Abstract
Heterotopic ossification (HO), a synonym for osseous metaplasia, is a pathological phenomenon, characterized by abnormal bone formation outside the skeletal system observed commonly in various neoplastic and non-neoplastic diseases. HO occurring in meningioma is exceptionally rare. We reportherein an unusual case of spinal meningioma containing numerous calcified psammoma bodies and extensive HO in a 75-year-old woman, who presented with progressive worsening bilateral lower limb weakness and numbness. The presence of remarkable bone formation within a meningioma is controversial among pathologists; while some regard them as psammomatous meningioma as the primary diagnosis, others prefer osteoblastic meningioma, a form of metaplastic meningioma. There is compelling molecular data to advocate that HO is an active disease process involving metaplastic (osseous) differentiation of meningioma stroma mesenchymal stem-like cells, but not the meningothelial-derived tumor cells. Henceforth, the term “metaplastic meningioma” may not be appropriate in this context. A plausible designation as “psammomatous meningioma with osseous metaplasia” defines this entity more accurately. This paper highlights the need for a unifying nomenclature to reduce diagnostic controversy caused by conflicting terms in the literature. The possible pathogenesis of this intriguing phenomenon is discussed.
Keywords: Diagnostic dilemma, heterotopic ossification, meningioma, osseous metaplasia, psammomatous
Introduction
Heterotopic ossification (HO), a synonym for osseous metaplasia, is a diverse pathologic phenomenon well described in various neoplastic and non-neoplastic diseases. It is characterized by abnormal bone formation outside the skeletal system, and usually occurs following traumatic injuries, local or systemic insults, and surgery [1]. HO in meningioma is exceedingly rare, with approximately 30 published cases worldwide [2]. We report herein an unusual case of spinal meningioma containing numerous calcified psammoma bodies and extensive HO in a 75-year-old woman with no prior history of spinal injury.
The issue raised was whether this phenomenon represents a true tumor metaplasia. The exact histogenesis of this devastating occurrence remains elusive to date. This confusion has created a diagnostic controversy among experienced pathologists; some regarded them as psammomatous meningioma, while others preferred osteoblastic meningioma, a form of metaplastic meningioma [2]. The most relevant key genes implicated in the osteogenesis pathways in meningiomas linked to the possible pathogenetic etiology of this intriguing phenomenon are discussed in the light of the literature.
Case presentation
A 75-year-old woman presented to us with two-year history of bilateral lower limb weakness and numbness. Her symptoms had progressively worsened two months prior to presentation. She had no other constitutional symptoms such as fever, urinary and faecal incontinence. There was no previous history of spinal injury. On examination, the motor strength of both lower extremities was reduced, with associated sensory deficit from the T2 up to S1 level. Both the ankle and knee jerks were hyperreflexic. Anal tone was normal.
Magnetic resonance imaging (MRI) of the spine revealed a 16 mm well-defined intradural extramedullary lesion at T10/T11 level, compressing the adjacent spinal cord. Remarkably, noticeable hypointense signal was seen centrally on T1- and T2-weighted sequences, representing intratumoral calcification (Figure 1). In addition, there were multilevel extensive degenerative disc changes of the spine with protrusion of osteophyte-discal complex from C3 to C7, most severe at the C5/C6 level with associated significant spinal canal stenosis and cord compression (not shown), corresponding to the neurological impairment elicited. Computed topography (CT) examination of the spine demonstrated a highly attenuating intratumoral calcification within the spinal canal, in agreement with the MRI finding (Figure 1). She underwent a left T10 hemilaminectomy and excision of the intradural extramedullary mass. The tumor was removed piecemeal successfully with minimal adherence to surrounding structures. No residual disease was detected by postoperative MRI.
Figure 1.
