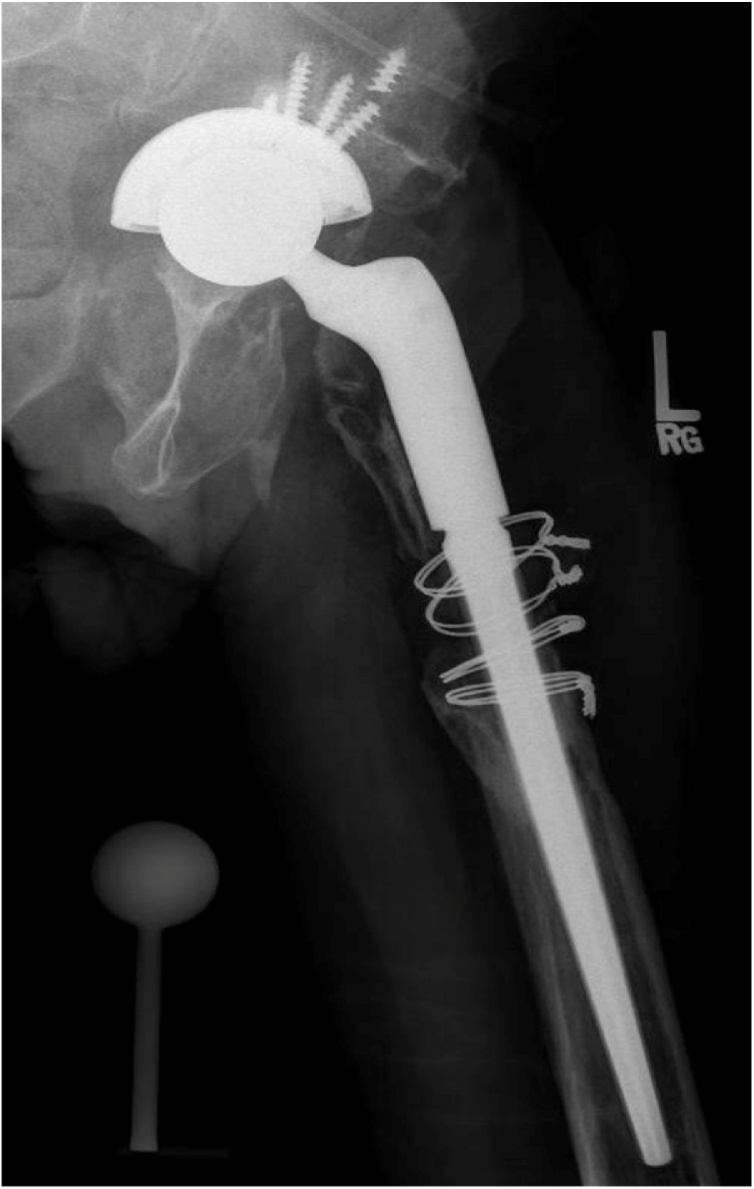
Fig. 2.
X-ray of the left hip showed arthroplasty with long stem implant and cerclage wires with cortical gap and no significant bone bridging in the proximal shaft. There is evidence of acetabular screw fracture and acetabular component loosening.
Discussion
The present case underscores the importance of being aware that Salmonella species can cause PJI and highlights that the infection can happen without any preceding gastrointestinal manifestations. The number of total joint arthroplasties procedures has been steadily increasing over the past several decades, linked to a rapidly aging population in the United States. More than 1 million total hip and knee arthroplasties are done annually. The total number of arthroplasties is expected to increase to 3.48 million by 2030 [1]. Prosthetic joint infection is one of the most dreaded complications of total joint arthroplasty, and it is seen in 1 %–2 % arthroplasty recipients [2]. PJI increases healthcare costs and morbidity because of prolonged hospitalizations, complex and prolonged antimicrobial therapies and serious impairment in quality of life for the patient [[2], [3], [4], [5], [6], [7], [8]].
PJIs can be classified based on time of onset after the implantation. PJI is classified as early when presentation is within 3 months of implantation; ‘delayed’ when presentation is between 3 and 24 months; and ‘late’ if presentation is greater than 24 months after implantation [5]. The most common causative agents of PJI are Gram-positive organisms such as Staphylococcus spp. and Streptococcus spp. Gram-negative organisms constitute <10 % of all episodes of PJI [4,5,7,8], with Escherichia coli and Pseudomonas spp. being most commonly identified [8]. Salmonella spp. as a causative organism of PJI is rare [[2], [3], [4],[7], [8], [9], [10], [11]] and the prevalence of PJI due to Salmonella spp. is reported less than 0.3 % of all cases of PJI [12]. Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Enteritidis are the species most commonly linked to bone and soft tissue infections [2,4].
