Abstract
Chemical compounds have recently been introduced as alternative and non‐integrating inducers of pluripotent stem cell fate. However, chemical reprogramming is hampered by low efficiency and the molecular mechanisms remain poorly characterized. Here, we show that inhibition of spleen tyrosine kinase (Syk) by R406 significantly promotes mouse chemical reprogramming. Mechanistically, R406 alleviates Syk / calcineurin (Cn) / nuclear factor of activated T cells (NFAT) signaling‐mediated suppression of glycine, serine, and threonine metabolic genes and dependent metabolites. Syk inhibition upregulates glycine level and downstream transsulfuration cysteine biosynthesis, promoting cysteine metabolism and cellular hydrogen sulfide (H2S) production. This metabolic rewiring decreased oxidative phosphorylation and ROS levels, enhancing chemical reprogramming. In sum, our study identifies Syk‐Cn‐NFAT signaling axis as a new barrier of chemical reprogramming and suggests metabolic rewiring and redox homeostasis as important opportunities for controlling cell fates.
Keywords: chemical reprogramming, hydrogen sulfide, R406, Syk, metabolism
Subject Categories: Metabolism, Regenerative Medicine
Block of Syk/Calcineurin/NFAT signalling facilitates murine iPSC induction via increased cysteine biosynthesis.
Introduction
Somatic cells can be reprogrammed to pluripotent stem cells (PSCs) by somatic cell nuclear transfer, cell fusion, and transcription factors (Gurdon, 1962; Tada et al, 2001; Takahashi & Yamanaka, 2006). Chemical compounds not only are more convenient and non‐integrating for manipulating cell fates, but also can provide a better understanding of cell‐fate transitions (Li et al, 2014; Theunissen & Jaenisch, 2014; Ma et al, 2017). Now, it is possible to generate chemical‐induced PSCs (ciPSCs) from mouse somatic cells (Hou et al, 2013; Long et al, 2015; Zhao et al, 2015; Cao et al, 2018; Fu et al, 2018; Yang et al, 2020). However, the process is still time consuming and labor intensive; and therefore, more efforts are required to identify additional small molecules for rapid and efficient chemical reprogramming.
Besides technical innovations and advances, many efforts were also made to elucidate the molecular mechanisms of pluripotent reprogramming (Hochedlinger & Jaenisch, 2015). Important cellular and molecular mechanisms have been identified as hallmarks of pluripotent reprogramming, including epigenetic modulations, mesenchymal‐to‐epithelial transition, and metabolic regulations (Theunissen & Jaenisch, 2014; Hochedlinger & Jaenisch, 2015). Particularly, the interplays between cellular metabolism and cell‐fate transition are being more appreciated in recent years. As early as 2010, we have identified the Warburg effect as a classical hallmark of pluripotent reprogramming, and that chemical compounds modulating the Warburg effect plus small molecules targeting epigenetics enabled single factor OCT4‐mediated pluripotent reprogramming (Zhu et al, 2010). Later, many studies supported our initial discovery, and further expanded the knowledge about metabolic regulations of pluripotency induction, including the Warburg effect, mitochondrial dynamics, autophagy, and lipid metabolism (Folmes et al, 2012; Zhang et al, 2012; Ma et al, 2015; Wu et al, 2015; Wu et al, 2016; Wang et al, 2017; Ying et al, 2018; Wu et al, 2019; Cheng et al, 2020; Zhu et al, 2020). Even with all these efforts, currently, the upstream signaling pathways regulating metabolism during reprogramming and how metabolic pathways regulate reprogramming remain incompletely understood.
Spleen tyrosine kinase (Syk) has a crucial role in immunity, such as B‐cell response and autoimmune diseases (Turner et al, 1995; Mocsai et al, 2010), and other biological processes, such as endothelial cell development and brown adipocyte differentiation (Yanagi et al, 2001; Knoll et al, 2017). Syk activation subsequently activates cellular calcium (Ca2+) signaling, and one downstream signaling axis of Syk‐Ca2+ is Cn‐NFAT (Crabtree & Olson, 2002). In the nucleus, NFAT can transcriptionally active or repress downstream genes (Baksh et al, 2002; Nguyen et al, 2009; Goodyer et al, 2012; Moreno et al, 2015; Yao et al, 2016). Cn‐NFAT modulates diverse physiological processes, including T‐cell and B‐cell activation, embryonic and adult stem cell self‐renewal and differentiation, aging, and tissue regeneration (Horsley et al, 2008; Kao et al, 2009; Li et al, 2011; Kujawski et al, 2014). Whether and how the Syk‐Cn‐NFAT signaling axis regulates somatic cell reprogramming remains largely unknown.
In this study, we conducted a small molecule screen and identified Syk inhibitor R406 that can significantly promote mouse chemical reprogramming, and then studied the Syk‐Cn‐NFAT signaling axis in chemical reprogramming. After further investigating the molecular mechanisms of R406, we identified that glycine, serine, and threonine metabolic genes were notably induced by R406. We found that endogenous H2S, as one downstream metabolite of glycine, serine, and threonine metabolism, transsulfuration cysteine biosynthesis pathway, and cysteine metabolism, was significantly upregulated after R406 treatment, and consequently modulated redox homeostasis and promoted chemical reprogramming.
Results
Identification of Syk inhibitor R406 that can significantly promote mouse chemical reprogramming
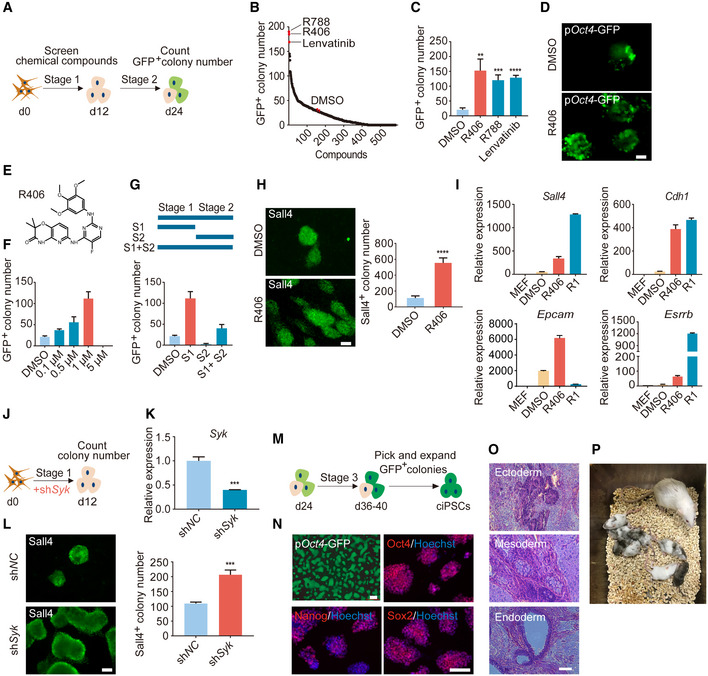
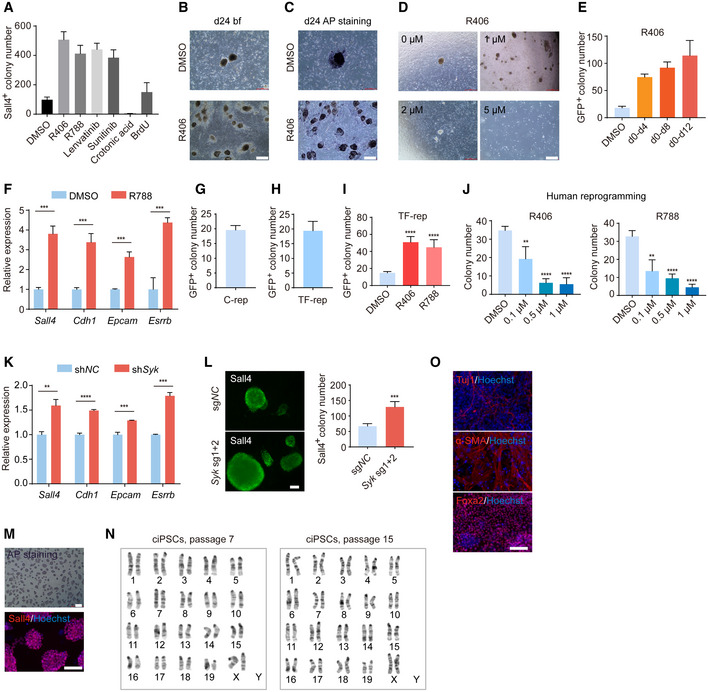
Mouse chemical reprogramming remains a slow and labor‐intensive process and takes about 40–60 days in total (Hou et al, 2013; Zhao et al, 2015; Cao et al, 2018), which prompted us to identify new small molecules that can improve the reprogramming process. As a starting point, we carried out a chemical screen using a chemical library mainly targeting kinases. Based on our recent work on successfully identifying small molecules that can promote CRISPR‐Cpf1‐based genome editing (Ma et al, 2018), we hypothesized that we can further explore this potent chemical library in the mouse chemical reprogramming system. We used MEFs with pOct4‐GFP reporter as starting materials, treated cells with Stage 1 medium from day 0 to day 12, and screened small molecules during Stage 1. Then, we cultured the reprogramming intermediates for another 12 days with Stage 2 medium and counted GFP+ colony number on day 24 (Fig 1A). Interestingly, the top three small molecules that we identified were R406 and R788, two Syk inhibitors, and Lenvatinib, a VEGFR inhibitor (Fig 1B). From independent replication experiments, we further confirmed that these three small molecules could significantly increase GFP+ colony number (Fig 1C). For further comparison, we tested previously reported small molecules that could promote chemical reprogramming at different stages and found that R406 had the highest effect on promoting chemical reprogramming when added at Stage 1 (Fig EV1A). Crotonic acid and BrdU, which were identified from the late stage (Stage 2), could not promote chemical reprogramming when they were added at Stage 1 (Fig EV1A) (Long et al, 2015; Fu et al, 2018). This result indicated that our screening helped us identify new small molecules that specifically worked at Stage 1, which so far has not been extensively explored. Then, we focused our studies on the roles of R406 in mouse chemical reprogramming. Both GFP+ and alkaline phosphatase‐positive (AP+) colony number were significantly increased after R406 treatment (Figs 1D, and EV1B and C). The optimal concentration of R406 was 1 μM (Figs 1E and F, and EV1D). Next, we confirmed that R406 functioned at the early stage of chemical reprogramming and could increase typical early pluripotent marker Sall4 (Fig 1G and H). Interestingly, the effect of R406 could be observed even when we treated cells during d0‐d4 (Fig EV1E). Based on these observations, we could conclude that the effect of R406 was stage‐specific, and the optimal stage of treatment was Stage 1. Using real‐time qualitative PCR (RT–qPCR), we observed that R406 and R788 promoted the induction of early pluripotent genes, including Sall4, Cdh1, Epcam, and Esrrb (Figs 1I and EV1F). We further tested the effects of R406 and R788 on mouse reprogramming induced by transcription factors (TFs). TF‐induced reprogramming by the lentiviral approach took about 8 days. When we counted iPSC colony number at day 24 for chemical reprogramming and at day 8 for TF‐induced reprogramming, we observed that the efficiencies were relatively comparable (Fig EV1G and H). We observed that R406 and R788 could also promote TF‐induced mouse reprogramming (Fig EV1I). Next, we tested the effects of R406 and R788 in human cells and found that R406 and R788 inhibited human somatic cell reprogramming (Fig EV1J). These results suggested that there are differences between mouse and human somatic cell reprogramming. Such observation is not unexpected, considering the difference between mouse and human PSCs, and the underlying molecular mechanisms can be explored in the future. In addition, genetically knocking down or knocking out Syk gene by shRNA or sgRNAs could also promote chemical reprogramming, indicating that R406 works through its target protein kinase Syk (Figs 1J–L, and EV1K and L).
Figure 1. Syk inhibitor R406 can significantly promote mouse chemical reprogramming.
-
ASchematic diagram depicting the procedure of compound screening during mouse chemical reprogramming.
-
BThe chemical screening result evaluated by GFP+ colony number on d24.
-
CGFP+ colony number of samples treated with R406, R788, and Lenvatinib. n = 3.
-
DFluorescence image of colonies with pOct4‐GFP expression after DMSO and R406 treatments. Scale bar, 100 μm.
-
E–GConcentration (F) and stage (G) test of R406 (E) during reprogramming. n = 3.
-
HImmunofluorescence of early pluripotent marker Sall4 in DMSO‐ and R406‐treated cells on d12. n = 5. Scale bar, 100 μm.
-
IRT–qPCR analysis of Sall4, Cdh1, Epcam, and Esrrb gene expression in MEFs, intermediate cells on d12 treated with DMSO and R406, and R1 (mESCs). n = 3.
-
JDiagram showing the procedure of shSyk virus infection at the early stage of reprogramming.
-
KRT–qPCR analysis of Syk expression in MEFs infected with shNC and shSyk viruses. n = 3.
-
LImmunofluorescence of Sall4 in reprogramming intermediates infected with shNC and shSyk. n = 3. Scale bar, 100 μm.
-
MSchematic diagram of the procedure of establishing ciPSC lines.
-
NciPSCs express key pluripotent markers Oct4, Sox2, and Nanog. Scale bar, 100 μm.
-
OciPSCs have developmental potentials to form teratomas containing tissues from all three germ layers. Scale bar, 100 μm.
