Abstract
To evaluate the clinical characteristics of unilateral open-angle glaucoma, patients diagnosed with unilateral open-angle glaucoma from January 2017 to October 2018 were divided into primary open-angle glaucoma and normal-tension glaucoma groups according to the type of glaucoma diagnosed. The glaucoma and the contralateral eyes were compared, and the contralateral eye was analyzed for conversion to glaucoma and its risk factors were assessed during the 2-year follow-up period. Among 99 patients, 36 were diagnosed with primary open-angle glaucoma and 63 with normal-tension glaucoma. When comparing the glaucoma eye with the contralateral eye, the visual field mean deviation value (all p<0.001), peripapillary retinal nerve fiber layer thickness (all p<0.001), macular ganglion cell layer-inner plexiform layer thickness (p<0.001, p=0.003), and optic nerve cup-disc ratio (p=0.005, p<0.001) were significantly different in both the primary open-angle glaucoma and normal-tension glaucoma groups. In normal-tension glaucoma, peripapillary retinal nerve fiber layer thickness was significantly thinner in the glaucoma conversion group than in the glaucoma non-conversion group (p=0.008). It was significantly associated with glaucoma conversion (odds ratio=0.97, p=0.023). In conclusion, in patients with unilateral open-angle glaucoma, the contralateral eye may develop glaucoma. In particular, if the peripapillary retinal nerve fiber layer thickness is decreased in normal-tension glaucoma, the possibility of glaucoma conversion is high; hence, careful examination is required.
Keywords: Unilateral Glaucoma, Open-Angle Glaucoma, Low Tension Glaucoma, Glaucoma Conversion
INTRODUCTION
Glaucoma is one of the leading causes of blindness worldwide and is characterized by progressive optic disc rim reduction and associated visual field defects.1,2 Open-angle glaucoma is a progressive optic neuropathy, showing optic nerve head cupping and visual field defects in the state of an open angle. It can be classified as normal-tension glaucoma if intraocular pressure (IOP) is less than 21 mmHg and primary open-angle glaucoma if IOP is more than 21 mmHg.3 In primary open-angle glaucoma, mechanical damage to the optic nerve occurs due to high IOP and is known to be an essential factor in the development and progression of glaucoma, whereas in normal-tension glaucoma, damage to the optic nerve is caused even at normal IOP; the pathophysiological mechanism underlying this damage at normal IOP is hypothesized to be reduced ocular blood flow, but the exact mechanism has not yet been clarified.3,4
Usually, glaucoma is a disease that occurs bilaterally, and the degree of glaucoma damage varies in both sides. Sometimes, glaucoma occurs in one eye and the other side remains normal but develops glaucoma over time.5,6 It is known that the rate of glaucoma conversion in the contralateral eye of patients with unilateral glaucoma is higher than that in normal subjects.7,8 There have been several previous studies on the risk factors for glaucoma conversion in the contralateral eye of patients with unilateral glaucoma, but only normal-tension glaucoma has been studied, and there studies on primary open-angle glaucoma are limited.9,10
Therefore, in this study, we divided patients into primary open-angle glaucoma and normal-tension glaucoma groups, compared the clinical differences between eyes with glaucoma and contralateral normal eyes in patients with unilateral open-angle glaucoma, and investigated glaucoma conversion and its risk factors during a 2-year follow-up period.
MATERIALS AND METHODS
From January 2017 to October 2018, medical records were retrospectively analyzed for patients over 18 years of age diagnosed with unilateral glaucoma at a glaucoma clinic and who could be followed up for more than two years. This study was conducted with the institutional review board's approval (IRB No. CNUH-2021-029).
