Summary
Objective
Prostate cancer (PCa) is the second most common malignancy in men. Radiotherapy and surgery successfully control organ-confined tumors, although, locally advanced/high-risk PCa frequently progress to the metastatic stage of the disease, which is uncurable. Identification of novel strategies to improve the efficacy of standard clinical protocols is a primary need. Among the molecular targets of potential clinical interest recently highlighted by accurate preclinical studies, the TRPM8 cation channel is particularly promising. In this study, we aim at establishing a standardized immunohistochemistry protocol to evaluate TRPM8 expression in normal and pathological prostate tissues.
Methods
The specificity and sensitivity of TRPM8 antibody ACC-049 was validated in different human prostate cell lines by western blot and immunocytochemistry analyses. Expression of the TRPM8 channel in normal and pathological prostate tissue was evaluated by immunohistochemistry using a tissue microarray containing 58 cases of prostate adenocarcinomas and in primary and lymph nodes metastatic human PCa matched specimens.
Results
TRPM8 expression marks luminal epithelial cells in benign prostate tissue. In malignant lesions of the prostate, TRPM8 expression is frequently more abundant in advanced stages of the disease (PCa stage III/IV). Finally, lymph node metastases and matched primary tumors show similar amounts of the channel.
Conclusions
Collectively, our results reinforce the importance of TRPM8 as prostate biomarker and emphasize the value of the channel as promising novel molecular target for the treatment of prostate adenocarcinoma.
Key words: prostate cancer, TRPM8, immunohistochemistry, biomarker, ion channel
Introduction
Prostate cancer (PCa) is the most frequently diagnosed malignancy affecting men and a major cause of cancer-related death 1. From a clinical point of view, 90% of patients present an organ-confined tumor at diagnosis, while the remaining 10% has already developed the metastatic stage of the disease. Particular attention is needed patients with locally advanced/high-risk PCa (stage III/IV). In these cases, clinical protocols include radical prostatectomy coupled with radiotherapy, or radiotherapy combined with adjuvant systemic treatments in order to eliminate possible local and distant residual disease 2. Unfortunately, locally advanced/high-risk PCa frequently progresses to the metastatic stage of the disease and, almost invariably, after one or two years of androgen deprivation therapy, to the lethal castration resistant metastatic condition (mCRPC) 3.
In this scenario, accurate functional genomic approaches are needed for the identification of novel biomarkers and molecular targets of potential clinical interest, and, in turn, to guide the clinician towards more personalized and effective therapeutic strategies especially for the aggressive forms of PCa.
In the last years, increasing evidence has revealed that ion channels play critical roles in the tumorigenesis of a wide range of human cancers 4. Consequently, these membrane proteins have emerged as putative biomarkers and molecular target candidates providing new opportunities to improve treatment response in patients with different types of cancers.
Among several channels expressed in normal and tumor prostate epithelial cells, TRPM8 is especially interesting. TRPM8, which was functionally characterized as a primary receptor for cold stimuli in humans 5,6, is a Ca2+-permeable channel belonging to the transient receptor potential (TRP) channel superfamily. This channel presents a restricted pattern of expression. In addition to cold-sensitive peripheral sensory neurons, TRPM8 was found in few epithelial tissues, with prostate showing in absolute the highest expression levels 7-9. Noteworthy, TRPM8 channel expression rises in primary prostate cancer, while its levels are frequently reduced in mCRPC 7-11. This expression pattern makes TRPM8 a valuable candidate as a diagnostic biomarker for localized and metastatic PCa and a potential target for therapeutic interventions in prostate cancer treatment 8-10,12. However, despite numerous studies, the physiological function of TRPM8 in luminal epithelial cells and, overall, the role in prostate tumorigenesis remains largely elusive and controversial 9,13,14.
This study presents an optimized immunostaining procedure to evaluate the expression of TRPM8 in normal and malignant prostatic tissues. Our results show TRPM8 almost invariably over-expressed in PCa compared to adjacent benign tissue, with very high TRPM8 staining frequently associated with advanced stages (III/IV) of the disease. Importantly, TRPM8 expression marks hormone sensitive metastatic cells seeded to local lymph nodes.
