Abstract
While there is growing evidence that perturbation of the gut microbiota can result in a variety of pathologies including gut tumorigenesis, the influence of commensal fungi remains less clear. In this issue, Zhu et al (2021) show that mycobiota dysbiosis stimulates energy metabolism changes in subepithelial macrophages promoting colon cancer via enhancing innate lymphoid cell activity. These findings provide insights into a role of the gut flora in intestinal carcinogenesis and suggest opportunities for adjunctive antifungal or immunotherapeutic strategies to prevent colorectal cancer.
Subject Categories: Cancer, Immunology, Metabolism
Recent work reports a role for the commensal gut flora in driving aberrant host immunity and malignant cytokine signaling.
There is growing evidence for an important role for the microbiota in influencing tumorigenesis (Helmink et al, 2019). It is now well documented that gut microbiota represents a highly diverse polymicrobial population of bacteria, fungi, viruses, and protozoa. Recent evidence highlights involvement of the bacterial component of the gut microbiota in protection or enhancement of colorectal tumorigenesis. In contrast, the importance of the mycobiota is less well understood although recently suggested to promote pancreatic oncogenesis and colitis‐associated colon cancer (CAC) (Wang et al, 2018; Aykut et al, 2019). Therefore, gut fungi may play a role in the development of other gastro‐intestinal cancer types, such as CRC. Notably, there is emerging evidence suggesting that mycobiota imbalance modulates immune cells and can trigger inflammatory bowel disease (IBD) (Richard & Sokol, 2019).
Here, Zhu et al (2021) provide new insight into the association between mycobiota dysbiosis, immunomodulation, and tumorigenesis in the mouse gut (Fig 1).
Figure 1. Dectin‐3 deficiency induces fungal dysbiosis and tumorigenesis in mice by orchestrating immune cell metabolism and cytokine signaling.
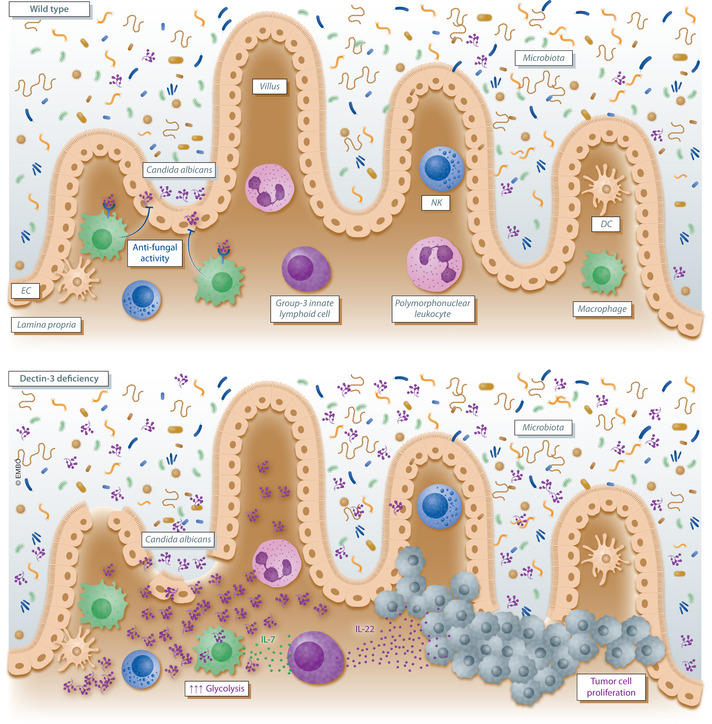
In the gut of wild‐type mice, the natural population of the commensal yeast Candida albicans is detected by the Dectin‐3 receptor located on the subepithelial macrophage cell surface. This recognition allows macrophages to maintain gut homeostasis by exerting an antifungal activity. In Dectin‐3‐deficient mice, the mycobiota becomes disrupted and aberrantly increased populations of C. albicans emerge. Elevated C. albicans load triggers increased glycolysis in macrophages and interleukin‐7 (IL‐7) secretion. Macrophage‐derived IL‐7 finally induces IL‐22 secretion by group‐3 innate lymphoid cells that in turn promote tumor cell proliferation in the gut epithelium.