Age, sex, and systemic diseases were noted in all patients. Best-corrected visual acuity (LogMAR), IOP (AT 900®, Haag-Streit, Bern, Switzerland), and refractive power (spherical equivalent, SE) were measured with an autorefractometer (KR-8900; Topcon, Tokyo, Japan); slit-lamp microscopy (Haag-Streit BQ900, Hagg-Streit AG, Switzerland), gonioscopy with a Goldmann-type contact lens according to standard methods, fundus photography (Kowa Nonmyd7 fundus camera, Kowa, Tokyo, Japan), optical coherence tomography (Cirrus® HD-OCT; Carl Zeiss Meditec, Dublin, CA, USA), optical low coherence reflectometry (Lenstar LS900, Haag-Streit, Mason, OH, USA), specular microscopy (NSP-9900, Konan medical Inc., Nishinomiya, Japan), and automated perimetry (Humphrey® Visual Field Analyzer; Carl Zeiss Ophthalmic System, Inc., USA) were performed. The peripapillary retinal nerve fiber layer (pRNFL), macular ganglion cell-inner plexiform layer (mGC-IPL) thickness, and optic nerve head cup-disc ratio (CDR) were measured by optical coherence tomography. Axial length (AXL) and central corneal thickness (CCT) were measured by optical low coherence reflectometry. Visual field mean deviation (MD) was measured by automated perimetry. We measured best-corrected visual acuity, IOP, and refractive power and performed slit-lamp microscopy and fundus photography every four months. Optical coherence tomography and automatic perimetry were performed every year, and low-coherence reflectometry and specular microscopy were performed every two years.
Two independent glaucoma specialists diagnosed unilateral glaucoma based on the above test results. Any disagreements were settled by consensus, and an additional grader was consulted, if necessary. The definition of glaucoma included (1) local or diffuse neuroretinal rim thinning or optic disc cupping, a difference in vertical CDR of 0.2 or more between both eyes, retinal nerve fiber layer defects, and glaucomatous optic nerve damage such as those with optic disc hemorrhage, and (2) on visual field examination performed at least two times, the mean sensitivity of three or more points in the arcuate region in the pattern deviation plot was less than 5% of normal, and one of them was less than 1% or outside normal on the glaucoma hemifield test. Glaucoma-related visual field damage, such as when the limits appear twice in a row or when the corrected pattern standard deviation was less than 5%, was considered (Fig. 1).11 When an open anterior chamber angle was seen on gonioscopic examination and if the IOP was 21 mmHg or less, normal-tension glaucoma was diagnosed, and if IOP was more than 21 mmHg, primary open-angle glaucoma was diagnosed and included in the study.
FIG. 1. Representative case of unilateral open-angle glaucoma. Disc photography, red-free fundus photography, peripapillary retina nerve fiber layer thickness of optical coherence tomography, and pattern deviation map of standard automated perimetry show glaucoma in the left eye; the contralateral eye shows normal finding.
Patients were excluded if they had used an IOP lowering agent at the time of visit; had other ophthalmic diseases that may cause glaucoma; had a history of trauma or surgery; had ophthalmic diseases other than glaucoma such as diabetic retinopathy or macular degeneration; had a best-corrected visual acuity of less than 0.5; had a refractive power of −6 diopters or less; had a history of ocular surgery excluding cataract surgery; or had other causative diseases that may cause visual field damage. If the visual field test result exceeded false positive by 15%, false-negative by 15%, or fixation loss by 20% or if optical coherence tomography signal strength was less than 6, then the patients were excluded. The glaucoma eye of a patient with unilateral open-angle glaucoma was treated with IOP-lowering eye drops. We educated patients not to use an IOP lowering agent on the other eye. Two years later, we evaluated patients for the development of glaucoma in the contralateral eye (Fig. 2).
FIG. 2. Representative case of glaucoma conversion in contralateral eyes of patients with unilateral open glaucoma. A patient whose contralateral eye showed normal findings at baseline. Two years later, peripapillary retina nerve fiber layer thickness on optical coherence tomography and pattern deviation map on standard automated perimetry indicate glaucoma conversion in the contralateral eye.
For statistical analysis, SPSS 18.0 for Windows (SPSS Inc. Chicago, IL, USA) was used. A student's t-test, Mann Whitney U-test, Wilcoxon signed rank test, and chi-square test were used to compare groups. Logistic regression analysis was performed to determine the risk factors for conversion to glaucoma in the opposite eye. A p-value than 0.05 was considered statistically significant.
RESULTS
A total of 99 patients with unilateral open-angle glaucoma were included, of which 36 had primary open-angle glaucoma and 63 had normal-tension glaucoma. Age, sex, laterality of glaucoma eye, hypertension, diabetes, and the rate of glaucoma conversion of the contralateral eye did not show significant differences between the primary open-angle glaucoma group and the normal-tension glaucoma group (Table 1).
TABLE 1. Comparison of demographics between primary open-angle glaucoma and normal-tension glaucoma groups.
Values are presented as mean±standard deviation unless otherwise indicated.
POAG: primary open angle glaucoma, NTG: normal-tension glaucoma.