Overall, our study highlights the potential value of TRPM8 as both biomarker and therapeutic target in prostate cancer.
Materials and methods
CELL LINES AND CELL CULTURE
The immortalized non-tumorigenic prostatic epithelial cell line RWPE-1 and the prostate cancer cell lines LNCaP and PC-3 were purchased from the American Type Culture Collection (ATCC, USA). Cells were cultured in a humidified incubator at 37°C and 5% CO2 and maintained according to manufacturer’s instructions. Plasmid construction and lentiviral transduction for TRPM8 overexpression, used to obtain the line RWPE-1 M8, was performed as described previously 9. For gene knockdown of TRPM8, cells were transfected with non-targeting (siCTR) or TRPM8 targeting (siRNA1 or siRNA2) small interfering RNA molecules (obtained from Ambion, Life Tech), following the approach described elsewhere 9.
WESTERN BLOT
Cells were lysed in ice-cold RIPA buffer supplemented with protease inhibitors cocktail (Halt™, ThermoFisher Sci). Equal amounts of protein were resolved on SDS-PAGE gels, transferred to PVDF membranes and detected by chemiluminescence after incubation with primary antibodies against TRPM8 (1:1000, Alomone Labs, #ACC-049) and GAPDH (1:5000, Cell Signaling, #5174). Western blots were performed in at least 3 independent biological replicates and representative data are shown.
PROSTATE ADENOCARCINOMA TISSUE MICROARRAY
The human prostate tissue microarray (TMA) PR208a was purchased from US Biomax, Inc. The TMA contains 58 cases of adenocarcinomas and 6 normal tissues (192 total cores, triplicate cores per case). The pathological features including pathology diagnosis, clinical stage, Gleason grade, Gleason score and the involvement of regional lymph nodes and distant metastasis are stated.
IMMUNOHISTOCHEMISTRY
RWPE-1 and the other prostate cell lines were grown at confluence in 10 cm Petri dishes, detached and collected by centrifugation. Cell pellets were fixed in 4% PFA, dehydrated with graded alcohols and finally embedded in paraffin. Paraffin blocks were cut at the microtome to obtain 5 μm–thick sections which were mounted on coated slides, deparaffinized in xylene, followed by a graded ethanol rehydration and used for immunohistochemistry. Deidentified primary and lymph node metastatic human samples were retrieved from the tissue bank archives of the Surgical Pathology Unit of the S. Chiara Hospital (Trento, Italy) upon approval of the Hospital Ethical Committee (Prot.:1946 I.D.:112786962). The TMA and other slides were subjected to a complete immunohistochemical analyses carried out at the Department of Histopathology (S. Chiara Hospital, Trento, Italy) using an automatic immunostainer (BOND-III platform, Leica Biosystems). Antigen retrieval was performed with BOND reagents (Bond epitope retrieval solution 1, Leica Biosystems) at pH 6 for 20 min. The primary antibodies used were: TRPM8 (1:300, Alomone Labs, #ACC-049) and Cytokeratin HMWs (34βE12, PA0134, Leica Biosystems) optimally diluted for use on the BOND system. BOND compact polymer detection solution (Leica Biosystems) was employed for the detection. High resolution images were acquired using an Axio Imager M2 microscope (Zeiss). Sample histology and intensity of TRPM8 immunostaining were reviewed by two trained pathologists (M.B. and F.G.C) to ensure appropriate assignment of the following scores: weak (0), moderate (1), high (2), and very high (3).
