Abstract
Stem cells are the essential source of building blocks for tissue homeostasis and regeneration. Their behavior is dictated by both cell‐intrinsic cues and extrinsic cues from the microenvironment, known as the stem cell niche. Interestingly, recent work began to demonstrate that hair follicle stem cells (HFSCs) are not only passive recipients of signals from the surroundings, but also actively send out signals to modulate the organization and function of their own niches. Here, we discuss recent findings, and briefly refer to the old, on the interaction of HFSCs and their niches with the emphasis on the outwards signals from HFSCs toward their niches. We also highlight recent technology advancements that further promote our understanding of HFSC niches. Taken together, the HFSCs emerge as a skin‐organizing center rich in signaling output for niche remodeling during various stages of adult skin homeostasis. The intricate crosstalk between HFSCs and their niches adds important insight to skin biology that will inform clinical and bioengineering fields aiming to build complete and functional 3D organotypic cultures for skin replacement therapies.
Keywords: hair follicle stem cells, skin endothelial cells, stem cell niche
Subject Categories: Skin, Regenerative Medicine, Vascular Biology & Angiogenesis
This review focuses on the emerging role of epidermal stem cells as outward signaling modulators of their niche environment and its intricate dynamics.
Introduction
Tissue stem cells are the foundation for adult tissue regeneration, and their cellular states during homeostasis are tightly regulated by (i) cell‐intrinsic mechanisms, such as chromatin structure, transcriptional control, and metabolism (Lee et al, 2021) and (ii) cell‐extrinsic mechanisms, which encompass the bi‐directional molecular crosstalking between stem cells and their functional niche cells. For the study of SC‐niche interactions, the mouse hair follicle stem cells (HFSCs) stand out as an excellent model system. The HFSCs periodically undergo activation and quiescence in a highly synchronized manner during the homeostatic hair follicle regeneration (Müller‐Röver et al, 2001; Alonso & Fuchs, 2006), and the location and molecular markers of HFSCs are well‐defined (Morris et al, 2004; Tumbar et al, 2004). Even more interesting, the whole skin and various cellular compartments within the dermis undergo dramatic remodeling along with the cycling hair follicles (Goldstein & Horsley, 2012; Hsu et al, 2014; Chen et al, 2020), therefore prompting intense investigation into the potential SC‐niche crosstalk. Cell–cell signaling from specific niche cells toward HFSCs has been the classical direction of niche‐SC communication for studies in the stem cell field. Several reviews considered this direction of communication in‐depth (Goldstein & Horsley, 2012; Hsu et al, 2014; Chen et al, 2020; Fuchs & Blau, 2020), and hence, we will only briefly summarize it here. Instead, here we will focus our attention onto the less understood communication with reverse signaling, from the HFSCs outward toward the niche cells in the skin. There is power in numbers, and the clustering of HFSCs in the bulge turns them into a strong signaling center, with influence far beyond cells in their immediate vicinity. In this process, the HFSCs secrete molecules that strongly pattern the organization and behavior of various adult skin compartments, which in turn act as HFSC‐niche components during normal skin homeostasis. The skin compartments modulated by HFSCs comprise a rapidly growing list and include such diverse components as the extracellular matrix, nerves, arrector pili muscle (APM), and more recently the skin vasculature to name a few. The emerging niche‐organizing function of HFSCs places these stem cells at the very center of skin organization and homeostasis, thus elevating their relevance in skin biology from their basic and somewhat frivolous role (at least in humans, where hair is not required for survival), that of simply producing a hair shaft.
Mouse skin is a well‐structured, layered tissue that can be divided into epidermis, dermis, and hypodermis (Figure 1). The hair follicle is a skin appendage that protrudes downwards from the epidermis into the dermis. HFSCs orchestrate a highly choreographed series of dramatic hair follicle morphological modifications during homeostasis, known as the hair cycle. The hair cycle consists of several successive and synchronized phases of telogen (rest), anagen (growth), and catagen (regression; Figure 2).
Figure 1. Structure of the skin and components of the HFSC niche.
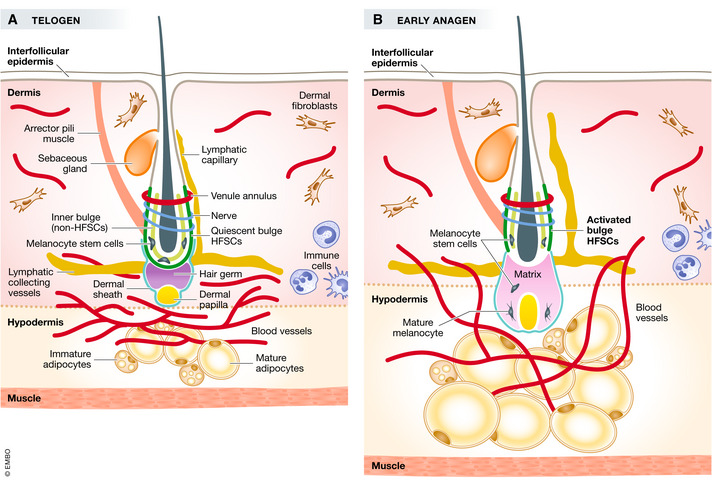
Skin is composed of epidermis, dermis, and hypodermis layers. Below the hypodermis layer is the subcutaneous muscle layer. The hair follicle is a downward protrusion from the epidermis. HFSCs reside in the outer layer of the bulge region (dark green), and in telogen, they are quiescent. Melanocyte stem cells (black star) are also in the bulge. The inner bulge layer is a non‐HFSC population (light green). Above the bulge is the sebaceous gland (orange). The venous annulus is around the upper bulge (dark purple). Sensory nerves innervate the middle bulge (dark blue). Arrector pili muscle (dark brown) connects the bulge and the epidermis. The lymphatic capillaries (yellow line) are along the side of the hair follicle and connect to the lymphatic collecting (yellow line) vessels that are at the bottom of the hair follicle and parallel to the epidermis. Hair germ (gray) locates below the bulge, housing primed HFSCs. Below the hair germ is the dermal papilla (yellow). Around the dermal papilla is the dermal sheath (teal). The hair follicle is closely associated with blood vessels (red), and at this stage, blood vessels concentrate beneath the hair germ and form a horizontal plexus in telogen, with few dispersed blood vessels in the dermis. Dermal fibroblasts (beige) fill the empty space in the dermis. In the hypodermis are adipose tissue (mature and immature adipocytes, yellow and brown, respectively). Immune cells (light blue) also reside in the skin, with some being more mobile than others. (B) In early anagen, HFSCs are activated. The hair germ proliferates and differentiates to make progenitor or transit‐amplifying cells that enclose the DP and make the matrix. Melanocyte stem cells migrate downward from the bulge. The sebaceous gland is expanded. Adipocytes undergo hyperplasia and hypertrophy. The horizontal vasculature plexus in telogen (A) proliferates and becomes more vertical toward the epidermis in anagen (B). The lymphatic capillary increases its caliber and disassociates from the bulge at anagen. The dermal sheath also extends to enclose the bottom of the hair.
Figure 2. Hair cycle and vasculature dynamics during the hair cycle.
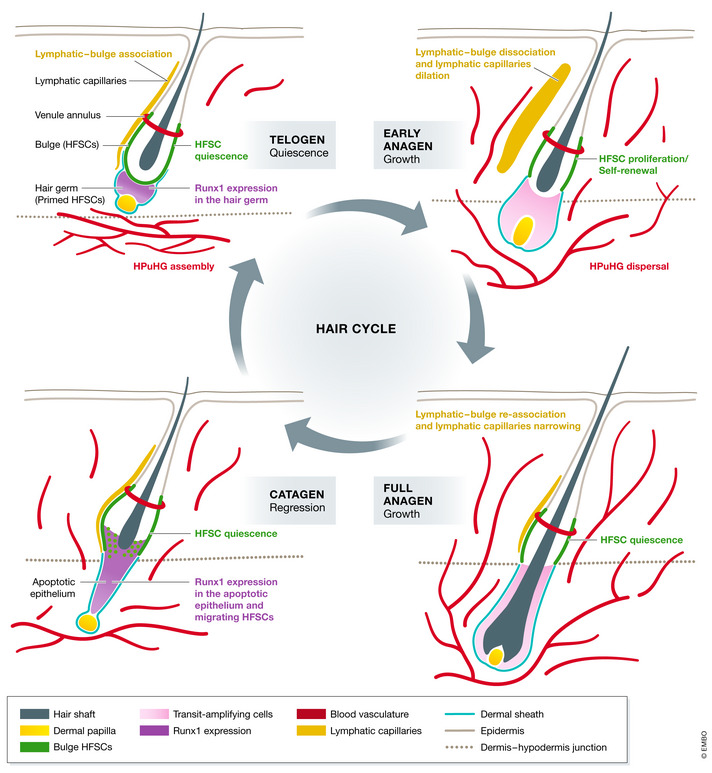
In telogen when HFSCs (green) and the primed HFSCs in the hair germ are both quiescent, blood vessels (red) concentrate to form a horizontal plexus under the hair germ (HPuHG). Runx1 (purple) is expressed by the hair germ at this stage and regulates the expression of vasculature‐related genes. Lymphatic capillaries (yellow) are associated with bulge. Once the hair germ receives the activation signals to enter anagen, the growth phase, hair germ cells start to proliferate and give rise to progenitor cells. Bulge HFSCs proliferate to replenish the stem cell pool. Runx1 expression is diminished. At the same time, blood vessels proliferate and disperse to become more vertical toward the epidermis. Lymphatic capillaries disassociate from the bulge and dilate more relative to their telogen morphology. At full anagen, bulge HFSCs return to quiescence. The progenitor cells that are generated earlier now terminally differentiate to make different inner hair lineages which include a new hair shaft. Morphologically, the hair follicle now spans the entire dermis. Blood vessels keep proliferating, and lymphatic capillaries re‐associate with the bulge and become narrow again. At the transition from anagen to catagen the regression stage, bulge cells migrate out of their niche to produce a new hair germ. Then in catagen, differentiated lineages produced in anagen will undergo apoptosis and die, and the hair germ will survive. Both of them have high Runx1 expression. Blood vessels also undergo apoptosis at this stage and begin to change their angle relative to epidermis to adopt a more horizontal orientation again. After all the differentiated lineages in the hair bulb are gone, only the hair germ and bulge cells remain. Hair follicle goes back to telogen and rest, ready for the next round of the hair cycle. The HPuHG is reassembled below the hair follicle.
In the hair follicle, a permanent region called bulge harbors the quiescent HFSCs in the outer most cellular layer. A second population of HFSCs called primed SCs which are derived from the bulge, reside temporarily in the structure underneath the bulge known as the hair germ during telogen (Panteleyev, 2018). In the bulge, besides HFSCs, there are also melanocyte stem cells (McSCs) that give rise to differentiated melanocytes for melanin deposition in the colored hair shaft. Surrounding the HFSCs is a mixed neighborhood, which is also the home for many other types of cells of the dermis (Figure 1). Cutaneous nerves are immediately adjacent to HFSCs as they wrap around the bulge for signal relay (Botchkarev et al, 1997). The arrector pili muscle (APM) connects the posterior side of the hair follicle also at the bulge area with the interfollicular epidermis (IFE; Müller‐Röver et al, 2001; Fujiwara et al, 2011). Its contraction results in piloerection which has implications in body temperature control and fight‐or‐flight responses. On the other side of the hair follicle is the lymphatic capillary that collects tissue fluid and drains into the lymphatic collecting vessels beneath the hair follicle (Gur‐Cohen et al, 2019; Peña‐Jimenez et al, 2019). Right above the bulge is the sebaceous gland filled with sebocytes that release the sebum into the hair canal spanning the skin surface (Niemann & Horsley, 2012). The hair follicle is closely surrounded by the dermal sheath, a population of specialized fibroblast with a recently uncovered contractile function, important for hair follicle regression (Martino et al, 2021). Below the hair germ, there is a ball of mesenchymal cells that is known as the dermal papilla (DP), with known relevance to HFSC activation at the telogen‐to‐anagen transition (Morgan, 2014; Chen et al, 2020). Outside of the hair follicle, there are fibroblasts in the dermis, adipose tissue in the hypodermis, and blood vessels interlaced in the whole skin. In addition to these tissue niches, the extracellular matrix between cells represents a scaffold that provides essential structural support for all these niche components.
When hair follicles begin to grow at the telogen‐to‐anagen transition, activating signalings such as Shh and Wnt are elevated whereas the inhibitory BMP signaling is suppressed (Alonso & Fuchs, 2006; Lee & Tumbar, 2012). Then, the primed HFSCs in the hair germ form a new hair bulb by rapidly proliferating to first make the hair matrix, a population of multipotent progenitor or transient amplifying cells that can temporarily self‐renew and also differentiate during anagen to make a new hair shaft that grows upward (Figure 1). In the meantime, the bulb grows downward pushing the matrix away from the bulge into the hypodermis, and the hypodermis expands at remarkable extent with newly appearing mature adipocytes and vasculature redistributing all around it (Hansen et al, 1984; Mecklenburg et al, 2000; Yano et al, 2001; Festa et al, 2011; Li et al, 2019). The DP is also pushed downward along with the matrix, while the dermal sheath expands around the hair follicle (Figure 1). In parallel, the quiescent HFSC in the bulge temporarily exits quiescence and divides symmetrically a few times while remaining in the bulge, thus expanding the SC pool. Eventually, the balance from activation signals shifts toward inhibitory signals, and together with contact inhibition, push back the bulge HFSCs into quiescence, where they would soon begin to migrate outside the bulge and into the outer root sheath (or layer) of the regressing hair follicle (Waghmare et al, 2008; Zhang et al, 2009; Zhang et al, 2010; Hsu et al, 2011). As the bulb cells are dying out during catagen, the HFSCs survive, and eventually by the next telogen, they will make a new hair germ and a new bulge around the newly formed hair shaft that has now stopped growing (Lee et al, 2021). The dynamic behavior of HFSCs, their maintenance, activation, self‐renewal, migration, and differentiation are tightly linked with the fate of various components of the SC niche (Goldstein & Horsley, 2012; Hsu et al, 2014; Chen et al, 2020; Fuchs & Blau, 2020).