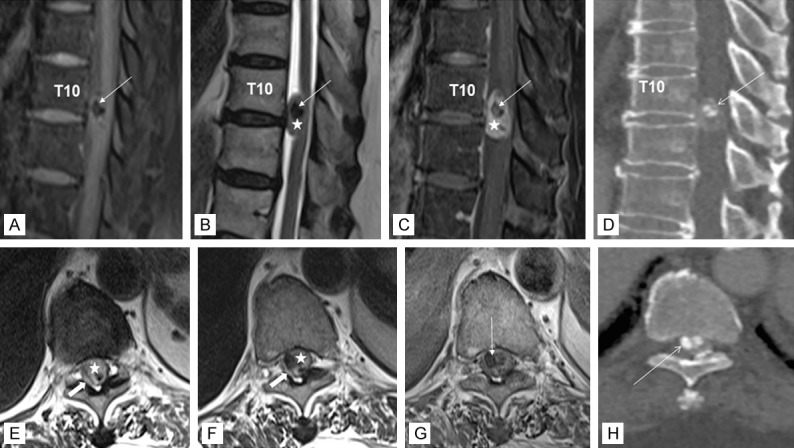
MR imaging of the thoracic spine in T1-weighted image with fat saturation (A), T2-weighted image (B), T1-weighted fat saturation post contrast image (C), and CT bone setting reconstruction image in sagittal plane (D) and T2-weighted image (E, F), T1-weighted post contrast image (G), and CT reconstruction image in bone setting in axial plane (H). The intradural extramedullary lesion (star) exhibits isointense signal on T1, is hyperintense on T2, and enhances homogeneously with contrast. There are hypointense foci within (arrow) that are markedly hypointense on T1 and T2 consistent with intratumoral calcification as shown in the CT images. The spinal cord is displaced and compressed right posterolaterally by the mass and returns high signal intensity on T2 indicating cord edema (block arrow).
Gross examination of tumor showed grey brownish “hard” tissue fragments which imparted a gritty sensation on cutting. Histopathologic examination displayed morphologic features of classical psammomatous meningioma (Figure 2A), composed of syncytial sheets and lobules of uniform neoplastic meningothelial cells arranged in a whorled configuration. The tumor cells had round to oval nuclei with delicate chromatin and inconspicuous nucleoli. Rare pseudonuclear inclusions were identified. Numerous psammoma bodies were readily apparent (Figure 2B). There was no increase in mitosis or tumor necrosis detected. Interestingly, a substantial interlacing network of immature woven bony trabeculae without osteoblastic rimming representing HO (Figure 2C) was intermingled intimately with these lobules of neoplastic meningothelial cells. No haematopoietic marrow element was present. An area suggestive of an endochondral ossification was also observed (Figure 2D).
Figure 2.

Microphotographs of psammomatous spinal meningioma with osseous metaplasia. (A) The tumor exhibits syncytial sheets and lobules of uniform neoplastic meningothelial cells arranged in a whorled configuration (H&E ×400); (B) Numerous psammoma bodies are readily apparent, consistent with a diagnosis of psammomatous meningioma (H&E ×200); (C) Intermingled closely with the tumor, there is a substantial interlacing network of immature woven bony trabeculae without osteoblastic rimming, signifying heterotopic ossification (H&E ×100); (D) An area suggestive of an endochondral ossification is seen (H&E ×400). Immunohistochemically, the neoplastic meningothelial cells are immunoreactive for (E) epithelial membrane antigen (EMA) (×400) and (F) progesterone receptor (PR) (×400).
Immunohistochemically, the meningothelial tumor cells exhibited epithelial membrane antigen (EMA) (Figure 2E), progesterone receptor (focal) (Figure 2F), vimentin and S-100 immunoreactivity, while the osteoblastic component was stained negative. Ki-67 proliferative and p53 labelling indices were low (approximately 1% and 10% respectively). After multidisciplinary discussion following extensive literature review, a diagnosis of psammomatous meningioma with extensive osseous metaplasia, World Health Organization (WHO) grade I was rendered.
The patient was discharged a week after surgery with no immediate post-operative complications. At six months post-surgical extirpation of tumor followed up, her neurological symptoms did not show significant improvement. She was subsequently counselled for C3-T1 posterior laminectomy and instrumentation.
