Abstract
Context:
Postoperative quadriceps strength weakness after knee surgery is a persistent issue patients and health care providers encounter.
Objective:
To investigate the effect of neuromuscular electrical stimulation (NMES) parameters on quadriceps strength after knee surgery.
Data Sources:
CINAHL, MEDLINE, SPORTDiscus, and PubMed were systematically searched in December 2018.
Study Selection:
Studies were excluded if they did not assess quadriceps strength or if they failed to report the NMES parameters or quadriceps strength values. Additionally, studies that applied NMES to numerous muscle groups or simultaneously with other modalities/treatments were excluded. Study quality was assessed with the Physiotherapy Evidence Database (PEDro) scale for randomized controlled trials.
Study Design:
Systematic review.
Level of Evidence:
Level 1.
Data Extraction:
Treatment parameters for each NMES treatment was extracted for comparison. Quadriceps strength means and standard deviations were extracted and utilized to calculate Hedge g effect sizes with 95% CIs.
Results:
Eight RCTs were included with an average Physiotherapy Evidence Database scale score of 5 ± 2. Hedge g effect sizes ranged from small (−0.37; 95% CI, −1.00 to 0.25) to large (1.13; 95% CI, 0.49 to 1.77). Based on the Strength of Recommendation Taxonomy Quality of Evidence table, the majority of the studies included were low quality RCTs categorized as level 2: limited quality patient-oriented evidence.
Conclusion:
Because of inconsistent evidence among studies, grade B evidence exists to support the use of NMES to aid in the recovery of quadriceps strength after knee surgery. Based on the parameters utilized by studies demonstrating optimal treatment effects, it is recommended to implement NMES treatment during the first 2 postoperative weeks at a frequency of ≥50 Hz, at maximum tolerable intensity, with a biphasic current, with large electrodes and a duty cycle ratio of 1:2 to 1:3 (2- to 3-second ramp).
Keywords: anterior cruciate ligament, total knee arthroplasty, meniscectomy, quadriceps impairment
Knee pathologies such as anterior cruciate ligament (ACL) tears, meniscal injuries, and chondral injuries are frequently treated with surgical interventions to address symptoms or the overall health of the joint. Subsequent quadriceps weakness and poor limb symmetry indices are common consequences after knee surgery and have been observed to persist for years after surgery.45,55,68 Furthermore, relationships between quadriceps strength and functional performance, such as gait, have been reported.37 Subsequently, long-term consequences, such as osteoarthritis, are a concern for patients with quadriceps strength deficits.26,39,50 Addressing postoperative quadriceps weakness is advantageous for positive short- and long-term patient outcomes.
Neuromuscular electrical stimulation (NMES) is one therapeutic modality utilized to improve postoperative quadriceps weakness. Neuromuscular electrical stimulation has demonstrated mixed success as a clinical modality for facilitating strength restoration. After ACL reconstruction specifically, 4- to 6-week bouts of NMES have been observed to assist in the recovery of quadriceps strength and functional performance as compared with rehabilitation without NMES.72,74 However, other ACL-related studies have reported no advantage in postoperative strength from NMES interventions implemented within similar time frames.35,57 Each of the above studies employed varying treatment parameters likely influencing the outcomes.
Throughout the literature, the NMES parameters reported for treatments vastly differ between studies, possibly due to the sizable amount of NMES parameters available for customization to clinicians. The inconsistency among the parameters has been theorized to contribute to the variable therapeutic effect of NMES on postoperative quadriceps strength previously described.4,27 Identifying the most effective NMES parameters for recovering quadriceps strength after surgery is essential for optimizing treatment effectiveness. Therefore, the purpose of this review was to investigate the most effective NMES parameters for targeting postoperative quadriceps weakness. The specific parameters investigated included the following: intensity, electrode size, frequency, initiation of treatment, waveform/current, pulse duration, duty cycle, ramp time, knee angle, active or passive muscle contraction, and treatment volume.
Methods
Searches were performed in December 2018 using the following electronic databases: PubMed, CINAHL, MEDLINE, and SPORTDiscus. Key terms were searched utilizing the search strategy presented in Table 1 and then reviewed for inclusion as outlined in Figure 1. Specifically, search results were exported to an electronic spreadsheet where duplicate references were deleted. The titles of all remaining articles were reviewed to determine study inclusion. If the title alone was not sufficient to determine study eligibility, the abstract was reviewed. The article was retrieved and reviewed in its entirety if a decision regarding inclusion or exclusion was unable to be made from the abstract. Last, a manual search by hand was performed from the references of the final articles included in the study to identify any additional articles.
Table 1.
Systematic search strategy and results
| Results | |||
|---|---|---|---|
| Search Terms | Ebsco Host (1979-2018) CINAHL with Full Text, SPORTDiscus, MEDLINE |
PubMed (1966-2018) | |
| #1 | Neuromuscular electrical stimulation | 2693 | 7491 |
| #2 | Electrical stimulation | 63,599 | 179,630 |
| #3 | Clinic* electrical stimulation | 749 | 25,650 |
| #4 | Home-based electrical stimulation | 72 | 125 |
| #5 | Battery-operated electrical stimulation | 6 | 49 |
| #6 | Portable electrical stimulation | 34 | 312 |
| #7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 63,601 | 179,630 |
| #8 | Anterior cruciate ligament | 39,391 | 19,946 |
| #9 | ACL | 28,166 | 23,769 |
| #10 | Anterior cruciate ligament reconstruction | 18,005 | 10,906 |
| #11 | Anterior cruciate ligament revision | 659 | 893 |
| #12 | Anterior cruciate ligament repair | 941 | 2282 |
| #13 | Anterior cruciate ligament surgery | 6946 | 15,143 |
| #14 | Total knee arthroplasty | 28,811 | 28,707 |
| #15 | Meniscectomy | 4680 | 2724 |
| #16 | Meniscal transplant | 84 | 1121 |
| #17 | Meniscal repair | 1367 | 2116 |
| #18 | Knee | 261,887 | 155,379 |
| #19 | Knee injury | 37,547 | 40,473 |
| #20 | Knee surgery | 30,817 | 73,969 |
| #21 | #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 | 278,456 | 163,687 |
| #22 | Rehabilitation | 615,335 | 568,700 |
| #23 | Therapy | 6,170,174 | 8,931,528 |
| #24 | #22 OR #23 | 6,582,635 | 9,020,021 |
| #25 | Muscle strength | 77,615 | 60,573 |
| #26 | Muscle weakness | 29,257 | 41,915 |
| #27 | Quadriceps weakness | 978 | 1364 |
| #28 | Quadriceps strength | 5222 | 5051 |
| #29 | #25 OR #26 OR #27 OR #28 | 103,796 | 97,986 |
| #30 | #7 AND #21 | 2391 | 2148 |
| #31 | #24 AND #30 | 1477 | 1342 |
| #32 | #29 AND #31 | 376 | 310 |
Figure 1.