The idea that HFSCs actively organize their surrounding niches through signaling originated in our two‐decade‐old work, when we purified H2B‐GFP label‐retaining HFSCs and obtained their transcriptional profile (Tumbar et al, 2004). This revealed many upregulated genes encoding secreted molecules that were known to regulate other cell types, many of which were co‐incidentally neighbors of HFSCs. This finding then led us to propose a speculative model where HFSCs actually crosstalk to their niches—both receiving signals from the niches and also actively modulating the niches (Fuchs et al, 2004). In addition, since niche organization involves multiple molecules and complex regulation, and HFSCs express a multitude of transcription factors such as Sox9 and Runx1 in accordance with their state, a follow‐up hypothesis is that HFSCs may use these transcription factors as “master regulators” for niche organization (Fuchs et al, 2004; Lee et al, 2014). Since we proposed this hypothesis, many pieces have been added to shed light to it, but the whole picture is still far from complete. In this review, we will focus on the outward signals from HFSCs to modulate the niche as we understand them today, while also briefly touching upon the reverse signals to clarify the importance of each specific niche to HFSCs.
Vasculature
We begin our journey with the vascular niche, where recent exciting evidence suggests a novel role of this niche in HFSC activation and skin homeostasis, opening up new avenues of investigation not only in skin, but also in vasculature and endothelial cell biology. The vascular niche has been previously studied in crosstalks with several tissue stem cells such as hematopoietic stem cells, neural stem cells, and muscle stem cells where it maintains homeostasis of the stem cell pool and tissue integrity (Otsuki & Brand, 2017; Sasine et al, 2017; Verma et al, 2018; Mammoto & Mammoto, 2019). Increasing evidence suggests that stem cells can actively recruit or modulate neighboring endothelial cells to create a functional vascular niche around them for stem cell homeostasis or to enhance wound healing (Chen et al, 2008; Donne et al, 2015; Verma et al, 2018).
In the skin, the HFSC‐vasculature niche consists of two major types of vessels: blood vessels that provide blood flow with oxygen and nutrients and lymphatic vessels that drain the tissue fluid and support immune surveillance (Skobe & Detmar, 2000; Hampton & Chtanova, 2019). Skin biologists have long known that vasculature organizes itself in a specific arrangement around the hair epithelium (Durward & Rudall, 1958; Ellis & Moretti, 1959). Some vasculature structures stay constant throughout the hair cycle in mice, such as the venule annulus which is a venous ring‐like vessel formed during hair morphogenesis to be associated with the K15‐positive upper bulge (Xiao et al, 2013) (Figures 1 and 2). On the other hand, skin vasculature as a whole is dynamic and keeps up with the morphological changes in hair follicles and in the hypodermis. During the adult hair cycle, in the anagen growth phase, skin vasculature undergoes angiogenesis and in turn may promote hair shaft growth through fueling O2 and nutrients to the matrix progenitor cells (Mecklenburg et al, 2000; Turksen, 2015). In catagen, the perifollicular vasculature collapses along with the regression of the hair follicle. Interestingly, this change in vasculature is associated with and potentially modulated by a down‐regulation of VEGF and upregulation of TSP‐1 in the follicular keratinocytes (Yano et al, 2001; Yano et al, 2003; Xiao et al, 2013). However, earlier work failed to examine a possible role of vasculature in HFSC activation at stages prior to anagen and the potential molecular crosstalk through signaling between vasculature and HFSCs remained unknown. In contrast to numerous studies done to understand the vascular niche of other tissue stem cells, relatively little effort has been made to understand the HFSC‐vasculature crosstalk, until a recent series of several publication on this subject aimed at filling this gap.
First, three independent studies all showed that genetic targeting of HFSCs specifically in the epithelium modulates the skin vascular niche organization (Gur‐Cohen et al, 2019; Li et al, 2019; Peña‐Jimenez et al, 2019; Figure 2). In one of these studies, we found that as HFSCs are activated, a hypodermal vasculature structure that is normally horizontally oriented and crowds vessels underneath the hair germ disperses and becomes more vertical, pointing into the dermis toward the hair follicles and epidermis, as it may proliferate more (Li et al, 2019). This dramatic reorganization of skin vasculature through the hair cycle has not been previously recognized, as earlier work focused on the minute interactions at point of contact between the vasculature and the lower hair bulb and DP (Ellis & Moretti, 1959; McLeod, 1970; Mecklenburg et al, 2000; Yano et al, 2001). Then, as HF pass through anagen and the hair bulbs degenerate at catagen, we found that the vasculature again becomes horizontal forming a dense vascular structure that we refer to as the horizontal(H) plexus(P) underneath (u) the hair germ (HG) during HFSC quiescence, the HPuHG (Figure 2) (Li et al, 2019). Interestingly, our epithelial knockout of Runx1, a transcription factor important for HFSC activation, which is expressed in the primed HFSC in the HG at late catagen and telogen (Figure 2), led to abnormal HPuHG patterning around the hair follicle, which was characterized by increased CD31+ area under the hair germ (Li et al, 2019). These data revealed an association between dense vasculature near the stem cell activation zone (hair germ) and increased quiescence of HFSCs, accompanied by delayed hair cycle progression (Osorio et al, 2008; Li et al, 2019). Microarray data of purified HFSCs from Runx1 epithelial overexpression mice further support the vascular niche‐organizing role of HFSCs. This showed altered expression of many genes encoding secreted molecules as Runx1 target genes, such as Ntn4, Sema3a, Figf, and Edn1, to name a few, known to have angiogenesis and vascular patterning functions (Lee et al, 2014; Li et al, 2019). With the exception of Ntn4 (Gur‐Cohen et al, 2019), direct targeting of these vasculature‐organizing molecules in the hair follicle is awaiting. We did not distinguish between blood vessels and lymphatics remodeling during hair cycle, but given the localization of the CD31‐high vasculature considered in our study and well‐documented now location of lymphatics, the HPuHG appears to have largely excluded the skin lymphatics.
The lymphatic vasculature was timely characterized by two subsequent studies focusing on the interaction between HFSCs and lymphatic capillaries. Both studies revealed that lymphatic capillaries transiently increase their caliber at HFSC activation (Gur‐Cohen et al, 2019; Peña‐Jimenez et al, 2019). In addition, the association between the lymphatic capillaries and HFSC changes dynamically in a way that the lymphatic capillaries are in close proximity to HFSCs during quiescence and dissociate during HFSC activation (Figure 2) (Gur‐Cohen et al, 2019). Closer examination of the lymphatic capillary morphology further revealed that lymphatic capillaries look more fenestrated at anagen onset, potentially conferring a difference in their drainage capacity (Peña‐Jimenez et al, 2019). The two studies also converge on finding a HFSCs’ role in regulating the lymphatic vascular niche. Specifically, Peña‐Jimenez et al (2019) showed that HFSC‐derived Wnt signaling was essential for lymphatic capillaries patterning and for their association with the bulge. In addition, anagen induced by modulation of macrophages (Castellana et al, 2014) recapitulates the increased caliber of lymphatic capillaries in homeostatic anagen, indicating that the hair cycle is a driver of this lymphatic remodeling (Peña‐Jimenez et al, 2019). Conversely, transcriptome analysis by Gur‐Cohen et al found that HFSCs act as a switch regulating their lymphatic vascular niche by expressing different factors in accordance with their cellular states. Specifically, quiescent HFSCs secrete Angptl7 to promote the lymphatic‐HFSC association, whereas activated HFSCs secrete Ntn4 and Angptl4 to promote dissociation (Gur‐Cohen et al, 2019). Intriguingly, Ntn4 and Angptl4 are also target genes downstream of transcription factors Runx1 and Tcf3, respectively (Nguyen et al, 2006; Lee et al, 2014; Li et al, 2019), and Runx1 deletion in the epithelium perturbs the nearby vasculature (Li et al, 2019), as discussed above (Figure 3). In combination with the finding from epithelial Runx1 KO mice, it is possible that transcription factors in the hair epithelium are the “master regulators” for vascular niche organization, thus allowing HFSCs to efficiently induce dramatic changes in global gene expression all at once, without tuning each signaling molecule individually.
Figure 3. HFSCs crosstalk with niches.
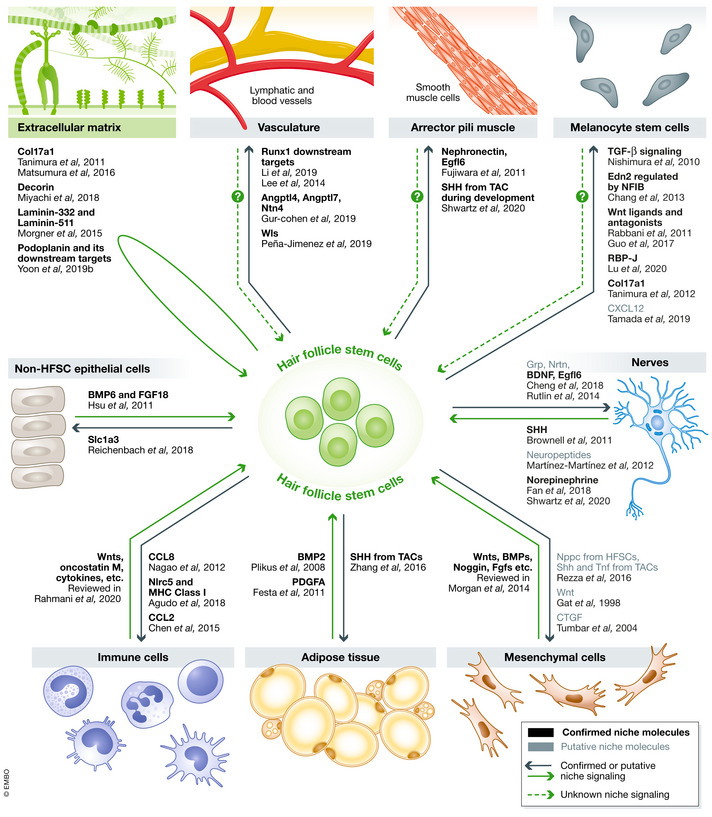
Hair follicle stem cells and niches crosstalk with each other using various signaling molecules. Black names represent confirmed niche molecules, and gray names represent putative molecules that need further confirmation. Solid arrows indicate confirmed or putative signaling, and dashed arrows indicate unknown signaling.
Why would HFSCs need to arrange the vascular niche in a specific organization? Intriguingly, the exact role the vascular niche plays on HFSC behavior is still debatable, and currently, saving some suggestive preliminary observations, there are no signals proven to flow from endothelial cells toward HFSCs to modulate their behavior. Our Alk1 endothelial knockout mice showed increased area of the CD31+ horizontal plexus under the hair germ (HPuHG; Li et al, 2019). This is associated with a hair cycle delay with HFSC activation defects, which we speculate might be due to increased local concentration of the quiescence factor BMP4 (Li et al, 2019). BMP4 is likely secreted by endothelial cells, as also shown in few other stem cell systems including alveolar stem cells, NSCs, and HSCs (Mathieu et al, 2008; Goldman et al, 2009; Chung et al, 2018). Of note, CD31+ vasculature may inhibit HFSC activation in the hair germ possibly via signaling, but at the same time promote hair follicle growth fueled by transit‐amplifying matrix cells (Yano et al, 2001). While endothelial cells are often found to maintain the quiescence of other tissue stem cells such as muscle stem cells and neural stem cells (Azevedo et al, 2017; Verma et al, 2018), the role played by lymphatic endothelial cells is more controversial as the ablation of lymphatic vessels and genetically induced changes in lymphatic vessel showed highly mixed outcomes on HFSC behaviors (Gur‐Cohen et al, 2019; Peña‐Jimenez et al, 2019; Yoon et al, 2019a). Therefore, an urgent question for the field is to more precisely dissect the functions of each type of vasculature on HFSC activity.
While recent discoveries shed new light on the HFSC‐vascular niche after a long silence in the field, it is just the beginning and a lot more questions on the HFSC‐vasculature niche are waiting for answers. For example, signaling from the vascular niche toward the hair remains entirely obscure and the chemokines HFSCs use to modulate the blood vascular niche are yet to be identified, with follow‐up functional studies being required. Furthermore, while age‐related functional alteration in the vascular niche has been investigated in HSC and NSC (Apple & Kokovay, 2017; Ya‐Hsuan & Simón, 2020), current studies on the HFSC‐vascular niche mostly focused on the homeostatic condition. Whether the molecular interaction between HFSCs and the vascular niche has any implication in stress conditions, such as injury, is still unknown. Given the importance of the vascular niche in tissue function, integration of endothelial cells into 3D organoid culture systems also greatly enhanced the outcome, to be a more faithful representation of the tissue (Grebenyuk & Ranga, 2019; Vargas‐Valderrama et al, 2020). On the other hand, defective crosstalk between stem cell and endothelial cells has an important role in disease progression. In the hematopoietic system, miscommunication between HSCs and their vascular niche can lead to more severe conditions like skewed lineage production in hematopoiesis and anemia (Perlin et al, 2017). In the skin, it has been suggested that abnormal vasculature alterations such as vascularization, perivasculitis, and dilated lymphatic vessels are related to alopecia (Pratt et al, 2017). In addition, neovascularization is often the hallmark of pathological conditions especially various cancers, in which the cancer stem cells can recruit endothelial cells as well as promote nearby endothelial sprouting (Ahn & Brown, 2008; Lugano et al, 2020). Thus, there is a crucial need to study this interaction in molecular details and understand its regulation.