Salmonella infections are classically associated with gastroenteritis syndromes caused by ingestion of contaminated food, poultry, milk and egg products [7]. However our patient did not have any clear preceding gastrointestinal complaints similar to other cases reported in the literature [[2], [3], [4]]. The source of his Salmonella bacteremia was not evident to us. However, it is notable that patient had acute abdominal symptoms during the hospitalization requiring cholecystectomy. We postulate he may have had mild gastroenteritis previously unnoticed and may have developed acute acalculous cholecystitis as a complication of this gastroenteritis and bacteremia.
Salmonella can infect any prosthetic joint, however a review of the literature notes that the hip is the most commonly affected joint as was the case in our patient [8,13]. Approximately 45 % of patients with Salmonella PJI had predisposing risk factors such as diabetes mellitus, hemoglobinopathies (sickle cell being a common association), HIV/AIDS or other immunosuppressed state (e.g., chronic corticosteroids use, history of solid organ transplantation, use of TNF-alpha inhibitors), malignancy, malnutrition and alcoholism [[2], [3], [4], [5],7,8,[10], [11], [12],[14], [15], [16]]. Although our patient was not clearly immunosuppressed, he had an alcohol use disorder and was malnourished as evidenced by his cachexia. Hematogenous spread is the most common route of joint seeding, especially in late PJI [2,4]. A rare case of intraoperative inoculation leading to an early joint infection has been described and it was unknown if this patient was a chronic carrier of the organism [2].
Treatment of Salmonella PJI includes antimicrobials and surgery [12,17,18]. Antimicrobials commonly prescribed for these infections include fluoroquinolones or third generation cephalosporins. Other reasonable antimicrobial alternatives include trimethoprim-sulfamethoxazole and ampicillin. However, reduced susceptibility and resistance to fluoroquinolones and third generation cephalosporins are being reported [19]. Surgical treatment depends on the clinical presentation and duration of symptoms in the patient. Acute infections (symptoms less than three weeks) can be treated with debridement and retention of the prosthesis, and chronic infections (> 3 weeks of symptoms) may require one-stage or two-stage revisions arthroplasties. Salmonella spp. are known to form biofilms and this feature likely plays a role in the pathogenesis and impacts management of PJI [8]. Oe et al. reported the success rate of control of infection without removal of the infected implant to be 27–40 % [12]. Our patient did not undergo any surgical intervention in the acute period and presented with treatment failure requiring surgery. The surgeons were unable to perform one or two stage exchange due to his comorbidities, and he had retained hardware necessitating chronic antimicrobial suppression. The risk of failure with retention of prosthesis rises after discontinuation of antimicrobials, but extending the duration of antimicrobial therapy may simply postpone, rather than prevent failure [7].
In conclusion, Salmonella spp. are capable of causing PJI. Although rare, Salmonella spp. should be included in the differential diagnosis when a patient has risk factors such as malignancy, hemoglobinopathies, diabetes mellitus, HIV/AIDS, alcohol dependency or immunosuppressed state, even without significant preceding gastrointestinal symptoms.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Sources of funding
No funding.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
Ethical approval
A case report is a medical/educational activity that does not meet the definition of “research”, which is: "a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge." Therefore, the activity does not have to be reviewed by ethics committee.
Author contribution
Fernando Casado-Castillo: Writing-original draft. Takaaki Kobayashi: Writing- review & editing. Poorani Sekar: Writing - review & editing. Judy Streit: Writing - review & editing. Ilonka Molano De Pena: Writing - review & editing.
Acknowledgements
None.