Results
SPECIFICITY OF TRPM8 ANTIBODY ACC049
The reliability of immunohistochemical studies critically depends on the specificity of the antibodies. To establish a consistent immunohistochemical procedure to score TRPM8 levels in prostate specimens, we meticulously tested a panel of commercially available TRPM8 antibodies. A combination of western blot studies and immunocytochemistry analyses demonstrated the specificity of the Alomone Labs ACC-049 antibody. Compared to untransfected and non-targeting siRNA (siCTR) transfected cells, western blot studies demonstrated a reduction of ~ 90% of endogenous TRPM8 protein in both RWPE-1 and LNCaP cell lines transfected with two different siRNAs against TRPM8 (Fig. 1A). Furthermore, the ACC-049 antibody confirmed the lack of TRPM8 expression in the metastatic PCa cell line PC-3 9,11, while it detected an increased amount of TRPM8 in the RWPE-1 M8 cells, which was obtained by stable insertion in their genome of exogenous cDNA encoding the channel 9 (Fig. 1B). Next, the Alomone ACC-049 antibody was tested for the ability to detect TRPM8 by immunocytochemistry in RWPE-1, RWPE-1 M8, LNCaP, and PC3 aldehyde fixed-paraffin embedded cellular pellets. The antibody showed a high specificity, nicely detecting the expected differences in TRPM8 amounts across the four samples (RWPE-1 M8 = high, RWPE-1 and LNCaP = weak, PC3 = absent; Fig. 1C). The secondary antibody alone was used as further internal control and showed no signal. Finally, by immunohistochemistry, ACC-049 antibody marked the epithelial compartment of the gland in human prostate specimens, with cancer cells (lumens without outer layer of HMWCKs positive cells) more strongly stained than benign (lumens with an outer layer of HMWCKs positive cells) (Fig. 2).
Figure 1.
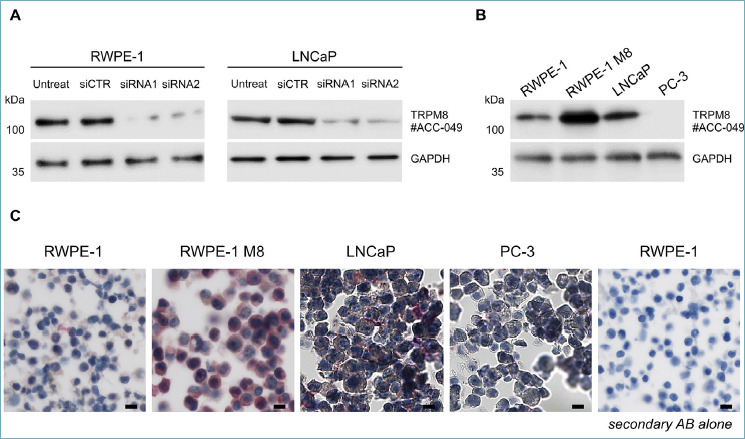
(A) Western blotting analysis with Alomone ACC-049 antibody detecting endogenous full length TRPM8 protein in both RWPE1 and LNCaP prostate cell lines. Antibody specificity was confirmed through TRPM8 knockdown. RWPE1 and LNCaP prostate cells were transfected with non-targeting (siCTR) or TRPM8 targeting (siRNA1 and siRNA2) small interfering RNA molecules as indicated. (B) Western blotting analysis with Alomone ACC049 antibody detecting endogenous amount of full length TRPM8 protein in RWPE1 and LNCaP but not in PC3 prostate cell lines. Increased levels of the channel are detectable in RWPE1 stably overexpressing exogenous TRPM8. (C) Immunohistochemistry of TRPM8 on paraffin embedded RWPE1, RWPE1 M8, LNCaP and PC3 cell pellets matching the biochemical data. Scale bars, 100 μm.
Figure 2.
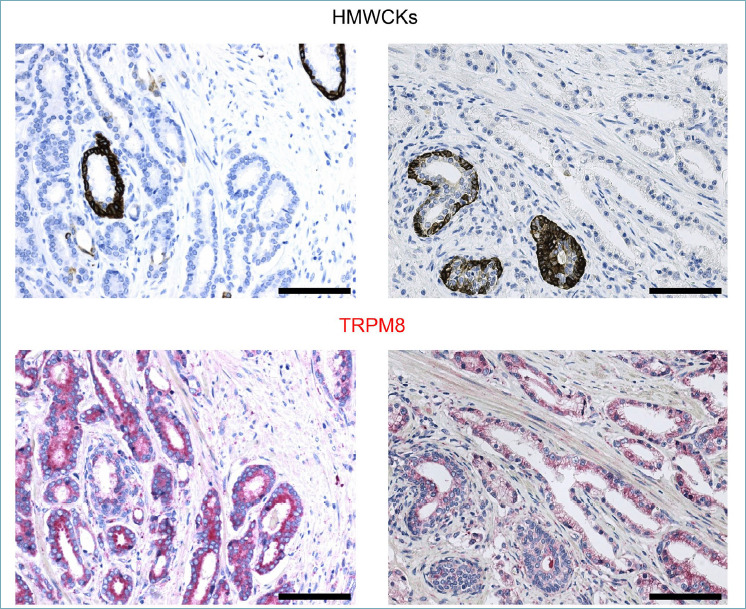
Representative immunostaining for High Molecular Weight Cytokeratins (HMWCKs) and TRPM8 in human PCa specimens showing greater amount of TRPM8 protein in HMWCKs negative malignant lumens. Scale bars, 100 μm.