Discussion
Spinal meningioma is relatively less frequent than its intracranial counterpart, accounting for approximately 12% of all meningiomas and 25% of all spinal neoplasms. A wide spectrum of morphologic appearances comprising 15 distinct histologic subtypes of spinal meningioma was illustrated in the latest WHO classification, with psammomatous, meningothelial and transitional being the commonest histologic variants [3]. Metaplastic meningioma is infrequently described in the spine. It is characterized histologically by the presence of focal or widespread osseous elements, singly or in combination with other mesenchymal differentiation including xanthomatous, cartilaginous, lipomatous, or myxoid components [4].
Ossification in meningioma was first described by Rogers [5] in 1928, and was rarely reported thereafter. A literature search from PubMed database using the search terms ((((((ossified) OR osteoblastic) OR osseous metaplasia) OR bone) OR psammomatous AND spinal) AND meningioma) retrieved 667 articles. After filtering our initial selection and excluding irrelevant articles, only 21 papers with 30 cases of ossified spinal meningioma were identified in the English literature, and five of them were psammomatous meningioma exhibiting osseous metaplasia. Clinicopathologic characteristics of these published cases [2,6-9] are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of the published cases of ossified psammomatous spinal meningiomas
| Reference | No. of cases | Age/Gender | Symptoms and duration | Tumor Laterality | Tumor size (mm) | Histologic subtype/WHO grade | Recurrence |
|---|---|---|---|---|---|---|---|
| Freidberg et al. (1972) [6] | 1 | 69/female | Progressive weakness lower limbs for 9 months, recent urinary incontinence | T1-T2, intradural extramedullary | 20 | Psammomatous/WHO grade I | NA |
| Uchida et al. (2009) [7] | 1 | 76/female | Painful lower limb weakness & numbness for 2 years | T8, T11/T12, extramedullary | NA | Psammomatous, fibrous & metaplastic/WHO grade I | No |
| Chotai et al. (2013) [8] | 1 | 61/female | Lower leg numbness & Weakness for 3 years, gait disturbances for 6 months | T4, intradural | NA | Psammomatous/WHO grade I | No |
| Prakash et al. (2017) [9] | 1 | 60/female | Progressive lower limbs weakness & numbness, urinary incontinence for 1 year | T7, intradural extramedullary | 15 | Psammomatous/WHO grade I | NA |
| Wang et al. (2019) [2] | 1 | 52/female | Chronic back pain for 5 years | T4, intradural extramedullary | 12 | Psammomatous/WHO grade I | No |
| Present case | 1 | 75/female | Bilateral lower limb weakness and numbness for 2 years | T10/T11, intradural extramedullary | 16 | Psammomatous/WHO grade I | No |
HO in meningioma should be distinguished from tumoral invasion of the overlying bone, a phenomenon commonly depicted in meningioma. Endochondral ossification or presence of woven (immature) bone is not a feature in the latter. In meningioma with bony invasion, the bony trabeculae usually appear thicker in parallel arrangement, commonly with osteoblastic rimming [10]. The characteristic MR imaging appearance of bony infiltration by meningioma, demonstrating gadolinium enhancement in the involved bone [11], may help in this distinction.
Another differential diagnosis worth mentioning is ossifying fibroma, especially the juvenile psammomatoid variant (JPOF). The presence of numerous psammomatoid ossicles uniformly distributed within a fibroblastic stroma admixed with small bone trabeculae with osteoblastic rim may mimic psammomatous meningioma with HO to perfection. Unlike psammomatous meningioma, which is predominantly found in the spine in older women, JPOF has a predilection for the craniofacial skeleton of the young [12]. A careful search for tumor cells showing unique meningothelial features, such as ovoid nuclei with delicate chromatin and nuclear pseudoinclusions, provides important clues to diagnosis.
