Abstract
Understanding the long-term kinetics of antibodies in coronavirus disease 2019 (COVID-19) is essential in interpreting serosurvey data. We investigated the antibody response one year after infection in 52 mildly symptomatic patients with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection, using three commercial immunoassays and a surrogate virus neutralization test (sVNT) kit. Anti-N pan-immunoglobulin (Ig), anti-S IgG, and anti-S1 IgG were detected in 43 (82.7%), 44 (84.6%), and 30 (57.7%), respectively. In 49 (94.2%), the antibody could be detected by either anti-N pan-Ig or anti-S IgG assay. In the sVNT, 30 (57.7%) had positive neutralizing activity. Despite waning immunity, SARS-CoV-2 antibodies can be detected up to one year after infection, even in mild COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, Antibody, ELISA, ECLIA
Graphical Abstract
Understanding how rapidly severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) antibody levels waned, especially in asymptomatic or mildly symptomatic infection is critical for interpreting serosurvey results; however, the long-term kinetics of antibody remains mostly unknown. In response to coronavirus disease 2019 (COVID-19) epidemic, Korean government established the non-hospital facilities called “community treatment centers (CTCs)” for isolation of mild patients.1 The CTCs provided a unique opportunity to conduct studies on COVID-19 patients presenting with mild symptoms,2 and serologic responses were previously reported 8 months after infection in patients isolated within a CTC.3 Here, we evaluated the antibody responses one year after infection in mildly symptomatic patients with COVID-19.
This cross-sectional survey's eligible participants were reverse transcription polymerase chain reaction-confirmed COVID-19 patients who had been isolated in the CTC operated by Seoul National University Hospital March 5–April 9, 2020. We collected serum samples one year after infection from all patients who provided written informed consent. We investigated a history of exposure to other COVID-19 patients and symptom development suggesting reinfection after recovery using self-questionnaire and physician's interview on the sampling day.
We measured SARS-CoV-2-specific antibodies with three commercial immunoassays: anti-N pan-immunoglobulin electrochemiluminescence immunoassay (anti-N pan-immunoglobulin [Ig] electrochemiluminescence immunoassay (ECLIA), Roche Diagnostics, https://diagnostics.roche.com), anti-S IgG enzyme-linked immunosorbent assay (anti-S IgG ELISA, InBios International, https://www.inbios.com), and anti-S subunit 1 IgG ELISA (anti-S1 IgG ELISA, Euroimmun, https://www.euroimmune.com). A surrogate virus neutralization test (sVNT, GenScript, https://www.genscript.com) was used to evaluate neutralizing activity targeting the spike receptor-binding domain. These four assays have received Food and Drug Administration Emergency Use Authorizations.
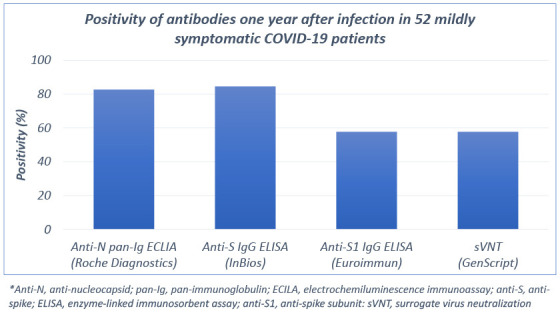
Data from 52 patients with mildly symptomatic COVID-19 were analyzed (Table 1). Sixteen (30.8%) were male with a median age of 26 years (interquartile range [IQR], 22–39.5). The median interval from symptom onset to sampling was 351 days (IQR, 349–352 days). None of the patients reported exposure to other COVID-19 patients or developing symptoms of COVID-19 after recovery. One year after infection, anti-N pan-Ig, anti-S IgG, and anti-S1 IgG were detected in 43 (82.7%), 44 (84.6%), and 30 (57.7%), respectively. In 49 (94.2%), the SARS-CoV-2 antibodies could be detected by either anti-N pan-Ig or anti-S IgG assay. In the sVNT, 30 (57.7%) had positive neutralizing activity. Twenty-seven patients (51.9%) showed positive results in all three binding antibody assays and sVNT.