Nerves
Nerves and neurons have been found to communicate with several tissue stem cells, such as hematopoietic stem cells (HSCs; Agarwala & Tamplin, 2018), intestinal stem cells (Lundgren et al, 2011; Davis et al, 2018; Puzan et al, 2018), and muscle stem cells (satellite cells; Mousavi & Jasmin, 2006; Tatsumi et al, 2009; Yin et al, 2013). In homeostatic condition and in aging, major research efforts have been in the field of HSCs, where innervation was found critical for blood regeneration and HSC mobilization (Agarwala & Tamplin, 2018). Nerves also contribute to intestinal stem cell proliferation and differentiation, and to the barrier function of the gut (Davis et al, 2018; Puzan et al, 2018). In adult muscle tissue, it is well‐established that denervation leads to a decline in satellite cell number in the long term (Yin et al, 2013). The satellite cells are also found to secrete neurotrophic factors such as Sema3A and BDNF to maintain a functional nervous niche (Mousavi & Jasmin, 2006; Tatsumi et al, 2009). The interdependence of stem cells and the nervous system is also alluded by cancer studies, revealing bi‐directional mutually enhancing interactions between cancer cells and the surrounding nerves (Jobling et al, 2015).
Skin is a highly innervated organ with multiple types of nerve fibers (Glatte et al, 2019). In mice, the skin innervation network can be largely divided into three major nerve bundles based on their anatomical location: the sub‐epidermal plexus that is right underneath the epidermis, the deep cutaneous plexus that is in the hypodermis, and the subcutaneous plexus that is below the muscle layer (Botchkarev et al, 1997). Out from the three major nerve plexuses, nerve fibers protrude and innervate skin components such as epidermis, the hair follicle, and the arrector pili muscle (APM; Botchkarev et al, 1997; Glatte et al, 2019). During the hair cycle, the whole skin remodels with the hair follicle, and nerves also exhibit plasticity in this process, as shown by earlier work in the nineties (Botchkarev et al, 1997; Botchkarev et al, 1999). Both the follicular innervation and the density of the surrounding nerves in the skin increase in anagen, and decline in catagen, along with changes in neuronal markers expression (Botchkarev et al, 1997; Botchkarev et al, 1999).
Sensory nerves have been identified as a niche that modulates HFSC behaviors in wound healing through hedgehog and putative neuropeptide signalings (Brownell et al, 2011; Martínez‐Martínez et al, 2012). Besides sensory nerves, sympathetic nerves that innervate the APM and project to the middle bulge play a role in promoting HFSC activation (Fan et al, 2018; Shwartz et al, 2020). Intriguingly, this nerve niche is supported by the APM, together with HFSCs forming a trilineage homeostatic unit (Shwartz et al, 2020). This exciting new finding provides insight on the crosstalk between different HFSC niches and their collective effort on regulating HFSCs. In addition, while 6‐OHDA‐induced sympathectomy leads to a pronounced delay in anagen entry (Shwartz et al, 2020), complete ablation of cutaneous nerves by surgical denervation do not have an effect on the hair cycle (Maurer et al, 1998; Brownell et al, 2011; Xiao et al, 2015), potentially due to compensation mechanism or opposing roles of different types of nerves. In any case, further dissection of the role that distinct kinds of nerves play in hair homeostasis could augment our understanding of the HFSC microenvironment and the fascinating crosstalk with neural networks.
Several tissue stem cells express neurotrophins to modulate their nerve niche (Kosinski et al, 2007; Yin et al, 2013; Hofer & Tuan, 2016), and HFSCs are no exception. Recently, the signaling from HFSCs to the nerve niche has been uncovered (Figure 3). During development, the nerve niche is defined by the initial formation of the APM niche (Shwartz et al, 2020). Then in adulthood, transcriptomic analysis on a subset of Gli1+ HFSCs associated with cutaneous nerves showed that this population expresses neurogenesis and neuron‐patterning‐related genes such as Grp, Nrtn, and BDNF, suggesting its nerve regulatory function (Cheng et al, 2018). Of note, BDNF was previously found important to attach the mechanosensory organ lanceolate complexes, an axonal ending and Schwann cell bundle required for touch response, on the caudal side of the hair follicle (Rutlin et al, 2014). BDNF is also highly expressed by the satellite cells of the muscle and mesenchymal stem cells, suggesting that it might be a common stem cell‐derived niche modulatory factor (Mousavi & Jasmin, 2006; Hofer & Tuan, 2016).
In addition to nerve regulatory genes, genes encoding ECM proteins were also enriched in the Gli1‐positive HFSCs, among which EGFL6 was found to ensure the proper patterning of lanceolate complexes through its interaction with αv‐integrin accumulated at the axonal terminals (Cheng et al, 2018). During hair regeneration, the old bulge, that harbors the quiescent HFSCs on the caudal side of the hair follicle, maintains the HFSC‐nerve interactive domain. Interestingly, club‐hair‐plucking‐induced HFSC apoptosis in the old bulge leads to relocation of the lanceolate complexes to the rostral side of the hair follicle which is the new bulge, but the Egfl6 expression on the rostral side was not immediately changed (Cheng et al, 2018). In de novo hair follicle regeneration by cell mixture chamber grafting, Egfl6 expression is restored in all hair follicles but only the hair follicle at the edge of the graft is innervated (Xiao et al, 2013). The inconsistency between Egfl6 expression and hair follicle innervation might indicate that other molecules are involved in attracting the nerves toward the hair follicle, which can be investigated in follow‐up studies. It is also worth investigating whether neighboring hair follicles are involved in helping attach the nerve through creating a gradient of secreted nerve regulatory molecules.
Hair follicle stem cells are both a source and a target of nervous regulation with the expression of various nerve regulatory factors and their receptors. The bi‐directional communication is critical for biological processes and interruptions can cause diseases. Clinical observation showed hyperactivation of sympathetic nerves leads to hypertrichosis, a condition marked by excessive hair growth (Chen et al, 2020). In other systems like the muscle, it has also been speculated that the level of neurotrophins secreted by the satellite cells is correlated with exercise duration and may play a role in elderly frailty that involves declined regeneration capacity of satellite cells (Mitsugu Sun et al, 2019; Hachisu et al, 2020). Nevertheless, the relationship between diseases and stem cell‐nervous niche communication is still obscure, and studies using the excellent HFSC model can inspire new therapeutic interventions and shed new light on other tissue stem cell biology.
Arrector pili muscle
Muscle is a contractile tissue that carries out the motility function. Besides its physiological functional importance, muscle is also a niche component of several tissue stem cells, such as intestinal, lung, and muscle stem cells, producing BMP antagonists (Kosinski et al, 2007), Fgf10 (Volckaert et al, 2011), JAK/STAT ligands (Lin et al, 2010), Wnt ligands (Lin et al, 2008), and many more molecules known to regulate stem cell behavior. The other signaling direction, from stem cells to the muscle, is currently under investigation. It has been suggested that intestinal stem cells may regulate their smooth muscle niche through Hedgehog signaling (Zacharias et al, 2011). Moreover, the club cells in the lung that act as stem cells for bronchiolar epithelium regeneration may signal to the smooth muscle niche through Wnt signaling (Cohen et al, 2009; Volckaert et al, 2011). For the skin, early finding uncovered the role of HFSC secreted ECM in regulating the muscular niche, which we elaborate below (Fujiwara et al, 2011).
The skin has at least two discrete classical muscle structures. While there is no evidence suggesting the striated muscle underneath the hypodermis is a niche for HFSCs, the arrector pili muscle (APM) is clearly identified as an important one. APM is a string‐like muscle structure that connects the bulge and interfollicular epidermis (Müller‐Röver et al, 2001). Its bulge attachment is at the K15‐positive region, slightly lower than the nerve attachment site (Brownell et al, 2011; Fujiwara et al, 2011) (Figure 1). Recently, it has been shown that APM is required for the establishment and maintenance of the sympathetic nerve niche, which in turn promotes HFSC activation (Shwartz et al, 2020). However, the independent function of APM on HFSCs is unclear and difficult to untangle from nerves, thus requiring further investigation.
For the reverse signaling from HFSCs to the APM, recent finding revealed that during hair follicle morphogenesis transit‐amplifying cells define the APM niche through SHH signaling (Shwartz et al, 2020; Figure 3). However, less is known about how adult HFSCs regulate the APM. To our knowledge, the only work that tackles this question was conducted by Fujiwara et al (2011). It is showed that HFSCs deposit a Wnt‐targeted ECM protein nephronectin to the basement membrane surrounding the bulge and the hair germ. Through nephronectin–Itga8 interactions, HFSCs regulate the anchoring of Itga8+ mesenchymal cells to the bulge and induce their differentiation to smooth muscle cells, thus creating a smooth muscle niche for the APM. Without nephronectin, APM forms fewer interaction sites with the bulge and loses its attachment site near the K15+ bulge region. Instead, APM attaches to the upper bulge area that upregulates Egfl6 in a compensatory manner (Fujiwara et al, 2011). This relocation suggests a hierarchy of niche regulatory molecules secreted by the bulge as the primary regulatory molecule is ablated; other molecules can function as compensatory chemokines. In addition, one molecule may be responsible for mediating multiple niches. One example is Egfl6, which regulates both nerve and smooth muscle/APM attachment (Fujiwara et al, 2011; Cheng et al, 2018), and may possibly be a factor for the vascular niche as well, since it has been previously shown important for angiogenesis (Chim et al, 2011; Kang et al, 2020). However, it is still unclear whether other ECM proteins are also involved in this SC‐niche interaction. In addition, what signaling is involved in the maintenance of the APM niche during adult tissue regeneration is also a subject of future investigations.
Together with the early work by Fujiwara et al, the new findings from Hsu laboratory remind us that the APM is not only a mechanical spring for piloerection, a relatively trivial function for humans, but also a niche of HFSCs. While there is some indication that APM is associated with alopecia, and aged hair follicle has mis‐attached APM, in‐depth understanding of the APM niche biology and its clinical relevance is lacking (Torkamani et al, 2017; Ge et al, 2020). In fact, many questions remain to be answered. For example, if APM is able to modulate HFSC behaviors, what molecules are involved? Besides molecular regulation, “is the physical contact between the APM and the hair follicle important for the stem cells and/or the APM?”. A better understanding of the APM and its communication with the HFSCs will be of great interest to the skin field in elucidating hair‐related disorders and to the stem cell community as a whole, as knowledge of the muscle niche from the HFSCs may also provide insights for other fields.
Melanocyte stem cells
The bulge not only harbors HFSCs, but also has melanocyte stem cells (McSCs) that give rise to melanocytes for melanin deposition. Being adjacent to each other, McSCs and HFSCs share many signaling pathways. The two heterotypic stem cell populations also often exhibit concerted behavior of activation and quiescence, suggesting a molecular crosstalk may exist between them (Nishimura et al, 2002; Chang et al, 2013).
Since the publication of the first paper on the crosstalk between HFSCs and McSCs in 2010 by Nishimura et al (2010) showing that TGf‐β derived from the bulge contributes to McSC quiescence, the field has never stopped making progress to understand how HFSCs regulate McSC behaviors through different niche factors (Figure 3). By knocking out the transcription factor NFIB from only the HFSCs, Chang et al (2013) showed that NFIB‐null HFSCs upregulate Edn2 and induce ectopic differentiation of neighboring McSCs. Wnt signaling is extensively studied and plays a vital role in HFSC activation and differentiation. While Wnt signaling is also important for McSC proliferation and differentiation, McSCs themselves are not the cellular source of Wnt ligand. Instead, primed HFSCs in hair germ at anagen onset produce Wnt ligands for McSC activation in an Edn‐EdnrB signaling‐dependent manner (Rabbani et al, 2011; Goldstein & Horsley, 2012). Similar to Wnt ligands, sFRP4, a Wnt antagonist, is also found to be preferentially expressed in the epithelial lineages including bulge HFSCs in telogen (Guo et al, 2017). Exogenous supply of sFRP4 inhibits McSC proliferation and differentiation. Since sFRP4 is constantly present in the lower portion of the hair follicle throughout the hair cycle, its control over McSC behavior is likely balanced by activating Wnt ligand also from the HFSCs (Guo et al, 2017). Together, these findings provide insight on how HFSCs coordinate McSC activities by fine‐tuning Wnt signaling pathway through the secretion of antagonists and activators. In addition to Wnt signaling, Notch signaling also emerges as a niche modulatory pathway. The key effector of Notch signaling RBP‐J in HFSCs was found to maintain McSCs in an undifferentiated state in which McSCs are insensitive to differentiation signal Kit‐ligand through metabolic regulation (Lu et al, 2020). ECM proteins also play an important role as mediators that HFSCs use to modulate the McSC neighbor (Goldstein & Horsley, 2012). Col17a1 is expressed by HFSCs but undetectable in McSCs. HFSCs that lack Col17a1 downregulate TGF‐β expression and promote adjacent McSCs to differentiate prematurely and eventually lead to the loss of McSCs and hair graying (Tanimura et al, 2011; Goldstein & Horsley, 2012). Another bulge‐derived niche factor that has been implied to have a regulatory function on McSC behavior is CXCL12, a chemokine that regulates the migration of various types of cells such as endothelial cells and melanocytes (Gupta et al, 1998; Belmadani et al, 2005). Recent study examining human hair follicle showed CXCL12 is expressed by the bulge whereas McSCs express its receptor CXCR7 (Yamada et al, 2019). CXCL12 also induced the migration and suppressed the differentiation of McSC modeling cells in vitro (Yamada et al, 2019). However, support for CXCL12 as a niche factor for McSC is needed from in vivo functional studies, especially since CXCL12 is upregulated in bulge overexpressing Runx1 (Lee et al, 2014; Li et al, 2019). This supports the idea that HFSCs modulate the behavior of neighboring cells around them through the regulation of transcription factors (Matsumura et al, 2016; Qiu et al, 2019).