-
PChimeric mice were generated from ciPSCs.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, **P < 0.01, ***P < 0.001, ****P < 0.0001. See also Fig EV1.
Figure EV1. Tests of R406 and other candidate small molecules on different reprogramming contexts and characterization of ciPSCs.
-
ASall4+ colony number on d12 after treatments with candidate small molecules. n = 5.
-
BBright field (bf) of colonies on d24 after DMSO and R406 treatments. Scale bar, 500 μm.
-
CAP staining of colonies on d24 after DMSO and R406 treatments. Scale bar, 500 μm. AP staining was done after the data acquisition of bright field (B) from the same sample.
-
DThe effects of R406 with different concentrations. Scale bar, 500 μm.
-
EThe effects of R406 in different time windows. n = 3.
-
FRT–qPCR analysis of early pluripotent genes expression in reprogramming intermediates treated with DMSO and R788. n = 3.
-
G, HGFP+ colony number on d24 of chemical reprogramming (C‐rep) and d8 of TF‐induced reprogramming (TF‐rep). n = 5.
-
IThe effects of R406 and R788 in TF‐induced reprogramming. n = 5.
-
JThe effects of R406 and R788 in human reprogramming. n = 4.
-
KRT–qPCR analysis of pluripotent genes expression in reprogramming intermediates treated with shNC and shSyk. n = 3.
-
LImmunofluorescence of Sall4 in reprogramming intermediates infected with sgNC and Syk sgRNA viruses. n = 4. Scale bar, 100 μm.
-
M–OCharacterization of ciPSCs: AP staining and Sall4 staining (M), karyotyping analysis of ciPSCs at passage 7 and passage 15 (N), and Tuj1, α‐SMA, and Foxa2 immunostaining after EB differentiation (O). Scale bar, 100 μm.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To generate ciPSCs after R406 treatment, we cultured the day‐24 cells for another 12–16 days and then established pOct4‐GFP+ ciPSC lines (Fig 1M). These ciPSCs were positive for AP and typical pluripotent makers Oct4, Sox2, Nanog, and Sall4 (Figs 1N and EV1M). Karyotyping results showed that these ciPSCs maintained a normal karyotype during expansion process (Fig EV1N). Using embryoid body differentiation, we could detect all three germ layer cells, including Tuj1+ ectoderm, α‐SMA+ mesoderm, and Foxa2+ endoderm (Fig EV1O). In addition, we did teratoma assay and found that these ciPSCs could generate typical teratomas containing derivatives of all three germ layers, suggesting that these ciPSCs are pluripotent (Fig 1O). Chimeric mice could also be generated from ciPSCs (Fig 1P), further supporting their in vivo developmental potentials. Collectively, above characterizations demonstrated that these ciPSCs are morphologically, cellularly, and functionally similar to mESCs.
Overall, through chemical screening, we have identified a novel small molecule R406, a specific Syk inhibitor, that could significantly promote the early stage of chemical reprogramming, overcoming one challenging issue of the mouse chemical reprogramming protocol. Furthermore, we confirmed that the mouse chemical reprogramming system was effective for deriving ciPSCs. Based on our observations, we decided to focus our studies on how R406 and its target Syk regulated mouse chemical reprogramming.
R406 works through the Syk‐Cn‐NFAT axis during chemical reprogramming
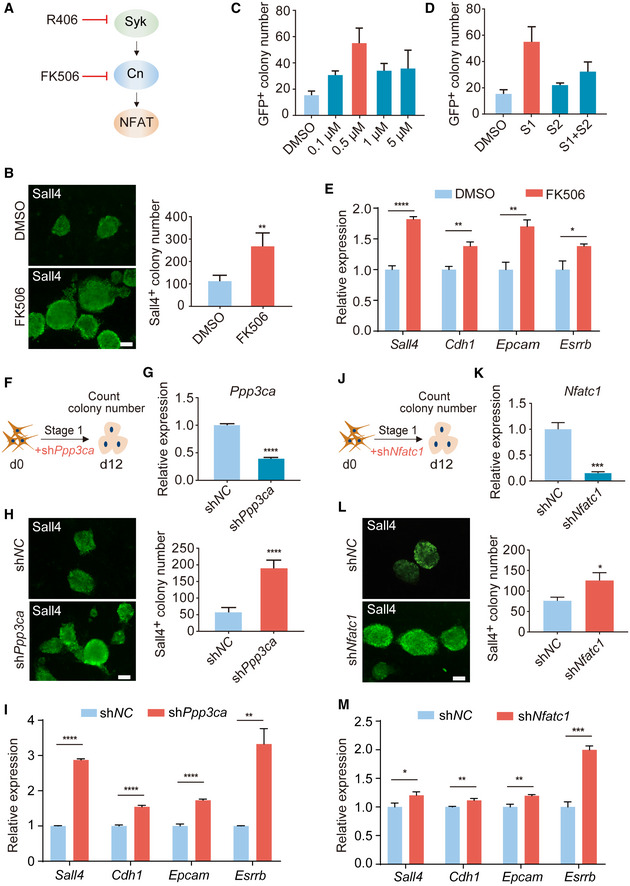
One main downstream signaling axis of Syk is Cn‐NFAT (Li et al, 2011; Moreno et al, 2015), and therefore, we tested whether Cn and NFAT could also regulate chemical reprogramming (Fig 2A). Firstly, we examined the functional effects of FK506, a specific Cn inhibitor, and found that FK506 treatment indeed could increase the number of Sall4+ colonies at day 12 and AP+ colonies at day 24 (Figs 2B and EV2A–C). The optimal concentration of FK506 was 0.5 μM (Fig 2C). Consistent with R406, FK506 also worked specifically at Stage 1 (Fig 2D). Using RT–qPCR, we observed that FK506 notably promoted the induction of early pluripotent genes Sall4, Cdh1, Epcam, and Esrrb (Fig 2E). We have also noticed the literatures indicating that calcineurin‐NFAT is activated and required for reprogramming (Sun et al, 2016; Khodeer & Era, 2017), so we detected NFAT activity during chemical reprogramming by immunostaining. We found that R406 treatment indeed inhibited NFATc1 and the fluorescence signal of NFATc1 could not be detected in colonies on d4 of reprogramming (Fig EV2D–F). Next, we genetically knocked down Ppp3ca, one of the genes that encode Cn proteins, and found that knocking down Ppp3ca by shRNA could increase Sall4+ colony number (Fig 2F–H) and promote the induction of early pluripotent genes Sall4, Cdh1, Epcam, and Esrrb (Fig 2I). In addition, we found that knocking down Nfatc1 could also enhance chemical reprogramming (Fig 2J–M). Conclusively, these results suggested that R406 works through the Syk‐Cn‐NFAT signaling cascade in chemical reprogramming.
Figure 2. Inhibition of Syk‐Cn‐NFAT signaling pathway promotes chemical reprogramming.
-
ASchematic diagram showing Syk‐Cn‐NFAT pathway axis.
-
BImmunofluorescence of Sall4 in reprogramming intermediates treated with DMSO and FK506. n = 5. Scale bar, 100 μm.
-
C, DConcentration (C) and stage (D) test of FK506 during reprogramming. n = 3.
-
ERT–qPCR analysis of Sall4, Cdh1, Epcam, and Esrrb gene expression in reprogramming intermediates treated with DMSO and FK506. n = 3.
-
FDiagram showing the procedure of Ppp3ca knockdown at the early stage of reprogramming.
-
GRT–qPCR analysis of Ppp3ca expression in MEFs infected with shNC and shPpp3ca viruses. n = 3.
-
HImmunofluorescence of Sall4 in WT cells and Ppp3ca knockdown cells on d12. n = 6. Scale bar, 100 μm.
-
IRT–qPCR analysis of pluripotent genes expression in cells treated with shNC and shPpp3ca. n = 3.
-
JDiagram showing the procedure of shNfatc1 virus infection at the early stage of reprogramming.
-
KRT–qPCR analysis of Nfatc1 expression in MEFs infected with shNC and shNfatc1 viruses. n = 3.
-
LImmunofluorescence of Sall4 in WT cells and Nfatc1 knockdown cells on d12. n = 3. Scale bar, 100 μm.
-
MRT–qPCR analysis of pluripotent genes expression in cells treated with shNC and shNfatc1. n = 3.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. See also Fig EV2.
Figure EV2. FK506 can promote chemical reprogramming and the regulation of NFAT by R406.
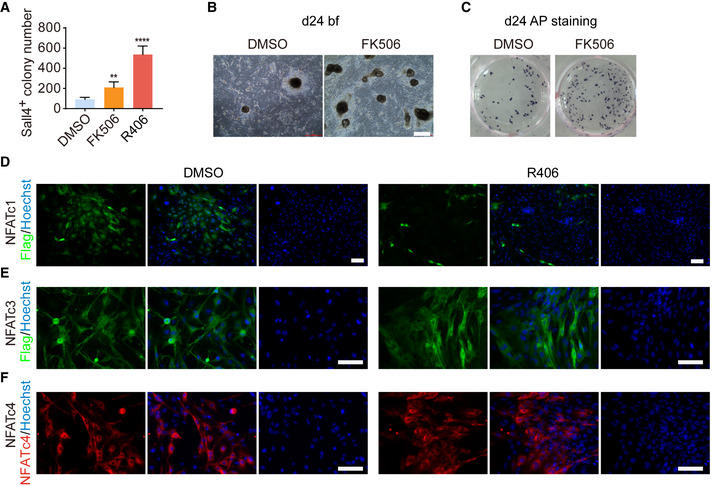
-
ASall4+ colony number on d12 after FK506 and R406 treatments. n = 5. Statistical significance was assessed by the two‐tailed Student’s t‐test, **P < 0.01, ****P < 0.0001.
-
BBright field (bf) of colonies on d24 after DMSO and FK506 treatments. Scale bar, 500 μm.
-
CAP staining of cells on d24 after DMSO and FK506 treatments. AP staining was done after the data acquisition of bright field (B) from the same sample.
-
D–FImmunofluorescence of Flag in Flag‐NFATc1 or Flag‐NFATc3 overexpressed cells (D, E) and NFATc4 in NFATc4 overexpressed cells (F) on d4 of reprogramming. Only NFATc1 was detected in the nucleus and affected by R406 treatment. Scale bar, 100 μm.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, **P < 0.01, ****P < 0.0001.
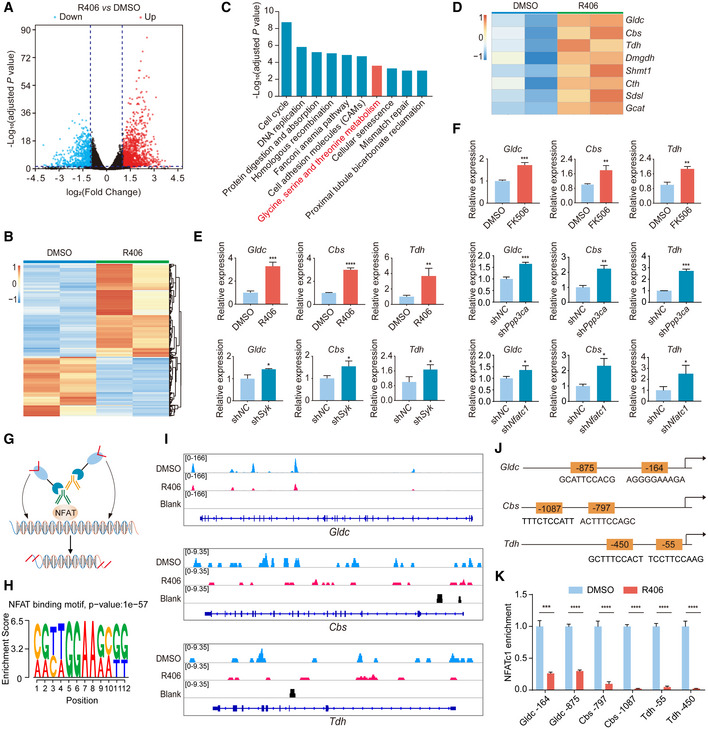
R406 can transcriptionally upregulate genes involved in glycine, serine, and threonine metabolism during chemical reprogramming
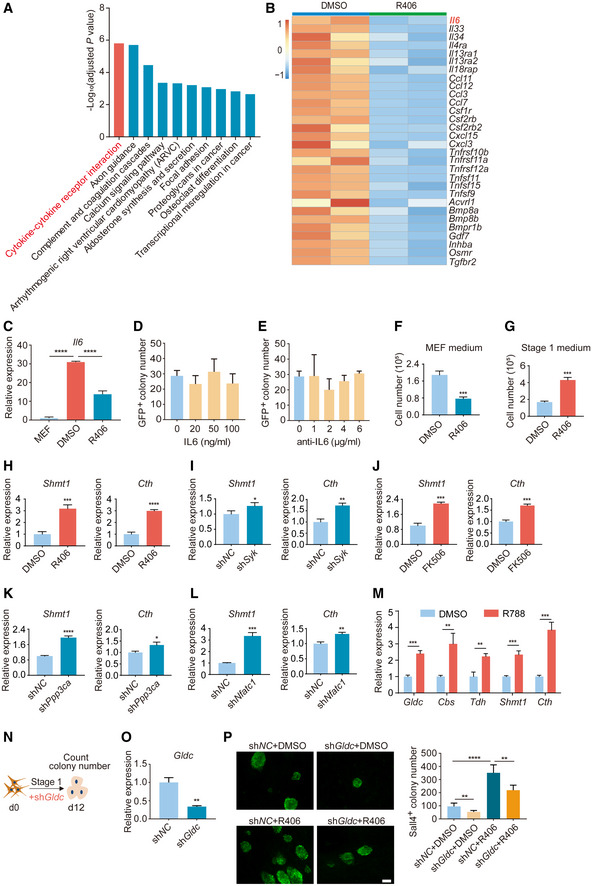
To further identify the molecular mechanisms of R406 in chemical reprogramming, the reprogramming intermediates treated with and without R406 were harvested to perform RNA‐seq. We conducted the differential expression analysis between the two treatment conditions. In total, 1,234 genes were upregulated and 781 genes were downregulated after R406 treatment (|log2(Fold change)| > 1, adjusted P‐value < 0.05) (Fig 3A and B). Next, we did Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. The KEGG terms among downregulated genes included cytokine–cytokine receptor interaction, axon guidance, complement and coagulation cascades, and calcium signaling pathway (Fig EV3A). Lots of genes encoding cytokines and chemokines were downregulated in the reprogramming intermediates with R406 treatment, including Il6, Il33, Ccl11, and Ccl12 (Fig EV3B). IL6 has been reported as a positive regulator for TF‐mediated reprogramming (Brady et al, 2013; Mosteiro et al, 2016; Chiche et al, 2017), and then, we suspected that IL6 might play distinct roles between chemical reprogramming and TF‐mediated reprogramming. Using RT–qPCR, we observed notable induction of Il6 gene during chemical reprogramming, and R406 could downregulate Il6 expression level (Fig EV3C). We checked the effect of IL6 in chemical reprogramming. However, neither addition of IL6 recombinant protein with several concentrations, nor treatment with an anti‐IL6 antibody, affected chemical reprogramming efficiency (Fig EV3D and E). These results indicated that there are distinct molecular mechanisms between chemical reprogramming and TF‐mediated reprogramming. Therefore, it is necessary to further explore the detailed molecular mechanisms of chemical reprogramming, which currently is very limited.