Table 1. Clinical characteristics of and positivity of antibodies one year after infection in 52 mildly symptomatic COVID-19 patients.
| Variables | Values (n = 52) | |
|---|---|---|
| Sex | ||
| Male | 16 (30.8) | |
| Female | 36 (69.2) | |
| Age, yr | 26 (22–39.5) | |
| Underlying diseasesa | 3 (5.8) | |
| Symptoms | ||
| Febrile/chilling sense | 8 (15.4) | |
| Myalgia | 6 (11.5) | |
| Headache | 14 (26.9) | |
| Cough | 24 (46.2) | |
| Sputum | 35 (67.3) | |
| Rhinorrhea | 28 (53.8) | |
| Sore throat | 6 (11.5) | |
| Chest discomfort/dyspnea | 6 (11.5) | |
| Oxygen requirement | 0 (0) | |
| Duration of PCR positivity, days | 25 (19–35) | |
| Contact with other COVID-19 patient after recovery | 0 (0) | |
| Time interval from symptom onset to blood sampling, days | 351 (349–352) | |
| Positivity of antibodies one year after infection | ||
| Anti-N pan-Ig ECLIA (Roche Diagnostics) | 43 (82.7) | |
| Anti-S IgG ELISA (InBios) | 44 (84.6) | |
| Anti-S1 IgG ELISA (Euroimmun) | 30 (57.7) | |
| sVNT (GenScript) | 30 (57.7) | |
Values are presented as number (%) or median (interquartile range).
Anti-N = anti-nucleocapsid, pan-Ig = pan-immunoglobulin, ECLIA = electrochemiluminescence immunoassay, Anti-S = anti-spike, ELISA = enzyme-linked immunosorbent assay, anti-S1 = anti-spike subunit: sVNT = surrogate virus neutralization test.
aUnderlying disease: hypertension (1), diabetes (1), and bronchitis (1) were included.
Understanding the longevity of humoral immunity to SARS-CoV-2 is essential for predicting herd immunity to SARS-CoV-2 and interpreting serosurvey data. In case of SARS-CoV-1, 90% and 50% of patients have been shown to maintain IgG antibodies for two and three years, respectively.4 Studies conducted in the early COVID-19 epidemic showed that the antibody titers of the patients with mild COVID-19 declined more quickly than those reported for SARS-CoV-1,5 and waning immunity has been confirmed five months after infection.6 Therefore, concerns about the usefulness of population-based seroprevalence studies have been raised because rapid waning immunity may lead to substantial false negatives in an immunoassay and underestimate the number of persons with previous SARS-CoV-2 infection.7
Recent studies showed antibodies against SARS-CoV-2 remained stable over time, declining moderately over 6–8 months after infection.3,8 In the present study, we showed that the antibody-positive rate was still high one year after infection in two of three commercial kits (82.7–84.6%), even in mildly symptomatic patients. By combining the anti-N pan-Ig and anti-S IgG assay results, we could identify ~94% of patients with mildly symptomatic SARS-CoV-2 infection one year after symptom onset.
Longitudinal seroprevalence study of healthcare workers in the United Kingdom showed that SARS-CoV-2 anti-N antibodies waned faster, but anti-S IgG remained stably detected,9 and other study showed that anti-N antibodies frequently became undetectable by 5–7 months.10 However, the present study demonstrated that despite the possible waning of anti-N antibodies, the anti-N pan-Ig assay could be useful to estimate immunity or seroprevalence up to one year after infection. It is of note that anti-N antibodies are useful to differentiate vaccination-induced immunity from that conferred by natural infection, because most vaccines except live attenuated or inactivated vaccines have been developed targeting the spike protein.