Though many studies have been done to decipher the mystery of how HFSCs regulate McSCs, surprisingly little is known about the opposite communication, and what influence if any, McSCs have on HFSCs. As far as we know, the only uncovered effect from the melanocyte lineages to the HFSCs is that the ectopic deposition of pigment from the melanocytes can kill the HFSCs by causing apoptosis (Chang et al, 2013), and lack of melanin does not seem to have major effect as albino laboratory mice still have a normal hair cycle. However, key experiments such as the ablation of the McSC population to examine effects on neighboring HFSCs have not yet been performed to our knowledge.
Mesenchymal cells
Among all cell types, mesenchymal lineages are a very unique one. They can be found almost anywhere in the body where they provide critical structural support and exert diverse signaling functions. Being a highly heterogeneous population, mesenchymal cells include mesenchymal stem cells, stromal cells, fibroblasts, and many other more specialized sub‐populations. While different mesenchymal cells exhibit distinct characteristics, evidence showed that many of them can communicate to nearby stem cells, and they are often an essential niche component both in vivo and in vitro (Anthony & Link, 2014; Horiguchi et al, 2017; Liao & Li, 2020). The flip side of communication, from the stem cells toward the mesenchymal cells, has also been revealed, especially in the lung (Ruiz et al, 2014; Volckaert et al, 2017). Specifically, the Lgr6+ lung stem cell population produces SDF‐1 to activate the stroma, which in turn recruit endothelial cells to establish a functional niche (Ruiz et al, 2014). More recent finding suggested that the basal stem cells in the lung downregulate the Hippo pathway to modulate the adjacent mesenchymal stromal cells that express Fgf10, which is critical for the development and maintenance of the basal stem cells (Volckaert et al, 2017).
Mesenchymal cells constitute a large portion of the skin dermis and hypodermis, and they tightly surround the hair follicle. There are several different types of mesenchymal cells in the skin, and they all serve different functions in wound healing and the induction of hair follicles (Thulabandu et al, 2018). The most studied mesenchymal cells are the dermal papilla (DP) cells that reside at the base of the hair follicle (Figure 1). DP is a well‐known signaling center for HFSCs with indispensable roles in homeostatic hair regeneration (Morgan, 2014; Chen et al, 2020). A viable number of DP cells are required for HFSC activation as they express various inductive signaling molecules including Wnts, BMPs, noggin, and FGFs to initiate and promote hair growth (Driskell et al, 2009; Greco et al, 2009; Goldstein & Horsley, 2012; Rompolas et al, 2012; Chi et al, 2013).
However, despite numerous studies focusing on how the DP coordinates HFSC behaviors (Morgan, 2014), it is currently unclear how adult HFSCs modulate the DP, if at all. Early work in which adult bulges were transplanted in embryonic skin in utero resulted in new hair follicles with the epithelium derived from donor cells and the DPs derived from the host cells (Oshima et al, 2001). This suggests that in the right context adult bulges have the ability to induce new DP formation. This notion is also supported by the phenotype of a truncated constitutively active β‐Catenin, which when overexpressed in adult HFSCs to maintain activated Wnt signaling induced a de novo DP formation with every new initiated hair cycle (Gat et al, 1998). This suggests that aberrant Wnt signaling in HFSCs can induce the surrounding dermal cells to assemble a new DP, though it does not inform us on the role of the HFSCs in maintaining or coordination DP functions in normal adult homeostasis. More recently, transcriptomic analysis on different skin components revealed putative ligand‐receptor interaction pairs between HFSCs and the DP, opening up new avenues for future investigations (Rezza et al, 2016).
In addition to the DP, another mesenchymal lineage closely associated with the hair follicle is the dermal sheath that encloses the DP at telogen and the hair follicle at anagen and catagen (Yang & Cotsarelis, 2010; Martino et al, 2021). Recent work demonstrated that the dermal sheath is made of cells with smooth muscle characteristics that contract to draw the DP upwards to bring it closer to the HFSCs, thus ensuring matrix cell apoptosis and proper hair cycle progression (Heitman et al, 2020). This may also suggest that mechanical forces are important for initiation or propagation of progenitor‐cell pruning (Martino et al, 2021). Conversely, our epithelial mutants in which progenitor‐cell death is prematurely induced along with accelerated catagen (Lee et al, 2014; Wang et al, 2017), the dermal sheath must follow suit as these follicles return to what appears as a morphologically normal telogen follicle. Together, these data suggest that crosstalking must exist in both direction between the dermal sheath and HFSCs and their progeny, but the particular drivers in this molecular communication remain entirely unknown. Therefore, more investigation is needed to understand this unique mesenchymal population and its relationship with HFSCs.
Finally, the extra‐follicular dermal fibroblast (DFs) is another type of dermal mesenchymal cells and they are the major cell types in the dermis (Figure 1). DF heterogeneity has been identified in embryonic as well as in adult skin, and these cells play essential roles in skin wound‐healing responses and in cancer (Rognoni & Watt, 2018; Abbasi et al, 2020; Jiang & Rinkevich, 2020). DF cells also secrete BMP4 at different hair cycle stages, which potentially help maintain the quiescence of HFSC (Plikus et al, 2008). It has been previously shown that HFSCs may secrete CTGF (Tumbar et al, 2004), a known fibroblast proliferating factor (Ramazani et al, 2018), but whether this has a functional role in a putative crosstalk with DF is currently unknown.
Overall, with the exception of the DP, an extensively studied HFSC niche widely accepted as a major signaling driver for hair regeneration, the mesenchymal niche of the HFSCs is relatively understudied. Especially, the signaling from the HFSCs toward all these niche components is largely unknown. Understanding the relationship between the mesenchymal niche and HFSCs relationship bears high therapeutic value as miscommunication between the mesenchymal compartment and tissue stem cells often leads to diseases, as in the case of multiple blood disorders (Shi et al, 2018; Mangolini & Ringshausen, 2020) and chronic obstructive pulmonary disease where primary epithelial cells induce a inflammatory phenotype in the fibroblasts (Osei et al, 2016). In addition, due to the easy accessibility of mesenchymal lineages in the body and established differentiation protocol to generate induced mesenchymal stem cells (Secunda et al, 2015; Abdal Dayem et al, 2019), this cell population is widely used in various stem cell therapies and organotypic co‐culturing systems (Brown et al, 2019). Therefore, elucidating the mesenchyme‐stem cell relationship not only adds important scientific knowledge, but it also facilitates future development in both the clinical and tissue engineering fields.
Adipose tissue
Adipose tissue is important for both metabolism and molecular signaling. The crosstalk between adipose tissue and stem cells takes on various means including metabolic regulation (Ya‐Hsuan & Simón, 2020), ECM protein deposition (Weaver & Drummond‐Barbosa, 2018), and diffusible signaling molecules (DiMascio et al, 2007; Poloni et al, 2013; Zhou et al, 2017).
The adipose tissue in the skin resides in the hypodermis (Figure 1). In terms of the signaling from the adipose tissue to HFSCs, mature adipocytes inhibit HFSC activation and maintain the hair follicle in a refractory telogen stage by secreting BMP2, a well‐established HFSC inhibitor (Plikus et al, 2008), while adipose progenitor cells have the opposite effect on the hair cycle (Festa et al, 2011; Schmidt & Horsley, 2012; Figure 3).
For the reverse signaling, though no evidence shows that the intradermal adipose tissue is directly regulated by HFSCs, it undergoes cyclic expansion and shrinkage synchronized with hair follicle remodeling during the hair cycle (Guerrero‐Juarez & Plikus, 2018) (Figure 1). It is very possible that the hyperplasia and hypertrophy in anagen and lipolysis in catagen of adipose tissue occur under the instruction of HFSCs, or at least of the rapidly changing hair epithelium. Furthermore, in our epithelial mutants that halt anagen entry the adipose tissue seems to remain behind as well (Osorio et al, 2008; Wang et al, 2017), suggesting a dominant role of hair follicles in the regeneration of the adipose tissue. Interestingly, the transit‐amplifying matrix cells in anagen induce adipogenesis by targeting PDGFRa+ adipose precursors through SHH signaling (Zhang et al, 2016). There is also speculation that in catagen, signals from the apoptotic epithelium direct the lipolysis of the adipose tissue (Guerrero‐Juarez & Plikus, 2018). Given the proximity between the lower cyclic portion and the adipose tissues that reside in the hypodermis, it is plausible that the matrix and the apoptotic epithelium, rather than the HFSCs, are probably the immediate effectors on the adipose tissue niche.
In the clinical field, defects in intradermal adipose tissue are often seen in hair and skin disorders (Schmidt & Horsley, 2012; Rivera‐Gonzalez et al, 2014). Lipodystrophy is closely associated with different types of alopecia, though whether the alopecia leads to the change in the adipose tissue or the opposite awaits further exploration (Schmidt & Horsley, 2012; Rivera‐Gonzalez et al, 2014). Dermal focal hyperplasia patients often exhibit skin adipose tissue abnormality with hair thinning and baldness (Goltz, 1992; Ishii et al, 1992). In cancer, adipose tissue is often considered as a pro‐malignancy niche for cancer cells as it promotes cancer cell “stemness”, metastasis, and resistance to chemotherapy (Lengyel et al, 2018). Clearly, more in‐depth understanding of the crosstalk between stem cells and adipose tissue will bring huge benefits for disease biology and therapeutic development.
Immune cells
Immune cells circulate in the body as guards against infections. Based on their distinct roles, immune cells can be divided into the following categories: lymphocytes that fight against infections through secreted molecules or direct killing of the infected cells, neutrophils that exert quick response and ingest microbes, and macrophages (monocytes) that phagocytose both cell debris and pathogens. Since the immune cells are a highly responsive population under stress conditions such as injury and infection, their activities are largely mediated by cellular crosstalk, including that with stem cells (Naik et al, 2018; Qi et al, 2018; Brazil et al, 2019; Tsai, 2020). Beyond their communication in non‐homeostatic injury conditions, the immune cells receive increasing appreciation on their crosstalk with homeostatic tissue stem cells (Naik et al, 2018; Qi et al, 2018; Tsai, 2020). Among different studies, several exciting new findings attested that stem cells in systems such as mammary gland, intestine, and co‐culture of NSC and hNSCs and peripheral blood mononuclear cells provide molecular cues to manage the immune community (Zhang et al, 2015b; Biton et al, 2018; Chakrabarti et al, 2018).
Skin harbors diverse immune cell populations. Interestingly, many of the immune cells are found to be associated with the follicular epithelium. The number of different types of immune cells fluctuates during the hair cycle and is not only critical for skin’s barrier function, but may also actively communicate with HFSCs to maintain tissue homeostasis (Cho et al, 2020; Rahmani et al, 2020; Wang & Higgins, 2020). In the immune cell‐rich milieu of the skin, some immune cells are known to crosstalk with HFSCs. Responsible for phagocytosis and the clearing of debris (Yanez et al, 2017), macrophages are a potent regulator of HFSC quiescence and wound‐induced hair regeneration (Castellana et al, 2014; Rahmani et al, 2018; Wang et al, 2019; Cho et al, 2020; Rahmani et al, 2020; Wang & Higgins, 2020). γδT cells that secret cytokines in early immune responses are shown to promote HFSC activations after wounding (Kloepper et al, 2013; Lee et al, 2017). Another category of epidermal T cells, αβT cells, can modulate HFSC behaviors both in homeostasis and in response to injury (Ali et al, 2017; Mathur et al, 2019). Since the intense regulation from immune cells to the hair follicle is well summarized in very recent reviews (Cho et al, 2020; Rahmani et al, 2020; Wang & Higgins, 2020), we will not elaborate it further here.
While our knowledge of the reverse signaling from the HFSCs to the immune cells is still limited, a few studies provided some clues (Figure 3). In the early work by Nagao et al (2012), HFSCs were shown to inhibit the recruitment of Langerhans cells via CCL8 production. A more recent study further revealed quiescent HFSCs suppress antigen presentation, thus creating an immune‐privileged environment (Agudo et al, 2018). Plucking‐induced local injury involves the production of CCL2 from HFSCs to recruit TNF‐α‐releasing macrophage that promotes hair regeneration (Duheron et al, 2011; Chen et al, 2015). Failure in mediating the immune cell niche is associated with various pathological conditions (Rahmani et al, 2020). In scarring alopecia, the expression of MHC class I and II is increased in HFSCs, thus causing a breach in HFSC immune privilege and making them vulnerable targets for immune attack (Hoang et al, 2009). Similarly, lichen psoriasis is also characterized by the immune privilege collapse in the bulge (Harries et al, 2013). Therefore, understanding the crosstalk between HFSCs and immune cells will greatly benefit the clinical field from the perspective of finding potential therapies for skin and hair diseases and modulating immune rejection of skin grafts. In addition, though injury causes the recruitment of immune cells to the wounded site through the secretion of signaling factors by the keratinocytes (Fuchs & Blau, 2020; Rahmani et al, 2020), the specific molecules utilized by HFSCs, if any, still need further elucidation.