Overall, these data demonstrated the specificity of the Alomone ACC-049 antibody and the reliability of our protocol.
HIGH TRPM8 SCORE IS FREQUENTLY ASSOCIATED WITH ADVANCED PCa STAGES
Based on the above results, TRPM8 expression was evaluated by immunohistochemistry on a commercially available PCa TMA (US Biomax Inc. PR208a) using the ACC-049 antibody. Fifty-eight cases of prostate adenocarcinoma and six cases of normal prostate tissue – triplicate cores per case, 192 total cores – were examined in this study. The mean age of the analyzed PCa cases was 69.8 years, and the youngest patient at time of diagnosis was 26-year-old, while the oldest was 87 years. Grade II adenocarcinoma was the most common histology stated in the microarray.
Immunohistochemistry of TRPM8 in normal prostatic tissues exhibited weak to moderate immunoreactivity in the cytoplasm and cell membrane of the luminal epithelial cells (Fig. 3). No appreciable TRPM8 immunoreactivity was detected in the basal cells or in the stroma. On the other hand, in adenocarcinoma lesions, the malignant cells showed significantly increased and diffused immunoreactivity for TRPM8 in their cytoplasm and plasma membranes (Fig. 3).
Figure 3.
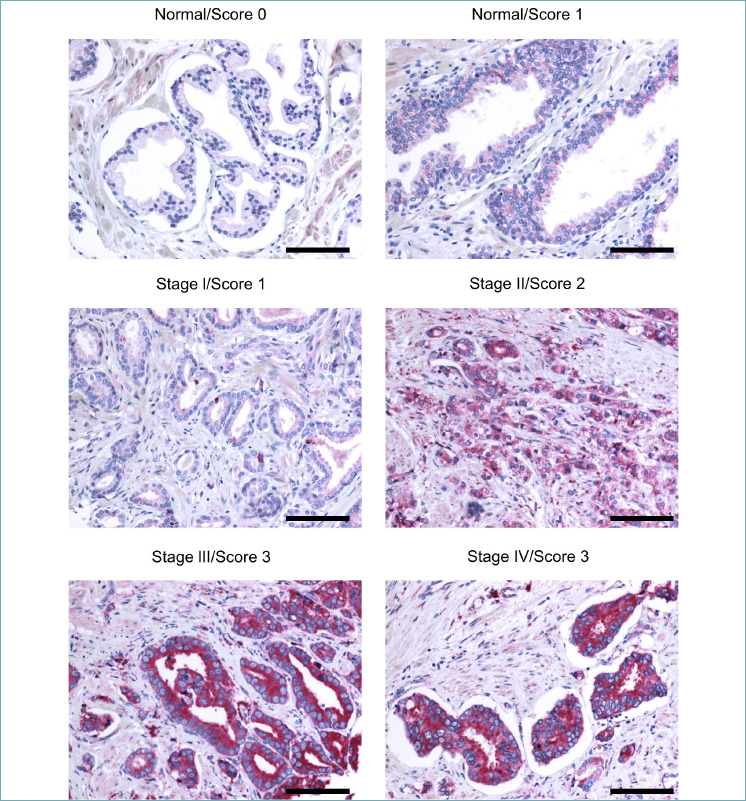
TRPM8 immunostaining of PCa specimens. TRPM8 immunostaining was scored as weak (0), moderate (1), high (2) or very high (3) on normal prostate cores and 174 PCa cores representing 58 different cases. Representative images are shown. Scale bars, 100 μm.
