Abstract
The antibody titer of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was observed in 289 healthy healthcare workers who had completed the second dose of the Pfizer-BioNTech coronavirus disease 2019 (COVID-19) vaccine. Antibody tests were performed using both the automated electrochemiluminescence immunoassay (ECLIA) and the chromatographic lateral flow immunoassay (LFIA). All subjects had antibodies against the receptor binding domain of the spike protein of SARS-CoV-2 only one week after completing the vaccination, and the antibody titer became significantly higher after another week (P < 0.001). Since there was a large amount of antibody formation within two weeks after completion of vaccination, the less sensitive method, LFIA, also showed high sensitivity. There was no significant difference between whole blood and serum in detecting SARS-CoV-2 antibodies after vaccination. This is an early study of vaccinations among Koreans and is expected to contribute to the establishment of national guidelines on COVID-19 vaccination.
Keywords: SARS-CoV-2, COVID-19, mRNA Vaccine, Korean, Antibody Formation, Immunoassay
Graphical Abstract
The novel respiratory virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged at the end of 2019, leading to the coronavirus disease 2019 (COVID-19) pandemic worldwide. As of May 1, 2021, there had been a cumulative 150,989,419 confirmed cases, including 3,173,576 deaths globally.1 Currently, approximately 300 vaccines are under development on various platforms, with five already in the market and in phase 4. The majority of vaccines target the receptor binding domain (RBD) of the spike (S) protein of SARS-CoV-2,2,3,4,5 because the virus is known to bind the receptor angiotensin-converting enzyme 2 of the host cell via the RBD of the S protein, and infects through it.6,7
The COVID-19 vaccine administration in Korea began on February 26, 2021 under the national vaccine project. Currently, there are two vaccines available in the country: the AstraZeneca COVID-19 vaccine inj. (adenovirus-vectored vaccine, AstraZeneca, Cambridge, UK), and the Comirnaty inj. (mRNA vaccine, Pfizer-BioNTech; Pfizer, New York, NY, USA and BioNTech, Mainz, Germany). Both vaccines require two doses; the second dose boosts the immune response.8 As of May 1, 2021, a total of 3,395,104 people received the first dose, and 236,188 people received the second dose.9
The Boramae Medical Center was designated as a COVID-19 treatment center, and vaccination of healthcare workers (HCWs) began in early March 2021. To date, 1,817 HCWs, have completed the second dose of Pfizer-BioNTech, and 350 others received the first dose of AstraZeneca. In this study, two separate blood samples from HCWs who received the second dose of the Pfizer-BioNTech vaccine were collected to evaluate the antibody production. Blood sampling was done 7 ± 2 days (1 week) and 14 ± 2 days (2 weeks) after the second dose. complete blood count (CBC; hemoglobin, white blood cells, and platelets), admission panel (blood urea nitrogen, creatinine, cholesterol, protein, albumin, bilirubin, alkaline phosphatase, aspartate transaminase, alanine aminotransferase), inflammation markers (C-reactive protein and procalcitonin), hepatitis B surface antigen/hepatitis B surface antibody, and SARS-CoV-2 antibody tests were performed. Antibody tests for SARS-CoV-2 were performed using both the automated electrochemiluminescence immunoassay (ECLIA) and the chromatographic lateral flow immunoassay (LFIA) in point-of-care testing (POCT), which are representative methods in general hospital laboratories.
A total of 289 people were enrolled in this study; however, six people did not participate in the second blood sampling, leaving only 283 blood samples in the second sampling. Demographic and basic hematologic characteristics are shown in Supplementary Table 1. Most values were within the reference ranges, with a few exceptions. Nevertheless, these out-of-range values were still not recorded critical or panic values. Therefore, all the subjects could be considered healthy adults.