*Student's t-test, †Chi square test and Fisher's exact test.
p<0.05 was considered to be significant.
When comparing the glaucoma eye and contralateral eye in patients with unilateral open-angle glaucoma, the glaucoma eye in the primary open-angle glaucoma group had higher IOP (23.53±7.05 mmHg vs 18.08±5.74 mmHg, p<0.001), lower visual field MD value (−11.77±10.05 dB vs −2.41±2.77 dB, p<0.001), pRNFL thickness (68.11±14.71 µm vs 88.50±9.92 µm, p<0.001), and mGC-IPL thickness (68.06±11.70 µm vs 83.00±9.48 µm, p<0.001), and larger CDR (0.75±0.13 vs 0.63±0.12, p<0.001) compared to those of the contralateral eye, but the best-corrected visual acuity, CCT, AXL, refractive power, and corneal endothelial cell density were not significantly different (Table 2). In the normal-tension glaucoma group, the glaucoma eye had a lower visual field MD value (−5.14±5.30 dB vs −1.88±2.28 dB, p<0.001), pRNFL thickness (74.48±12.93 µm vs 85.40±15.13 µm, p<0.001), and mGC-IPL thickness (70.43±9.55 µm vs 76.70±14.52 µm, p=0.003), and larger CDR (0.71±0.10 vs 0.53±0.11, p<0.001) compared to those of the contralateral eye, but the best-corrected visual acuity, IOP, CCT, AXL, refractive power, and corneal endothelial cell density were not significantly different (Table 2).
TABLE 2. Comparison of glaucoma eyes and non-glaucoma eyes in patients with unilateral open-angle glaucoma.
Values are presented as mean±standard deviation.
POAG: primary open angle glaucoma, NTG: normal-tension glaucoma, BCVA: best corrected visual acuity, IOP: intraocular pressure, CCT: central corneal thickness, AXL: axial length, MD: mean deviation, SE: spherical equivalent, pRNFL: peripapillary retinal nerve fiber layer, mGC-IPL: macular ganglion cell-inner plexiform layer.
*Paired t-test p<0.05 was considered to be significant.
The groups were further divided based on conversion to glaucoma in the contralateral eye after two years; on comparing the ophthalmic findings at the first visit in the contralateral eye of a patient with unilateral open-angle glaucoma, there was no significant difference in all parameters in the primary open-angle glaucoma group, and the thickness of the pRNFL was thinner than that of the non-glaucoma conversion group in the normal-tension glaucoma group (Table 3).
TABLE 3. Comparison between glaucoma converted eyes and non-converted eyes in the two groups at baseline.
Values are presented as mean±standard deviation.
POAG: primary open angle glaucoma, NTG: normal-tension glaucoma, HTN: hypertension, DM: diabetes mellitus, BCVA: best corrected visual acuity, IOP: intraocular pressure, CCT: central corneal thickness, AXL: axial length, MD: mean deviation, SE: spherical equivalent, pRNFL: peripapillary retinal nerve fiber layer, mGC-IPL: macular ganglion cell-inner plexiform layer.
*Mann Whitney U-test, †Chi square test and Fisher's exact test p<0.05 was considered to be significant.
When comparing the first visit and two year follow-up ophthalmological findings of the eyes that developed glaucoma in patients with primary open-angle glaucoma, higher IOP (21.47±5.37 mmHg vs 18.53±7.66 mmHg, p=0.047), lower MD values (−6.64±1.98 dB vs −2.33±2.32 dB, p=0.031), pRNFL thickness (75.73±7.88 µm vs 87.73±7.88 µm, p=0.037), and mGC-IPL thickness (72.60±6.15 µm vs 80.60±9.30 µm, p=0.048) and larger CDR (0.68±0.14 vs 0.52±0.14, p=0.050) were seen at the two year follow-up; however the best-corrected visual acuity, CCT, AXL, refractive power, and corneal endothelial cell density were not significantly different (Table 4). In the eyes that showed conversion in patients with normal-tension glaucoma, compared to the first visit values, lower MD values (−4.27±0.58 dB vs −2.27±2.44 dB, p=0.041), pRNFL thickness (79.04±9.24 µm vs 81.04±11.42 µm, p=0.043), and mGC-IPL thickness (72.61±12.19 µm vs 74.61±12.19 µm, p=0.039) were seen at the two year follow-up, but the best-corrected visual acuity, IOP, CCT, AXL, refractive power, corneal endothelial cell density, and CDR were not significantly different (Table 4).