Our study has several limitations. First, the relatively small sample size and the predominantly young population reduce the generalization of the results. Second, due to the cross-sectional study design, we could not get the baseline or longitudinal serological samples, although of 52 patients, 44 patients also participated in an 8-month cross-sectional survey previously reported.3 There was no significant difference between 8 and 12 months after infection in positivity to anti-N pan-Ig, anti-S IgG, and sVNT activity, except anti-S1 IgG (Supplementary Table 1).
In conclusion, despite waning immunity, SARS-CoV-2 antibodies can be detected up to one year after infection, even in mild COVID-19 patients. Especially, anti-N pan-Ig assay or its combination with anti-S IgG assay could be used to detect past SARS-CoV-2 infection at least one year after infection even in a vaccinated population.
Ethics statement
The Institutional Review Boards (IRB) of Seoul National University Hospital and Pusan National University Hospital approved the study (IRB No. H-2009-168-1160 and 2010-013-096). Written informed consent was obtained from all patients.
ACKNOWLEDGMENTS
We appreciated Kyung Sook Ahn, MD, the Director of Gyeongsan City Health Center, for the administrative support, and Areum Jo, Su Jin Choi, from Seoul National University Hospital Biomedical Research Institute, and Mee Kyung Ko, from Pusan National University Hospital Biomedical Research Institute, for the technical support.
Footnotes
Funding: Funding for this project was supported by the research fund of Seoul National University Hospital (Grant No. 04-2021-0010). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Park WB, Yi J.
- Data curation: Choe PG, Kang CK, Suh HJ, Kang E, Lee SY.
- Formal analysis: Choe PG, Kim KH, Kim NJ, Yi J, Park WB, Oh MD.
- Funding acquisition: Oh MD.
- Investigation: Choe PG, Kang CK.
- Methodology: Kim KH, Yi J, Park WB.
- Supervision: Yi J, Park WB.
- Validation: Choe PG, Kim KH, Kang CK, Kim NJ, Yi J, Park WB, Oh MD.
- Writing - original draft: Choe PG, Park WB.
- Writing - review & editing: Choe PG, Kim KH, Kang CK, Suh HJ, Kang E, Lee SY, Kim NJ, Yi J, Park WB, Oh MD.
SUPPLEMENTARY MATERIAL
Comparison of antibody positivity between 8 and 12 months after mild SARS-CoV-2 infection (n = 44)
References
- 1.Choe PG, Kang EK, Lee SY, Oh B, Im D, Lee HY, et al. Selecting coronavirus disease 2019 patients with negligible risk of progression: early experience from non-hospital isolation facility in Korea. Korean J Intern Med. 2020;35(4):765–770. doi: 10.3904/kjim.2020.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choe PG, Kang CK, Suh HJ, Jung J, Kang E, Lee SY, et al. Antibody responses to SARS-CoV-2 at 8 weeks postinfection in asymptomatic patients. Emerg Infect Dis. 2020;26(10):2484–2487. doi: 10.3201/eid2610.202211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choe PG, Kim KH, Kang CK, Suh HJ, Kang E, Lee SY, et al. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg Infect Dis. 2021;27(3):928–931. doi: 10.3201/eid2703.204543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe PG, Kang CK, Suh HJ, Jung J, Song KH, Bang JH, et al. Waning antibody responses in asymptomatic and symptomatic SARS-CoV-2 infection. Emerg Infect Dis. 2021;27(1):327–329. doi: 10.3201/eid2701.203515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Self WH, Tenforde MW, Stubblefield WB, Feldstein LR, Steingrub JS, Shapiro NI, et al. Decline in SARS-CoV-2 antibodies after mild infection among frontline health care personnel in a multistate hospital network - 12 states, April-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(47):1762–1766. doi: 10.15585/mmwr.mm6947a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumley SF, Wei J, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, et al. The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ripperger TJ, Uhrlaub JL, Watanabe M, Wong R, Castaneda Y, Pizzato HA, et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53(5):925–933.e4. doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of antibody positivity between 8 and 12 months after mild SARS-CoV-2 infection (n = 44)