Intensity of staining was semi-quantified through pathologist visual analysis as score 0 (weak), 1 (moderate), 2 (high) and 3 (very high). This analysis showed substantial inter-tumor heterogeneity of TRPM8 levels across PCa samples (Fig. 3, Tab. I), with advanced PCa (stage III/IV) frequently showing higher amounts of the channel compared to early stages (I/II) of the disease.
Table I.
The percent coverage for expression of TRPM8 in prostate cancer respect to tumor stage.
| Intensity of anti-TRPM8 immunoreactivity (Score) | |||
|---|---|---|---|
| Tumor stage | 1 | 2 | 3 |
| I | 36% | 36% | 28% |
| II | 9% | 64% | 27% |
| III | 8% | 38% | 54% |
| IV | 12% | 25% | 63% |
Finally, to complete the immunohistochemical analysis we extended our study to metastatic PCa tissues. As predicted by immunoblot evaluation on hormone naïve metastatic LNCaP cell line (Fig. 1A and 1B), parallel TRPM8 immunostaining of lymph node metastases and paired primary prostate tumors shows a comparable amount of the channel (Fig. 4).
Figure 4.
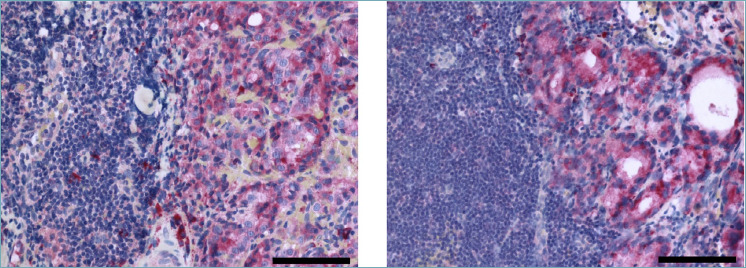
TRPM8 immunostaining of hormone naïve lymph nodes PCa metastases. Representative images are shown. Scale bars, 100 μm.
Discussion
Precision oncology is paving new routes for the clinical management of cancer patients 15. Definition of innovative molecular strategies aimed at defeating castration resistant metastatic PCa is the primarily goal in the field, although the development of novel clinical protocols for a more effective treatment of PCa patients diagnosed with locally advanced/high risk tumors would represent a critical achievement to counteract disease progression and, in turn, mortality. In this scenario, the almost exclusive expression of TRPM8 in luminal cells of the prostate, the increased amount of the channel in primary and hormone naïve lymph node metastatic PCa, and, finally, the availability of well-characterized molecules acting as agonists or antagonists, make TRPM8 a very promising potential new target.
Here we established a reliable immunohistochemistry protocol to score TRPM8 protein amounts in human prostate samples. In line with previous findings 9,11,12,16-18, we found that TRPM8 expression specifically marks the luminal compartment in both normal and malignant prostate tissues and that it is almost invariably over-expressed in PCa cells compared to contiguous benign glands. Differently from others 16-18, we did not find any TRPM8 immunoreactivity in basal epithelial cells or in hyperplastic stromal cells.
Collectively, our study revealed a heterogeneous distribution of TRPM8 amount across PCa specimens with very high TRPM8 staining frequently, but not exclusively, associated with advanced stage (III/IV) of the disease. Notably, matched primary PCa and hormone naïve metastatic samples derived from the same patient score very similar for TRPM8 immunostaining.
Conclusions
Our findings strength the value of TRPM8 as biomarker of prostate tissue and pave the way for “precise” pre-clinical and clinical studies testing the relevance of this channel as potential therapeutic target for locally advanced/high-risk prostate cancer.