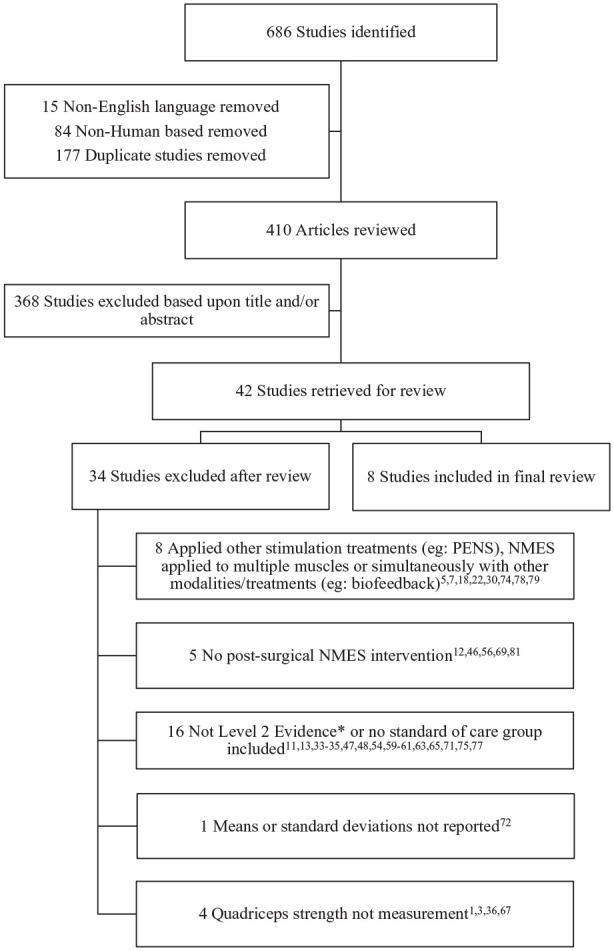
Study selection flowchart for all studies returned in the search. *Based on the Oxford Center for Evidence-Based Medicine (CEBM) 2011. NMES, neuromuscular electrical stimulation; PENS, patterned electrical neuromuscular stimulation.
Selection Criteria
Article inclusion and exclusion criteria were as follows: studies examining NMES treatment benefits classified as a level 2, randomized controlled trials (per The Oxford Center for Evidence-Based Medicine [CEBM] 2011 Levels of Evidence) were included in this review.51 The CEBM hierarchy ranges from 1 to 5, where a level 5 represents a low level of evidence and a level 1 represents the best level of evidence.51 English-language and human-based articles reporting randomized controlled trials that measured volitional postoperative quadriceps strength, included a postoperative standard-of-care control group for comparison, and who reported the NMES parameters utilized were eligible for review. Volitional quadriceps strength could be measured through isometric or isokinetic testing. Quadriceps strength means and standard deviations were required to be reported. The standard-of-care control group did not receive any form of a NMES treatment and instead performed postoperative voluntary quadriceps muscle contractions. Studies were excluded if they did not apply an NMES treatment or measure volitional quadriceps strength, applied NMES to other muscles in addition to the quadriceps, applied NMES simultaneously with other modalities/treatments, and/or did not report means and standard deviations. These exclusions were chosen to isolate the effect of an NMES treatment applied directly to the quadriceps on postoperative quadriceps strength. In the instance authors reported adjusted means and standard deviation, the authors were contacted to request the unadjusted means and standard deviation to allow the authors to calculate effect size values.
Assessment of Methodological Quality
Two independent reviewers assessed each articles’ eligibility and the quality of evidence. The assessment of the methodological quality was performed utilizing the Physiotherapy Evidence Database (PEDro) scale.42 The PEDro scale consists of 11 questions; however, only questions 2 to 11, a total of 10 questions, are utilized for the total score calculation. Therefore, the PEDro is a 10-point scale with a high score (10) reflecting a high-quality study. A study with a score greater than or equal to 6 was considered to be of moderate to high quality.58 Once each reviewer had completed independent assessment of the articles, they met to discuss any disagreements in score. If there was a disagreement between the 2 reviewers, a third reviewer would assess the quality of evidence for the point of disagreement. There were no disagreements between the 2 independent reviewers.
Strength of Recommendation
Strength of recommendation was assessed utilizing the Strength of Recommendation Taxonomy (SORT).15 The strength of recommendation is evaluated with grades A, B, and C.15 According to the taxonomy, a C is a recommendation founded on case series, consensus, disease-oriented evidence, or expert opinion.15 A B recommendation is given when there is inconsistent or limited quality patient-oriented evidence.15 Last, a recommendation strength of an A is given to consistent good-quality patient-oriented evidence.15
Data Extraction
All data were extracted by the primary author. The intervention parameters, administration instructions, and quadriceps strength measures (isometric or isokinetic) were extracted from each study and input into a standardized electronic spreadsheet. The NMES treatment intervention parameters extracted consisted of intensity, electrode size, frequency, initiation of treatment, waveform/current, pulse duration, duty cycle, ramp time, knee angle active or passive muscle contraction, and treatment volume. Subsequently, quadriceps strength means and standard deviations at pre- and posttreatment were extracted. All articles presented group means and standard deviations in text except for 2 articles.16,38 One article38 presented means and standard deviations in a graph. The means and standard deviations were extracted from the graph by hand utilizing a digital caliper (Mitutoyo).49 The other article16 reported baseline-adjusted means and standard deviations. The posttreatment means and standard deviations were obtained from the article’s authors.16
Data Analysis
A variable of total active treatment time was calculated. The number of repetitions (on portion of the duty cycle) performed in a total duty cycle was computed. This amount was then multiplied by the length of time the contraction was performed to obtain the total time active contractions occurred in a single treatment session. This was then multiplied by the total treatment volume for a total active treatment time over the entire prescribed treatment.
Between group quadriceps strength effect sizes were the primary outcome of interest. Hedges g effect size (g) and 95% CIs were calculated using all available data to determine the effect of the treatment on quadriceps strength. Effect size calculations were interpreted as small 0.2, moderate 0.5, and large 0.8.6 Statistically significant treatment effects occurred if the posttreatment confidence interval did not contain zero. An overall effect size was calculated using Comprehensive Meta-Analysis software (Version 3.3.070; Biostat). In a situation where an article had multiple time points or multiple strength assessment, the highest effect size was utilized for the overall effect size calculation. A sensitivity analysis was conducted by calculating an effect size with the lowest effect sizes if an article had multiple time points.
Results
The search strategy resulted in a total of 686 articles from the specified databases (Table 1). A total of 99 non–English language and nonhuman-based studies were excluded through search filters. A total of 177 duplicate articles were excluded and the titles for the remaining 410 articles were reviewed. After reviewing titles and abstracts, an additional 368 articles were excluded. From the remaining 42 articles, a total of 8 studies were included for review16,17,38,70,76,82-84 and the remaining 34 studies were excluded1,3,5,7,11-13,18,22,30,33-36,46-48,54,56,59-61,63,65,67,69,71,72,74,75,77-79,81 (Figure 1).