Non‐HFSC epithelial cells
The epithelium itself is composed of highly diverse cell populations, and the interplay among them is emerging (Donne et al, 2015). Lately, the luminal cells that line the lumen of gland tissues were shown to be critical for lineage fidelity of the surrounding basal stem cells, shedding light on the communication within the heterogeneous epithelium (Centonze et al, 2020). As special residents of the epithelium, HFSCs are also neighbored by different kinds of non‐HFSC epithelial cells. Early on, it has been shown that the K6+ inner bulge cells that do not act as stem cells can send repressive signals to the HFSCs (Hsu et al, 2011). More recent findings called attention to sebocytes, which are cells in the sebaceous gland, and epidermal cells in the IFE.
Sebocytes act from the sebaceous gland (SG) to secrete sebum into the hair canal and to the skin surface for the barrier and thermoregulation function. During the hair cycle, HFSCs dynamically tune the expression of glutamate transporter Slc1a3 (Reichenbach et al, 2018), which is also shown to be a marker for the non‐label‐retaining population in the basal IFE (Sada et al, 2016). When HFSCs are activated in the growth phase, IFE and the sebaceous gland (SG) undergo synchronized expansion along with the hair follicle, accompanying an increase in Slc1a3+ cells in these regions (Reichenbach et al, 2018) (Figure 3). The absence of Slc1a3 delays anagen entry and results in the uncoupling of the three compartments (Reichenbach et al, 2018). In addition to metabolism, transcription factors that control HFSC homeostasis may also regulate SG. We and others found that Gata6 is expressed in early HF progenitors during development and primed HFSCs in hair germ (Wang et al, 2017; Oulès et al, 2019). Though so far Gata6 has only been shown to be involved in SG morphogenesis (Oulès et al, 2019), since Gata6 is continuously expressed in adult HF, it may also have a role in adult SG maintenance.
HFSCs may also choreograph the turnover of the epidermis. Clonal analysis revealed that the clonal expansion in the IFE in anagen happens more prominently near hair follicles (Roy et al, 2016), and this expansion is not a result of direct bulge contribution as HFSCs do not contribute to the epidermis under homeostatic condition (Ito et al, 2005). Therefore, though the detailed molecular interaction between the HFSCs and the basal cells in the IFE has not been elucidated, this study points toward a possibility that HFSCs may provide diffusible molecules to the IFE cells near them and induce such a range‐dependent effect.
The highly coordinated behaviors between HFSCs and the non‐HFSC epithelium open up new windows for future investigations. What are the molecular mechanisms behind the coupled activity of epithelium? Are some cyclically expressed transcription factors involved? What is the function of the cyclic expansion and regression of non‐hair follicle epithelial compartments? In terms of clinical relevance, dysregulation of the sebaceous gland results in acne, the most common skin concern (Lolis et al, 2009; Makrantonaki et al, 2011). Plus, there are numerous medical conditions involved in impaired epidermal turnover (Turner et al, 2012; Zhang et al, 2015a). Therefore, answers to the abovementioned scientific questions will provide new insights into skin biology and potential therapeutics targeting HFSCs to harness their niche modulatory function.
Extracellular matrix
Extracellular matrix (ECM) is the tissue scaffold that provides structural support and is critical for cell adhesion, migration, and cell signaling. Its major components include collagens, integrins, proteoglycans, and other structural macromolecules (Frantz et al, 2010). The myriad interplays between stem cells and the ECM have been a hot topics for stem cell niche investigations with deep implications in diseases and cancer metastasis (Ahmed & ffrench‐Constant, 2016; Nallanthighal et al, 2019).
In the skin, mutations in ECM proteins can result in life‐threatening diseases, such as epidermolysis bullosa (Chermnykh et al, 2018). In addition to its clinical relevance, in‐depth understanding of the ECM also greatly benefits tissue engineering as many reports showed enhanced hair follicle maintenance and regeneration in culture with the addition of ECM components (Wang et al, 2016; Chacón‐Martínez et al, 2017; Chermnykh et al, 2018). In the hair follicle, ECM proteins are highly expressed by the HFSCs as niche factors (Tumbar et al, 2004; Joost et al, 2016; Yang et al, 2017). In the above paragraphs, we have discussed nephronectin in muscle niche regulation, EGFL6 in nerve and possibly vasculature and smooth muscle niche regulation, and Col17a1 in McSC niche regulation, all ECM proteins produced by the HFSCs (Fujiwara et al, 2011; Tanimura et al, 2011; Cheng et al, 2018). A recent study comparing young and aged HFSCs further emphasized the importance of ECM proteins to HFSC niches including the epithelium, nerves, APM, immune cells, and possibly more skin components by showing that though aged HFSCs are able to maintain their cell identity with unaltered expression of key transcription factors such as Sox9 and stable chromatin landscape, ECM‐related genes showed dramatic changes accompanied by a compromised niche environment (Ge et al, 2020).
While ECM proteins from the hair epithelium are important as a mediator of various components in the HFSC microenvironment, we cannot ignore the fact that ECM is itself a bonafide niche of HFSCs, as it is involved in various molecular signaling pathways (Chermnykh et al, 2018). Col17a1 is one such example, important for HFSC self‐renewal. Loss of Col17a1 in HFSCs leads to their exit from quiescence and promotes an epidermal fate, which eventually causes the depletion of the HFSC pool (Tanimura et al, 2011; Matsumura et al, 2016). Decorin is another bulge‐expressed ECM protein that has been shown to maintain HFSCs in an undifferentiated state (Miyachi et al, 2018). Interestingly, decorin is also found to be differentially expressed by the lymphatic capillaries at different HFSC cellular states (Peña‐Jimenez et al, 2019). Considering the dynamic association between HFSC and the lymphatic capillaries (Gur‐Cohen et al, 2019), this result points to an interesting spatiotemporal regulatory mechanism from lymphatic vessels to HFSCs (Peña‐Jimenez et al, 2019). Laminins are the major component of the basement membrane and are critical for ensuring the proper attachment of keratinocytes to the basement membrane. In addition to its structural support function, laminins are also found to regulate HFSC activation and quiescence. The expression of laminin‐332 (LN332) and laminin‐511 (LN511) is downstream of integrin‐linked kinase that is highly expressed in the bulge and the hair germ (Morgner et al, 2015). Unique spatial distribution of LN332 that inhibits Wnt signaling and LN511 that promotes TGF‐β signaling along the hair follicle maintains HFSC in quiescence. Perturbation of the deposition pattern of these two laminins leads to the de‐regulation of these two key signaling pathways for stem cell behavior (Morgner et al, 2015). Podoplanin is a transmembrane glycoprotein recently found to be expressed by HFSCs in late anagen (Yoon et al, 2019b). Epithelial knockout of podoplanin leads to slightly prolonged anagen and increased bulb diameter. Interestingly, many focal adhesion‐related and ECM genes are downstream of podoplanin, including collagens, integrins, and LAMA4. This finding points toward the role of HFSC‐derived ECM in regulating anagen progression possibly via regulation of cell migration (Yoon et al, 2019b).
All these new discoveries attest to ECM as a critical niche for the HFSCs—not only in the sense of providing mechanical support and mediating the organization of various niche components, but also by actively and directly regulating SC behavior by itself. A whole slur of ECM proteins seems to be made by the HFSCs, and it would be interesting to further dissect the role they play both as mediators of different niche components in the microenvironment and its direct contribution to HFSC signaling. This is especially interesting given the apparent implications of ECM molecules in long‐term maintenance of stem cells and in skin aging. Could ECM plausibly be the major “fountain of youth” people have been seeking all the time, with which we can reverse the skin aging by varying the ECM composition? Knowledge on these questions can lead to new progress in tissue engineering as well as breakthroughs in real‐world applications, such as cosmetics and pharmaceutical development.
Technology advancement
In recent years, the study of HFSC niches has benefited from technology advancement and new tools that are now available to researchers. A major leap was the fast‐evolving intravital imaging techniques that allow direct visualization of HFSC niches (Brown & Greco, 2014; Park et al, 2016). Recently, it has been employed to visualize the dermal lymphatic vessels and revealed a continuous flow in the lymphatic vascular network (Peña‐Jimenez et al, 2019). Another widely used imaging technique is whole mount imaging that can render a similarly accurate image of HFSC and their niches, though relatively static compared with intravital imaging. With better clearing technique and high‐resolution two‐photon microscope, recent work using whole mount imaging revealed the spatial relationship between HFSCs and the surrounding nerves, vasculature, and mesenchyme (Cheng et al, 2018; Gur‐Cohen et al, 2019; Peña‐Jimenez et al, 2019; Heitman et al, 2020; Shwartz et al, 2020). By checking different time points, whole mount imaging can also shed light on dynamic tissue changes. For example, vasculature arrays seem to associate more frequently with more proliferative regions of the epidermis (Sada et al, 2016), and lymphatic capillaries vary their association with HFSCs during the hair cycle (Gur‐Cohen et al, 2019; Peña‐Jimenez et al, 2019). Whole mount imaging also revealed nerve attachment to the bulge during hair regeneration and the interaction between ECM proteins and its binding partner (Cheng et al, 2018). All these findings are not possible, or at least will require a lot more work to reconstruct 3D architecture from thin sections, with more conventional imaging techniques.
Single‐cell sequencing technique also gained increasing popularity lately. Along with the algorithms that construct lineage trajectories, single‐cell RNA‐seq allows precise definition of unique gene expression by HFSCs and their niches at different stages. For instance, in a mixed HFSC pool, only a subset express Angptl7 in early anagen HFSCs but not in telogen HFSCs (Gur‐Cohen et al, 2019). In the same work by Gur‐Cohen et al (2019), lymphatic vessels and blood vessels are highly divergent in terms of the niche factors they produce, hence providing a better resolution on the interaction between HFSCs with each sub‐populations in a certain niche. Other studies from the Kasper laboratory revealed epithelium heterogeneity that is linked with both spatial location and differentiation states, and further characterized the transcriptomic profile in each sub‐population. This information provides a platform for the more precise dissection of molecular interaction between the epithelium and the surrounding environment (Joost et al, 2016). Besides purifying only the epithelium, single‐cell RNA‐seq on full‐thickness skin provides a bird‐eye view at transcriptomic level of different skin components including the epithelium, vasculatures, immune cells, neural crest‐derived lineages, DF, and others (Joost et al, 2020). Though some rare populations might be missed in such study, this global expression profiling detects numerous receptor‐ligand interaction pairs between different tissue components (Joost et al, 2020). In situ mRNA hybridization attested that the heterogeneity within the epithelium and DF population correspond to its anatomical location (Joost et al, 2020). In fact, the DF has also been probed at the single‐cell level by various groups under stress condition such as injury, not only in mice but also in humans (Hu et al, 2018; Philippeos et al, 2018; Guerrero‐Juarez et al, 2019; Joost et al, 2020; Solé‐Boldo et al, 2020). These studies helped elucidate heterogeneity that can drive various functional studies related to wound healing. Perhaps the future will bring more light into the heterogeneity of various other skin components, such as the endothelium, and how specialized subsets of cells may interact with the HFSCs. We are awaiting exciting times ahead of us!
Another rapidly evolving technology is tissue engineering. In fact, cultured skin has been employed in clinical setting to treat burn patients since the last century (Gallico et al, 1984; Rheinwald, 1989). Earlier skin culture lacked the 3D structure of the actual skin in the 2D “on‐the‐plastic” culturing system (Oh et al, 2013) and was largely limited to the epidermis without other critical skin components such as hair follicle, sweat and sebaceous glands, nerves, and vasculature. Recent effort aims to reconstruct the fully functional 3D skin with additional components (Boyce & Lalley, 2018; Castro & Logarinho, 2020; Fuchs & Blau, 2020; Weng et al, 2020). It is well‐established that hair follicle neogenesis requires inductive DP cells. Thus, hypoxia culture (Upton et al, 2015), trichostatin A treatment (Guo et al, 2019), and various other methods have been introduced in an attempt to maintain the inductive property of DP cells in vitro. In addition to the DP, other niche components have been added for a more faithful reconstruction of the skin (Blais et al, 2013; Boyce & Lalley, 2018). Importantly, in tissue engineering, hair follicle and some niche components have a mutually enhancing relationship. For example, vascularization of human skin constructed with hair follicles enhances engraftment efficiency and ensures hair follicle growth (Abaci et al, 2018). Hair follicle has also been shown to pattern cutaneous nerves in engineered skin (Gagnon et al, 2011; Blais et al, 2013). Based on these results, it is very clear that a better understanding of the niche‐stem cell crosstalk can facilitate the engineering of the fully functional 3D skin for future transplantation therapies.
Conclusions and outlook for the future
The regeneration of the hair follicle is closely associated with the turnover of the whole skin, and HFSCs seem to be at the center of this dynamic process. HFSC behavior is choreographed by complicated intrinsic (Lee et al, 2021) and extrinsic signaling pathways, the later of which we discussed in‐depth in this review and summarized concisely in Figure 3. At the same time, HFSCs also signal to their neighbors to create a favorable environment for themselves. Their role as an organizing signaling center in the skin has become increasingly evident in the past decade and has inspired many studies to understand the intricate mechanisms underlying the HFSC unique activities.
Currently, a major question in the field is how HFSCs regulate so many different tissue niches in such a highly synchronized and coordinated manner. One possible answer is through the expression of core transcription factors such as Gata6, Runx1, and Tcf3, which can modulate the concerted expression of hundreds and even thousands of niche modulatory factors at the same time. In addition, the existence of dual‐component niche, such as those composed of sympathetic nerves and the APM (Shwartz et al, 2020), sheds light on another possibility that different HFSC niches may communicate among themselves to form a joint niche, thus coordinating their function. In fact, changing one niche component may lead to a cascade in the skin microcosm remodeling.