Figures and tables
Acknowledgements
This work has been supported by the Giovanni Armenise - Harvard Foundation Career Development Award granted to A. Lunardi and by Lega Italiana Lotta ai Tumori (LILT-Bolzano). A. Alaimo was funded by a postdoctoral fellowship from the Fondazione Umberto Veronesi and by Starting Grants Young Researchers 2019 from the University of Trento. We thank the technical staff at the Department of Histopathology (S. Chiara Hospital, Trento, Italy) for their support with the histological work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. https://doi.org/10.3322/caac.21551 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415-1424. https://doi.org/10.1056/NEJMoa1606220 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 3.Moris L, Cumberbatch MG, Van den Broeck T, et al. Benefits and risks of primary treatments for high-risk localized and locally advanced prostate cancer: an international multidisciplinary systematic review. Eur Urol 2020;77:614-627. https://doi.org/10.1016/j.eururo.2020.01.033 10.1016/j.eururo.2020.01.033 [DOI] [PubMed] [Google Scholar]
- 4.Prevarskaya N, Skryma R, Shuba Y. Ion channels in cancer: are cancer hallmarks oncochannelopathies? Physiol Rev 2018;98:559-621. https://doi.org/10.1152/physrev.00044.2016 10.1152/physrev.00044.2016 [DOI] [PubMed] [Google Scholar]
- 5.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002;416(6876):52-58. https://doi.org/10.1038/nature719 10.1038/nature719 [DOI] [PubMed] [Google Scholar]
- 6.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell 2002;108:705-715. [DOI] [PubMed] [Google Scholar]
- 7.Tsavaler L, Shapero MH, Morkowski S, et al. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 2001;61:3760-3769. [PubMed] [Google Scholar]
- 8.Henshall SM, Afar DEH, Hiller J, et al. Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res 2003;63:4196-4203. [PubMed] [Google Scholar]
- 9.Alaimo A, Lorenzoni M, Ambrosino P, et al. Calcium cytotoxicity sensitizes prostate cancer cells to standard-of-care treatments for locally advanced tumors. Cell Death Dis 2020;11:1039. https://doi.org/10.1038/s41419-020-03256-5 10.1038/s41419-020-03256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuessel S, Sickert D, Meye A, et al. Multiple tumor marker analyses (PSA, hK2, PSCA, trp-p8) in primary prostate cancers using quantitative RT-PCR. Int J Oncol 2003;23:221-228. https://doi.org/10.1016/s1569-9056(03)80234-8 10.1016/s1569-9056(03)80234-8 [DOI] [PubMed] [Google Scholar]
- 11.Bidaux G, Flourakis M, Thebault S, et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest 2007;117:1647-1657. https://doi.org/10.1172/JCI30168 10.1172/JCI30168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer 2006;13:27-38. https://doi.org/10.1677/erc.1.01093 10.1677/erc.1.01093 [DOI] [PubMed] [Google Scholar]
- 13.Grolez GP, Gkika D. TRPM8 puts the chill on prostate cancer. Pharmaceuticals 2016;9:44. https://doi.org/10.3390/ph9030044 10.3390/ph9030044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noyer L, Grolez GP, Prevarskaya N, et al. TRPM8 and prostate: a cold case? Pflugers Arch 2018;470:1419-1429. https://doi.org/10.1007/s00424-018-2169-1 10.1007/s00424-018-2169-1 [DOI] [PubMed] [Google Scholar]
- 15.Grandori C, Kemp CJ. Personalized cancer models for target discovery and precision medicine. Trends Cancer 2018;4:634-642. https://doi.org/10.1016/j.trecan.2018.07.005 10.1016/j.trecan.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, Xu Z, Zou C, et al. Ion channel TRPM8 promotes hypoxic growth of prostate cancer cells via an O2-independent and RACK1-mediated mechanism of HIF-1α stabilization. J Pathol 2014;234:514-525. https://doi.org/10.1002/path.4413 10.1002/path.4413 [DOI] [PubMed] [Google Scholar]
- 17.Asuthkar S, Elustondo PA, Demirkhanyan L, et al. The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions. J Biol Chem 2015;290:2659-2669. https://doi.org/10.1074/jbc.M114.610824 10.1074/jbc.M114.610824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asuthkar S, Demirkhanyan L, Mueting SR, et al. High-throughput proteome analysis reveals targeted TRPM8 degradation in prostate cancer. Oncotarget 2017;8:12877-12890. https://doi.org/10.18632/oncotarget.14178 10.18632/oncotarget.14178 [DOI] [PMC free article] [PubMed] [Google Scholar]