The PEDro scores for the 8 articles ranged from 2 to 7 with an average of 5 (Table 2). All studies lacked 1 or more aspects of blinding, for no study blinded the participants or the treatment administrators (criteria 5 and 6). Additionally, a majority of studies failed to conceal group allocation (criteria 3), blind the assessor of the key outcome (criteria 7), or include or report a baseline group assessment (criteria 4). Examining criterion 4, 5 studies did not provide baseline group assessments.17,38,70,82,83 Two of the 5 studies17,70 did not include any baseline information while 3 of the 5 studies38,82,83 reported baseline values but did not report a statistical comparison between groups.
Table 2.
Physiotherapy Evidence Database scale (PEDro) Methodological Quality Assessment Scores for each included article a
| 1 b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feil et al16 | × | × | – | × | – | – | × | – | × | × | × | 6/10 |
| Fitzgerald et al17 | × | × | – | – | – | – | × | × | × | × | × | 6/10 |
| Lieber et al38 | × | × | – | – | – | – | – | × | × | × | × | 5/10 |
| Sisk et al70 | × | × | – | – | – | – | – | × | – | × | × | 4/10 |
| Stevens-Lapsley76 | × | × | × | × | – | – | – | × | × | × | × | 7/10 |
| Wigerstad-Lossing et al82 | × | × | – | – | – | – | – | × | × | × | × | 5/10 |
| Williams et al83 | × | × | – | – | – | – | – | – | – | – | × | 2/10 |
| Yoshida et al84 | × | × | × | × | – | – | × | × | – | × | × | 7/10 |
“×” denotes criterion was satisfied, “–” denotes criterion was not satisfied.
Question 1 is not included in the score total.
Study Characteristics
Individual study characteristics are presented in Table 3. In 5 articles,16,17,38,70,82 ACL reconstruction patients were treated, in 2 articles,76,84 total knee arthroplasty patients were treated, and in 1 article,83 meniscectomy patients were treated. Quadriceps strength was measured through isometric testing via a dynamometer at various knee angles,17,38,70,76,82,84 including 30°, 60°, 75°, and/or 90° and with isokinetic testing16,83 at speeds of 90, 120, 180, 240, and/or 300 deg/s.
Table 3.
Study demographic characteristics for each included article a
| NMES, n | Age, b y | Control, n | Age, y | Procedure | Strength Measurement | |
|---|---|---|---|---|---|---|
| Feil et al16 | ||||||
| G1 | 42 c | 31.1 ± 1.52 | 44 | 31.6 ± 1.36 | ACL | Isokinetic 90 deg/s, 180 deg/s (N·m/kg) |
| G2 | 45 d | 34.8 ± 1.49 | ||||
| Fitzgerald et al17 | 21 | 29.2 ± 10.1 | 22 | 31.9 ± 10.9 | ACL | Isometric index at 60° |
| Lieber et al38 | 20 | 28.0 ± 8.2 | 20 | 27.3 + 8.5 | ACL | Isometric at 90° (N·m) |
| Sisk et al70 | 11 | 23.4 ± 7.5 | 11 | 23.9 ± 9.2 | ACL | Isometric at average of 75° (N·m/kg) |
| Stevens-Lapsley76 | 35 | 66.2 ± 9.1 | 31 | 64.8 ± 7.7 | TKA | Isometric at 60° (N·m/kg) |
| Wigerstad-Lossing et al82 | 13 | 28 (21-45) | 10 | 26 (21-33) | ACL | Isometric at 30° (N·m) |
| Williams et al83 | 13 | 32.8 ± 7.9 | 8 | 32.9 ± 7.7 | Menis-cectomy | Isokinetic 120 deg/s, 180 deg/s, 240 deg/s, 300 deg/s (ft·lb) |
| Yoshida et al84 | ||||||
| G1 | 22e | 75.9 ± 4.7 | 22 | 72.6 ± 6.2 | TKA | Isometric at 90° (kgf/kg) |
| G2 | 22 f | 71.6 ± 7.0 |
ACL, anterior cruciate ligament reconstruction; NMES, neuromuscular electrical stimulation; TKA, total knee arthroplasty.
All comparisons were between NMES and control groups receiving standard of care, except Feil et al16 and Yoshida et al,84 which had multiple treatment groups compared with a control group.
Age presented as means ± SDs with the exception of Wigerstad-Lossing et al,82 which reported median and range.
Feil et al16 group 1 (G1): Kneehab NMES.
Feil et al16 group 2 (G2): Ploystim NMES.
Yoshida et al84 group 1 (G1): motor-level NMES.
Yoshida et al84 group 2 (G2): sensory-level NMES.
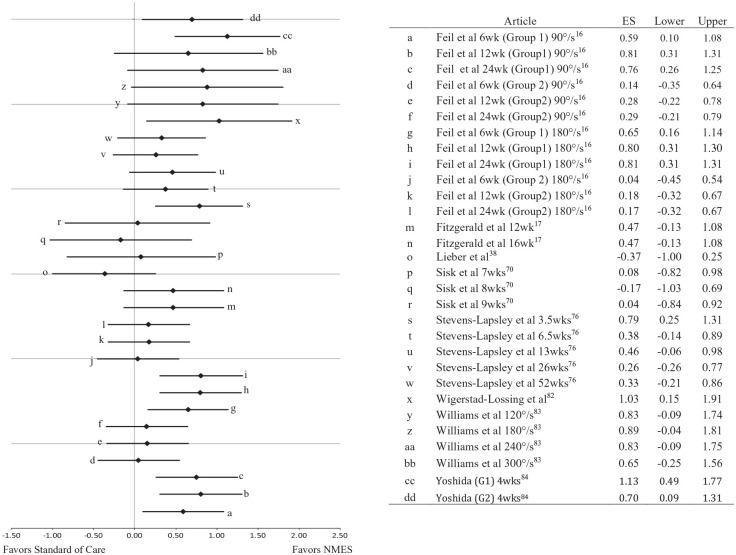
Postintervention quadriceps strength measures were reported to statistically improve in 5 of the 8 studies.16,17,76,82,84 Between group effect sizes calculated for each time point tested and each strength assessment reported in the 8 articles resulted in effect sizes ranging from −0.37 to 1.13 (Figure 2).16,17,38,70,76,82-84 Moderate to large effect sizes with confidence intervals that did not cross zero were found in 4 studies.16,76,82,84 The posttreatment confidence intervals for a portion of the calculated effect sizes did cross zero; however, overall there was a favorable trend for the effect of NMES on postoperative quadriceps strength when compared with the standard of care treatment. The overall NMES effect was 0.55 (0.31, 0.75). An effect size of 0.41 (0.21, 0.60) was found in the sensitivity analysis.
Figure 2.
Hedge g effect sizes and 95% CIs for the effect of the neuromuscular electrical stimulation (NMES) treatment on postoperative quadriceps strength. Every time point at which postoperative quadriceps strength was measured is presented. All comparisons were between NMES and control groups. Feil et al16 G1, group 1 (Kneehab); G2, group 2 (Polystim) and Yoshida e al84 G1, motor-level NMES; G2, sensory-level NMES.
Treatment Parameters
The NMES administration setup and treatment parameters can be found in Tables 4 and 5, respectively. There was a high degree of variability in both the amount of NMES treatment parameters reported and the specific parameter settings utilized. Regarding the amount of NMES treatment parameters reported, 4 studies16,38,83,84 failed to report at least 2 parameters. The parameters commonly absent were current type, waveform shape, and electrode size. Only 4 studies17,70,76,82 reported all the NMES treatment parameters included in this review.