Figure 3. Inhibition of Syk‐Cn‐NFAT signaling axis by R406 can transcriptionally upregulate genes involved in glycine, serine, and threonine metabolism.
-
AVolcano plot of differentially expressed genes (DEGs) in DMSO‐ and R406‐treated cells on d12. Red dots represent upregulated genes, and blue dots represent downregulated genes in R406‐treated cells. Dash lines represent twofold change, adjusted P‐value < 0.05. Statistical significance was assessed by the method described previously (Love et al, 2014).
-
BHeatmap showing the normalized expression (z score) of DEGs. Red indicates upregulated, whereas blue indicates downregulated genes.
-
CKEGG analysis of upregulated genes in R406‐treated reprogramming intermediates. Statistical significance was assessed by Benjamini–Hochberg method.
-
DHeatmap of RNA‐seq data for the normalized expression (z score) of genes involved in glycine, serine, and threonine metabolism. Red indicates upregulated genes, whereas blue indicates downregulated genes.
-
E, FRT–qPCR analysis of Gldc, Cbs, and Tdh expression in R406‐ and shSyk‐treated cells (E) and FK506‐, shPpp3ca‐, and shNfatc1‐treated cells (F) on d8. n = 3.
-
GSchematic of CUT&Tag experiments to map the genomic occupancy of NFATc1.
-
HNFAT motif enrichment and associated P‐value are shown. Statistical significance was assessed by the method described previously (Heinz et al, 2010).
-
INFATc1 occupancy at Gldc, Cbs, and Tdh gene loci in cells treated with DMSO and R406 and no‐antibody control.
-
JThe predicted NFAT‐binding sites at the promoters of Gldc, Cbs, and Tdh.
-
KqPCR assay of the binding of NFATc1 at Gldc, Cbs, and Tdh gene loci in cells treated with DMSO and R406. n = 3.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. See also Fig EV3.
Figure EV3. Further characterization of RNA‐seq data and the tests of IL6 and Gldc on chemical reprogramming.
-
AKEGG analysis of downregulated genes in R406‐treated reprogramming intermediates. Statistical significance was assessed by Benjamini–Hochberg method.
-
BHeatmap of RNA‐seq data for the normalized expression (z score) of genes involved in cytokine–cytokine receptor interaction (A).
-
CRT–qPCR analysis of Il6 expression in MEFs and reprogramming intermediates treated with DMSO and R406. n = 3.
-
DGFP+ colony number on d24 of samples treated with IL6 protein. n = 3.
-
EGFP+ colony number on d24 of samples treated with anti‐IL6 antibody. n = 3.
-
FCell number on d4 of samples cultured in MEF medium supplemented with DMSO or R406. n = 3.
-
GCell number on d4 of samples cultured in Stage 1 medium supplemented with DMSO or R406. n = 3.
-
H–LRT–qPCR analysis of Shmt1 and Cth gene expression after treatments of R406, shSyk, FK506, shPpp3ca, and shNfatc1 on d8 of reprogramming. n = 3.
-
MRT–qPCR analysis of Gldc, Cbs, Tdh, Shmt1, and Cth gene expression after R788 treatment on d8 of reprogramming. n = 3.
-
NDiagram showing the procedure of shGldc virus infection at the early stage of reprogramming.
-
ORT–qPCR analysis of Gldc expression in MEFs infected with shNC and shGldc viruses. n = 3.
-
PImmunofluorescence of Sall4 in shNC‐ and shGldc‐infected cells with and without R406 treatment on d12. n = 5. Scale bar, 100 μm.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The KEGG terms among upregulated genes included cell cycle, DNA replication, homologous recombination, and fanconi anemia pathway (Fig 3C). Upregulation of cell cycle and DNA replication supported the conclusion that R406 could promote cellular reprogramming. We also checked the effect of R406 treatment on cell proliferation. First, R406 inhibited the growth of MEF cells cultured in MEF medium (Fig EV3F). Second, R406 could increase the cell number by twofolds when cells were cultured in Stage 1 medium and underwent reprogramming (Fig EV3G). As shown above, R406 could increase reprogramming efficiency by about fivefolds to sixfolds, suggesting that R406 enhanced chemical reprogramming not simply through promoting cell proliferation. Therefore, we tried to identify other novel molecular mechanisms. Interestingly, among the KEGG terms for upregulated genes, we observed that genes involved in glycine, serine, and threonine metabolism were significantly upregulated (Fig 3C). These genes included Gldc, Cbs, Tdh, Dmgdh, Shmt1, Cth, Sdsl, and Gcat (Fig 3D). Next, we confirmed this observation by RT–qPCR. Indeed, Gldc, Cbs, Tdh, Shmt1, and Cth were all significantly upregulated after treatment of R406, R788, and FK506, or knockdown of Syk, Ppp3ca, and Nfatc1 by shRNAs (Figs 3E and F, and EV3H–M). We knocked down Gldc using shRNA and found that shGldc significantly blocked chemical reprogramming (Fig EV3, EV4, EV5).
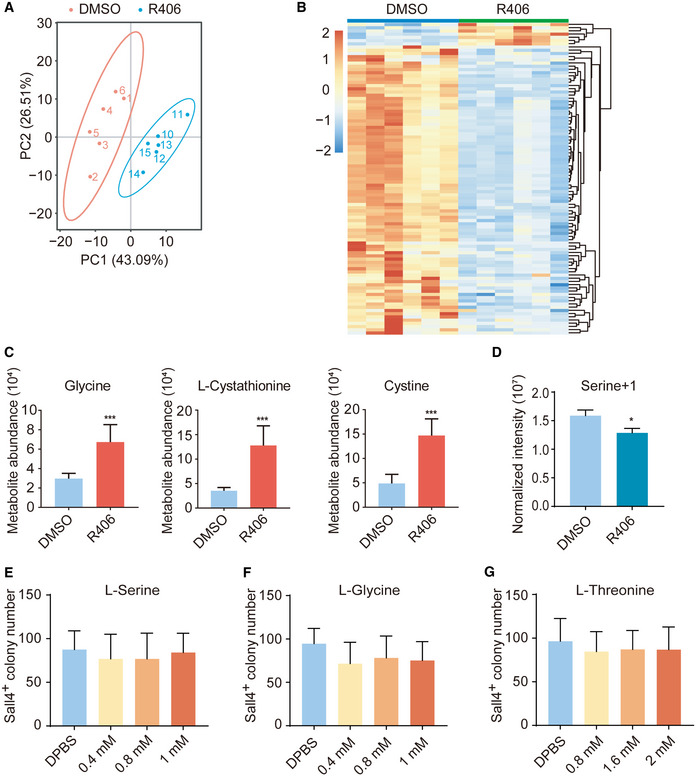
Figure EV4. More characterization of metabolomics.
-
APrincipal component analysis of analyzed metabolites in DMSO‐treated samples and R406‐treated samples on d8. n = 6.
-
BHeatmap showing the normalized abundance (z score) of the differential metabolites between DMSO‐treated and R406‐treated samples. Red indicates upregulated, whereas blue indicates downregulated metabolites. n = 6.
-
CAbundance of R406‐upregulated metabolites in glycine, serine, and threonine metabolism in cells treated with DMSO and R406. n = 6.
-
DStable isotope tracing mass spectrometry using 13C‐labeled Serine in cells treated with DMSO and R406. n = 3.
-
E–GSall4+ colony number on d12 after treatments with l‐serine, l‐glycine, and l‐threonine. n = 5.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, *P < 0.05, ***P < 0.001.
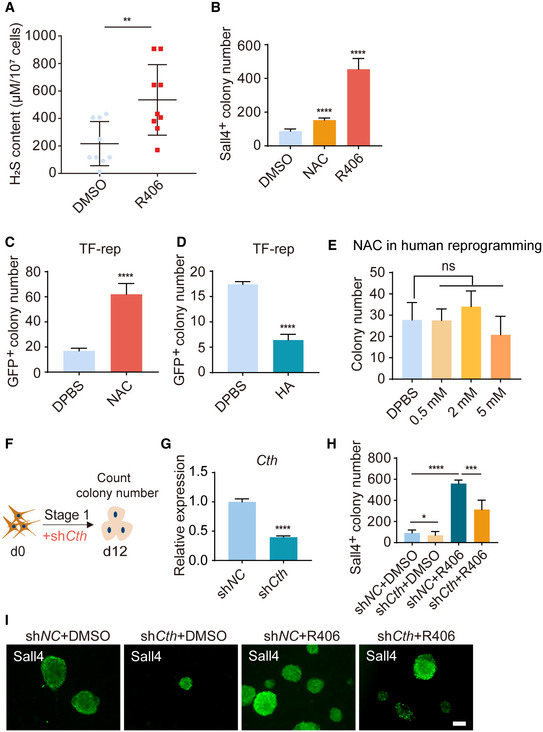
Figure EV5. Further characterization of H2S on chemical reprogramming.
-
AH2S levels in DMSO‐ and R406‐treated reprogramming intermediates by methylene blue method. n = 9.
-
BSall4+ colony number on d12 after treatments of NAC and R406. n = 5.
-
C, DGFP+ colony number of samples treated with NAC (C) and HA (D) on d8 of TF‐induced reprogramming. n = 5.
-
EThe effect of NAC in human reprogramming. n = 4.
-
FDiagram showing the procedure of shCth virus infection at the early stage of reprogramming.
-
GRT–qPCR analysis of Cth expression in MEFs infected with shNC and shCth viruses. n = 3.
-
H, IImmunofluorescence of Sall4 in shNC‐ and shCth‐infected cells with and without R406 treatment on d12. n = 5. Scale bar, 100 μm.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To determine whether these metabolic genes are the direct targets of NFATc1, we performed the CUT&Tag assay (Kaya‐Okur et al, 2019) (Fig 3G). We found that NFATc1 could directly bind to the genomic regions of Gldc, Cbs, and Tdh, and R406 treatment significantly decreased the binding of NFATc1 at these genomic loci (Fig 3H and I). This conclusion was further confirmed by qPCR assay (Fig 3J and K). Thus, we have identified NFATc1 as a new direct suppressor of essential genes involved in glycine, serine, and threonine metabolism.
Collectively, these data demonstrated that inhibition of Syk‐Cn‐NFAT signaling axis by R406 can directly alleviate the repression of the essential genes involved in glycine, serine, and threonine metabolism at the transcriptional level.