The ECLIA tests were analyzed with two types of SARS-CoV-2 antibody kits using the Cobas 8000 e801 unit (Roche Diagnostics, Mannheim, Germany). The first kit was the quantitative Elecsys Anti-SARS-CoV-2 S assay (Elecsys Anti-S; Roche Diagnostics) and the second kit was the qualitative Elecsys Anti-SARS-CoV-2 assay (Elecsys Anti-N; Roche Diagnostics). The Elecsys Anti-S assay uses a recombinant protein representing the RBD of the S antigen, which favors quantitative determination of high-affinity antibodies against SARS-CoV-2.10 The Elecsys Anti-N assay uses a recombinant protein representing the nucleocapsid (N) antigen for the determination of antibodies against SARS-CoV-2.11 The clinical sensitivities (days post PCR COVID-19 confirmation ≥ 7) were 97.6% and 93.6% for anti-S and anti-N, respectively.10,11 The clinical specificities were 100.0% and 99.8% for anti-S and anti-N, respectively.10,11
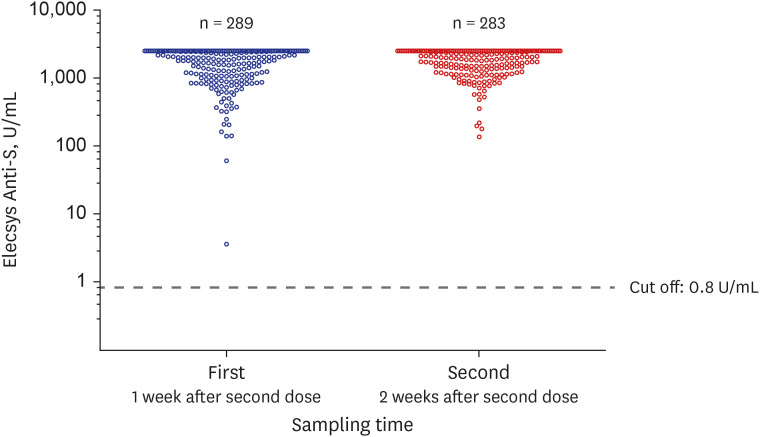
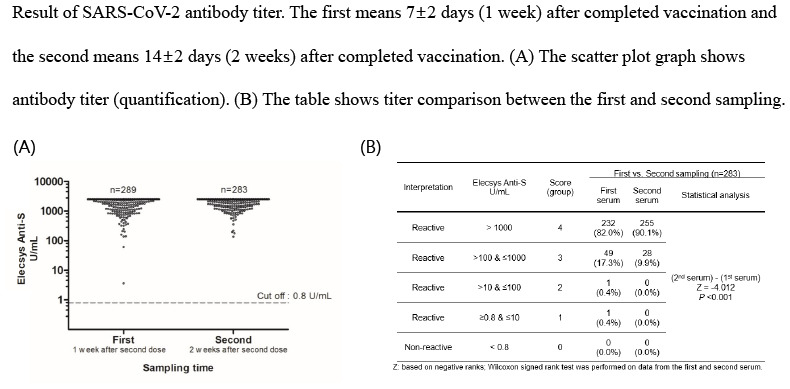
The Elecsys Anti-S results are shown in Fig. 1. The reactive (positive) value is ≥ 0.8 U/mL, and all subjects (100%; first, n = 289; second, n = 283) showed reactivity (Fig. 1); that is, antibodies to the RBD of the S protein were produced in all subjects' serum at 1 or 2 weeks after the second dose. Among them, 137 (137/289 = 47.4%) in the first sampling and 144 (144/283 = 50.9%) in the second sampling recorded excessive antibodies, exceeding the upper limits of the measurable range (2,500 U/mL). The values of Elecsys Anti-S could be divided into five groups (score 0–4) according to log10: score 0, non-reactive (< 0.8 U/mL); score 1, reactive (≥ 0.8 U/mL and ≤ 10 U/mL); score 2, reactive (> 10 U/mL and ≤100 U/mL); score 3, reactive (> 100 U/mL and ≤ 1,000 U/mL); and score 4, reactive (> 1,000 U/mL). When grouped as above and compared between the first and second serum sampling (Table 1), a significantly higher antibody titer was observed in the second sampling (P < 0.001). In the first sampling, two patients with relatively low titers (≤ 100 U/mL) did not have any specific laboratory findings. The antibodies to the RBD of the S protein were detected in all subjects; however, antibodies to the nucleocapsid (Elecsys Anti-N) were detected in only two HCWs with a history of SARS-CoV-2 infection (data not shown).
Fig. 1. Results of the automated electrochemiluminescence immunoassay, Elecsys Anti-SARS-CoV-2 S assay (Elecsys Anti-S assay) of the first (1 week after second dose Pfizer-BioNTech vaccination) and second sampling (2 weeks after second dose Pfizer-BioNTech vaccination). This scatter plot graph shows the antibody titer of SARS-CoV-2.
SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Table 1. Comparison of the antibody titer between the first (1 week after second dose Pfizer-BioNTech vaccination) and second sampling (2 weeks after second dose Pfizer-BioNTech vaccination).
| Interpretation | Elecsys Anti-S, U/mL | Score (group) | First vs. Second sampling (n = 283) | ||
|---|---|---|---|---|---|
| First serum | Second serum | Statistical analysis | |||
| Reactive | > 1,000 | 4 | 232 (82.0%) | 255 (90.1%) | (2nd serum)–(1st serum) Z = −4.012 P < 0.001 |
| Reactive | > 100 & ≤ 1,000 | 3 | 49 (17.3%) | 28 (9.9%) | |
| Reactive | > 10 & ≤ 100 | 2 | 1 (0.4%) | 0 (0.0%) | |
| Reactive | ≥ 0.8 & ≤ 10 | 1 | 1 (0.4%) | 0 (0.0%) | |
| Non-reactive | < 0.8 | 0 | 0 (0.0%) | 0 (0.0%) | |
Z: based on negative ranks; Wilcoxon signed rank test was performed on data from the first and second serum.