Table 4.
Neuromuscular electrical stimulation (NMES) treatment administration and setup parameters utilized in the included articles a
| Treatment Volume | Total Active Treatment Time, min | NMES | Time Initiated Postoperatively | Muscle Contraction | Knee Angle | |
|---|---|---|---|---|---|---|
| Feil et al16 | ||||||
| G1 b | 12 wk (20 min 3×/d, 5 d/wk) | 1200 | NMES (battery) | 3rd to 4th day | Active | Full extension |
| G2c | 12 wk (20 min 3×/d, 5 d/wk) | 1200 | Polystim (battery) | 3rd to 4th day | Active | Full extension |
| Fitzgerald et al17 | 11 wk (11-12 min/d, 2 d/wk) | 40 min 19 s to 44 min | NMES (AC power) | Average 12 d | Passive | Full extension |
| Lieber et al38 | 4 wk (30 min/d, 5 d/wk) | 200 | NMES (AC power) | 2-6 wk | — | — |
| Sisk et al70 | 6 wk (8 h/d, 7 d/wk) | 5040 | NMES (battery) | 2nd day | Active or passive | Week 1-4: 45°-50° of flexion Week 5-6: 45°-90° of flexion |
| Stevens-Lapsley et al76 | 6 wk (15 min 2×/d, 7 d/wk) | 157 min 30 s | NMES (battery) | 2nd day | Passive | 60° of flexion |
| Wigerstad-Lossing et al82 | 6 wk (40 min/d, 3d /wk) | 270 | NMES (battery) | 2nd day | Active | 20°-30° of flexion |
| Williams et al83 | 3 wk (10 min/d, 5 d/wk) | 34 min 36 s | NMES (AC power) | Average 31 d | — | 65° of flexion |
| Yoshida et al84 | ||||||
| G1 d | 2 wk (30 min/d, 5 d/wk) | 100 | NMES (unknown) | 2 wk | Passive | — |
| G2 e | 2 wk(45 min/d, 5 d/wk) | 2700 | NMES (unknown) | 2 wk | Passive | — |
Table 5.
Neuromuscular electrical stimulation (NMES) treatment parameters utilized in each included article a
| Frequency | Duty Cycle, s (on/off) | Ramp Time | Intensity | Pulse Duration | Waveform/Current | Electrode Size | |
|---|---|---|---|---|---|---|---|
| Feil et al16 | |||||||
| G1 b | 50 | 5/10 | 2 s/1 s down | — | 300-400 μs | — | G1
b
: 10 × 20 cm, 3 × 18 cm, 10 × 7.5 cm, 7 × 14 cm |
| G2 c | 50 | 10/20 | 1.5 s/1 s down | — | — | — | G2c: 4 × 70 mm round |
| Fitzgerald et al17 | 75 | 10/50 | 2 s up | MT | N/A | 2.5 kHz triangular alternating burst | 6.98 × 12.7 cm |
| Lieber et al38 | 50 | 10/20 | 2 s up | MT unadjusted d | 250 μs | Asymmetrical balanced | — |
| Sisk et al70 | 40 | 10/30 | 0.5 s up | MT | 300 μs | Rectangular waveform | 5 × 10 cm |
| Stevens-Lapsley et al76 | 50 | 15/45 | 3 s up | MT | 250 μs | Symmetrical biphasic | 7.6 × 12.7 cm |
| Wigerstad-Lossing et al82 | 30 | 6/10 | 2 s up | MT (65-100 mA) | 300 μs | Rectangular asymmetrical balanced biphasic | 4 × 10 cm |
| Williams et al83 | 50 | 15/50 | 3.5 s up | MT | N/A | 2.5 kHz sinusoidal alternating | — |
| Yoshida et al84 | |||||||
| G1 f | 100 | 10/20 | — | MT e | 1 ms | Symmetrical biphasic | 5 × 9 cm |
| G2 g | 100 | Continuous | — | Sensory-level | 1 ms | Symmetrical biphasic | 5 × 9 cm |
MT, maximal tolerance; N/A, not applicable.
“—” indicates information not provided.
Feil et al16: group 1 (G1): Kneehab NMES.
Feil et al16: group 2 (G2): Polystim NMES.
Required to produce visible muscle contraction.
Intensity level was set to maximum toleration during the first treatment setup. This level was then utilized throughout the treatment sessions.
Yoshida et al84: group 1 (G1): motor-level NMES.
Yoshida et al84: group 2 (G2): sensory-level NMES.
The varying parameters reported and utilized in each article prevented a meta-analysis from being conducted. Despite the variability, a narrative synthesizing of the parameters from the included studies was conducted. The majority of studies prescribed the intensity to be at a level of maximal tolerance. All but 2 studies16,38 instructed the patients to continually increase the intensity level over time. One study84 specifically excluded patients (n = 3) who were unable to tolerate an intensity that produced a visible muscle contraction. A 2-electrode placement generally was implemented, with the exception of Feil et al,16 who utilized a 4-electrode placement in 1 intervention group (group 1). Predominantly, a frequency of 50 Hz was utilized.16,38,76,83 When reported, a pulse duration of 250 to 300 μs was most common.16,38,70,76,82 The NMES treatment was commonly implemented during the first16,70,76,82 and second17,84 postoperative weeks. Duty cycle on/off times were inconsistent between studies. When duty cycle times were expressed as a ratio, contraction/relaxation ratios of 1:216,38,82,84 and 1:370,76,83 were most frequently applied.
Discussion
Regaining quadriceps strength after surgery is a paramount goal during rehabilitation, for quadriceps weakness has been found to increase joint loading44 and contribute to the development of osteoarthritis.26 In this review, the effect sizes for NMES on postoperative quadriceps strength, when compared with a control group, ranged from small (−0.37) to large (1.13). While these results align with other reviews supporting NMES8,27,28 as a positive postoperative treatment directed at regaining quadriceps strength, little focus has been placed on the most effective parameter settings.
This review sought to ascertain the most effective NMES parameters for recovering postoperative quadriceps strength. Evaluation of all the included articles revealed large variations in the parameters selected for the NMES treatments. In the studies with positive effect sizes, some similarities were observed regarding intensity, electrode size, frequency, treatment initiation time, and current of the NMES treatment.16,76,82,84 Among those studies reporting positive effects, NMES treatment was consistently implemented during the first 2 postoperative weeks16,76,82,84 at an intensity level of maximum toleration76,82,84 with a biphasic current using electrodes ≥40 cm2. Furthermore, Feil et al16 and Stevens-Lapsley et al76 both used at least 2 large electrodes (>96 cm2) at a frequency of 50 Hz and prescribed NMES multiple times per day. Of the remaining parameters, there were several inconsistencies among the 4 studies; thus, a definitive consensus about the effects of each parameter on quadriceps strength was not possible. However, a summary of the similarities among the available parameters was compiled to provide a recommendation of the optimal parameter selections for recovering quadriceps strength after surgery. All these parameters will be discussed in further detail.