Metabolic analysis of chemical reprogramming intermediates after R406 treatment
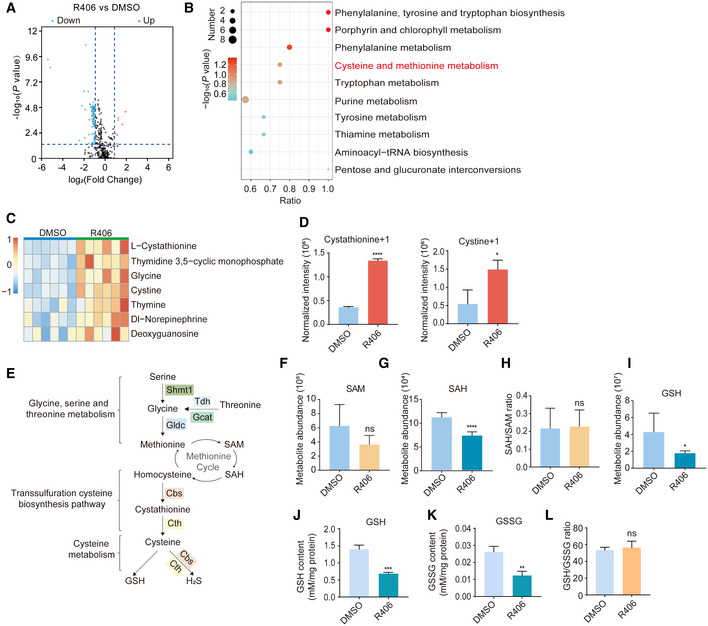
We speculated that metabolites of glycine, serine, and threonine metabolic pathway, for example, S‐adenosylmethione (SAM) and S‐adenosylhomocysteine (SAH), might be upregulated after R406 treatment during chemical reprogramming. Such metabolic pathway can provide obligate substrates for the epigenetic modifications of histones and DNA (Shyh‐Chang et al, 2013; Ren et al, 2017; Ly et al, 2020). We carried out metabolic analysis of reprogramming intermediates treated with and without R406. We checked the levels of about 345 metabolites by Quasi‐Targeted Metabolomics detection technology based on LC‐MS/MS. In total, 7 metabolites were upregulated and 90 metabolites were downregulated after R406 treatment (Fig 4A, and EV4A and B). KEGG analysis showed that cysteine and methionine metabolism was among the significantly changed KEGG terms (Fig 4B). Consistent with the RNA‐seq results, we could detect the upregulation of metabolites in glycine, serine, and threonine metabolism as well as the downstream pathways, including glycine, cystine, and l‐cystathionine (Figs 4C and EV4C). We performed stable isotope tracing mass spectrometry using 13C‐labeled serine and observed that R406 increased the labeled levels of cystine and cystathionine, supporting that de novo synthesis was indeed the basis for increased intracellular cystine levels (Fig 4D). Consistently, we observed that labeled serine was downregulated, which supported the upregulated activity of glycine, serine, and threonine metabolism (Fig EV4D). Because the culture medium has already contained excess levels of glycine, serine, and threonine, we found that simply adding additional cell‐permeable glycine, serine, or threonine to the reprogramming medium formulation had no effect (Fig EV4, EV5).
Figure 4. Metabolic analysis of chemical reprogramming intermediates after R406 treatment.
-
AVolcano plot showing the differential metabolites between DMSO‐treated and R406‐treated samples. Red dots represent significantly upregulated metabolites, and blue dots represent significantly downregulated metabolites in R406‐treated cells. Dash lines represent twofold change, P‐value < 0.05. Statistical significance was assessed by the method described previously (Love et al, 2014).
-
BKEGG analysis of these differential metabolites. Statistical significance was assessed by Benjamini–Hochberg method.
-
CHeatmap showing the normalized abundance (z score) of upregulated metabolites in R406‐treated cells. Red indicates upregulated metabolites, whereas blue indicates downregulated metabolites.
-
DStable isotope tracing mass spectrometry using 13C‐labeled serine in cells treated with DMSO and R406. n = 3.
-
ESchematic diagram showing glycine, serine, and threonine metabolism and downstream metabolic pathways.
-
F–IMetabolite abundance of SAM, SAH, and GSH (F, G, and I), and the SAH/SAM ratio (H) in samples treated with DMSO and R406. n = 6.
-
J–LThe GSH and GSSG levels (J, K) and the ratio of GSH/GSSG (L) in samples treated with DMSO and R406. n = 3.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 and not significant (ns) indicates P > 0.05. See also Fig EV4.
Our initial hypothesis was that SAM and SAH might be upregulated after R406 treatment and then contributed to epigenetic regulations (Fig 4E). However, the level of SAH was slightly decreased while the level of SAM and the SAH/SAM ratio were not notably changed (Fig 4F–H). Based on the observation that metabolites of the transsulfuration cysteine biosynthesis pathway were upregulated, we checked the level of glutathione (GSH), which is a well‐known downstream product of transsulfuration pathway and cysteine metabolism (Zhu et al, 2019), but we found the decrease of GSH after R406 treatment (Fig 4I). Using a GSH and GSSG assay kit, we observed that the levels of GSH and GSSG were decreased, but the ratio of GSH/GSSG was not altered (Fig 4J–L).
R406 can upregulate cellular H2S level during chemical reprogramming
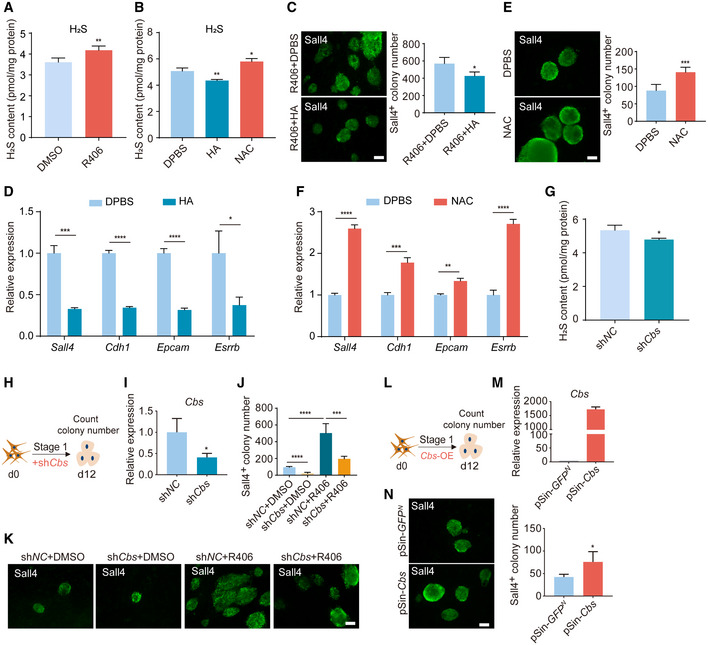
Because H2S is another downstream product of the transsulfuration cysteine biosynthesis pathway and cysteine metabolism, and the two main genes Cbs and Cth for the production of H2S were also significantly upregulated (Fig 3D) (Rose et al, 2017), we next analyzed the cellular H2S levels. We used the monobromobimane (MBB) method, which is a direct and confident measurement for H2S quantification (Shen et al, 2014; Koike et al, 2017; Tan et al, 2017). The MBB assay uses monobromobimane for efficient H2S capture, followed by LC–MS quantification of bimanylated H2S. We observed that the cellular H2S level was upregulated by R406 treatment (Fig 5A). This result was confirmed by methylene blue method, which is another assay for measuring the cellular H2S level (Fig EV5A). Then, we asked whether H2S could regulate chemical reprogramming. We treated cells with hydroxylamine (HA), an inhibitor of cystathionine γ‐lyase (Cth), and cystathionine β synthase (Cbs) (Asimakopoulou et al, 2013) and found that HA could decrease the cellular level of H2S (Fig 5B) and significantly inhibit reprogramming (Fig 5C). In addition, HA notably decreased the expression of early pluripotent genes Sall4, Cdh1, Epcam, and Esrrb (Fig 5D). On the other hand, N‐acetyl cysteine (NAC), which acts as a direct precursor of cysteine and can trigger intracellular H2S production mediated by Cth, Cbs, and 3‐mercaptopyruvate sulfurtransferase (Mpst) (Ezerina et al, 2018), increased cellular H2S level, and promoted chemical reprogramming (Figs 5B, E and F, and EV5B). We also found that NAC or HA treatment could promote or inhibit TF‐induced reprogramming, respectively (Fig EV5C and D). Next, we tested the effects of NAC in human cells and observed that NAC treatment could not increase human reprogramming efficiency (Fig EV5E), which was consistent with the previous observation (Ji et al, 2014). This result again suggested that there are differences between mouse and human somatic cell reprogramming. We knocked down Cbs using shRNA, and found that shCbs reduced H2S levels, and blocked chemical reprogramming (Fig 5G–K). Knockdown of Cth also significantly inhibited chemical reprogramming (Fig EV5F–I). In addition, overexpression of Cbs could promote chemical reprogramming (Fig 5L–N). Collectively, upregulation of cellular H2S level promotes chemical reprogramming.
Figure 5. R406 can upregulate cellular H2S level and H2S can promote chemical reprogramming.
-
AH2S levels in DMSO‐ and R406‐treated reprogramming intermediates detected by monobromobimane method. n = 4.
-
BH2S levels in reprogramming intermediates treated with DPBS, HA, and NAC. n = 3.
-
CImmunofluorescence of Sall4 in reprogramming intermediates treated by R406 + HA and R406 + DPBS on d12. n = 3. Scale bar, 100 μm.
-
DRT–qPCR analysis of Sall4, Cdh1, Epcam, and Esrrb gene expression in DPBS‐ and HA‐treated cells on d12. n = 3.
-
EImmunofluorescence of Sall4 in NAC‐ and DPBS‐treated cells on d12. n = 5. Scale bar, 100 μm.
-
FRT–qPCR analysis of Sall4, Cdh1, Epcam, and Esrrb gene expression in DPBS‐ and NAC‐treated cells on d12. n = 3.
-
GH2S levels in reprogramming intermediates treated with shNC and shCbs. n = 3.
-
HDiagram showing the procedure of reprogramming infected with shRNA for Cbs.
-
IRT–qPCR analysis of Cbs expression in MEFs infected with shNC and shCbs. n = 3.
-
J, KImmunofluorescence of Sall4 in shNC‐ and shCbs‐infected cells with and without R406 treatment on d12. n = 5. Scale bar, 100 μm.
-
LDiagram showing the procedure of reprogramming infected with Cbs‐OE (pSin‐Cbs) virus.
-
MRT–qPCR analysis of Cbs expression in MEFs infected with pSin‐GFPN and pSin‐Cbs viruses. n = 3.
-
NImmunofluorescence of Sall4 in WT cells and Cbs overexpressing cells on d12. n = 4. Scale bar, 100 μm.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. See also Fig EV5.
R406 addition and H2S production promote chemical reprogramming through modulating redox homeostasis
H2S has profound biological effects within living organisms and is now increasingly considered as an important gaseous signaling molecule (Rose et al, 2017). Cellular H2S plays many essential roles in cell signaling, metabolic regulation, and post‐translational modifications. Recent studies have revealed new insights into the biology of H2S within the cardiovascular organs, neurobiology, inflammation, aging, and age‐related diseases (Liu et al, 2014; Hine et al, 2015; Yang et al, 2015; Das et al, 2018; Longchamp et al, 2018; Paul et al, 2018). H2S has been reported to regulate mitochondrial complex IV and inhibit oxidative phosphorylation (OXPHOS) (Gao et al, 2015; Longchamp et al, 2018). Whether H2S production after R406 treatment can regulate redox homeostasis during reprogramming is unknown.
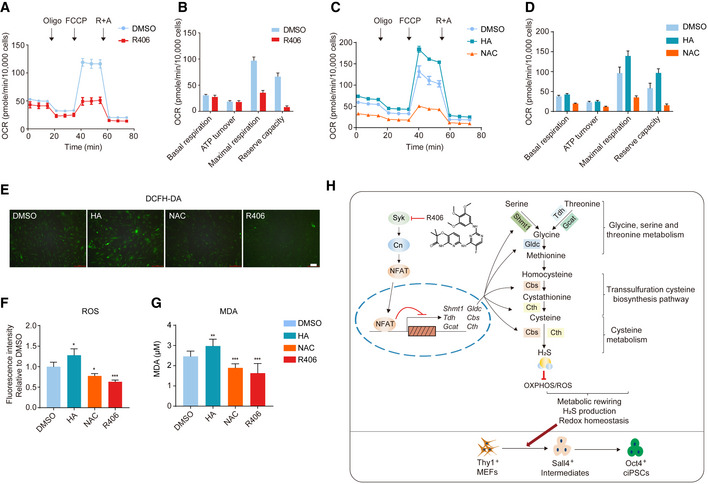
We checked the OXPHOS activity of chemical reprogramming intermediates after R406 treatment by detecting the oxygen consumption rate (OCR). As shown in Fig 6, the basal respiration level was slightly lower after R406 treatment (Fig 6A and B). Notably, maximal respiration and reserve capacity were significantly repressed by R406 (Fig 6A and B). On the other hand, HA treatment upregulated OCR (Fig 6C and D). We could also observe that OCR was significantly repressed by NAC treatment (Fig 6C and D). These results suggested that cellular H2S upregulated by R406 in turn inhibited OXPHOS activity. Mitochondrial OXPHOS is the major cellular source of ROS production. Next, we checked the ROS levels after R406, NAC, and HA treatments. Consistently, we found that R406 and NAC reduced, while HA increased the ROS levels during chemical reprogramming (Fig 6E and F). Furthermore, we checked lipid peroxidation, a hallmark of oxidative stress, by determining the levels of malondialdehyde (MDA) (Fafian‐Labora et al, 2020). The result demonstrated that R406 and NAC treatments decreased, while HA treatment increased the levels of MDA (Fig 6G). Thus, R406 and its downstream H2S production could modulate redox homeostasis during chemical reprogramming.
Figure 6. R406 and H2S modulate redox homeostasis during chemical reprogramming.
-
A–DQuantification of oxygen consumption rate (OCR) of reprogramming intermediates treated with small molecules (A, C) and the indicated OCR parameters (B, D). n = 9.
-
E, FFluorescence images of DCFH‐DA staining for ROS levels in cells treated with DMSO, HA, NAC, and R406 on d12 (E) and the quantification of the ROS levels (F). n = 4. Scale bar, 100 μm.