The LFIA method tests used the GeneFinder COVID-19 IgG/IgM Rapid Test (Osang Healthcare, Anyang, Korea) to detect antibodies targeting the RBD of the S protein and/or the N protein. We evaluated the clinical performance of the LFIA with 97.9% sensitivity (days from symptom onset ≥ 8) and 98.0% specificity. LFIA tests were performed with both whole blood and serum. All results were determined in the five groups by three specialized readers based on the intensity of the test line according to the reference color sheet. The results were reviewed until all the specialized readers reached agreement, and the result of the agreement was recorded as the final result (Supplementary Table 2). A total of 286 (286/289 = 99.0%) and 282 (282/283 = 99.6%) HCWs in the first and second sampling, respectively, showed positive antibody findings. There was no significant difference between whole blood and serum (P = 0.248); however, the second serum sampling showed significantly intensive signals compared to the first sampling (P < 0.001).
This study should be interpreted considering the following limitations.
First, the gold standard for quantifying soluble materials such as antibodies has been enzyme-linked immunosorbent assay (ELISA),12,13 but it was not included in this study because it is difficult to implement in a general hospital laboratory setting. Nevertheless, this was not a major issue because the S protein antibodies were excessively produced in all subjects, hence leading to approximately 100% sensitivity for ECLIA and LFIA. Second, in this study, there was no significant difference between ECLIA and LFIA because of the excessive antibodies produced. ECLIA can provide a quantitative evaluation; moreover, it has higher screening power and is much more advantageous than LFIA when bulky samples are treated in a laboratory. However, because ECLIA needs an analytic equipment with a higher cost than LFIA, it is better to evaluate the advantages and disadvantages of both methods with respect to the laboratory environment.14,15,16,17 If LFIA is chosen as the main method, it is recommended that an additional, more sensitive ECLIA or ELISA method be performed when the LFIA results are negative or inconclusive (including grey zone). Third, antibody tests were not performed before vaccination and between the first and second dose. It is regrettable that if both tests were performed, it would have been possible to obtain more objective and scientific analysis by separating the first and second dose. Finally, this study evaluated the production of antibodies against the RBD of the S protein and/or the N protein of SARS-CoV-2; however, this does not fully represent the immunity of the vaccinated subjects. There is controversy as to whether these antibody titers have a positive correlation with overall adaptive humoral immunity (including neutralizing antibody titer) and adaptive cellular immunity (including T cell activity).18,19,20,21,22 Therefore, complicated and expensive experiments, such as virus plaque reduction tests, cytokine quantification tests, and flow cytometry of T cell markers, are further needed to provide convincing evidence on the immunity from vaccination.
In summary, the healthy adults in our study reacted to the Pfizer-BioNTech vaccine; All participants produced SARS-CoV-2 S protein antibodies only 1 week after completion of vaccination, and the antibody titer became significantly higher a week after (i.e., 2 weeks after completion of vaccination). These results are similar to those of previous studies on the mRNA vaccine; approximately 1–2 weeks after completion of vaccination, the antibody titers were found to be high and the total vaccine efficacy exceeded 95%.21,23,24,25,26 Moreover, because of the large amount of antibody formation within 2 weeks after completion of vaccination, the LFIA method also showed high sensitivity. Compared to the ECLIA test, the sensitivity reached 99.6% (based on the second sampling serum). Finally, there was no significant difference between whole blood and the serum in detecting SARS-CoV-2 antibodies after vaccination. Therefore, in a small laboratory without an ECLIA analyzer and centrifuge for serum separation, antibody formation (seroprevalence) test for vaccine efficacy could be performed using whole blood through LFIA in the POCT.
Currently, Korea is speeding up COVID-19 vaccination according to its priorities. In addition, the individual prevention and isolation guidelines for COVID-19 are being revised only for people who have completed vaccination. Despite the limitations, our findings intuitively demonstrate SARS-CoV-2 antibody formation in Korean healthy adults who have completed Pfizer-BioNTech vaccination, using the ECLIA and LFIA, which can easily be performed in a general laboratory. Therefore, it is considered useful for future policy decisions regarding vaccination.
Ethics statement
This study was approved by the Institutional Review Board of the Boramae Medical Center (No. 30-2021-31) and written informed consent was obtained from all participants.
ACKNOWLEDGMENTS
The reagent kits were provided by Roche Diagnostics and Osang Healthcare.