Intensity (current amplitude) is emerging as a critical parameter for regaining quadriceps strength.28,77 The studies with large treatment effects76,82,84 identified in this review prescribed intensity at a level of maximal tolerance with an emphasis on progressive intensity escalation.76,82,84 Specifically, Yoshida et al,84 who had the largest treatment effect, required the participants to maintain an intensity level that produced a visible muscle contraction during the entire treatment for inclusion in the study. These observations are consistent with other literature reporting a linear relationship between the level of intensity during an NMES treatment and the quadriceps strength.43,73,77 The only study to specifically report that intensity level was unadjusted, not increased within or across treatment sessions, and did not find a statistical difference between groups.38 Therefore, to maximize motor unit recruitment and achieve a forceful, sustained muscle contraction, a high intensity level should be applied and progressively increased throughout treatment.
Undoubtedly, intensity is one of the more difficult parameters to control due to the limiting factor of patients’ perceived comfort.66 Patients also generally have control of this parameter during an NMES treatment; thus, if the patient experiences too much discomfort during the treatment, the patient may refrain from increasing the intensity or even decrease the intensity. In addition to educating patients on the anticipated treatment discomfort and the effects of accommodation and habituation,40,52,64 one strategy to address patient discomfort is the use of large electrodes to decrease current density.2,31,66 All studies demonstrating a large posttreatment effect utilized electrodes ≥40 cm2 in size.16,76,82,84 It is recommended to use large electrodes and to routinely encourage the patient to increase the intensity both within and between treatment sessions in order to maximize the current intensity a patient can tolerate during NMES.
Another parameter of consensus for those studies showing a large treatment effect was the use of a frequency high enough to achieve a sustained tetanic contraction. Three of the studies16,76,84 with a positive treatment effect implemented a frequency ≥50 Hz while the other study82 utilized a frequency of 30 Hz. Examining the recommendations within the literature for recovering muscle strength, clinicians have been advised to utilize a frequency around 50 Hz in order to minimize excessive fatigue.41,66 Furthermore, the contraction produced by the higher frequencies (50 and 100 Hz) is reported to be smoother,66 more comfortable,29 and resulting in increased muscular force production.14 All the frequencies reported in this review are supported based on the property of summation, where a frequency >30 Hz is necessary to sustain a tetanic contraction.66
All the NMES treatments within the studies with large treatment effects were implemented within the first 2 postoperative weeks.16,76,82,84 It is theorized that the early positive effect of NMES on regaining quadriceps strength is attributed to characteristics immediately after surgery, such as muscle activation failure and neuroplastic changes at the cortical level, that impair the ability to generate a muscle contraction after surgery.62,80 The external stimulation generated by an NMES treatment is believed to assist the muscle in achieving a full contraction when activation failure is present.55,80 Thus, it is recommended to implement the NMES treatment as early as feasible during the first 2 postoperative weeks.
Current type and waveform shape are also likely to influence NMES effectiveness. The results in this study support the use of a biphasic current. Three76,82,84 of the 4 studies with positive treatment effects utilized a biphasic current, while the remaining study with positive effect sizes16 did not report the type of current generated by the investigated devices. Previous research regarding current has been inconclusive, with both a biphasic current and an alternating current (typically “Russian” current) being supported for quadriceps recovery.8,32 Less information is available for the waveform shape. Only 1 study with a positive treatment effect reported the waveform shape, rectangular.82 While there is minimal research documenting the effect of waveform shape on regaining quadriceps strength, the shape of the waveform does appear to have an impact on an individual patient’s comfort level.10 Furthermore, the preferred waveform varies between indviduals.10 Applying a biphasic current with a waveform shape individualized to the patient’s perceived comfort is recommended for an NMES treatment.
In our review, the treatment protocols with positive effects implemented longer pulse durations (250 μs, 300 μs, 400 μs, and 1 ms).16,17,38,70,76,82,83 A long pulse duration is favored to achieve a greater quadriceps torque.19,21 A torque-duration curve across pulse durations of 100 to 600 illustrates the curvilinear nature between both variables, with torque increasing with the rise in the pulse duration.21 It has been reported that a larger area of the muscle is stimulated when using a longer pulse duration (450 μs) compared with a short pulse duration (150 μs).20 This review supports the use of long pulse durations.
All studies with a large positive effect implemented a duty cycle ratio of 1:216,82,84 or 1:376 with a ramp time of 2 to 3 seconds. It has been reported that the shorter the rest (off) time applied, the greater the level of muscle fatigue experienced23,53; although little information is available on the effect of specific duty cycle times on regaining strength. The ramp time does not appear to have an effect on strength values but rather patient comfort. Increasing ramp time results in a gradual increase in stimulus rather than abruptly administering a strong stimulus.66 Based on patient endurance and comfort, clinicians may consider a duty cycle between 1:2 and 1:3 with a ramp time of 2 to 3 seconds.
Knee flexion angles ranged from 0° to 60° in the studies with positive effects. An angle of 60° has been shown to produce the largest voluntary knee extension torque during an exercise.9 However, not all patients can achieve a flexed position immediately after surgery and require position modifications. Thus, clinicians may wish to consider a patient position close to 60° of knee flexion but can consider full knee extension if medically necessary.
Lastly, less information is known about the remaining parameters. Unfortunately, there was insufficient evidence to reach a consensus regarding NMES treatment volume and the long-term effect of the NMES treatment. Treatment sessions ranged from 15 to 40 minutes per session 1 to 3 times per day for 2 to 12 weeks in the studies reviewed with positive effects.16,17,70,76 Similarly, there was no consensus if adding a voluntary contraction with the stimulus was advantageous. The studies with positive treatment effects were split with 2 studies16,82 of the 4 studies16,76,82,84 implementing active contractions during the treatment. Based on neuroplasticity principles, the act of performing a volitional contraction during NMES stimulation may be beneficial for the quadriceps muscle. Introducing a new activity and placing attention on the given task, such as contracting the quadriceps, can increase the motor maps within the cortex.24,25 The development of this additional motor pattern may assist the participant after the stimulation treatment is discontinued. While these theories are promising, similar to the treatment volume parameter, the results of this review are inconclusive on the inclusion of a voluntary muscular contraction in an NMES treatment.