Since the niche regulation often happens via diffusible signaling molecules, it is likely that the same type of cells at different distances from the HFSCs receives the molecular instruction in a gradient, and thus behave differently. Therefore, another possible direction to take for understanding the HFSC‐niche interaction is to integrate the genomic data with high‐resolution spatial information. This type of study can be made possible by combining single‐cell sequencing and intravital/whole mount imaging, or other novel techniques. One example is spatial single‐cell RNA‐seq, which has already been employed to understand the complex bone marrow niche (Baccin et al, 2020).
Furthermore, it is clear that stem cell niches are tightly related to stem cell aging but the interplay among them is still unclear. Several studies put emphasis on the niche and consider it as a putative driver for aging, as transplantation assays of HF into young environment functionally rejuvenate HFSCs (Cao et al, 2016; Ge et al, 2020). On the other hand, the expression of genes encoding ECM proteins in aged HFSCs is altered, further leading to dysregulation of the niche (Ge et al, 2020). Though whether it is the aging HFSCs that drive the aging microenvironment, or the opposite, may be a chicken‐and‐egg question, figuring out the real driver of aging is fascinating. Such work will also provide insights for other stem cell fields, to understand whether rejuvenation might need to focus more on niches or on the stem cells themselves.
Similar to aging, HFSCs under stress conditions, such as injury and diseases, also have dramatically altered behaviors. Though the niche contribution to the wound‐healing process has been revealed and summarized by Chen et al (2020), whether and how the alterations in HFSCs lead to change in niches, and whether niches may contribute to some of the HFSC alterations are largely unknown. Since niches are involved in the stress response of many other stem cells (Chacón‐Martínez et al, 2018), the HFSC niches in the skin should also be investigated from those perspectives. After all, the skin is our first line of defense and thus may easily encounter various stresses.
Since the HFSC field is a relatively “young” stem cell field, the niches of the HFSCs are understudied compared with other stem cells such as the hematopoietic stem cells and neural stem cells. While the molecular crosstalk between the HFSCs and the niches remains to be further elucidated, one classic way to get started is to utilize the currently available gene expression data to screen for potential candidate molecules to perform functional studies. By probing deeper into this question, the role of HFSCs as a skin signaling center not only benefits skin biologists, but may also provide valuable insight for other stem cell fields, in revealing potentially common tissue stem cell signaling mechanisms.
In addition to basic science merit, the understanding of HFSC niches is exceptionally handy for tissue engineering of the skin. Skin transplantation has been used to treat burn patients for decades, and yet, it still faces the challenge of successfully engrafting fully functional skin with all the dermal components including spontaneously cycling hair follicles. Moreover, patients with chronic wounds and diabetic ulcers also benefit from skin transplants as these wounds are difficult to heal and can lead to severe consequences such as amputation. In fact, as of 2019, approximately 463 millions adults have diabetes, and the number is projected to be 579 millions in 2025, among which 15–25% will develop diabetic foot ulcers (Federation, 2019; Oliver & Mutluoglu, 2020). While enormous efforts have been paid to develop promising skin transplantation therapies, given that the complete wound closure rate is lower than 50% (Santema et al, 2016), there is still a long way to go. One reason causing the significantly delayed healing for this type of wound is the impaired angiogenesis (Ahmed & Antonsen, 2016; Okonkwo & DiPietro, 2017), peripheral neuropathy (Volmer‐Thole & Lobmann, 2016), and dysregulation of immune cells (Ahmed & Antonsen, 2016; Sawaya et al, 2020), all of which are components of the skin environment. Altogether, it is crucial to understand the skin and hair follicle microenvironment for better engineering of skin grafts and for management of chronic wound healing. Excitingly, with the increasing knowledge we gained from mouse research, we are not too far from translating our findings from mouse to humans.
In conclusion, with the involvement of the niche environment in aging and diseases, the study of HFSC niches will advance the tissue engineering field of organotypic cultures and pave the way for future clinical and cosmetic applications.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
We thank all our colleagues for their seminal contributions to knowledge of skin and hair follicle biology and for making this an exciting and rewarding field of research. This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR070157 and R01AR073806) to TT.
The EMBO Journal (2021) 40: e107135.
References
- Abaci HE, Coffman A, Doucet Y, Chen J, Jacków J, Wang E, Guo Z, Shin JU, Jahoda CA, Christiano AM (2018) Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun 9: 5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbasi S, Sinha S, Labit E, Rosin NL, Yoon G, Rahmani W, Jaffer A, Sharma N, Hagner A, Shah P et al (2020) Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell 27: 396–412 [DOI] [PubMed] [Google Scholar]
- Abdal Dayem A, Lee SB, Kim K, Lim KM, Jeon T‐I, Seok J, Cho SG (2019) Production of Mesenchymal stem cells through stem cell reprogramming. Int J Mol Sci 20: 1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwala S, Tamplin OJ (2018) Neural crossroads in the hematopoietic stem cell niche. Trends Cell Biol 28: 987–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A, Merad M et al (2018) Quiescent tissue stem cells evade immune surveillance. Immunity 48: 271–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AS, Antonsen EL (2016) Immune and vascular dysfunction in diabetic wound healing. J Wound Care 25: S35–S46 [DOI] [PubMed] [Google Scholar]
- Ahmed M, ffrench‐Constant C (2016) Extracellular matrix regulation of stem cell behavior. Curr Stem Cell Rep 2: 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn GO, Brown JM (2008) Matrix metalloproteinase‐9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow‐derived myelomonocytic cells. Cancer Cell 13: 193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong H‐A, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC et al (2017) Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169: 1119–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso L, Fuchs E (2006) The hair cycle. J Cell Sci 119: 391 [DOI] [PubMed] [Google Scholar]
- Anthony BA, Link DC (2014) Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol 35: 32–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple DM, Kokovay E (2017) Vascular niche contribution to age‐associated neural stem cell dysfunction. Am J Physiol Heart Circ Physiol 313: H896–H902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo PO, Lousado L, Paiva AE, Andreotti JP, Santos GSP, Sena IFG, Prazeres PHDM, Filev R, Mintz A, Birbrair A (2017) Endothelial cells maintain neural stem cells quiescent in their niche. Neuroscience 363: 62–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccin C, Al‐Sabah J, Velten L, Helbling PM, Grünschläger F, Hernández‐Malmierca P, Nombela‐Arrieta C, Steinmetz LM, Trumpp A, Haas S (2020) Combined single‐cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol 22: 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ (2005) The chemokine stromal cell‐derived factor‐1 regulates the migration of sensory neuron progenitors. J Neurosci 25: 3995–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, Ashenberg O, Su C‐W, Smillie C, Shekhar K et al (2018) T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 175: 1307–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais M, Parenteau‐Bareil R, Cadau S, Berthod F (2013) Concise review: tissue‐engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med 2: 545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Eichmüller S, Johansson O, Paus R (1997) Hair cycle‐dependent plasticity of skin and hair follicle innervation in normal murine skin. Journal of Comparative Neurology 386: 379–395 [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Peters EMJ, Botchkareva NV, Maurer M, Paus R (1999) Hair cycle‐dependent changes in adrenergic skin innervation, and hair growth modulation by adrenergic drugs. J Investig Dermatol 113: 878–887 [DOI] [PubMed] [Google Scholar]
- Boyce ST, Lalley AL (2018) Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma 6: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil JC, Quiros M, Nusrat A, Parkos CA (2019) Innate immune cell–epithelial crosstalk during wound repair. J Clin Investig 129: 2983–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR (2019) Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med 13: 1738–1755 [DOI] [PubMed] [Google Scholar]
- Brown S, Greco V (2014) Stem cells in the wild: understanding the World of stem cells through intravital imaging. Cell Stem Cell 15: 683–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL (2011) Nerve‐derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8: 552–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Li L, Kajiura S, Amoh Y, Tan Y, Liu F, Hoffman RM (2016) Aging hair follicles rejuvenated by transplantation to a young subcutaneous environment. Cell Cycle 15: 1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana D, Paus R, Perez‐Moreno M (2014) Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol 12: e1002002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro AR, Logarinho E (2020) Tissue engineering strategies for human hair follicle regeneration: how far from a hairy goal? Stem Cells Transl Med 9: 342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze A, Lin S, Tika E, Sifrim A, Fioramonti M, Malfait M, Song Y, Wuidart A, Van Herck J, Dannau A et al (2020) Heterotypic cell–cell communication regulates glandular stem cell multipotency. Nature 584: 608–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón‐Martínez CA, Klose M, Niemann C, Glauche I, Wickström SA (2017) Hair follicle stem cell cultures reveal self‐organizing plasticity of stem cells and their progeny. The EMBO journal 36: 151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón‐Martínez CA, Koester J, Wickström SA (2018) Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development 145: dev165399 [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Celià‐Terrassa T, Kumar S, Hang X, Wei Y, Choudhury A, Hwang J, Peng J, Nixon B, Grady JJ et al (2018) Notch ligand Dll1 mediates cross‐talk between mammary stem cells and the macrophageal niche. Science 360: eaan4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C‐Y, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, Fuchs E (2013) NFIB is a governor of epithelial–melanocyte stem cell behaviour in a shared niche. Nature 495: 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C‐L, Huang W‐Y, Wang EHC, Tai K‐Y, Lin S‐J (2020) Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration. J Biomed Sci 27: 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C‐C, Wang L, Plikus M, Jiang T, Murray P, Ramos R, Guerrero‐Juarez C, Hughes M, Lee O, Shi S et al (2015) Organ‐level quorum sensing directs regeneration in hair stem cell populations. Cell 161: 277–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PYG, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3: e1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C‐C, Tsutsui K, Taguchi T, Sanzen N, Nakagawa A, Kakiguchi K, Yonemura S, Tanegashima C, Keeley SD, Kiyonari H et al (2018) Hair follicle epidermal stem cells define a niche for tactile sensation. eLife 7: e38883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermnykh E, Kalabusheva E, Vorotelyak E (2018) Extracellular matrix as a regulator of epidermal stem cell fate. Int J Mol Sci 19: 1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Wu E, Morgan BA (2013) Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 140: 1676–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chim SM, Qin A, Tickner J, Pavlos N, Davey T, Wang H, Guo Y, Zheng MH, Xu J (2011) EGFL6 promotes endothelial cell migration and angiogenesis through the activation of extracellular signal‐regulated kinase. J Biol Chem 286: 22035–22046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I, Lui PP, Ali N (2020) Treg regulation of the epithelial stem cell lineage. J Immunol Regen Med 8: 100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M‐I, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM (2018) Niche‐mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 145: dev163014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Ihida‐Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE (2009) Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Investig 119: 2538–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EA, Zhou W, Dailey MJ (2018) Evidence for a direct effect of the autonomic nervous system on intestinal epithelial stem cell proliferation. Physiological Reports 6: e13745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U, Reya T (2007) Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol 178: 3511 [DOI] [PubMed] [Google Scholar]
- Donne ML, Lechner AJ, Rock JR (2015) Evidence for lung epithelial stem cell niches. BMC Dev Biol 15: 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM (2009) Sox2‐positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 136: 2815–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duheron V, Hess E, Duval M, Decossas M, Castaneda B, Klöpper JE, Amoasii L, Barbaroux J‐B, Williams IR, Yagita H et al (2011) Receptor activator of NF‐κB (RANK) stimulates the proliferation of epithelial cells of the epidermo‐pilosebaceous unit. Proc Natl Acad Sci 108: 5342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durward A, Rudall KM (1958) CHAPTER 9 – The vascularity and patterns of growth of hair follicles. In The biology of hair growth, Montagna W, Ellis RA (eds), pp 189–218. New York, NY: Academic Press; [Google Scholar]
- Ellis RA, Moretti G (1959) Vascular patterns associated with catagen hair follicles in the human scalp. Ann N Y Acad Sci 83: 448–457 [DOI] [PubMed] [Google Scholar]
- Fan SM‐Y, Chang Y‐T, Chen C‐L, Wang W‐H, Pan M‐K, Chen W‐P, Huang W‐Y, Xu Z, Huang H‐E, Chen T et al (2018) External light activates hair follicle stem cells through eyes via an ipRGC–SCN–sympathetic neural pathway. Proc Natl Acad Sci 115: E6880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federation ID (2019) IDF Diabetes Atlas, 9 th edn. Brussels, Belgium: International Diabetes Federation; [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V (2011) Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146: 761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123: 4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Blau HM (2020) Tissue stem cells: architects of their niches. Cell Stem Cell 27: 532–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116: 769–778 [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM (2011) The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144: 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon V, Larouche D, Parenteau‐Bareil R, Gingras M, Germain L, Berthod F (2011) Hair follicles guide nerve migration In Vitro and In Vivo in tissue‐engineered skin. Journal of Investigative Dermatology 131: 1375–1378 [DOI] [PubMed] [Google Scholar]