TABLE 4. Comparison between baseline and two year follow-up data in the glaucoma converted eyes.
Values are presented as mean±standard deviation.
POAG: primary open angle glaucoma, NTG: normal tension glaucoma, BCVA: best corrected visual acuity, IOP: intraocular pressure, CCT: central corneal thickness, AXL: axial length, MD: mean deviation, SE: spherical equivalent, pRNFL: peripapillary retinal nerve fiber layer, mGC-IPL: macular ganglion cell-inner plexiform layer.
*Wilcoxon signed rank test p<0.05 was considered to be significant.
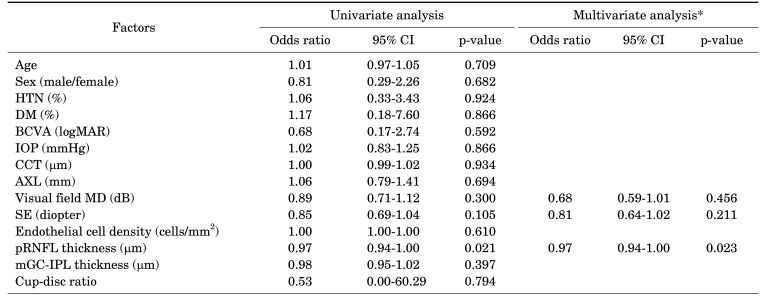
When the logistic regression analysis was performed to determine the risk factors for conversion to glaucoma in contralateral eyes of patients with unilateral open-angle glaucoma, no significant factors were found in the primary open-angle glaucoma group. When the univariate analysis was performed in the normal-tension glaucoma group, the results showed that the lower the thickness of the pRNFL (p=0.021; OR, 0.97; 95% CI, 0.94-1.00), the higher the risk of conversion to glaucoma, and the multivariate analysis showed consistent results (p=0.023; OR, 0.97; 95% CI, 0.94-1.00) (Tables 5, 6).
TABLE 5. Risk factor analyses for glaucoma conversion in non-glaucoma eyes of patients with unilateral POAG.
POAG: primary open angle glaucoma, CI: confidence interval, HTN: hypertension, DM: diabetes mellitus, BCVA: best corrected visual acuity, IOP: intraocular pressure, CCT: central corneal thickness, AXL: axial length, MD: mean deviation, SE: spherical equivalent, pRNFL: peripapillary retinal nerve fiber layer, mGC-IPL: macular ganglion cell-inner plexiform layer.
*Only variables with p<0.3 in the univariate analysis were included in the multivariate model.
TABLE 6. Risk factor analyses for glaucoma conversion in non-glaucoma eyes of patients with unilateral NTG.
NTG: normal tension glaucoma, CI: confidence interval, HTN: hypertension, DM: diabetes mellitus, BCVA: best corrected visual acuity, IOP: intraocular pressure, CCT: central corneal thickness, AXL: axial length, MD: mean deviation, SE: spherical equivalent, pRNFL: peripapillary retinal nerve fiber layer, mGC-IPL: macular ganglion cell-inner plexiform layer.
*Only variables with p<0.3 in the univariate analysis were included in the multivariate model.
DISCUSSION
Glaucoma is defined as a progressive optic neuropathy characterized by visual field defects associated with damage to the optic nerve, and it can appear in various features.1,2 Open-angle glaucoma occurs in 3.5% of Koreans and shows optic disc cupping and visual field defects when the anterior angle is open. If the untreated IOP is less than 21 mmHg, glaucoma is classified as normal-tension glaucoma, and if it is over 21 mmHg, it is classified as primary open-angle glaucoma.3,11 It is known that primary open-angle glaucoma is common in Caucasians and normal-tension glaucoma in East Asians, and genetic factors, risk factors, and clinical characteristics are different for the two diseases.12,13 Therefore, when analyzing patients with unilateral open-angle glaucoma in this study, the two diseases were analyzed separately.