There are a few potential explanations for the dissimilarities between the reviewed articles or patient groups with small effect sizes and those with larger effect sizes. To start, the intensity level was either only at a sensory level for a group,84 not reported for a group,16 or never adjusted during the treatment session.38 In addition to not changing the intensity level, the control group in Lieber et al38 was exercised at torque levels that progressively increased to match what would be elicited in the stimulation group, potentially diluting differences between groups. The patients in Sisk et al70 were instructed to set the intensity during treatment to a level that produced a palpable contraction, but at a maximum comfortable intensity level for 8 hours a day, 7 days a week. Given the extensive treatment duration and that the patients controlled the intensity, it is unknown for how much time the patients utilized a maximum strong intensity level. Both Williams et al83 and Fitzgerald et al17 had a smaller total time actively in contraction, ranging from 34 to 40 minutes total, compared with the other studies with positive effects that were over 150 minutes total. Additionally, Williams et al83 and Lieber et al38 had a late average start time, respectively, averaging 31 days postoperatively and between 2 and 6 weeks postoperatively. Last, the treatment effect was large initially for Steven-Lapsley et al76 but gradually diminished over time, which may be explained by the change in the rehabilitation setting. The treatment was initiated and continued inpatient for the first 3 postoperative days before transitioning into the home setting where the patients conducted the treatment for the remaining duration of the treatment program. Furthermore, once transitioned to the home setting, if there was concern about a patient’s utilization of the treatment, a research physical therapist visited the patient during the first week postdischarge. The variations in intensity level, time actively in contraction, treatment initiation time, and treatment setting implemented in the studies outlined in this paragraph may have contributed to some of the differences in the reported effectiveness.
Limitations
The number of studies that met inclusion criteria was small, limiting the amount of data available for comparison in the review. Additionally, in some circumstances, parameters were not consistently reported or highly varied between studies. This resulted in a reduction in the number of studies available for synthesis and prohibited a meta-analysis from being performed. Lastly, the majority of the studies reviewed did not monitor treatment adherence or include a compliance diary. Thus, it is difficult to know if the lack of statistical differences between the groups is due to the parameters selected or adherence to the prescribed treatment.
Conclusion
There is SORT level B evidence to support NMES for improving postoperative quadriceps strength. Based on the parameters for which a consensus was observed in those studies demonstrating a large treatment effect, clinicians are encouraged to utilize large electrodes (≥40 cm2) to deliver a biphasic current with the waveform individualized to the patient’s comfort level or an alternating current. The recommended setup parameters are a frequency of 50 Hz or greater with a long pulse duration(250 μs to 1 ms) accompanied by a duty cycle ratio between 1:2 and 1:3 that includes a ramp time of 2 to 3 seconds for patient comfort. The intensity of the stimulation treatment ought to be set at the patient’s maximal tolerance level and continually increased. Last, the patient’s position can range from full extension to 60° of flexion; however, it is advised to position the patients as close to 60° of flexion as medically safe.
Footnotes
The following authors declared potential conflicts of interest: C.E.W.C.’s institution received financial support from International Cartilage Regeneration and Joint Preservation Society Patient Registry. C.L. is a paid consultant for Joint Restoration Foundation, Samumed, and Vericel.
References
- 1. Akkaya N, Ardic F, Ozgen M, Akkaya S, Sahin F, Kilic A. Efficacy of electromyographic biofeedback and electrical stimulation following arthroscopic partial meniscectomy: a randomized controlled trial. Clin Rehabil. 2012;26:224-236. [DOI] [PubMed] [Google Scholar]
- 2. Alon G, Kantor G, Ho HS. Effects of electrode size on basic excitatory responses and on selected stimulus parameters. J Orthop Sports Phys Ther. 1994;20:29-35. [DOI] [PubMed] [Google Scholar]
- 3. Avramidis K, Karachalios T, Popotonasios K, Sacorafas D, Papathanasiades AA, Malizos KN. Does electric stimulation of the vastus medialis muscle influence rehabilitation after total knee replacement? Orthopedics. 2011;34:175. [DOI] [PubMed] [Google Scholar]
- 4. Bax L, Staes F, Verhagen A. Does neuromuscular electrical stimulation strengthen the quadriceps femoris? Sports Med. 2005;35:191-212. [DOI] [PubMed] [Google Scholar]
- 5. Boucher A, Wang S, Trudelle-Jackson E, Olson S. Effectiveness of surface electromyographic biofeedback-triggered neuromuscular electrical stimulation on knee rehabilitation. N Am J Sports Phys Ther. 2009;4:100-109. [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Earlbaum; 1988. [Google Scholar]
- 7. Currier D, Ray J, Nyland J, Rooney J, Noteboom J, Kellogg R. Effects of electrical and electromagnetic stimulation after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1993;17:177-184. [DOI] [PubMed] [Google Scholar]
- 8. da Silva VZM, Durigan JLQ, Arena R, de Noronha M, Gurney B, Cipriano G., Jr. Current evidence demonstrates similar effects of kilohertz-frequency and low-frequency current on quadriceps evoked torque and discomfort in healthy individuals: a systematic review with meta-analysis. Physiother Theory Pract. 2015;31:533-539. [DOI] [PubMed] [Google Scholar]
- 9. De Ruiter C, Kooistra R, Paalman M, De Haan A. Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol. 2004;97:1693-1701. [DOI] [PubMed] [Google Scholar]
- 10. Delitto A, Rose SJ. Comparative comfort of three waveforms used in electrically eliciting quadriceps femoris muscle contractions. Phys Ther. 1986;66:1704-1707. [DOI] [PubMed] [Google Scholar]
- 11. Delitto A, Rose SJ, McKowen JM, Lehman RC, Thomas JA, Shively RA. Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate ligament surgery. Phys Ther. 1988;68:660-663. [DOI] [PubMed] [Google Scholar]
- 12. Dirks ML, Wall BT, Snijders T, Ottenbros CLP, Verdijk LB, van Loon LJC. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol (Oxf). 2014;210:628-641. [DOI] [PubMed] [Google Scholar]
- 13. Draper V, Ballard L. Electrical stimulation versus electromyographic biofeedback in the recovery of quadriceps femoris muscle function following anterior cruciate ligament surgery. Phys Ther. 1991;71:455-461. [DOI] [PubMed] [Google Scholar]
- 14. Dreibati B, Lavet C, Pinti A, Poumarat G. Influence of electrical stimulation frequency on skeletal muscle force and fatigue. Ann Phys Rehabil Med. 2010;53:266-277. [DOI] [PubMed] [Google Scholar]
- 15. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract. 2004;17:59-67. [DOI] [PubMed] [Google Scholar]