- Gallico GG, O'Connor NE, Compton CC, Kehinde O, Green H (1984) Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 311: 448–451 [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E (1998) De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β‐catenin in skin. Cell 95: 605–614 [DOI] [PubMed] [Google Scholar]
- Ge Y, Miao Y, Gur‐Cohen S, Gomez N, Yang H, Nikolova M, Polak L, Hu Y, Verma A, Elemento O et al (2020) The aging skin microenvironment dictates stem cell behavior. Proc Natl Acad Sci 117: 5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatte P, Buchmann SJ, Hijazi MM, Illigens BM‐W, Siepmann T (2019) Architecture of the cutaneous autonomic nervous system. Front Neurol 10: 970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DC, Bailey AS, Pfaffle DL, Al Masri A, Christian JL, Fleming WH (2009) BMP4 regulates the hematopoietic stem cell niche. Blood 114: 4393–4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J, Horsley V (2012) Home sweet home: skin stem cell niches. Cell Mol Life Sci 69: 2573–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltz RW (1992) Focal dermal hypoplasia syndrome: an update. Arch Dermatol 128: 1108–1111 [PubMed] [Google Scholar]
- Grebenyuk S, Ranga A (2019) Engineering organoid vascularization. Front Bioeng Biotechnol 7: 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz‐Racelis J, Fuchs E (2009) A two‐step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero‐Juarez CF, Dedhia PH, Jin S, Ruiz‐Vega R, Ma D, Liu Y, Yamaga K, Shestova O, Gay DL, Yang Z et al (2019) Single‐cell analysis reveals fibroblast heterogeneity and myeloid‐derived adipocyte progenitors in murine skin wounds. Nat Commun 10: 650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero‐Juarez CF, Plikus MV (2018) Emerging nonmetabolic functions of skin fat. Nat Rev Endocrinol 14: 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Lei M, Li Y, Liu Y, Tang Y, Xing Y, Deng F, Yang K (2017) Paracrine secreted frizzled‐related protein 4 inhibits melanocytes differentiation in hair follicle. Stem Cells Int 2017: 2857478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Wang X, Yuan J, Zhu M, Fu X, Xu R‐H, Wu C, Wu Y (2019) TSA restores hair follicle‐inductive capacity of skin‐derived precursors. Sci Rep 9: 2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM (1998) Chemokine receptors in human endothelial cells. J Biol Chem 273(7): 4282–4287. [DOI] [PubMed] [Google Scholar]
- Gur‐Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, Liu X, de la Cruz‐Racelis J, Mehrara BJ, Fuchs E (2019) Stem cell–driven lymphatic remodeling coordinates tissue regeneration. Science 366: 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachisu M, Obayashi M, Kogo M, Ihara K (2020) Psychosomatic health in elderly people for preventing frailty: the role of IGF‐1 and BDNF in the muscle and the brain. J Aging Sci 8: 223 [Google Scholar]
- Hampton HR, Chtanova T (2019) Lymphatic migration of immune cells. Front Immunol 10: 1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LS, Coggle JE, Wells J, Charles MW (1984) The influence of the hair cycle on the thickness of mouse skin. The Anatomical Record 210: 569–573 [DOI] [PubMed] [Google Scholar]
- Harries MJ, Meyer K, Chaudhry I, E Kloepper J, Poblet E, Griffiths CEM, Paus R (2013) Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol 231: 236–247 [DOI] [PubMed] [Google Scholar]
- Heitman N, Sennett R, Mok K‐W, Saxena N, Srivastava D, Martino P, Grisanti L, Wang Z, Ma’ayan A, Rompolas P et al (2020) Dermal sheath contraction powers stem cell niche relocation during hair cycle regression. Science 367: 161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang MP, Keady M, Mahalingam M (2009) Stem cell markers (cytokeratin 15, CD34 and nestin) in primary scarring and nonscarring alopecia. Br J Dermatol 160: 609–615 [DOI] [PubMed] [Google Scholar]
- Hofer HR, Tuan RS (2016) Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther 7: 131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi H, Endo M, Kawane K, Kadomatsu T, Terada K, Morinaga J, Araki K, Miyata K, Oike Y (2017) ANGPTL2 expression in the intestinal stem cell niche controls epithelial regeneration and homeostasis. The EMBO Journal 36: 409–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y‐C, Li L, Fuchs E (2014) Emerging interactions between skin stem cells and their niches. Nat Med 20: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y‐C, Pasolli HA, Fuchs E (2011) Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144: 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MS, Moore AL, Longaker MT (2018) A fibroblast is not a fibroblast is not a fibroblast. Journal of Investigative Dermatology 138: 729–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Baba N, Anaizuka IK, Nakajima H, Ono S, Amemiya F (1992) Histopathological study of focal dermal hypoplasia (Goltz syndrome). Clin Exp Dermatol 17: 24–26 [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354 [DOI] [PubMed] [Google Scholar]
- Jiang D, Rinkevich Y (2020) Scars or Regeneration?‐Dermal Fibroblasts as Drivers of Diverse Skin Wound Responses. Int J Mol Sci 21: 617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling P, Pundavela J, Oliveira SMR, Roselli S, Walker MM, Hondermarck H (2015) Nerve‐cancer cell cross‐talk: a novel promoter of tumor progression. Can Res 75: 1777 [DOI] [PubMed] [Google Scholar]
- Joost S, Annusver K, Jacob T, Sun X, Dalessandri T, Sivan U, Sequeira I, Sandberg R, Kasper M (2020) The molecular anatomy of mouse skin during hair growth and rest. Cell Stem Cell 26: 441–457 [DOI] [PubMed] [Google Scholar]
- Joost S, Zeisel A, Jacob T, Sun X, La Manno G, Lönnerberg P, Linnarsson S, Kasper M (2016) Single‐cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst 3: 221–237.e229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Wang J, Tian J, Shi R, Jia H, Wang Y (2020) The emerging role of EGFL6 in angiogenesis and tumor progression. Int J Med Sci 17: 1320–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepper JE, Kawai K, Bertolini M, Kanekura T, Paus R (2013) Loss of γδ T cells results in hair cycling defects. J Invest Dermatol 133: 1666–1669 [DOI] [PubMed] [Google Scholar]
- Kosinski C, Li VSW, Chan ASY, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST et al (2007) Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA 104: 15418–15423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Tumbar T (2012) Hairy tale of signaling in hair follicle development and cycling. Semin Cell Dev Biol 23: 906–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Gund R, Dutta A, Pincha N, Rana I, Ghosh S, Witherden D, Kandyba E, MacLeod A, Kobielak K et al (2017) Stimulation of hair follicle stem cell proliferation through an IL‐1 dependent activation of γδT‐cells. eLife 6: e28875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Li KN, Tumbar T (2021) Stem cell‐intrinsic mechanisms regulating adult hair follicle homeostasis. Exp Dermatol 30: 430–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Sada A, Zhang M, McDermitt David J, Lu Shu Y, Kemphues Kenneth J, Tumbar T (2014) High Runx1 levels promote a reversible, more‐differentiated cell state in hair‐follicle stem cells during quiescence. Cell Rep 6: 499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel E, Makowski L, DiGiovanni J, Kolonin MG (2018) Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer 4: 374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KN, Jain P, He CH, Eun FC, Kang S, Tumbar T (2019) Skin vasculature and hair follicle cross‐talking associated with stem cell activation and tissue homeostasis. eLife 8: e45977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Li H (2020) Dissecting the niche for alveolar type II cells with alveolar organoids. Front Cell Dev Biol 8: 419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R (2008) Paracrine Wingless signalling controls self‐renewal of Drosophila intestinal stem cells. Nature 455: 1119–1123 [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R (2010) Paracrine unpaired signaling through the JAK/STAT pathway controls self‐renewal and lineage differentiation of Drosophila intestinal stem cells. J Mol Cell Biol 2: 37–49 [DOI] [PubMed] [Google Scholar]
- Lolis MS, Bowe WP, Shalita AR (2009) Acne and systemic disease. Med Clin North Am 93: 1161–1181 [DOI] [PubMed] [Google Scholar]
- Lu Z, Xie Y, Huang H, Jiang K, Zhou B, Wang F, Chen T (2020) Hair follicle stem cells regulate retinoid metabolism to maintain the self‐renewal niche for melanocyte stem cells. eLife 9: e52712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugano R, Ramachandran M, Dimberg A (2020) Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 77: 1745–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren O, Jodal M, Jansson M, Ryberg AT, Svensson L (2011) Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS One 6: e16295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrantonaki E, Ganceviciene R, Zouboulis C (2011) An update on the role of the sebaceous gland in the pathogenesis of acne. Dermatoendocrinol 3: 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Mammoto T (2019) Vascular niche in lung alveolar development, homeostasis, and regeneration. Front Bioeng Biotechnol 7: 318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangolini M, Ringshausen I (2020) Bone marrow stromal cells drive key hallmarks of B cell malignancies. Int J Mol Sci 21: 1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Martínez E, Galván‐Hernández CI, Toscano‐Márquez B, Gutiérrez‐Ospina G (2012) Modulatory role of sensory innervation on hair follicle stem cell progeny during wound healing of the rat skin. PLoS One 7: e36421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino PA, Heitman N, Rendl M (2021) The dermal sheath: An emerging component of the hair follicle stem cell niche. Exp Dermatol 30: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C, Sii‐Felice K, Fouchet P, Etienne O, Haton C, Mabondzo A, Boussin FD, Mouthon M‐A (2008) Endothelial cell‐derived bone morphogenetic proteins control proliferation of neural stem/progenitor cells. Mol Cell Neurosci 38: 569–577 [DOI] [PubMed] [Google Scholar]
- Mathur AN, Zirak B, Boothby IC, Tan M, Cohen JN, Mauro TM, Mehta P, Lowe MM, Abbas AK, Ali N et al (2019) Treg‐cell control of a CXCL5‐IL‐17 inflammatory axis promotes hair‐follicle‐stem‐cell differentiation during skin‐barrier repair. Immunity 50: 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Mohri Y, Binh NT, Morinaga H, Fukuda M, Ito M, Kurata S, Hoeijmakers J, Nishimura EK (2016) Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 351: aad4395 [DOI] [PubMed] [Google Scholar]
- Maurer M, Peters EMJ, Botchkarev VA, Paus R (1998) Intact hair follicle innervation is not essential for anagen induction and development. Arch Dermatol Res 290: 574–578 [DOI] [PubMed] [Google Scholar]
- McLeod WA (1970) Observations of fenestrated capillaries in the human scalp. J Invest Dermatol 55: 354–357 [DOI] [PubMed] [Google Scholar]
- Mecklenburg L, Tobin DJ, Müller‐Röver S, Handjiski B, Wendt G, Peters EMJ, Pohl S, Moll I, Paus R (2000) Active hair growth (Anagen) is associated with angiogenesis. J Invest Dermatol 114: 909–916 [DOI] [PubMed] [Google Scholar]
- Miyachi K, Yamada T, Kawagishi‐Hotta M, Hasebe Y, Date Y, Hasegawa S, Arima M, Iwata Y, Kobayashi T, Numata S et al (2018) Extracellular proteoglycan decorin maintains human hair follicle stem cells. The Journal of Dermatology 45: 1403–1410 [DOI] [PubMed] [Google Scholar]
- Morgan BA (2014) The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med 4: a015180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgner J, Ghatak S, Jakobi T, Dieterich C, Aumailley M, Wickström SA (2015) Integrin‐linked kinase regulates the niche of quiescent epidermal stem cells. Nat Commun 6: 8198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417 [DOI] [PubMed] [Google Scholar]
- Mousavi K, Jasmin BJ (2006) BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J Neurosci 26: 5739–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller‐Röver S, Foitzik K, Paus R, Handjiski B, van der Veen C, Eichmüller S, McKay IA, Stenn KS (2001) A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 117: 3–15 [DOI] [PubMed] [Google Scholar]
- Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K et al (2012) Stress‐induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 13: 744–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Cowley CJ, Fuchs E (2018) Two to tango: dialog between immunity and stem cells in health and disease. Cell 175: 908–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallanthighal S, Heiserman JP, Cheon D‐J (2019) The role of the extracellular matrix in cancer stemness. Front Cell Dev Biol 7: 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E (2006) Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127: 171–183 [DOI] [PubMed] [Google Scholar]
- Niemann C, Horsley V (2012) Development and homeostasis of the sebaceous gland. Semin Cell Dev Biol 23: 928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S‐I (2002) Dominant role of the niche in melanocyte stem‐cell fate determination. Nature 416: 854–860 [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, Roes J, Beermann F, Fisher DE (2010) Key roles for transforming growth factor β in melanocyte stem cell maintenance. Cell Stem Cell 6: 130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JW, Hsi T‐C, Guerrero‐Juarez CF, Ramos R, Plikus MV (2013) Organotypic skin culture. J Invest Dermatol 133: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo UA, DiPietro LA (2017) Diabetes and wound angiogenesis. Int J Mol Sci 18: 1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver TI, Mutluoglu M (2020) Diabetic foot ulcer. Treasure Island, FL: StatPearls Publishing; [PubMed] [Google Scholar]
- Osei ET, Noordhoek JA, Hackett TL, Spanjer AIR, Postma DS, Timens W, Brandsma C‐A, Heijink IH (2016) Interleukin‐1α drives the dysfunctional cross‐talk of the airway epithelium and lung fibroblasts in COPD. Eur Respir J 48: 359 [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y (2001) Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104: 233–245 [DOI] [PubMed] [Google Scholar]
- Osorio KM, Lee SE, McDermitt DJ, Waghmare SK, Zhang YV, Woo HN, Tumbar T (2008) Runx1 modulates developmental, but not injury‐driven, hair follicle stem cell activation. Development 135: 1059 [DOI] [PubMed] [Google Scholar]
- Otsuki L, Brand AH (2017) The vasculature as a neural stem cell niche. Neurobiology of Disease 107: 4–14 [DOI] [PubMed] [Google Scholar]