-
GMDA quantification in cells treated with DMSO, HA, NAC, and R406 on d12. n = 9.
-
HA summary diagram of this study.
Data information: All data are presented as mean ± SD. Statistical significance was assessed by the two‐tailed Student’s t‐test, *P < 0.05, **P < 0.01, ***P < 0.001.
Collectively, from all these data, we discovered a novel mechanism of cell‐fate conversion that Syk inhibitor R406 alleviated the Syk‐Cn‐NFAT signaling cascade‐mediated suppression of glycine, serine and threonine metabolic genes, upregulated metabolites of glycine, serine and threonine metabolism and downstream transsulfuration cysteine biosynthesis pathway and cysteine metabolism, and increased cellular H2S level in particular. In turn, upregulated cellular H2S modulated OXPHOS activity and redox homeostasis, and promoted chemical reprogramming (Fig 6H).
Discussion
In this study, through chemical screening, we identified R406 as a new chemical compound that can significantly promote mouse chemical‐induced pluripotent reprogramming. An efficient chemical reprogramming system will save time and reagents, make experiments more controllable and consistent, and help us generate the roadmaps of chemical reprogramming and further investigate the underlying molecular mechanisms. In the future, besides pluripotent reprogramming, it is also interesting to test whether R406 can promote lineage‐specific direct reprogramming and directed differentiation (Zhou et al, 2020).
After further mechanistic investigations, we have identified a previously undiscovered connection between Syk‐Cn‐NFAT and glycine, serine and threonine metabolism, transsulfuration cysteine biosynthesis pathway, and cysteine metabolism. Our data demonstrated that NFAT can directly bind and transcriptionally suppress the essential genes involved in these metabolic pathways, which has not been reported in literature. Considering the important roles of Syk‐Cn‐NFAT plays in various biological processes, including immunity and tissue homeostasis, it will be interesting to test these downstream metabolic pathways in other biological processes.
Previously, glycine, serine, and threonine metabolism has been studied in pluripotent maintenance by generating SAM, which can promote histone methylations (Shyh‐Chang et al, 2013), but its roles in reprogramming remain largely unknown. We initially suspected that SAM would be upregulated, but further metabolic analysis suggested the upregulation of metabolites of the downstream transsulfuration cysteine biosynthesis pathway and cysteine metabolism. Only very recently, transsulfuration activity has been identified to restore cellular GSH level and support cancer cell growth upon extracellular cysteine limitation (Zhu et al, 2019). Interestingly, we found that after R406 treatment, H2S rather than GSH was notably increased. In turn, upregulated cellular H2S modulated redox homeostasis, and promoted chemical reprogramming. In recent years, H2S is increasingly considered as an important gaseous signaling molecule (Rose et al, 2017). Many studies have revealed new insights into the biology of H2S within various biological processes and diseases (Hine et al, 2015; Das et al, 2018; Longchamp et al, 2018; Paul et al, 2018). Recent studies suggested that H2S can mediate protein sulfhydration, and the sulfhydration of cysteine residues in proteins plays important roles in diverse biological processes (Liu et al, 2014; Gao et al, 2015; Yang et al, 2015; Paul et al, 2018; Zivanovic et al, 2019). New technologies for evaluating protein sulfhydration need to be developed, will undoubtedly provide better understanding of this evolutionarily conserved post‐translational modification, and help discover important functions of H2S in various biological processes, including cardiovascular diseases, neurodegeneration, metabolic diseases, cancer, and aging.
In summary, our studies not only have application in improving the chemical reprogramming technique, but also identified novel molecular mechanisms of chemical‐induced pluripotent reprogramming, which opens new areas for investigation. The pluripotent reprogramming itself represents a valuable approach to study general mechanisms of cell‐fate transition. Deciphering the molecular mechanisms of mouse chemical reprogramming will generate fundamental insights into cell‐fate decisions and in turn will provide innovative strategies to make the pluripotent and lineage reprogramming process rapid and efficient (Xu et al, 2015; Liu et al, 2016). More chemical screenings are required for achieving human chemical reprogramming, which will not only stimulate innovations for producing functional cells for regenerative medicine, but also generate novel strategies to pharmaceutically stimulate in vivo rejuvenation and regeneration.
Materials and Methods
Mice
Mice were housed in specific pathogen‐free conditions at the Laboratory Animal Center of Zhejiang University and fed a normal chow diet. All animal‐related procedures were performed under an approved protocol by Experimental Animal Ethics Committee of Zhejiang University.
The OG2 mice were mated with 129 mice to generate offsprings carrying Oct4 promoter‐driven GFP reporter (129xOG2 mice). Mouse embryonic fibroblasts (MEFs) were isolated from E13.5 embryos of 129xOG2 mice.
Cell culture
Mouse embryonic fibroblasts were cultured in MEF medium containing DMEM with 10% fetal bovine serum (FBS) and 1% penicillin‐streptomycin (P/S). Mouse ESCs and ciPSCs were maintained on feeder layers of mitomycin C‐treated CF1 MEFs in mESC medium containing DMEM, 10% knockout serum replacement (KSR), 10% FBS, 1% nonessential amino acids (NEAA), 0.055 mM 2‐mercaptoethanol (β‐ME), 1% P/S, 1,000 U/ml leukemia inhibitory factor (LIF), 3 µM CHIR99021, and 0.2 µM PD0325901. 293T cells were cultured in MEF medium. All cells were maintained at 37°C with 5% CO2.
Mouse chemical reprogramming
About 4 × 105 MEFs (at passage 3) were seeded onto one plate and cultured in MEF medium. One day later (d0), medium was changed to Stage 1 medium. Stage 1 medium contains DMEM, 10% KSR, 10% FBS, 1% NEAA, 1% P/S, 0.055 mM β‐ME and 20 ng/ml bFGF, 0.5 mM VPA, 20 μM CHIR99021, 10 μM 616452, 5 μM Parnate, 50 μM Forskolin, 0.5 μM AM580, 5 μM EPZ004777, and 250 μM vitamin C (Vc). Stage 1 medium was changed every 4 days for 12 days. Next, the reprogramming intermediates were treated with Stage 2 medium. Stage 2 medium contains DMEM, 10% KSR, 10% FBS, 1% NEAA, 1% P/S, 0.055 mM β‐ME, 20 ng/ml bFGF, 0.5 mM VPA, 10 μM CHIR99021, 10 μM 616452, 5 μM Parnate, 10 μM Forskolin, 0.5 μM AM580, 0.05 μM DZNep, 0.5 μM 5‐aza‐dC, 5 μM SGC0946, and 250 μM Vc. Stage 2 medium was changed every 4 days for 12 days. On day 24, Stage 3 medium was applied. Stage 3 medium contains 47% DMEM/F12, 47% Neurobasal medium, 1 × N2 supplement, 1 × B27 supplement, 1% NEAA, 1% P/S, 0.055 mM β‐ME, 1,000 U/ml LIF, 3 µM CHIR99021, and 0.2 µM PD0325901. Stage 3 medium was changed every 4 days for 12–16 days. Mouse ciPSC lines were established from GFP+ colonies with typical iPSC morphology. The information of reagents is listed in Table 1.
Table 1.
Reagents and tools
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Anti‐Sall4 | Santa Cruz | Cat#sc‐166033 |
| Anti‐Sox2 | Stemgent | Cat#09‐0024 |
| Anti‐Oct4 | Santa Cruz | Cat#sc‐5279 |
| Anti‐Nanog | Abcam | Cat#ab80892 |
| Anti‐Tuj1 | BioLegend | Cat#801201 |
| Anti‐ɑ‐SMA | Sigma‐Aldrich | Cat#A2547 |
| Anti‐Foxa2 | Sigma‐Aldrich | Cat#07‐633 |
| Alexa Fluor 488 donkey anti‐mouse lgG (H + L) | Invitrogen | Cat#A21202 |
| Alexa Fluor 555 donkey anti‐mouse lgG (H + L) | Invitrogen | Cat#A31570 |
| Alexa Fluor 555 donkey anti‐rabbit lgG (H + L) | Invitrogen | Cat#A31572 |
| Anti‐IL6 | Invitrogen | Cat#14‐7061‐85 |
| Anti‐Flag | Sigma‐Aldrich | Cat#F1804 |
| Anti‐NFATc4 | Invitrogen | Cat# PA1‐021 |
| Goat anti‐mouse IgG | Kitgen | Cat#GMI06 |
| Bacterial strains | ||
| DH5ɑ | ANGYUBIO | Cat#AYBIO‐G6016 |
| Chemicals, peptides, and recombinant proteins | ||
| R406 | APExBIO | Cat#A5880 |
| FK506 | TargetMol | Cat#T2144 |
| R788 | MCE | Cat#HY‐13038B |
| Lenvatinib | MCE | Cat#HY‐10981 |
| Sunitinib | MCE | Cat#HY‐10255A |
| Crotonic acid | Sigma‐Aldrich | Cat#802650 |
| BrdU | TargetMol | Cat#T6794 |
| Polybrene | Santa Cruz | Cat#sc‐134220 |
| Valproic acid | MCE | Cat#HY‐10585A |
| CHIR99021 | TargetMol | Cat#T2310 |
| 616452 | TargetMol | Cat#T6337 |
| Parnate | TargetMol | Cat#T7942 |
| Forskolin | TargetMol | Cat#T2939 |
| AM580 | MCE | Cat#HY‐10475 |
| EPZ004777 | APExBIO | Cat#A4170 |
| Vitamin C | Sigma‐Aldrich | Cat#A8960 |
| 3‐deazaneplanocin A | APExBIO | Cat#A1905 |
| 5‐aza‐dC | TargetMol | Cat#T1339 |
| SGC0946 | TargetMol | Cat#T3082 |
| PD0325901 | TargetMol | Cat#T6189 |
| Hydroxylamine | Sigma‐Aldrich | Cat#467804 |
| NAC | Sigma‐Aldrich | Cat#A9165 |
| l‐serine (1–13C) | Cambridge Isotope Laboratories | Cat#CLM‐1573‐PK |
| l‐Serine | Sigma‐Aldrich | Cat#S4500 |
| l‐Glycine | Sigma‐Aldrich | Cat#G7126 |
| l‐Threonine | Sigma‐Aldrich | Cat#T8441 |
| Monobromobimane | Sigma‐Aldrich | Cat#B4380 |
| Sulfide dibimane | DOJINDO | Cat#SB15 |
| Recombinant mouse IL‐6 | R&D system | Cat#406‐ML‐005 |
| Mouse LIF | Sigma‐Aldrich | Cat#ESG1107 |
| bFGF | PeproTech | Cat#100‐18B |
| DMEM basic | Gibco | Cat#C11995500CP |
| Penicillin‐Streptomycin | Gibco | Cat#15140‐122 |
| Non‐essential amino acids | Gibco | Cat#11140‐050 |
| 2‐Mercaptoethanol | Sigma‐Aldrich | Cat#M3148 |
| Fetal bovine serum | Gibco | Cat#10270‐106 |
| Knockout serum replacement | Gibco | Cat#10828‐028 |
| Assay kits | ||
| FastPure Cell/Tissue Total RNA Isolation Kit | Vazyme | Cat#RC101‐01 |
| Endo‐Free Plasmid Mini Kit | Omega | Cat#D6950 |
| Agarose Gel DNA Extraction Kit | Easydo | Cat#0103050 |
| BCIP/NBT Alkaline Phosphatase Color Development Kit | Beyotime | Cat#C3206 |
| NovoNGS® CUT&Tag 2.0 High‐Sensitivity Kit (for Illumina®) | Novoprotein | Cat#N259‐YH01 |
| GSH and GSSG Assay Kit | Beyotime | Cat#S0053 |
| BCA Protein Assay Kit | Beyotime | Cat#P0012 |
| Reactive Oxygen Species Assay Kit | Beyotime | Cat#S0033S |
| XFp Cell Mito Stress Test Kit | Agilent Technologies | Cat#103010‐100 |
| Lipid Peroxidation MDA Assay Kit | Beyotime | Cat#S0131S |
| Deposited data | ||
| RNA‐seq data | This paper | GEO: GSE154687 |
| CUT&Tag data | This paper | GEO: GSE154687 |