- 16. Feil S, Newell J, Minogue C, Paessler HH. The effectiveness of supplementing a standard rehabilitation program with superimposed neuromuscular electrical stimulation after anterior cruciate ligament reconstruction: a prospective, randomized, single-blind study. Am J Sports Med. 2011;39:1238-1247. [DOI] [PubMed] [Google Scholar]
- 17. Fitzgerald G, Piva SR, Irrgang JJ. A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2003;33:492-501. [DOI] [PubMed] [Google Scholar]
- 18. Glaviano NR, Langston WT, Hart JM, Saliba S. Influence of patterned electrical neuromuscular stimulation on quadriceps activation in individuals with knee joint injury. Int J Sports Phys Ther. 2014;9:915-923. [PMC free article] [PubMed] [Google Scholar]
- 19. Gorgey AS, Dudley GA. The role of pulse duration and stimulation duration in maximizing the normalized torque during neuromuscular electrical stimulation.J Orthop Sports Phys Ther. 2008;38:508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gorgey AS, Mahoney E, Kendall T, Dudley GA. Effects of neuromuscular electrical stimulation parameters on specific tension. Eur J Appl Physiol. 2006;97:737-744. [DOI] [PubMed] [Google Scholar]
- 21. Gregory CM, Dixon W, Bickel CS. Impact of varying pulse frequency and duration on muscle torque production and fatigue. Muscle Nerve. 2007;35:504-509. [DOI] [PubMed] [Google Scholar]
- 22. Hasegawa S, Kobayashi M, Arai R, Tamaki A, Nakamura T, Moritani T. Effect of early implementation of electrical muscle stimulation to prevent muscle atrophy and weakness in patients after anterior cruciate ligament reconstruction.J Electromyogr Kinesiol. 2011;21:622-630. [DOI] [PubMed] [Google Scholar]
- 23. Holcomb WR, Rubley MD, Miller MG, Girouard TJ. The effect of rest intervals on knee-extension torque production with neuromuscular electrical stimulation.J Sport Rehabil. 2006;15:116-124. [Google Scholar]
- 24. Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82-104. [DOI] [PubMed] [Google Scholar]
- 25. Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15:94-101. [DOI] [PubMed] [Google Scholar]
- 26. Keays SL, Newcombe PA, Bullock-Saxton JE, Bullock MI, Keays AC. Factors involved in the development of osteoarthritis after anterior cruciate ligament surgery. Am J Sports Med. 2010;38:455-463. [DOI] [PubMed] [Google Scholar]
- 27. Kim K-M, Croy T, Hertel J, Saliba S. Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: a systematic review. J Orthop Sports Phys Ther. 2010;40:383-391. [DOI] [PubMed] [Google Scholar]
- 28. Kittelson AJ, Stackhouse SK, Stevens-Lapsley JE. Neuromuscular electrical stimulation after total joint arthroplasty: a critical review of recent controlled studies. Eur J Phys Rehabil Med. 2013;49:909-920. [PubMed] [Google Scholar]
- 29. Kramer JF. Effect of electrical stimulation current frequencies on isometric knee extension torque. Phys Ther. 1987;67:31-38. [DOI] [PubMed] [Google Scholar]
- 30. Labanca L, Rocchi JE, Laudani L, et al. Neuromuscular electrical stimulation superimposed on movement early after ACL surgery. Med Sci Sports Exerc. 2018;50:407-416. [DOI] [PubMed] [Google Scholar]
- 31. Lake DA. Neuromuscular electrical stimulation. Sports Med. 1992;13:320-336. [DOI] [PubMed] [Google Scholar]
- 32. Laufer Y, Ries JD, Leininger PM, Alon G. Quadriceps femoris muscle torques and fatigue generated by neuromuscular electrical stimulation with three different waveforms. Phys Ther. 2001;81:1307-1316. [PubMed] [Google Scholar]
- 33. Laufer Y, Snyder-Mackler L. Factors affecting responsiveness of the quadriceps femoris muscle to neuromuscular electrical stimulation following total knee arthroplasty. J Isr Phys Ther Soc. 2010;12:2. [Google Scholar]
- 34. Laufer Y, Snyder-Mackler L. Response of male and female subjects after total knee arthroplasty to repeated neuromuscular electrical stimulation of the quadriceps femoris muscle. Am J Phys Med Rehabil. 2010;89:464-472. [DOI] [PubMed] [Google Scholar]
- 35. Lepley LK, Wojtys EM, Palmieri-Smith RM. Combination of eccentric exercise and neuromuscular electrical stimulation to improve quadriceps function post-ACL reconstruction. Knee. 2015;22:270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine M, McElroy K, Stakich V, Cicco J. Comparing conventional physical therapy rehabilitation with neuromuscular electrical stimulation after TKA. Orthopedics. 2013;36:e319-e324. [DOI] [PubMed] [Google Scholar]
- 37. Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2002;17:56-63. [DOI] [PubMed] [Google Scholar]
- 38. Lieber R, Silva P, Daniel D. Equal effectiveness of electrical and volitional strength training for quadriceps femoris muscles after anterior cruciate ligament surgery. J Orthop Res. 1996;14:131-138. [DOI] [PubMed] [Google Scholar]
- 39. Louboutin H, Debarge R, Richou J, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee. 2009;16:239-244. [DOI] [PubMed] [Google Scholar]
- 40. Maffiuletti NA, Bramanti J, Jubeau M, Bizzini M, Deley G, Cometti G. Feasibility and efficacy of progressive electrostimulation strength training for competitive tennis players. J Strength Cond Res. 2009;23:677-682. [DOI] [PubMed] [Google Scholar]
- 41. Maffiuletti NA, Gondin J, Place N, Stevens-Lapsley J, Vivodtzev I, Minetto MA. Clinical use of neuromuscular electrical stimulation for neuromuscular rehabilitation: what are we overlooking? Arch Phys Med Rehabil. 2018;99:806-812. [DOI] [PubMed] [Google Scholar]
- 42. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713-721. [PubMed] [Google Scholar]
- 43. Marmon AR, Snyder-Mackler L. Quantifying neuromuscular electrical stimulation dosage after knee arthroplasty. J Life Sci Res. 2011;5:581-583. [PMC free article] [PubMed] [Google Scholar]
- 44. Mikesky AE, Meyer A, Thompson KL. Relationship between quadriceps strength and rate of loading during gait in women. J Orthop Res. 2000;18:171-175. [DOI] [PubMed] [Google Scholar]
- 45. Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty. J Bone Joint Surg Am. 2005;87:1047-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mizner RL, Stevens JE, Snyder-Mackler L. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83:359-365. [PubMed] [Google Scholar]
- 47. Monaghan B, Caulfield B, O’Mathúna DP. Surface neuromuscular electrical stimulation for quadriceps strengthening pre and post total knee replacement. Cochrane Database Syst Rev. 2010;1:CD007177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morrissey MC, Brewster CE, Shields CL, Brown M. The effects of electrical stimulation on the quadriceps during postoperative knee immobilization.Am J Sports Med. 1985;13:40-45. [DOI] [PubMed] [Google Scholar]
- 49. Myers NL, Sciascia AD, Westgate PM, Kibler WB, Uhl TL. Increasing ball velocity in the overhead athlete: a meta-analysis of randomized controlled trials.J Strength Cond Res. 2015;29:2964-2979. [DOI] [PubMed] [Google Scholar]
- 50. Øiestad BE, Holm I, Gunderson R, Myklebust G, Risberg MA. Quadriceps muscle weakness after anterior cruciate ligament reconstruction: a risk factor for knee osteoarthritis? Arthritis Care Res. 2010;62:1706-1714. [DOI] [PubMed] [Google Scholar]
- 51. Oxford Centre for Evidence-Based Medicine. Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence. Accessed April 13, 2014. https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf
- 52. Owens J, Malone T. Treatment parameters of high frequency electrical stimulation as established on the Electro-Stim 180. J Orthop Sports Phys Ther. 1983;4:162-168. [DOI] [PubMed] [Google Scholar]