The current study (Zhu et al, 2021) is based on previous observations suggesting that human pathogenic fungi are recognized by the C‐type lectin receptor Dectin‐3. This led Zhu et al (2021) to test whether the mycobiota influenced gut tumor formation and is linked to immune recognition mediated by Dectin‐3. First, the authors demonstrated that mice lacking the Dectin‐3 receptor had increased colonic tumorigenesis in response to the azoxymethane (AOM) and dextran sodium sulfate (DSS). This was evident histologically in marked differences in tumor number, size, and burden in Dectin‐3‐deficient mice. Of note, immunohistochemical staining revealed that the lack of Dectin‐3 induced gut tumor formation by triggering epithelial cell proliferation rather than preventing cell apoptosis. In fact, first insight into the impact of microbes in CAC was suggested by the observation that co‐housed WT and Dectin‐3‐deficient mice displayed no difference in tumorigenesis. The pivotal role of the microbiota was then underlined in fecal transplantation experiments. Chemically induced germ‐free mice that received feces from Dectin‐3 tumor‐bearing mice displayed exacerbated tumor development compared to wild‐type controls. In addition, the fungal burden was specifically increased in tumor‐bearing Dectin‐3‐deficient animals. Deep profiling of the mycobiota alterations demonstrated an increase in a single yeast species, i.e., Candida albicans, that normally behaves as commensal in the gut (Papon et al, 2013; Wilson, 2019). Preliminary experiments suggested that the increased burden of C. albicans in Dectin‐3‐deficient tumor‐bearing mice is due to impaired antifungal killing by macrophages. Consistently, elevated C. albicans populations triggered glycolysis and inflammatory IL‐7 secretion from lamina propria macrophages, suggesting that Dectin‐3 deficiency‐induced fungal dysbiosis resulted in modulation of gut macrophage metabolism, promoting tumorigenesis. Exploring the molecular and cellular mechanisms that linked macrophage‐derived IL‐7 secretion and CRC development, Zhu et al (2021) showed in vitro that IL‐7 produced by subepithelial macrophages induced IL‐22 secretion by group‐3 innate lymphoid cells (ILC3s). In turn, up‐regulation of IL‐22 in Dectin‐3‐deficient mice contributed to the oncogenesis seen in these animals. Finally, a detailed analysis of tumor tissues collected from 172 patients with CRC showed correlation and poorer clinical outcome in patients with decreased expression of Dectin‐3, but increased expression of IL‐22 and mycobiota burden, although they did not directly link this to the presence of C. albicans in these patients.
Overall, Zhu et al (2021) define a new cell paradigm linking mycobiota dysbiosis, macrophage energy metabolism, and innate lymphoid cell function to tumor development in the mouse gut. In this context, this study also sheds additional light on a new role of ILC3s, a recently described type of lymphoid effectors (Serafini et al, 2015). Indeed, ILC3s have been shown in the present article to act as cornerstone cells orchestrating cytokine‐regulated tumorigenesis in the gut. Beyond these pathophysiological considerations, the study opens up new opportunities for developing adjunctive antifungal or immunotherapeutic strategies for the prevention of high morbidity in CRC. Importantly, this enlightening article provides firm evidence that colonic C. albicans populations promote metabolic reprogramming in lamina propria macrophages and tumor cell formation. Metabolic reprogramming has been observed with other fungi, such as Aspergillus fumigatus, which induces metabolic rewiring of alveolar macrophages in the lung epithelium (Gonçalves et al, 2020). In line, the report by Zhu et al (2021) adds to previous work suggesting that mycobiota promotes pancreatic oncogenesis via activation of mannose‐binding lectins (Aykut et al, 2019). Mycobiota dysbiosis therefore stands out as an important new field of investigation in cancer research that is ripe for future exploration.
Conflict of interest
The authors declare that they have no conflict of interest.
The EMBO Journal (2021) 40: e108175.
See also: Y Zhu et al (2021)
References
- Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N et al (2019) The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574: 264–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves SM, Duarte‐Oliveira C, Campos CF, Aimanianda V, Ter Horst R, Leite L, Mercier T, Pereira P, Fernández‐García M, Antunes D et al (2020) Phagosomal removal of fungal melanin reprograms macrophage metabolism to promote antifungal immunity. Nat Commun 11: 2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA (2019) The microbiome, cancer, and cancer therapy. Nat Med 25: 377–388 [DOI] [PubMed] [Google Scholar]
- Papon N, Courdavault V, Clastre M, Bennett RJ (2013) Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog 9: e1003550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard ML, Sokol H (2019) The gut mycobiota: insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol 16: 331–345 [DOI] [PubMed] [Google Scholar]
- Serafini N, Vosshenrich CA, Di Santo JP (2015) Transcriptional regulation of innate lymphoid cell fate. Nat Rev Immunol 15: 415–428 [DOI] [PubMed] [Google Scholar]
- Wang T, Fan C, Yao A, Xu X, Zheng G, You Y, Jiang C, Zhao X, Hou Y, Hung MC et al (2018) The adaptor protein CARD9 protects against colon cancer by restricting mycobiota‐mediated expansion of myeloid‐derived suppressor cells. Immunity 49: 504–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D (2019) Candida albicans . Trends Microbiol 27: 188–189 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Shi T, Lu X, Xu Z, Qu J, Zhang Z, Shi G, Shen S, Hou Y, Chen Y et al (2021) Fungal‐induced glycolysis in macrophages promotes colon cancer by enhancing innate lymphoid cell secretion of IL‐22. EMBO J 40: e105320 [DOI] [PMC free article] [PubMed] [Google Scholar]