| Experimental models: Cell & mouse lines | ||
| Mouse: S2/SvPasCrl (129) | Beijing Vital River Laboratory, Animal Technology | Stock#217 |
| Mouse: CBA‐Tg (Pou5f1‐EGFP)2Mnn/J (OG2) | Xiaoyang Zhao Lab, School of Basic Medical Sciences, Southern Medical University, China | NA |
| Mouse: CB17. Cg‐PrkdcscidLystbg‐J/Crl (SCID Beige) | Beijing Vital River Laboratory, Animal Technology | Cat#405 |
| Oligonucleotides | ||
| shRNA: CTR (sense): 5′‐CCGGCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTG‐3′ | This paper | N/A |
| shRNA: CTR (antisense): 5′‐AATTCAAAAACCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG‐3′ | This paper | N/A |
| shRNA: Syk (sense): 5′‐CCGGGGAGAACCTCATCAGGGAATACTCGAGTATTCCCTGATGAGGTTCTCCTTTTTG‐3′ | This paper | N/A |
| shRNA: Syk (antisense): 5′‐AATTCAAAAAGGAGAACCTCATCAGGGAATACTCGAGTATTCCCTGATGAGGTTCTCC‐3′ | This paper | N/A |
| shRNA: Ppp3ca (sense): 5′‐CCGGTGTGATGAACATCAGGCAGCTCGAGCTGCCTGATGTTCATCACATTTTTG‐3′ | This paper | N/A |
| shRNA: Ppp3ca (antisense): 5′‐AATTCAAAAATGTGATGAACATCAGGCAGCTCGAGCTGCCTGATGTTCATCACA‐3′ | This paper | N/A |
| shRNA: Nfatc1 (sense): 5′‐CCGGGGTCAGTGTGACCGAAGATACCTCGAGGTATCTTCGGTCACACTGACCTTTTTG‐3′ | This paper | N/A |
| shRNA: Nfatc1 (antisense): 5′‐AATTCAAAAAGGTCAGTGTGACCGAAGATACCTCGAGGTATCTTCGGTCACACTGACC‐3′ | This paper | N/A |
| shRNA: Gldc (sense): 5′‐CCGGTAGGGTCTTCATTCAAGAGAACTCGAGTTCTCTTGAATGAAGACCCTATTTTTG‐3′ | This paper | N/A |
| shRNA: Gldc (antisense): 5′‐ AATTCAAAAATAGGGTCTTCATTCAAGAGAACTCGAGTTCTCTTGAATGAAGACCCTA‐3′ | This paper | N/A |
| shRNA: Cbs (sense): 5′‐CCGGATCACAGGGATCGCCAGAAAGCTCGAGCTTTCTGGCGATCCCTGTGATTTTTTTG‐3′ | This paper | N/A |
| shRNA: Cbs (antisense): 5′‐AATTCAAAAAAATCACAGGGATCGCCAGAAAGCTCGAGCTTTCTGGCGATCCCTGTGAT‐3′ | This paper | N/A |
| shRNA: Cth (sense): 5′‐CCGGGCCCAGGGATGGTCAGTTTCTCGAGAAACTGACCATCCCTGGGCTTTTTTG‐3′ | This paper | N/A |
| shRNA: Cth (antisense): 5′‐AATTCAAAAAAGCCCAGGGATGGTCAGTTTCTCGAGAAACTGACCATCCCTGGGC‐3′ | This paper | N/A |
| sgRNA: Syk sg‐1 (sense): 5′‐CACCGCGCCAGAGCCGCAATTACCT–3′ | This paper | N/A |
| sgRNA: Syk sg‐1 (antisense): 5′‐AAACAGGTAATTGCGGCTCTGGCG‐3′ | This paper | N/A |
| sgRNA: Syk sg‐2 (sense): 5′‐CACCGACTCCCGGGGGCCGGTTGAA‐3′ | This paper | N/A |
| sgRNA: Syk sg‐2 (antisense): 5′‐AAACTTCAACCGGCCCCCGGGAGT‐3′ | This paper | N/A |
| Primers for RT–qPCR, see Table 2 | This paper | N/A Software and Algorithms |
| Software and algorithms | ||
| GraphPad Prism | GraphPad Software | http://www.graphpad.com |
| SnapGene | https://www.snapgene.com/ | |
| Rstudio | https://rstudio.com/ | |
| Wave | Agilent | https://www.agilent.com/zh‐cn/products/cell‐analysis/software‐download‐for‐wave‐desktop |
| HISAT2 (v2.1.0) | http://ccb.jhu.edu/software/hisat2/manual.shtml | |
| Stringtie (v2.0) | http://ccb.jhu.edu/software/stringtie/ | |
| Bowtie2 (v2.2.5) | http://bowtie‐bio.sourceforge.net/bowtie2/index.shtml | |
| MACS2 (v2.1.2) | https://pypi.org/project/MACS2/ | |
Mouse TF‐induced reprogramming
Mouse embryonic fibroblasts were plated onto 6‐well plates and cultured in MEF medium to 60–80% confluence, and infected twice with lentivirus expressing Oct4, Sox2, Klf4, and c‐Myc. After 24 h of infection, cells were replated onto 24‐well plates (3 × 105 cells/plate) and cultured with MEF medium. Next day, medium was switched to reprogramming medium (DMEM, 10% KSR, 10% FBS, 1% NEAA, 1% P/S, 0.055 mM β‐ME, and 1,000 U/ml mLIF) with 2 μg/ml doxycycline (Dox). Medium was changed again on day 4, and GFP+ colonies were counted on day 8.
Human TF‐induced reprogramming
Human fibroblasts were derived from H9 (WA09, which were purchased from WiCell Research Institute) as previously reported (Park et al, 2008). Human fibroblasts were cultured in MEF medium supplemented with vitamin C (MEF + Vc medium) and infected twice with lentivirus expressing OCT4, SOX2, KLF4, and c‐MYC. After 24 h of infection, cells were replated onto 24‐well plates (4 × 105 cells/plate) and cultured in MEF + Vc medium. Next day, cultures were changed to reprogramming medium (DMEM, 10% KSR, 10% FBS, 1% NEAA, 1% P/S, 0.055 mM β‐ME, 10 ng/ml bFGF, 0.5 mM VPA, 10 μM 616452, and 0.2 μM PD0325901) with 2 μg/ml Dox, and the medium was changed on day 4. Colony number was counted on day 8.
High throughput chemical screening
Mouse embryonic fibroblasts were seeded onto 24‐well plates and treated with small molecules from the kinase inhibitor library (MCE) for 12 days (one well for each compound). Then, Stage 2 medium was applied for another 12 days. Finally, GFP+ colonies were counted on day 24.
RT–qPCR assay
Total RNA was isolated using FastPure Cell/Tissue Total RNA Isolation Kit (Vazyme) and converted to cDNA using Reverse Transcriptase Kit (Takara). RT–qPCR was performed with SYBR RT‐qPCR Kit (Takara) on CFX ConnectTM Real‐Time system (Bio‐Rad). Primer sequences are listed in Table 2.
Table 2.
Primer sequences
| Gene locus | Forward primer | Reverse primer |
|---|---|---|
| Sall4 | TGGCAGACGAGAAGTTCTTTC | TCCAACATTTATCCGAGCACAG |
| Cdh1 | CAGCCTTCTTTTCGGAAGACT | GGTAGACAGCTCCCTATGACTG |
| Esrrb | GTGGCTGAGGGCATCAATG | AACCGAATGTCGTCCGAAGAC |
| Epcam | GCGGCTCAGAGAGACTGTG | CCAAGCATTTAGACGCCAGTTT |
| Gapdh | CCTTCATTGACCTCAACTACA | TAGACTCCACGACATACTCA |
| Il6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| Syk | CCTGATGTGGGAAGCGTTCT | GGTCACTTCGCTCCCTTTCA |
| Ppp3ca | TTCGCGGAAACCATGAATGTA | GGCAGTCGAAGGCATCCAT |
| Nfatc1 | GGAGAGTCCGAGAATCGAGAT | TTGCAGCTAGGAAGTACGTCT |
| Cbs | GGCCTGATACCCCAAGCAG | GGTGTTCCCAATTTTCCTCAGAA |
| Gldc | AGCATCCGCTTGAAGAGACC | CCCATGCCAATATACGACCTC |
| Tdh | CTGGCTGTTTCACTACAGTGC | GGAGAGGTAGGTCCAAAGGC |
| Shmt1 | CAGGGCTCTGTCTGATGCAC | CGTAACGCGCTCTTGTCAC |
| Cth | TTCCTGCCTAGTTTCCAGCAT | GGAAGTCCTGCTTAAATGTGGTG |
| Gldc −164 | ATCAGGCAGGGGACTCCA | ATGGGAATGGGTTGCAGGG |
| Gldc −875 | GCCAATATACCAACAGCATACTCA | TCTCCCTCCCTCCTTTGTATGT |
| Cbs −797 | AAACAGGCTCTCCTTCCAGC | AGGGCCACATCTTGTTCCAG |
| Cbs −1087 | TGGCAGCTTGTGTGTCTACAG | TACGGTGCCCTTAAGAGAAGA |
| Tdh −55 | AGATGGGAGCCCTTTGGTCG | GCAAGCACTAAGCAGGATGG |
| Tdh −450 | GGGCCATAAAAGGCTACCCA | TTCCCAAGGCCGATTGACTT |
| Gapdh +294 | GGTCTAGGGATGCTGGTGC | AGATTAGCGTGGCCCGAAG |
Immunofluorescence
Cells were fixed with 4% PFA for 10 min, washed with PBST for 3 times, then blocked in blocking buffer (PBST + 5% BSA) for 0.5–1 h. Primary antibodies were diluted as the manufacturer’s recommendation in blocking buffer (anti‐Sall4, 1:1,000; anti‐Sox2, 1:2,000; anti‐Oct4, 1:500; anti‐Nanog, 1:500; anti‐Tuj1, 1:5,000; anti‐SMA, 1:2,000; anti‐Foxa2, 1:1,000; anti‐Flag, 1:100; anti‐NFATc4, 1:100) (Table 1). Cells were incubated with primary antibodies overnight at 4°C, then washed with PBST for 3 times. Cells were then incubated with secondary antibodies (1: 2,000) in blocking buffer for 1 h at RT. The cells were washed twice with PBST and imaged using fluorescence microscope (Zeiss).
AP staining
AP staining was performed using Alkaline Phosphatase Assay Kit (Beyotime) according to the manufacturer’s instruction.
Plasmid construction and lentivirus preparation
For shRNA or sgRNA plasmid construction, single‐strand DNA was synthesized from Sangon Biotech. After annealing, the insert DNA was ligated with pLKO.1 or plentiCRISPRv2 vector, and then transformed into DH5α. For gene expression, CDS sequences were amplified from cDNA library and linked with pSin vector or TetO‐Flag‐FUW vector. Nfatc1 CDS was amplified from PLEX‐HA‐NFATc1 plasmid. A fragment of 462 bp at N‐terminal of GFP was amplified and linked to pSin vector (pSin‐GFPN) as a control for gene expression. For lentivirus production, 293T cells were plated onto 6‐well plates and cultured with MEF medium to 60–80% confluence. For each well, dilute 2 μg DNA (1 μg lentivirus plasmid, 0.75 μg psPAX packaging plasmid and 0.25 μg pMD envelope plasmid) and 6 μl Fugene transfection reagent separately with 150 μl Opti‐MEM medium, add the DNA mixture into the Fugene mixture 5 min later. After 15 min incubation, add the mixture into the well drop by drop and mix gently. After 8 h, change to MEF medium. At 48 and 72 h, viruses were collected, filtered, and stocked at −80°C.
Stable gene knockdown and overexpression
About 4 × 105 MEFs were seeded onto a 24‐well plate and cultured in Stage 1 medium. On day 2, cells were infected with shRNA viruses, sgRNA viruses or pSin‐Cbs overexpression viruses with 5 μg/ml polybrene for 4 h. On day 12, Sall4+ colony number was counted as readout.
Embryoid body differentiation
Mouse ciPSCs (at passage 7) were seeded onto low‐attached plates to form aggregates and cultured in EB medium containing DMEM, 10% KSR, 10% FBS, 1% P/S, 1% NEAA, and 0.055 mM β‐ME for 7 days. Next, embryoid bodies were transferred onto 6‐well plates, cultured in EB medium for another 2 weeks. The differentiation capacity was evaluated by immunostaining.
Teratoma assay
About 1 × 106 ciPSCs were mixed with 100 μl Matrigel and subcutaneously injected into the dorsal flank of SCID Beige mice. After 4 weeks, teratomas were surgically dissected and analyzed with H&E staining.
Chimera assay
Chimera assay was done as described previously (Yuan et al, 2011; Liu et al, 2018).
Karyotyping analysis
ciPSCs at passage 7 and passage 15 were analyzed using G‐banding at the Department of Prenatal Diagnosis (Screening) Center of Hangzhou Women's Hospital (Hangzhou Maternity and Child Health Care Hospital).
RNA‐seq analysis
Total RNA was isolated and used for RNA‐seq analysis. cDNA library construction and high‐throughput sequencing were performed by Novogene. A total amount of 3 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB, USA) following manufacturer’s recommendations, and paired‐end sequencing was carried out with the Illumina NovaSeq 6000 with read length of 150 bp. The raw reads were aligned to the mouse genome (GRCm38, mm10) using HISAT2 (v2.1.0) with the default parameter settings. Transcript assembly was performed by stringtie (v2.0) and expression of transcripts sharing each gene_id was quantified as transcripts Per Million (TPM). The differential expression analysis was performed by R package DESeq2 (Love et al, 2014) from the bioconductor project (http://www.bioconductor.org/). KEGG pathway enrichment analysis was performed with R package clusterProfiler (Yu et al, 2012) from the bioconductor project. Heatmaps were generated by R package pheatmap from CRAN (https://cran.r‐project.org/).