- 53. Packman-Braun R. Relationship between functional electrical stimulation duty cycle and fatigue in wrist extensor muscles of patients with hemiparesis. Phys Ther. 1988;68:51-56. [DOI] [PubMed] [Google Scholar]
- 54. Paillard T. Combined application of neuromuscular electrical stimulation and voluntary muscular contractions. Sports Med. 2008;38:161-177. [DOI] [PubMed] [Google Scholar]
- 55. Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27:405-424. [DOI] [PubMed] [Google Scholar]
- 56. Pantović M, Popović B, Madić D, Obradović J. Effects of neuromuscular electrical stimulation and resistance training on knee extensor/flexor muscles. Coll Antropol. 2015;39(suppl 1):153-157. [PubMed] [Google Scholar]
- 57. Paternostro-Sluga T, Fialka C, Alacamliogliu Y, Saradeth T, Fialka-Moser V. Neuromuscular electrical stimulation after anterior cruciate ligament surgery. Clin Orthop. 1999;368:166-175. [PubMed] [Google Scholar]
- 58. PEDro Physiotherapy Evidence Database. PEDro statistics. Published 2014. Accessed April 13, 2014. https://pedro.org.au/english/learn/pedro-statistics/59/
- 59. Petterson S, Snyder-Mackler L. The use of neuromuscular electrical stimulation to improve activation deficits in a patient with chronic quadriceps strength impairments following total knee arthroplasty. J Orthop Sports Phys Ther. 2006;36:678-685. [DOI] [PubMed] [Google Scholar]
- 60. Petterson SC, Barrance P, Marmon AR, Handling T, Buchanan TS, Snyder-Mackler L. Time course of quad strength, area, and activation after knee arthroplasty and strength training. Med Sci Sports Exerc. 2011;43:225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Petterson SC, Mizner RL, Stevens JE, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Care Res. 2009;61:174-183. [DOI] [PubMed] [Google Scholar]
- 62. Pietrosimone BG, McLeod MM, Lepley AS. A theoretical framework for understanding neuromuscular response to lower extremity joint injury. Sports Health. 2012;4:31-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rainsford G. The use of neuromuscular electrical stimulation as an adjunctive therapy for muscle strengthening in knee rehabilitation. Physiother Ireland. 2012;33:36-41. [Google Scholar]
- 64. Randolph SM, Holcomb WR, Rubley MD, Miller MG. Assessment of torque and perceived pain during ten repetitions of neuromuscular electrical stimulation. Athl Train Sports Health Care. 2009;1:162-168. [Google Scholar]
- 65. Rebai H, Barra V, Laborde A, Bonny J-M, Poumarat G, Coudert J. Effects of two electrical stimulation frequencies in thigh muscle after knee surgery. Int J Sports Med. 2002;23:604-609. [DOI] [PubMed] [Google Scholar]
- 66. Reed B. The physiology of neuromuscular electrical stimulation. Pediatr Phys Ther. 1997;9:96-102. [Google Scholar]
- 67. Ross M. The effect of neuromuscular electrical stimulation during closed kinetic chain exercise on lower extremity performance following anterior cruciate ligament reconstruction. Res Sports Med. 2000;9:239-251. [Google Scholar]
- 68. Schmitt LC, Quatman CE, Paterno MV, Best TM, Flanigan DC. Functional outcomes after surgical management of articular cartilage lesions in the knee: a systematic literature review to guide postoperative rehabilitation. J Orthop Sports Phys Ther. 2014;44:565-578. [DOI] [PubMed] [Google Scholar]
- 69. Scott W, Adams C, Cyr S, et al. Electrically elicited muscle torque: comparison between 2500-Hz burst-modulated alternating current and monophasic pulsed current. J Orthop Sports Phys Ther. 2015;45:1035-1041. [DOI] [PubMed] [Google Scholar]
- 70. Sisk TD, Stralka SW, Deering MB, Griffin JW. Effect of electrical stimulation on quadriceps strength after reconstructive surgery of the anterior cruciate ligament. Am J Sports Med. 1987;15:215-220. [DOI] [PubMed] [Google Scholar]
- 71. Snyder-Mackler L, Binder-Macleod SA, Williams PR. Fatigability of human quadriceps femoris muscle following anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 1993;25:783-789. [DOI] [PubMed] [Google Scholar]
- 72. Snyder-Mackler L, Delitto A, Bailey S, Stralka S. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1995;77:1166-1173. [DOI] [PubMed] [Google Scholar]
- 73. Snyder-Mackler L, Delitto A, Stralka SW, Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74:901-907. [DOI] [PubMed] [Google Scholar]
- 74. Snyder-Mackler L, Ladin Z, Schepsis AA, Young J. Electrical stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament: effects of electrically elicited contraction of the quadriceps femoris and hamstring muscles on gait and on strength of thigh muscles. J Bone Joint Surg Am. 1991;73:1025-1036. [PubMed] [Google Scholar]
- 75. Spector P, Laufer Y, Elboim Gabyzon M, Kittelson A, Stevens Lapsley J, Maffiuletti NA. Neuromuscular electrical stimulation therapy to restore quadriceps muscle function in patients after orthopaedic surgery: a novel structured approach. J Bone Joint Surg Am. 2016;98:2017-2024. [DOI] [PubMed] [Google Scholar]
- 76. Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther. 2012;92:210-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stevens-Lapsley JE, Balter JE, Wolfe P, et al. Relationship between intensity of quadriceps muscle neuromuscular electrical stimulation and strength recovery after total knee arthroplasty. Phys Ther. 2012;92:1187-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Takahashi K, Hayashi M, Fujii T, Kawamura K, Ozaki T. Early rehabilitation with weight-bearing standing-shaking-board exercise in combination with electrical muscle stimulation after anterior cruciate ligament reconstruction. Acta Med Okayama. 2012;66:231-237. [DOI] [PubMed] [Google Scholar]
- 79. Taradaj J, Halski T, Kucharzewski M, et al. The effect of neuromuscular electrical stimulation on quadriceps strength and knee function in professional soccer players: return to sport after ACL reconstruction. Biomed Res Int. 2013;2013:802534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Thomas AC, Stevens-Lapsley JE. Importance of attenuating quadriceps activation deficits after total knee arthroplasty. Exerc Sport Sci Rev. 2012;40:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Walls RJ, McHugh G, O’Gorman DJ, Moyna NM, O’Byrne JM. Effects of preoperative neuromuscular electrical stimulation on quadriceps strength and functional recovery in total knee arthroplasty. A pilot study. BMC Musculoskel Disord. 2010;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wigerstad-Lossing I, Grimby G, Jonsson T, Morelli B, Peterson L, Renström P. Effects of electrical muscle stimulation combined with voluntary contractions after knee ligament surgery. Med Sci Sports Exerc. 1988;20:93-98. [DOI] [PubMed] [Google Scholar]
- 83. Williams RA, Morrissey MC, Brewster CE. The effect of electrical stimulation on quadriceps strength and thigh circumference in meniscectomy patients. J Orthop Sports Phys Ther. 1986;8:143-146. [DOI] [PubMed] [Google Scholar]
- 84. Yoshida Y, Ikuno K, Shomoto K. Comparison of the effect of sensory-level and conventional motor-level neuromuscular electrical stimulations on quadriceps strength after total knee arthroplasty: a prospective randomized single-blind trial. Arch Phys Med Rehabil. 2017;98:2364-2370. [DOI] [PubMed] [Google Scholar]