The differential between psammomatous meningioma with HO and calcifying pseudoneoplasm of the neuraxis (CAPNON), a benign non-neoplastic calcified lesion of the central nervous system can be problematic. CAPNON represents a spectrum of unusual reactive processes involving the meninges and/or adjacent bone. It is characterized histologically by nodules of chondromyxoid matrix with palisading spindle to epithelioid cells embedded within a fibrous stroma. In addition, the presence of variably calcified or even ossified basophilic amorphous to fibrillated material, frequently described as “chicken-feet calcifications” is a fairly distinctive feature [13]. The presence of rare meningothelial hyperplasia in this entity, however, should not be mistaken for a meningioma.
The enigmatic association of meningioma with HO remains obscure to date. In the past, the process of HO was perceived as passive dystrophic mineralization of the degenerating neoplastic cells [1]. The hypothesis speculated that the presence of psammoma bodies could serve as a nidus for initial ossification was debunked. There was no clear association between bone formation and psammoma bodies, as HO did occur in cases devoid of psammoma body formation [14].
In general, HO develops by intramembranous ossification or endochondral ossification. Intramembranous ossification involves direct differentiation of a mesenchymal template into bone; whereas in endochondral ossification, replacement of a pre-existing hyaline cartilage model with mineralized bone occurs [1]. The presence of endochondral bone in the present case might indicate that endochondral ossification had taken place, a feature seldom emphasized in the literatures.
HO in meningioma was regarded as a metaplastic process and was covered by the term “metaplastic meningiomas” in the recent WHO classification [4]. Metaplasia by definition is characterized by reversible substitution of one mature cell type for another. It was once believed that these meningothelial-derived tumor cells retained their pluripotential aptitude to differentiate, in which they had transformed into osteoblasts, a mesenchymal derivation. Emerging literature supported our postulation that HO is indeed an active disease process encompassing metaplastic (osseous) transformation of the meningiomatous stroma mesenchymal stem-like cells (MS-MSLCs), a component in the microenvironment, but not the meningothelial-derived tumor cells themselves [2,15]. Henceforth, the term “metaplastic meningioma” may not be appropriate in this context. A plausible designation as “psammomatous meningioma with osseous metaplasia” defines this entity more accurately, given the abundance of intralesional psammoma bodies as well as extensive HO present within the same lesion.
The metaplastic process of MS-MSLCs requires active secretion of osteogenic factors by the meningothelial-derived tumor cells [2,15]. This is further substantiated by the expression of osteogenic lineage genes in the neoplastic meningothelial cells, such as bone morphogenetic proteins (BMPs), BMP receptor type 2 (BMPR2), transforming growth factor-β2 (TGF-β2), and SMADs. BMPs are a heterogeneous group of proteins belonging to the larger TGF-β superfamily, together with other members including TGF-βs (1-3). To date, there are more than 30 BMPs being recognized, with BMP-2, BMP-4, and BMP-7 directly implicated in the occurrence of HO [16]. TGF-β2, in particular, acts synergistically with BMPs in promoting heterotopic bone formation by TGF-β signalling (through the SMAD pathway) and non-canonical p38MAPK pathway. In addition, the overexpression of BMPR2 in meningioma may facilitate BMP-2-induced osteoblastic metaplasia and calcium mineralization [16] of the MS-MSLCs.
In summary, we describe an exceedingly rare case of HO in psammomatous meningioma. This highlights a need for a unifying nomenclature to reduce diagnostic controversy caused by the variety of terms in the current literature.