CUT&Tag assay
MEFs were infected with rtTA and TetO‐Flag‐Nfatc1 lentiviruses and passaged at 4 × 105 per plate. MEFs were cultured with Stage 1 + Dox medium and treated with DMSO or R406 for 4 days. CUT&Tag assay was performed using NovoNGS® CUT&Tag High‐Sensitivity Kit (Novoprotein) according to the manufacturer’s recommendation. Briefly, NovoNGS® ConA Beads were pipetted into a new tube, 10 μl per sample, and washed by ConA Binding Buffer. 1 × 105 cells on d4 were harvested and washed by Wash buffer. The cells were mixed with ConA Beads and incubated with Monoclonal ANTI‐FLAG® M2 antibody (Sigma) (1:50) or without primary antibody (Blank) overnight at 4°C. Goat anti‐mouse IgG antibody (Kitgen) was diluted at 1:500 and added into the sample; then, the sample was incubated for 1 h at RT. After washing away the unbounded secondary antibody, NovoNGS® ChiTag™ 2.0 Transposome was added and incubated with cells for 1 h at RT. The sample was washed by ChiTag Buffer and followed with tagmentation by Tagmentation Buffer. Next, DNA was isolated by NovoNGS® DNA Clean Beads and dissolved in TE‐RA‐Buffer. DNA was amplified with N5 and N7 primers and purified with NovoNGS® DNA Clean Beads for high‐throughput sequencing. Paired‐end sequencing was carried out with the Illumina NovaSeq 6000 with read length of 150 bp (Novogene). Paired‐end reads were aligned using Bowtie2 (v2.2.5) with options: ‐‐local ‐‐very‐sensitive‐local ‐‐no‐unal ‐‐no‐mixed ‐‐no‐discordant ‐‐phred33 ‐I 10 ‐X 700. For peak calling, parameters used were macs2 (v2.1.2) callpeak ‐p 1e‐5 ‐f BAMPE ‐g hs ‐n sample‐name ‐B ‐‐keep‐dup all. The peaks visualization in genome was showed by IGV software (Robinson et al, 2011). And the peaks annotations and heatmaps were performed with R package ChIPseeker (Yu et al, 2015) from the bioconductor project. Motif enrichment was done using HOMER with options: ‐fdr 0.25 ‐len 8,10,12, and motif visualization was generated by using R package Logolas (Dey et al, 2018) from the bioconductor project.
CUT&Tag qPCR
Part of the purified DNA from CUT&Tag assay was diluted 8 times and used for qPCR. The NFATc1 binding sites at the gene promoter regions were predicted by JASPAR (http://jaspar.genereg.net/) and primers were designed by NCBI (https://www.ncbi.nlm.nih.gov/tools/primer‐blast/). Given that R406 treatment did not influence the expression of Gapdh gene and the binding of NFATc1, NFATc1 binding site at the Gapdh gene locus (Gapdh +294) was amplified and used as internal reference. Primer sequences are listed in Table 2.
Metabolite quantification
About 8 × 106 cells were harvested, frozen by liquid nitrogen, and preserved at −80°C. 6 replicates were prepared for each sample. Quasi‐Targeted Metabolomics analysis was performed by Novogene based on LC‐MS/MS.
Isotope labeling and [1–13C]‐serine tracing using liquid chromatography‐mass spectrometry (LC‐MS)
DMEM with isotopic serine was prepared from scratch following the standard DMEM formula except without serine and supplemented with 1–13C serine (400 μM), and the isotope labeled DMEM was used to prepare Stage 1 medium with 13C labeling. Cells were isotope labeled on d12. 2 h later, cells were lysed for LC‐MS analysis.
Intracellular metabolites were extracted using a method described previously (Tu et al, 2007; Ye et al, 2019) with some modifications. Briefly, after aspiring culture medium completely, cells were quenched immediately by floating culture dishes on liquid nitrogen. −40°C 60% methanol was added, and cells were scraped off and transferred to 1.5 ml screw‐cap tubes. Cells then were lysed and extracted with 5 freeze‐and‐thaw cycles. Soluble metabolites were separated by centrifugation and dried with a CentriVap Concentrator system (Labconco). For running samples, dried extracts were resuspended in 100 μl 60% acetonitrile for injection. Labeled serine, cystine, and cystathionine were analyzed with the targeted parent and daughter ions specific to the 13C forms of the metabolites using reversed‐phase HPLC coupled to a triple quadrupole mass spectrometer (Triple Quad 6500+, AB SCIEX). Metabolites were separated chromatographically on a C18‐based column with polar embedded groups (Synergi Fusion‐RP, 150 × 2.00 mm 4 µm, Phenomenex). Flow rate was 0.2 ml/min using the following method: Buffer A: 99.9% H2O/0.1% formic acid, Buffer B: 99.9% acetonitrile /0.1% formic acid. T = 0 min, 0% B; T = 2 min, 0% B; T = 7 min, 30% B; T = 9 min, 100% B; T = 11 min, 100% B, T = 12 min, 0% B; T = 15 min, stop. The retention time for each MRM peak was compared to an appropriate standard. The area under each peak was then quantitated by using Analyst software and reinspected for accuracy.
H2S detection
Monobromobimane (MBB) method: cells on d12 were harvested with three replicates for H2S detection and 1 replicate for protein detection. Intracellular H2S contents were detected as described previously (Shen et al, 2014; Koike et al, 2017; Tan et al, 2017) with some modifications. Cells were resuspended in 50% methanol and were repeatedly snap frozen in liquid nitrogen and thawed at 37°C three times. Samples were centrifuged at 1,000 g for 15 min at 4°C. 30 μl supernatant was mixed with 10 μl Tris–HCl buffer (100 mM, pH = 8.5), 10 μl EDTA solution (2 mg/ml, pH = 8.5), and 70 μl MBB in acetonitrile (20 mM), and the mixture was incubated for 30 min in the dark at room temperature. 10 μl 20% formic acid was added to stop the reaction, and samples then were incubated on ice for 15 min in the dark. Centrifuge samples at 12,000 g for 10 min at 4°C and take the supernatant to repeat the centrifugation. The supernatant was directly analyzed by LC‐MS/MS.
LC‐MS/MS analysis: Chromatography was performed using a Shimadzu 20A System with a Kinetex® C18 column (100 × 2.1 mm, 2.6 μm) from Phenomenex maintained at 40°C. The components were eluted by a gradient of A) 5 nM ammonium acetate solution and B) 100% acetonitrile. The gradient conditions of the mobile phase over 8 min were as follows: 0–1 min, 20% B; 1–4 min, 20–80% B; 4–5.9 min, 80% B; 5.9–6 min, 80–20% B; and 6–8 min, 20% B. The flow rate was 0.2 ml/min. The autosampler was maintained at 4°C throughout the analyses.
The Shimadzu 20A System was coupled with an AB Sciex Qtrap 6500+ mass spectrometer. Positive ionization mode for electrospray ionization source was used for detection. Nitrogen was used for the curtain and collision gas. Data were collected in multiple reaction monitoring (MRM) mode using transitions of m/z 415.1 → m/z 192.9, m/z 415.1 → m/z 223.1, and m/z 415.1 → m/z 113.2. The dwell time used was 300 ms per channel. Optimized MS acquisition parameters were as follows: collision gas (CAD) medium, curtain gas (CUR) 30 psi, ion source gas 1 (GS1) 35 psi, ion source gas 2 (GS2) 40 psi, ion spray voltage (IS) 5,500 V, source temperature 350°C, declustering potential (DP) 143 eV (m/z 415.1 → m/z 192.9), 122 eV (m/z 415.1 → m/z 223.1), and 138 eV (m/z 415.1 → m/z 113.2), entrance potential (EP) 10 eV, collision energy (CE) 26.1 eV (m/z 415.1 → m/z 192.9), 32.6 eV (m/z 415.1 → m/z 223.1) and 56.8 eV (m/z 415.1 → m/z 113.2), and collision cell exit potential (CXP) 10 eV. The data were acquired and processed using AB Sciex Analyst Processing 1.7 and SCIEX OS 1.7 Software.
Methylene blue method: cells treated with DMSO and R406 on day 8 of reprogramming were used for measuring intracellular H2S levels, with 9 replicates. H2S levels were measured as described previously (Tian et al, 2016).
OCR assay
Seahorse XFp extracellular flux analyzer was used to measure OCR. Cells on d12 of reprogramming were seeded onto 1% gelatin‐coated XFp Cell Culture Miniplate at 2 × 104 cells per well in 80 μl Stage 1 medium (supplemented with DMSO, HA, NAC, or R406, respectively) and incubated overnight at 37°C with 5% CO2. The medium was changed to unbuffered medium supplemented with 2 mM l‐glutamine, 1 mM sodium pyruvate, and 10 mM glucose, and cells were incubated for 1 h at 37°C without CO2 supply. OCR was measured using the XFp Cell Mitochondrial Stress Test kit in response to 2 μM oligomycin, 0.5 μM carbonyl cyanide 4‐(trifluoromethoxy)phenylhydrazone (FCCP), and 2 μM rotenone plus 2 μM antimycin A.
ROS detection
The intracellular ROS generation was measured using the Reactive Oxygen Species Assay Kit (Beyotime). After treatment with small molecules for 12 days, cells were washed with DPBS for 3 times and incubated in DMEM with 5 μM DCFH‐DA for 30 min in the dark at 37°C, followed by three washes with DPBS. Cells were observed immediately with inverted fluorescence microscopy (Olympus). Fluorescence intensity was quantified by ImageJ.
MDA detection
About 1 × 106 cells on d12 of reprogramming were harvested and lysed using 100 μL lysis buffer (Beyotime) following manufacturer’s recommendation supplemented with 100 × protease inhibitor cocktail (Beyotime). MDA was measured based on the color reaction with thiobarbituric acid (TBA) using the Lipid Peroxidation MDA Assay Kit (Beyotime) and detected at 532 nm.
Statistical analysis
The number of independent experimental replications is reported in the figure legends (n). Statistical analysis was performed in GraphPad Prism 7. Statistical significance was assessed by the two‐tailed Student’s t‐test, and P‐value < 0.05 was considered as statistically significant. The data are presented as the mean ± SD.
Author contributions
Study conception and manuscript writing: WYW and SYZ; Experiments: WYW; Bioinformatic analysis: YKL; Chimera assay: SFR and XYZ; H2S detection: JJQ and ZQL; Karyotyping analysis: NNY and HW; Help in experiments: XC, XJM, QD, ZSH, YJ, ZYZ, WYG, YBZ, QL, JQP, GC, and CQY.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Review Process File
Acknowledgments
We thank members of the Zhu laboratory for helpful discussions. We also thank Dr. Lingyong Jiang for providing PLEX‐HA‐Nfatc1 plasmid, and Dr. Xin‐Hua Feng, Dr. Junfang Ji, Dr. Shixian Lin, Dr. Jin Jin, Dr. Xing Guo, Dr. Chao Tong, Dr. Dong Fang, Dr. Bing Yang, and Dr. Dante Neculai for providing experiment reagents and helpful discussions. We thank Xueying Jiang and Core Facilities, Life Sciences Institute of Zhejiang University for providing technical supports. This work was supported by the grants from National Natural Science Foundation of China (No. 31970818), the National Key R&D Program of China (No. 2016YFC1305300 and 2017YFA0105001), and the Outstanding Youth Fund of Zhejiang Province (No. R17C120002).
The EMBO Journal (2021) 40: e106771.
Data availability
RNA‐seq and CUT&Tag sequencing data were deposited at Gene Expression Omnibus under GSE154687 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE154687).