- Oulès B, Rognoni E, Hoste E, Goss G, Fiehler R, Natsuga K, Quist S, Mentink R, Donati G, Watt FM (2019) Mutant Lef1 controls Gata6 in sebaceous gland development and cancer. EMBO J 38: e100526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleyev AA (2018) Functional anatomy of the hair follicle: The Secondary Hair Germ. Exp Dermatol 27: 701–720 [DOI] [PubMed] [Google Scholar]
- Park S, Greco V, Cockburn K (2016) Live imaging of stem cells: answering old questions and raising new ones. Curr Opin Cell Biol 43: 30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña‐Jimenez D, Fontenete S, Megias D, Fustero‐Torre C, Graña‐Castro O, Castellana D, Loewe R, Perez‐Moreno M (2019) Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J 38: e101688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin JR, Sporrij A, Zon LI (2017) Blood on the tracks: hematopoietic stem cell‐endothelial cell interactions in homing and engraftment. J Mol Med 95: 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD et al (2018) Spatial and single‐cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol 138: 811–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong C‐M (2008) Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451: 340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloni A, Maurizi G, Serrani F, Mancini S, Zingaretti MC, Frontini A, Cinti S, Olivieri A, Leoni P (2013) Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol 41: 558–566 [DOI] [PubMed] [Google Scholar]
- Pratt CH, King LE Jr, Messenger AG, Christiano AM, Sundberg JP (2017) Alopecia areata. Nat Rev Dis Primers 3: 17011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzan M, Hosic S, Ghio C, Koppes A (2018) Enteric nervous system regulation of intestinal stem cell differentiation and epithelial monolayer function. Sci Rep 8: 6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi K, Li N, Zhang Z, Melino G (2018) Tissue regeneration: The crosstalk between mesenchymal stem cells and immune response. Cell Immunol 326: 86–93 [DOI] [PubMed] [Google Scholar]
- Qiu W, Chuong C‐M, Lei M (2019) Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: Biological patterning and therapeutic potentials. Exp Dermatol 28: 395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, Taketo MM, Ito M (2011) Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 145: 941–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani W, Liu Y, Rosin NL, Kline A, Raharjo E, Yoon J, Stratton JA, Sinha S, Biernaskie J (2018) Macrophages promote wound‐induced hair follicle regeneration in a CX3CR1‐ and TGF‐β1‐dependent manner. J Invest Dermatol 138: 2111–2122 [DOI] [PubMed] [Google Scholar]
- Rahmani W, Sinha S, Biernaskie J (2020) Immune modulation of hair follicle regeneration. NPJ Regen Med 5: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramazani Y, Knops N, Elmonem MA, Nguyen TQ, Arcolino FO, van den Heuvel L, Levtchenko E, Kuypers D, Goldschmeding R (2018) Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol 68–69: 44–66 [DOI] [PubMed] [Google Scholar]
- Reichenbach B, Classon J, Aida T, Tanaka K, Genander M, Göritz C (2018) Glutamate transporter Slc1a3 mediates inter‐niche stem cell activation during skin growth. EMBO J 37: e98280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza A, Wang Z, Sennett R, Qiao W, Wang D, Heitman N, Mok K, Clavel C, Yi R, Zandstra P et al (2016) Signaling networks among stem cell precursors, transit‐amplifying progenitors, and their niche in developing hair follicles. Cell Rep 14: 3001–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG (1989) Human epidermal keratinocyte cell culture and xenograft systems: applications in the detection of potential chemical carcinogens and the study of epidermal transformation. Prog Clin Biol Res 298: 113–125. [PubMed] [Google Scholar]
- Rivera‐Gonzalez G, Shook B, Horsley V (2014) Adipocytes in skin health and disease. Cold Spring Harb Perspect Med 4: a015271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Watt FM (2018) Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol 28: 709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V (2012) Live imaging of stem cell and progeny behaviour in physiological hair‐follicle regeneration. Nature 487: 496–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy E, Neufeld Z, Cerone L, Wong HY, Hodgson S, Livet J, Khosrotehrani K (2016) Bimodal behaviour of interfollicular epidermal progenitors regulated by hair follicle position and cycling. EMBO J 35: 2658–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz EJ, Oeztuerk‐Winder F, Ventura J‐J (2014) A paracrine network regulates the cross‐talk between human lung stem cells and the stroma. Nat Commun 5: 3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutlin M, Ho C‐Y, Abraira Victoria E, Cassidy C, Bai L, Woodbury CJ, Ginty David D (2014) The cellular and molecular basis of direction selectivity of Aδ‐LTMRs. Cell 159: 1640–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada A, Jacob F, Leung E, Wang S, White BS, Shalloway D, Tumbar T (2016) Defining the cellular lineage hierarchy in the interfollicular epidermis of adult skin. Nat Cell Biol 18: 619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santema TB, Poyck PPC, Ubbink DT (2016) Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev 2: CD011255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasine JP, Yeo KT, Chute JP (2017) Concise review: paracrine functions of vascular niche cells in regulating hematopoietic stem cell fate. Stem Cells Transl Med 6: 482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya AP, Stone RC, Brooks SR, Pastar I, Jozic I, Hasneen K, O’Neill K, Mehdizadeh S, Head CR, Strbo N et al (2020) Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun 11: 4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Horsley V (2012) Unravelling hair follicle–adipocyte communication. Exp Dermatol 21: 827–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secunda R, Vennila R, Mohanashankar AM, Rajasundari M, Jeswanth S, Surendran R (2015) Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology 67: 793–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang Y, Li R, Xing W, Yang F‐C, Bai J, Zhou Y (2018) Alteration in the cytokine secretion of bone marrow stromal cells from patients with chronic myelomonocytic leukemia contribute to impaired hematopoietic supportive activity. Stem Cells Int 2018: 5921392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwartz Y, Gonzalez‐Celeiro M, Chen C‐L, Pasolli HA, Sheu S‐H, Fan S‐Y, Shamsi F, Assaad S, Lin E‐Y, Zhang B et al (2020) Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182: 578–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobe M, Detmar M (2000) Structure, function, and molecular control of the skin lymphatic system. J Invest Dermatol Symp Proc 5: 14–19 [DOI] [PubMed] [Google Scholar]
- Solé‐Boldo L, Raddatz G, Schütz S, Mallm J‐P, Rippe K, Lonsdorf AS, Rodríguez‐Paredes M, Lyko F (2020) Single‐cell transcriptomes of the human skin reveal age‐related loss of fibroblast priming. Commun Biol 3: 188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X‐L, Hao Q‐K, Tang R‐J, Xiao C, Ge M‐L, Dong B‐R (2019) Frailty and rejuvenation with stem cells: therapeutic opportunities and clinical challenges. Rejuvenation Res 22: 484–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, Nakauchi H, Tanaka Y, McMillan JR, Sawamura D et al (2011) Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 8: 177–187 [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Sankoda Y, Anderson JE, Sato Y, Mizunoya W, Shimizu N, Suzuki T, Yamada M, Rhoads RP, Ikeuchi Y et al (2009) Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol 297: C238–C252 [DOI] [PubMed] [Google Scholar]
- Thulabandu V, Chen D, Atit RP (2018) Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev Dev Biol 7: e307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkamani N, Rufaut N, Jones L, Sinclair R (2017) The arrector pili muscle, the bridge between the follicular stem cell niche and the interfollicular epidermis. Anat Sci Int 92: 151–158 [DOI] [PubMed] [Google Scholar]
- Tsai SL (2020) The molecular interplay between progenitors and immune cells in tissue regeneration and homeostasis. J Immunol Regenerative Med 7: 100024 [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E (2004) Defining the epithelial stem cell niche in skin. Science 303: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K (2015) Tissue‐specific stem cell niche. Cham: Springer; [Google Scholar]
- Turner GA, Hoptroff M, Harding CR (2012) Stratum corneum dysfunction in dandruff. Int J Cosmet Sci 34: 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JH, Hannen RF, Bahta AW, Farjo N, Farjo B, Philpott MP (2015) Oxidative stress‐associated senescence in dermal papilla cells of men with androgenetic alopecia. J Invest Dermatol 135: 1244–1252 [DOI] [PubMed] [Google Scholar]
- Vargas‐Valderrama A, Messina A, Mitjavila‐Garcia MT, Guenou H (2020) The endothelium, a key actor in organ development and hPSC‐derived organoid vascularization. J Biomed Sci 27: 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M, Asakura Y, Murakonda BSR, Pengo T, Latroche C, Chazaud B, McLoon LK, Asakura A (2018) Muscle satellite cell cross‐talk with a vascular niche maintains quiescence via VEGF and notch signaling. Cell Stem Cell 23: 530–543.e539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP (2011) Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest 121: 4409–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T, Yuan T, Chao C‐M, Bell H, Sitaula A, Szimmtenings L, El Agha E, Chanda D, Majka S, Bellusci S et al (2017) Fgf10‐Hippo epithelial‐mesenchymal crosstalk maintains and recruits lung basal stem cells. Dev Cell 43: 48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volmer‐Thole M, Lobmann R (2016) Neuropathy and Diabetic Foot Syndrome. Int J Mol Sci 17: 917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare SK, Bansal R, Lee J, Zhang YV, McDermitt DJ, Tumbar T (2008) Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J 27: 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AB, Zhang YV, Tumbar T (2017) Gata6 promotes hair follicle progenitor cell renewal by genome maintenance during proliferation. EMBO J 36: 61–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ECE, Dai Z, Ferrante AW, Drake CG, Christiano AM (2019) A subset of TREM2+ dermal macrophages secretes oncostatin m to maintain hair follicle stem cell quiescence and inhibit hair growth. Cell Stem Cell 24: 654–669 [DOI] [PubMed] [Google Scholar]
- Wang ECE, Higgins CA (2020) Immune cell regulation of the hair cycle. Exp Dermatol 29: 322–333 [DOI] [PubMed] [Google Scholar]
- Wang X, Wang J, Guo L, Wang X, Chen H, Wang X, Liu J, Tredget EE, Wu Y (2016) Self‐assembling peptide hydrogel scaffolds support stem cell‐based hair follicle regeneration. Nanomedicine 12: 2115–2125 [DOI] [PubMed] [Google Scholar]
- Weaver LN, Drummond‐Barbosa D (2018) Maintenance of proper germline stem cell number requires adipocyte collagen in adult Drosophila females. Genetics 209: 1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T, Wu P, Zhang W, Zheng Y, Li Q, Jin R, Chen H, You C, Guo S, Han C et al (2020) Regeneration of skin appendages and nerves: current status and further challenges. J Transl Med 18: 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Thoresen DT, Williams JS, Wang C, Perna J, Petrova R, Brownell I (2015) Neural Hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium. Proc Natl Acad Sci USA 112: 7195–7200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Woo W‐M, Nagao K, Li W, Terunuma A, Mukouyama Y‐S, Oro AE, Vogel JC, Brownell I (2013) Perivascular hair follicle stem cells associate with a venule annulus. J Invest Dermatol 133: 2324–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ya‐Hsuan H, Simón M‐F (2020) Microenvironmental contributions to hematopoietic stem cell aging. Haematologica 105: 38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Hasegawa S, Hasebe Y, Kawagishi‐Hotta M, Arima M, Iwata Y, Kobayashi T, Numata S, Yamamoto N, Nakata S et al (2019) CXCL12 regulates differentiation of human immature melanocyte precursors as well as their migration. Arch Dermatol Res 311: 55–62 [DOI] [PubMed] [Google Scholar]
- Yanez DA, Lacher RK, Vidyarthi A, Colegio OR (2017) The role of macrophages in skin homeostasis. Pflugers Arch 469: 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C‐C, Cotsarelis G (2010) Review of hair follicle dermal cells. J Dermatol Sci 57: 2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Adam RC, Ge Y, Hua ZL, Fuchs E (2017) Epithelial‐mesenchymal micro‐niches govern stem cell lineage choices. Cell 169: 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Brown LF, Detmar M (2001) Control of hair growth and follicle size by VEGF‐mediated angiogenesis. J Clin Invest 107: 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Detmar M, Brown LF, Lawler J, Miyakawa T (2003) Thrombospondin‐1 plays a critical role in the induction of hair follicle involution and vascular regression during the catagen phase. J Invest Dermatol 120: 14–19 [DOI] [PubMed] [Google Scholar]
- Yin H, Price F, Rudnicki MA (2013) Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S‐Y, Dieterich LC, Karaman S, Proulx ST, Bachmann SB, Sciaroni C, Detmar M (2019a) An important role of cutaneous lymphatic vessels in coordinating and promoting anagen hair follicle growth. PLoS One 14: e0220341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S‐Y, Dieterich LC, Tacconi C, Sesartic M, He Y, Brunner L, Kwon O, Detmar M (2019b) An important role of podoplanin in hair follicle growth. PLoS One 14: e0219938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias WJ, Madison BB, Kretovich KE, Walton KD, Richards N, Udager AM, Li X, Gumucio DL (2011) Hedgehog signaling controls homeostasis of adult intestinal smooth muscle. Dev Biol 355: 152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tsai P‐C, Gonzalez‐Celeiro M, Chung O, Boumard B, Perdigoto CN, Ezhkova E, Hsu Y‐C (2016) Hair follicles’ transit‐amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog. Genes Dev 30: 2325–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hou W, Henrot L, Schnebert S, Dumas M, Heusèle C, Yang J (2015a) Modelling epidermis homoeostasis and psoriasis pathogenesis. J R Soc Interface 12: 20141071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Shao B, Zhuge Q, Wang P, Zheng C, Huang W, Yang C, Wang B, Su D‐M, Jin K (2015b) Cross‐talk between human neural stem/progenitor cells and peripheral blood mononuclear cells in an allogeneic Co‐culture model. PLoS One 10: e0117432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T (2009) Distinct self‐renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 5: 267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, White BS, Shalloway DI, Tumbar T (2010) Stem cell dynamics in mouse hair follicles: a story from cell division counting and single cell lineage tracing. Cell Cycle 9: 1504–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ (2017) Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol 19: 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]