Acknowledgements
Informed consent was obtained from the enrolled patient. This work was supported by the Universiti Kebangsaan Malaysia (Fundamental Research Grant UKM, FF-2020-201). The funders had no role in data interpretation, preparation and writing of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, Sono T, McCarthy E, Levi B, James AW. Heterotopic ossification: a comprehensive review. JBMR Plus. 2019;3:e10172. doi: 10.1002/jbm4.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Chen Y, Zhang L, Ma X, Chen B, Li S. Thoracic psammomatous meningioma with osseous metaplasia: a controversial diagnosis of a case report and literature review. World J Surg Oncol. 2019;17:150. doi: 10.1186/s12957-019-1694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adogwa O, Fessler RG. Intradural extramedullary spinal tumors. Brain and spine surgery in the elderly. In: Moncef B, Krolak-Salmon P, editors. Switzerland: Springer; 2017. pp. 289–304. [Google Scholar]
- 4.Perry A, Louis D, Budka H, von Deimling A, Sahm F, Rushing EJ, Mawrin C, Claus EB, Loeffler J, Sadetzki S. Meningioma. WHO Classification of Tumours of the Central Nervous System. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D, Perry A, Reifenberger G, von Deimling A, editors. 4th edition. Lyon: IARC; 2016. pp. 237–240. [Google Scholar]
- 5.Rogers L. A spinal meningioma containing bone. British J Surg. 1928;15:675–677. [Google Scholar]
- 6.Freidberg SR. Removal of an ossified ventral thoracic meningioma. Case report. J Neurosurg. 1972;37:728–730. doi: 10.3171/jns.1972.37.6.0728. [DOI] [PubMed] [Google Scholar]
- 7.Uchida K, Nakajima H, Yayama T, Sato R, Kobayashi S, Mwaka ES, Imamura Y, Baba H. Immunohistochemical findings of multiple ossified en plaque meningiomas in the thoracic spine. J Clin Neurosci. 2009;16:1660–1662. doi: 10.1016/j.jocn.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Chotai SP, Mrak RE, Mutgi SA, Medhkour A. Ossification in an extra-intradural spinal meningioma-pathologic and surgical vistas. Spine J. 2013;13:e21–26. doi: 10.1016/j.spinee.2013.06.102. [DOI] [PubMed] [Google Scholar]
- 9.Prakash A, Mishra S, Tyagi R, Attri PC, Bhatnagar A, Kansal S. Thoracic psammomatous spinal meningioma with osseous metaplasia: a very rare case report. Asian J Neurosurg. 2017;12:270–272. doi: 10.4103/1793-5482.150222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Caro R, Giordano R, Parenti A, Zuccarello M. Osteomatous meningioma. Report of two cases. Acta Neurochir. 1982;60:313–317. doi: 10.1007/BF01406315. [DOI] [PubMed] [Google Scholar]
- 11.Terstegge K, Schörner W, Henkes H, Heye N, Hosten N, Lanksch WR. Hyperostosis in meningiomas: MR findings in patients with recurrent meningioma of the sphenoid wings. AJNR Am J Neuroradiol. 1994;15:555–560. [PMC free article] [PubMed] [Google Scholar]
- 12.Rao S, Nandeesh BN, Arivazhagan A, Moiyadi AV, Yasha TC. Psammomatoid juvenile ossifying fibroma: report of three cases with a review of literature. J Pediatr Neurosci. 2017;12:363–366. doi: 10.4103/jpn.JPN_78_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho ML, Eschbacher KL, Paolini MA, Raghunathan A. New insights into calcifying pseudoneoplasm of the neuraxis (CAPNON): a 20-year radiological-pathological study of 37 cases. Histopathology. 2020;76:1055–1069. doi: 10.1111/his.14066. [DOI] [PubMed] [Google Scholar]
- 14.Murakami T, Tanishima S, Takeda C, Kato S, Nagashima H. Ossified metaplastic spinal meningioma without psammomatous calcification: a case report. Yonago Acta Med. 2019;62:232–235. doi: 10.33160/yam.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim HY, Kim KM, Kim BK, Shim JK, Lee JH, Huh YM, Kim SH, Kim EH, Park EK, Shim KW, Chang JH, Kim DS, Kim SH, Hong YK, Lee SJ, Kang SG. Isolation of mesenchymal stem-like cells in meningioma specimens. Int J Oncol. 2013;43:1260–1268. doi: 10.3892/ijo.2013.2053. [DOI] [PubMed] [Google Scholar]
- 16.Kan C, Chen L, Hu Y, Ding N, Lu H, Li Y, Kessler JA, Kan L. Conserved sigdnaling pathways underlying heterotopic ossification. Bone. 2018;109:43–48. doi: 10.1016/j.bone.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