References
- Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A (2013) Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br J Pharmacol 169: 922–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksh S, Widlund HR, Frazer‐Abel AA, Du J, Fosmire S, Fisher DE, DeCaprio JA, Modiano JF, Burakoff SJ (2002) NFATc2‐mediated repression of cyclin‐dependent kinase 4 expression. Mol Cell 10: 1071–1081 [DOI] [PubMed] [Google Scholar]
- Brady JJ, Li M, Suthram S, Jiang H, Wong WH, Blau HM (2013) Early role for IL‐6 signalling during generation of induced pluripotent stem cells revealed by heterokaryon RNA‐Seq. Nat Cell Biol 15: 1244–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Yu S, Li D, Ye J, Yang X, Li C, Wang X, Mai Y, Qin Y, Wu J et al (2018) Chromatin accessibility dynamics during chemical induction of pluripotency. Cell Stem Cell 22: 529–542.e525 [DOI] [PubMed] [Google Scholar]
- Cheng Z‐L, Zhang M‐L, Lin H‐P, Gao C, Song J‐B, Zheng Z, Li L, Zhang Y, Shen X, Zhang H et al (2020) The Zscan4‐Tet2 transcription nexus regulates metabolic rewiring and enhances proteostasis to promote reprogramming. Cell Rep 32: 107877 [DOI] [PubMed] [Google Scholar]
- Chiche A, Le Roux I, von Joest M, Sakai H, Aguin SB, Cazin C, Salam R, Fiette L, Alegria O, Flamant P et al (2017) Injury‐induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell 20: 407–414.e404 [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN (2002) NFAT signaling: choreographing the social lives of cells. Cell 109(Suppl): S67–S79 [DOI] [PubMed] [Google Scholar]
- Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim L‐J, Osborne B, Joshi S, Lu Y et al (2018) Impairment of an endothelial NAD(+)‐H2S signaling network is a reversible cause of vascular aging. Cell 173: 74–89.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey KK, Xie D, Stephens M (2018) A new sequence logo plot to highlight enrichment and depletion. BMC Bioinformatics 19: 473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezerina D, Takano Y, Hanaoka K, Urano Y, Dick TP (2018) N‐Acetyl cysteine functions as a fast‐acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem Biol 25: 447–459.e444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafian‐Labora JA, Rodriguez‐Navarro JA, O'Loghlen A (2020) Small extracellular vesicles have GST activity and ameliorate senescence‐related tissue damage. Cell Metab 32: 71–86.e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmes CD, Dzeja PP, Nelson TJ, Terzic A (2012) Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 11: 596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Tian CL, Ye X, Sheng X, Wang H, Liu Y, Liu L (2018) Dynamics of telomere rejuvenation during chemical induction to pluripotent stem cells. Stem Cell Rep 11: 70–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XH, Krokowski D, Guan BJ, Bederman I, Majumder M, Parisien M, Diatchenko L, Kabil O, Willard B, Banerjee R et al (2015) Quantitative H2S‐mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. Elife 4: e10067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer WR, Gu X, Liu Y, Bottino R, Crabtree GR, Kim SK (2012) Neonatal beta cell development in mice and humans is regulated by calcineurin/NFAT. Dev Cell 23: 21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB (1962) Adult frogs derived from the nuclei of single somatic cells. Dev Biol 4: 256–273 [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK (2010) Simple combinations of lineage‐determining transcription factors prime cis‐regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee B, Brace L, Longchamp A, Treviño‐Villarreal J, Mejia P, Ozaki C et al (2015) Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160: 132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Jaenisch R (2015) Induced pluripotency and epigenetic reprogramming. Cold Spring Harb Perspect Biol 7: a019448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E (2008) NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132: 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, Li Y, Zhang Xu, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K et al (2013) Pluripotent stem cells induced from mouse somatic cells by small‐molecule compounds. Science 341: 651–654 [DOI] [PubMed] [Google Scholar]
- Ji J, Sharma V, Qi S, Guarch M, Zhao P, Luo Z, Fan W, Wang Yu, Mbabaali F, Neculai D et al (2014) Antioxidant supplementation reduces genomic aberrations in human induced pluripotent stem cells. Stem Cell Rep 2: 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, Crabtree GR (2009) Calcineurin/NFAT signaling is required for neuregulin‐regulated Schwann cell differentiation. Science 323: 651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya‐Okur HS, Wu SJ, Codomo CA, Pledger ES, Bryson TD, Henikoff JG, Ahmad K, Henikoff S (2019) CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat Commun 10: 1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodeer S, Era T (2017) Identifying the biphasic role of Calcineurin/NFAT signaling enables replacement of Sox2 in somatic cell reprogramming. Stem Cells 35: 1162–1175 [DOI] [PubMed] [Google Scholar]
- Knoll M, Winther S, Natarajan A, Yang H, Jiang M, Thiru P, Shahsafaei A, Chavarria TE, Lamming DW, Sun L et al (2017) SYK kinase mediates brown fat differentiation and activation. Nat Commun 8: 2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Kawamura K, Kimura Y, Shibuya N, Kimura H, Ogasawara Y (2017) Analysis of endogenous H2S and H2Sn in mouse brain by high‐performance liquid chromatography with fluorescence and tandem mass spectrometric detection. Free Radic Biol Med 113: 355–362 [DOI] [PubMed] [Google Scholar]
- Kujawski S, Lin W, Kitte F, Bormel M, Fuchs S, Arulmozhivarman G, Vogt S, Theil D, Zhang Y, Antos CL (2014) Calcineurin regulates coordinated outgrowth of zebrafish regenerating fins. Dev Cell 28: 573–587 [DOI] [PubMed] [Google Scholar]
- Li K, Zhu S, Russ HA, Xu S, Xu T, Zhang Y, Ma T, Hebrok M, Ding S (2014) Small molecules facilitate the reprogramming of mouse fibroblasts into pancreatic lineages. Cell Stem Cell 14: 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu L, Yang A, Lin J, Tang F, Jin S, Wei Z, Li J, Jin Y (2011) Calcineurin‐NFAT signaling critically regulates early lineage specification in mouse embryonic stem cells and embryos. Cell Stem Cell 8: 46–58 [DOI] [PubMed] [Google Scholar]
- Liu K, Yu C, Xie M, Li K, Ding S (2016) Chemical modulation of cell fate in stem cell therapeutics and regenerative medicine. Cell Chem Biol 23: 893–916 [DOI] [PubMed] [Google Scholar]
- Liu P, Chen M, Liu Y, Qi LS, Ding S (2018) CRISPR‐based chromatin remodeling of the endogenous Oct4 or Sox2 locus enables reprogramming to pluripotency. Cell Stem Cell 22: 252–261.e254 [DOI] [PubMed] [Google Scholar]
- Liu Yi, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar I et al (2014) Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell 15: 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Wang M, Gu H, Xie X (2015) Bromodeoxyuridine promotes full‐chemical induction of mouse pluripotent stem cells. Cell Res 25: 1171–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longchamp A, Mirabella T, Arduini A, MacArthur MR, Das A, Treviño‐Villarreal JH, Hine C, Ben‐Sahra I, Knudsen NH, Brace LE et al (2018) Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production. Cell 173: 117–129.e114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol 15: 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly CH, Lynch GS, Ryall JG (2020) A metabolic roadmap for somatic stem cell fate. Cell Metab 31: 1052–1067 [DOI] [PubMed] [Google Scholar]
- Ma T, Li J, Xu Y, Yu C, Xu T, Wang H, Liu K, Cao N, Nie B‐M, Zhu S‐Y et al (2015) Atg5‐independent autophagy regulates mitochondrial clearance and is essential for iPSC reprogramming. Nat Cell Biol 17: 1379–1387 [DOI] [PubMed] [Google Scholar]
- Ma X, Chen X, Jin Y, Ge W, Wang W, Kong L, Ji J, Guo X, Huang J, Feng X‐H et al (2018) Small molecules promote CRISPR‐Cpf1‐mediated genome editing in human pluripotent stem cells. Nat Commun 9: 1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Kong L, Zhu S (2017) Reprogramming cell fates by small molecules. Protein Cell 8: 328–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocsai A, Ruland J, Tybulewicz VL (2010) The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 10: 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Fernandez V, Monllau JM, Borrell V, Lerin C, de la Iglesia N (2015) Transcriptional profiling of hypoxic neural stem cells identifies Calcineurin‐NFATc4 signaling as a major regulator of neural stem cell biology. Stem Cell Rep 5: 157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteiro L, Pantoja C, Alcazar N, Marión RM, Chondronasiou D, Rovira M, Fernandez‐Marcos PJ, Muñoz‐Martin M, Blanco‐Aparicio C, Pastor J et al (2016) Tissue damage and senescence provide critical signals for cellular reprogramming in vivo . Science 354: aaf4445 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Lindner R, Tedeschi A, Forsberg K, Green A, Wuttke A, Gaub P, Di Giovanni S (2009) NFAT‐3 is a transcriptional repressor of the growth‐associated protein 43 during neuronal maturation. J Biol Chem 284: 18816–18823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451: 141–146 [DOI] [PubMed] [Google Scholar]
- Paul BD, Sbodio JI, Snyder SH (2018) Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol Sci 39: 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Ocampo A, Liu GH, Izpisua Belmonte JC (2017) Regulation of stem cell aging by metabolism and epigenetics. Cell Metab 26: 460–474 [DOI] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nat Biotechnol 29: 24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose P, Moore PK, Zhu YZ (2017) H2S biosynthesis and catabolism: new insights from molecular studies. Cell Mol Life Sci 74: 1391–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Chakraborty S, Dugas TR, Kevil CG (2014) Hydrogen sulfide measurement using sulfide dibimane: critical evaluation with electrospray ion trap mass spectrometry. Nitric Oxide 41: 97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh‐Chang N, Locasale JW, Lyssiotis Ca, Zheng Y, Teo RY, Ratanasirintrawoot S, Zhang J, Onder T, Unternaehrer JJ, Zhu H et al (2013) Influence of threonine metabolism on S‐adenosylmethionine and histone methylation. Science 339: 222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Liao B, Tao Y, Chen H, Xiao F, Gu J, Gao S, Jin Y (2016) Calcineurin‐NFAT signaling controls somatic cell reprogramming in a stage‐dependent manner. J Cell Physiol 231: 1151–1162 [DOI] [PubMed] [Google Scholar]
- Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T (2001) Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol 11: 1553–1558 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Tan Bo, Jin S, Sun J, Gu Z, Sun X, Zhu Y, Huo K, Cao Z, Yang P, Xin X et al (2017) New method for quantification of gasotransmitter hydrogen sulfide in biological matrices by LC‐MS/MS. Sci Rep 7: 46278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen TW, Jaenisch R (2014) Molecular control of induced pluripotency. Cell Stem Cell 14: 720–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Qiao Z, Zhang L, Li H, Pei Y (2016) Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedlings. Plant Physiol Biochem 109: 293–299 [DOI] [PubMed] [Google Scholar]
- Tu BP, Mohler RE, Liu JC, Dombek KM, Young ET, Synovec RE, McKnight SL (2007) Cyclic changes in metabolic state during the life of a yeast cell. Proc Natl Acad Sci USA 104: 16886–16891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL (1995) Perinatal lethality and blocked B‐cell development in mice lacking the tyrosine kinase Syk. Nature 378: 298–302 [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang T, Wang L, Cai Y, Zhong X, He X, Hu L, Tian S, Wu M, Hui L et al (2017) Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission. EMBO J 36: 1330–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ocampo A, Belmonte JCI (2016) Cellular metabolism and induced pluripotency. Cell 166: 1371–1385 [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen K, Xing G, Li L, Ma B, Hu Z, Duan L, Liu X (2019) Phospholipid remodeling is critical for stem cell pluripotency by facilitating mesenchymal‐to‐epithelial transition. Sci Adv 5: eaax7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li Y, Zhang H, Huang Y, Zhao P, Tang Y, Qiu X, Ying Y, Li W, Ni Su et al (2015) Autophagy and mTORC1 regulate the stochastic phase of somatic cell reprogramming. Nat Cell Biol 17: 715–725 [DOI] [PubMed] [Google Scholar]
- Xu J, Du Y, Deng H (2015) Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell 16: 119–134 [DOI] [PubMed] [Google Scholar]
- Yanagi S, Inatome R, Ding J, Kitaguchi H, Tybulewicz VL, Yamamura H (2001) Syk expression in endothelial cells and their morphologic defects in embryonic Syk‐deficient mice. Blood 98: 2869–2871 [DOI] [PubMed] [Google Scholar]
- Yang L, Liu X, Song L, Di A, Su G, Bai C, Wei Z, Li G (2020) Transient Dux expression facilitates nuclear transfer and induced pluripotent stem cell reprogramming. EMBO Rep 21: e50054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Qu C, Zhou Yu, Konkel JE, Shi S, Liu Yi, Chen C, Liu S, Liu D, Chen Y et al (2015) Hydrogen sulfide promotes Tet1‐ and Tet2‐mediated Foxp3 demethylation to drive regulatory T Cell differentiation and maintain immune homeostasis. Immunity 43: 251–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JJ, Zhao QR, Liu DD, Chow CW, Mei YA (2016) Neuritin Up‐regulates Kv4.2 alpha‐subunit of potassium channel expression and affects neuronal excitability by regulating the calcium‐calcineurin‐NFATc4 signaling pathway. J Biol Chem 291: 17369–17381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C, Sutter BM, Wang Y, Kuang Z, Zhao X, Yu Y, Tu BP (2019) Demethylation of the protein phosphatase PP2A promotes demethylation of histones to enable their function as a methyl group sink. Mol Cell 73: 1115–1126.e1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Xiang Ge, Zheng L, Tang H, Duan L, Lin X, Zhao Q, Chen K, Wu Yi, Xing G et al (2018) Short‐term mitochondrial permeability transition pore opening modulates histone lysine methylation at the early phase of somatic cell reprogramming. Cell Metab 28: 935–945.e935 [DOI] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16: 284–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, He QY (2015) ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31: 2382–2383 [DOI] [PubMed] [Google Scholar]
- Yuan X, Wan H, Zhao X, Zhu S, Zhou Q, Ding S (2011) Brief report: combined chemical treatment enables Oct4‐induced reprogramming from mouse embryonic fibroblasts. Stem Cells 29: 549–553 [DOI] [PubMed] [Google Scholar]
- Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA (2012) Metabolic regulation in pluripotent stem cells during reprogramming and self‐renewal. Cell Stem Cell 11: 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhao T, Guan J, Zhang Xu, Fu Y, Ye J, Zhu J, Meng G, Ge J, Yang S et al (2015) A XEN‐like state bridges somatic cells to pluripotency during chemical reprogramming. Cell 163: 1678–1691 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Ma X, Zhu S (2020) Recent advances and potential applications of human pluripotent stem cell‐derived pancreatic beta cells. Acta Biochim Biophys Sin 52: 708–715 [DOI] [PubMed] [Google Scholar]
- Zhu J, Berisa M, Schworer S, Qin W, Cross JR, Thompson CB (2019) Transsulfuration activity can support cell growth upon extracellular cysteine limitation. Cell Metab 30: 865–876.e865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Cheng X, Cheng Y, Chen J, Xu H, Gao Y, Duan X, Ji J, Li X, Yi W (2020) O‐GlcNAcylation regulates the methionine cycle to promote pluripotency of stem cells. Proc Natl Acad Sci USA 117: 7755–7763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S (2010) Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 7: 651–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivanovic J, Kouroussis E, Kohl JB, Adhikari B, Bursac B, Schott‐Roux S, Petrovic D, Miljkovic JL, Thomas‐Lopez D, Jung Y et al (2019) Selective persulfide detection reveals evolutionarily conserved antiaging effects of S‐Sulfhydration. Cell Metab 30: 1152–1170.e1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Review Process File
Data Availability Statement
RNA‐seq and CUT&Tag sequencing data were deposited at Gene Expression Omnibus under GSE154687 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE154687).