Although glaucoma is known as a bilateral disease, the degree of glaucoma-related damage varies in both eyes, and sometimes glaucoma appears in only one eye.14 It is known that the risk of glaucoma conversion in patients with unilateral glaucoma over time is higher than that in healthy individuals, and several studies on the risk factors for this conversion have been reported.5,6
When comparing patients with primary open-angle glaucoma and normal-tension glaucoma, there was no significant difference in age, sex, underlying disease, and conversion to glaucoma in the contralateral eye. After two years, the glaucoma conversion rate for the contralateral eye was 41.67% in the primary open-angle glaucoma group and 36.51% in the normal-tension glaucoma group. The high prevalence seen in this study was consistent with those of previous studies showing that patients diagnosed with unilateral glaucoma had a higher risk of developing glaucoma in the other eye than did normal individuals.7,8,11
When comparing the glaucoma eye and contralateral eye of patients with unilateral open-angle glaucoma, in both the primary open-angle glaucoma and normal-tension glaucoma groups, glaucoma eyes showed lower MD values, pRNFL thickness, and mGC-IPL thickness and larger CDR than did the contralateral eye, but IOP was significantly higher in the glaucoma eyes only in the primary open-angle glaucoma group. These findings are similar to those of previous studies, which reported that IOP acts as a significant risk factor in the development of primary open-angle glaucoma, and factors independent of IOP play an essential role in the development of normal-tension glaucoma.3
In patients with unilateral open-angle glaucoma, when comparing the contralateral eye's ophthalmological findings at the first visit to the glaucoma converted eye and the glaucoma non-converted eye, the pRNFL thickness was lower than those of the eyes that developed glaucoma in the normal-tension glaucoma group. There was no significant difference in any ophthalmological parameter in the primary open-angle glaucoma group. This may have occurred because patients who were suspected of having glaucoma or had pre-perimetric glaucoma were included; these patients may not have presented with as a visual field defect at the first visit, even though structural damage to the other eye had already occurred.
When comparing the first visit and 2 year follow-up visit ophthalmological findings of the contralateral eyes that developed glaucoma, both primary open-angle glaucoma and normal-tension glaucoma groups showed lower MD value, pRNFL thickness, and mGC-IPL thickness two years later. However, IOP and CDR increased after two years only in the primary open-angle glaucoma group, and there was no significant difference in the normal-tension glaucoma group. This result coincided with those of previous studies, which showed that glaucoma progression in normal-tension glaucoma is influenced by factors other than IOP. Besides, there was a difference in previous studies that showed that the degree of increase in the cup disc ratio was steep among patients with normal-tension glaucoma in Korea, which was thought to be due to the relatively short follow-up period of these studies.15,16,17
In this study, a significant risk factor for glaucoma conversion in the contralateral eye in patients with primary open-angle glaucoma was not defined. However, in previous studies, old age, high IOP, CDR of 0.5 or more, optic disc hemorrhage, thin CCT, and large pattern standard deviation on visual field examination were reported as significant risk factors.7,18 Instead, in this study, the risk of glaucoma conversion was found to significantly increase as the pRNFL thickness of the contralateral eye was decreased in patients with normal-tension glaucoma. This result was different from previous studies showing that severe visual field defects in glaucoma eyes, IOP above 14 mmHg in normal eyes, diabetes, and systemic vascular diseases such as cerebrovascular disease are related to glaucoma conversion.10 In this study, it was considered that structural damage to the optic nerve and visual field damage must be satisfactory to diagnose glaucoma. When referring to previous studies that have already known that structural damage precedes visual field defects in visual field exam, it is thought that these results were included in patients with glaucoma suspect or pre-perimetric glaucoma.19
Limitations of this study include 1) a relatively small number of patients from a single institution, 2) a short follow-up period, 3) its retrospective design, 4) lack of analysis of the ocular parameters such as β-zone PPA and various systemic diseases that have been reported to be related to glaucoma. It is thought that among the patients who did not show conversion to glaucoma, patients suspected of having glaucoma or those with pre-perimetric glaucoma might have been included, which influenced the outcomes. Additionally, it was impossible to analyze the contralateral eye's glaucoma conversion according to the degree of damage to the glaucoma eye. Among the patients who did not show conversion to glaucoma, glaucoma symptoms or prefield glaucoma patients were included, which may have influenced the outcome.
In the future, a large-scale, long-term study should be conducted to supplement our findings and to analyze the risk factors for conversion to glaucoma in the contralateral eye of patients with unilateral glaucoma as it will be of great help in determining the prognosis and treatment time for the contralateral eye.