References
- 1.Beam E., Osmon D. Prosthetic joint infection update. Infect Dis Clin North Am. 2018;32(4):843–859. doi: 10.1016/j.idc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Jeroense K.T., Kuiper J.W., Colen S., Schade R.P., Saouti R. One-stage revision in two cases of Salmonella prosthetic hip infection. World J Clin Cases. 2014;2(7):304–308. doi: 10.12998/wjcc.v2.i7.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Torre B., Tena D., Arias M., O Romanillos. Recurrent prosthetic joint infection due to Salmonella enteritidis: case report and literature review. Eur J Orthop Surg Traumatol. 2012;22(Suppl 1):89–97. doi: 10.1007/s00590-012-0955-6. [DOI] [PubMed] [Google Scholar]
- 4.Sebastian S., Dhawan B., Malhotra R., Gautam D., Kapil A. Salmonella typhimurium infection in total knee arthroplasty: a case report with review of literature. J Lab Physicians. 2017;9(3):217–219. doi: 10.4103/0974-2727.208254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett L., Atkins B. The clinical presentation of prosthetic joint infection. J Antimicrob Chemother. 2014;69(Suppl 1):i25–27. doi: 10.1093/jac/dku250. [DOI] [PubMed] [Google Scholar]
- 6.Dryden M. Prosthetic joint infection: managing infection in a bionic era. J Antimicrob Chemother. 2014;69(Suppl 1):i3–4. doi: 10.1093/jac/dku246. [DOI] [PubMed] [Google Scholar]
- 7.Dojode C.M.R., Hemingway J.S., Damodaran P., Shah N.N. Total hip arthroplasty infection caused by an unusual organism, Salmonella; its successful management and literature review. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2018-224792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajgopal A., Panda I., Gupta A. Unusual Salmonella typhi periprosthetic joint infection involving bilateral knees: management options and literature review. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-221221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A., Berbari E.F., Osmon D.R., Virk A. Prosthetic joint infection due to Salmonella species: a case series. BMC Infect Dis. 2014;14:633. doi: 10.1186/s12879-014-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tassinari A.M., Romaneli M., Pereira R.M., Tresoldi A.T. Septic arthritis caused by Salmonella enterica serotype Rubislaw: a case report. Rev Soc Bras Med Trop. 2019;52:e20180253. doi: 10.1590/0037-8682-0253-2018. [DOI] [PubMed] [Google Scholar]
- 11.McAnearney S., McCall D. Salmonella osteomyelitis. Ulster Med J. 2015;84(3):171–172. [PMC free article] [PubMed] [Google Scholar]
- 12.Oe K., Wada T., Ohno H., Kushida T., Iida H. Salmonella septic arthritis following total knee arthroplasty for rheumatoid arthritis in a patient receiving etanercept. J Orthop Sci. 2011;16(2):258–262. doi: 10.1007/s00776-011-0023-9. [DOI] [PubMed] [Google Scholar]
- 13.Day L.J., Qayyum Q.J., Kauffman C.A. Salmonella prosthetic joint septic arthritis. CMI-ESCMID. 2002;8(7):427–430. doi: 10.1046/j.1469-0691.2002.00466.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi K.M., Park C.S., Song G.W., Lee S.G. Non-typhoid salmonella septic arthritis in dual living liver transplant recipient: a case report. Korean J Hepatobiliary Pancreat Surg. 2014;18(1):29–32. doi: 10.14701/kjhbps.2014.18.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pak S., Pham C. Chronic Salmonella osteomyelitis in a diabetic patient. Cureus. 2017;9(5):e1285. doi: 10.7759/cureus.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould E.S., Gilet A.G., Vigorita V.J. Granulomatous salmonella osteomyelitis associated with anti-tumor necrosis factor therapy in a non-sickle cell patient: a case report. Skeletal Radiol. 2010;39(8):821–825. doi: 10.1007/s00256-010-0894-4. [DOI] [PubMed] [Google Scholar]
- 17.Uygur E., Reddy K., Ozkan F.U., Soylemez S., Aydin O., Senol S. Salmonella enteridis septic arthritis: a report of two cases. Case Rep Infect Dis. 2013;2013:642805. doi: 10.1155/2013/642805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Ss, Perino G., Boettner F., Miller A., Goodman S. Salmonella septic arthritis of the knees in a patient with systemic lupus erythematosus. Lupus. 2013;22(7):740–743. doi: 10.1177/0961203313491022. [DOI] [PubMed] [Google Scholar]
- 19.Humphries R.M., Fang F.C., Aarestrup F.M., Hindler J.A. In vitro susceptibility testing of fluoroquinolone activity against Salmonella: recent changes to CLSI standards. Clin Inf Dis. 2012;55(8):1107–1113. doi: 10.1093/cid/cis600. [DOI] [PubMed] [Google Scholar]