We thank all members including COVID-19 vaccination team of the Seoul Metropolitan Government-Seoul National University Boramae Medical Center who struggled with COVID-19.
Footnotes
Disclosure: The authors have no conflicts of interest to declare.
- Conceptualization: Kim N, Roh EY, Yoon JH, Park H, Shin S.
- Data curation: Roh EY, Yoon JH.
- Formal analysis: Kim N, Minn D, Park S.
- Investigation: Kim N.
- Methodology: Kim N, Park H, Shin S.
- Software: Kim N, Minn D, Park S.
- Validation: Kim N, Minn D, Park S.
- Writing - original draft: Kim N.
- Writing - review & editing: Kim N, Park H, Shin S.
SUPPLEMENTARY MATERIALS
Demographic characteristics and laboratory findings of the enrolled subjects
Results of chromatographic lateral flow immunoassay of the first (1 week after second dose Pfizer-BioNTech vaccination) and second (2 weeks after second dose Pfizer-BioNTech vaccination) sampling
References
- 1.World Health Organization. The coronavirus (COVID-19) dashboard. [Updated 2021]. [Accessed May 2, 2021]. https://covid19.who.int/
- 2.World Health Organization. The COVID-19 candidate vaccine landscape and tracker. [Updated 2021]. [Accessed May 2, 2021]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 3.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5(1):237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Food and Drug Safety. Drug information website. [Updated 2021]. [Accessed May 2, 2021]. https://nedrug.mfds.go.kr/index.
- 9.Korea Centers for Disease Control and Prevention Agency. COVID-19 vaccine dashboard. [Updated 2021]. [Accessed May 2, 2021]. https://ncv.kdca.go.kr/
- 10.Elecsys®. Anti-SARS-CoV-2 S. Insert (material number 09289267190 and 09289275190) [Updated 2020]. [Accessed May 2, 2021]. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2-s.html.
- 11.Elecsys®. Anti-SARS-CoV-2. Insert (material numbers 09203095190 and 09203079190) [Updated 2020]. [Accessed May 2, 2021]. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html.
- 12.Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 2015;72:4–15. doi: 10.1016/j.peptides.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Engvall E. The ELISA, enzyme-linked immunosorbent assay. Clin Chem. 2010;56(2):319–320. doi: 10.1373/clinchem.2009.127803. [DOI] [PubMed] [Google Scholar]
- 14.Nicol T, Lefeuvre C, Serri O, Pivert A, Joubaud F, Dubée V, et al. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech) J Clin Virol. 2020;129:104511. doi: 10.1016/j.jcv.2020.104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lassaunière R, Frische A, Harboe ZB, Nielsen ACY, Fomsgaard A, Krogfelt KA, et al. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv. 2020 doi: 10.1101/2020.04.09.20056325. Forthcoming. [DOI] [Google Scholar]
- 16.Serrano MM, Rodríguez DN, Palop NT, Arenas RO, Córdoba MM, Mochón MD, et al. Comparison of commercial lateral flow immunoassays and ELISA for SARS-CoV-2 antibody detection. J Clin Virol. 2020;129:104529. doi: 10.1016/j.jcv.2020.104529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shincy MR, Govindan V, Sudhakar HH, Venkatesha VT, Padmapriya K, Ravikumar KL. Comparison of performance characteristics between lateral flow, ELISA and electrochemiluminescence immunoassays for the detection of SARS-CoV-2 antibodies among healthcare workers. medRxiv. 2021 doi: 10.1101/2021.04.29.21256260. Forthcoming. [DOI] [Google Scholar]
- 18.Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J Clin Virol. 2020;129:104480. doi: 10.1016/j.jcv.2020.104480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes CO, Jette CA, Abernathy ME, Dam KA, Esswein SR, Gristick HB, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588(7839):682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang LX, Miao SY, Qin ZH, Wu JP, Chen HY, Sun HB, et al. Preliminary analysis of B- and T-cell responses to SARS-CoV-2. Mol Diagn Ther. 2020;24(5):601–609. doi: 10.1007/s40291-020-00486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 22.Criscuolo E, Diotti RA, Strollo M, Rolla S, Ambrosi A, Locatelli M, et al. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J Med Virol. 2021;93(4):2160–2167. doi: 10.1002/jmv.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 24.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doria-Rose N, Suthar MS, Makowski M, O'Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMc2103916. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel RR, Apostolidis SA, Painter MM, Mathew D, Pattekar A, Kuthuru O, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58):eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic characteristics and laboratory findings of the enrolled subjects
Results of chromatographic lateral flow immunoassay of the first (1 week after second dose Pfizer-BioNTech vaccination) and second (2 weeks after second dose Pfizer-BioNTech vaccination) sampling