In conclusion, in patients with unilateral open-angle glaucoma, the contralateral eye may develop glaucoma. In particular, if pRNFL thickness is decreased in normal-tension glaucoma, the possibility of glaucoma conversion is high; therefore, careful examination is required.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Thylefors B, Négrel AD. The global impact of glaucoma. Bull World Health Organ. 1994;72:323–326. [PMC free article] [PubMed] [Google Scholar]
- 2.Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001;85:1277–1282. doi: 10.1136/bjo.85.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields MB. Normal-tension glaucoma: is it different from primary open-angle glaucoma? Curr Opin Ophthalmol. 2008;19:85–88. doi: 10.1097/ICU.0b013e3282f3919b. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki Y, Drance SM. The relationship between progression of visual field defects and retrobulbar circulation in patients with glaucoma. Am J Ophthalmol. 1997;124:287–295. doi: 10.1016/s0002-9394(14)70820-7. [DOI] [PubMed] [Google Scholar]
- 5.Niziol LM, Gillespie BW, Musch DC. Association of fellow eye with study eye disease trajectories and need for fellow eye treatment in Collaborative Initial Glaucoma Treatment Study (CIGTS) participants. JAMA Ophthalmol. 2018;136:1149–1156. doi: 10.1001/jamaophthalmol.2018.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Öhnell H, Heijl A, Brenner L, Anderson H, Bengtsson B. Structural and functional progression in the early manifest glaucoma trial. Ophthalmology. 2016;123:1173–1180. doi: 10.1016/j.ophtha.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susanna R, Drance SM, Douglas GR. The visual prognosis of the fellow eye in uniocular chronic open-angle glaucoma. Br J Ophthalmol. 1978;62:327–329. doi: 10.1136/bjo.62.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gliklich RE, Steinmann WC, Spaeth GL. Visual field change in low-tension glaucoma over a five-year follow-up. Ophthalmology. 1989;96:316–320. doi: 10.1016/s0161-6420(89)33070-3. [DOI] [PubMed] [Google Scholar]
- 9.Poinoosawmy D, Fontana L, Wu JX, Bunce CV, Hitchings RA. Frequency of asymmetric visual field defects in normal-tension and high-tension glaucoma. Ophthalmology. 1998;105:988–991. doi: 10.1016/S0161-6420(98)96049-3. [DOI] [PubMed] [Google Scholar]
- 10.Kim C, Kim TW. Comparison of risk factors for bilateral and unilateral eye involvement in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2009;50:1215–1220. doi: 10.1167/iovs.08-1886. [DOI] [PubMed] [Google Scholar]
- 11.Kim CS, Seong GJ, Lee NH, Song KC Namil Study Group, Korean Glaucoma Society. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011;118:1024–1030. doi: 10.1016/j.ophtha.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Sung KR, Lee S, Park SB, Choi J, Kim ST, Yun SC, et al. Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Invest Ophthalmol Vis Sci. 2009;50:5266–5274. doi: 10.1167/iovs.09-3716. [DOI] [PubMed] [Google Scholar]
- 13.Stein JD, Kim DS, Niziol LM, Talwar N, Nan B, Musch DC, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118:1031–1037. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong D, Sung KR, Na JH. Comparison of clinical characteristics and progression rates of bilaterally and unilaterally progressing glaucoma. Korean J Ophthalmol. 2015;29:40–46. doi: 10.3341/kjo.2015.29.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park HY, Shin HY, Park CK. Imaging the posterior segment of the eye using swept-source optical coherence tomography in myopic glaucoma eyes: comparison with enhanced-depth imaging. Am J Ophthalmol. 2014;157:550–557. doi: 10.1016/j.ajo.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Carbonaro F, Hysi PG, Fahy SJ, Nag A, Hammond CJ. Optic disc planimetry, corneal hysteresis, central corneal thickness, and intraocular pressure as risk factors for glaucoma. Am J Ophthalmol. 2014;157:441–446. doi: 10.1016/j.ajo.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Jeoung JW, Bitrian E, Supawavej C, Mock D, Park KH, et al. Comparison of clinical characteristics between Korean and Western normal-tension glaucoma patients. Am J Ophthalmol. 2013;155:852–857. doi: 10.1016/j.ajo.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Gordon MO, Torri V, Miglior S, Beiser JA, Floriani I, Miller JP, et al. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–19. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommer A, Katz J, Quigley HA, Miller NR, Robin AL, Richter RC, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]