Abstract
Tobacco and cannabis co-users (T+CUs) have poor cannabis cessation outcomes, but the mechanisms underlying this are not well understood. This laboratory study examined the effects of: (1) the partial nicotinic agonist, varenicline, on tobacco cessation among T+CUs, and (2) varenicline, alone, and when combined with the cannabinoid agonist nabilone, on cannabis withdrawal and a laboratory model of cannabis relapse. Non-treatment-seeking T+CUs were randomized to active-varenicline or placebo-varenicline, and completed a 15-day outpatient phase; varenicline was titrated to 1 mg BID during days 1–8, and participants were instructed to abstain from tobacco during days 9–15. Participants then moved inpatient for 16 days, where they continued their outpatient medication and tobacco abstinence. Inpatient testing included two, 8-day medication periods, where active-nabilone and placebo-nabilone were administered in counterbalanced order, and measures of acute cannabis effects (days 1–2), withdrawal (days 4–5), and ‘relapse’ (days 6–8) were collected. Participants in the active-varenicline group were more likely to achieve cotinine-verified tobacco abstinence during the outpatient period vs. placebo-varenicline group (46% vs. 24%, respectively), and also reported less mood disturbance and cigarette craving while inpatient. Active-nabilone attenuated cannabis withdrawal in both groups, but did not affect cannabis relapse. Regression analyses revealed that two tobacco-related variables, i. e., age of first cigarette use, and cigarette craving while inpatient, were independent predictors of cannabis relapse outcomes. Thus, varenicline holds promise in this population, as a tool to examine the effects of tobacco abstinence on cannabis use outcomes, and as a component of smoking cessation treatments targeting T+CUs.
Keywords: Tobacco, Cannabis, Pharmacotherapy, Relapse, Varenicline, Withdrawal
INTRODUCTION
Tobacco cigarette and cannabis co-use (T+CU) is increasingly common, and associated with a constellation of poor clinical outcomes. In the U.S., the prevalence of daily tobacco cigarette and cannabis has doubled since 2002 (Goodwin et al., 2018). Currently, almost 3 million persons aged 12 years or older are daily T+CUs, including about half of all daily cannabis users (Goodwin et al., 2018; Pacek et al., 2018). The developmental trajectories of tobacco and cannabis use are closely intertwined (Badiani et al., 2015) with shared risk factors (Chen et al., 2008; Brook et al., 2010), and pharmacological interactions (Hindocha et al., 2017; Panlilio et al., 2013; Penetar et al., 2005) driving high rates of co-use. Relative to cannabis-only users, T+CUs have higher rates of Cannabis Use Disorder (CUD), more CUD-related problems and other psychiatric co-morbidities (Diekrer et al., 2018; Peters et al., 2014), and lower rates of cannabis cessation (Moore and Budney et al., 2001; Gray et al., 2017).
Tobacco smoking is clearly a critical factor in cannabis use behaviors, including frequency of use, risk of developing CUD, and cannabis cessation attempt outcomes. Data from recent studies examining the mechanisms behind these associations provide growing support for causal hypotheses (Diekrer et al., 2018; Hindocha et al., 2015). Understanding the nature of interactions between cannabis and nicotine is essential to determining the best treatment approaches T+CUs. Is concurrent tobacco use best conceptualized as (1) a prognostic indicator of CUD treatment outcome, with utility as a treatment-matching variable, or as (2) an intervention target, whereby tobacco cessation may be a means to improve cannabis cessation outcomes.
Four recent pilot studies examined the feasibility of interventions targeting tobacco and cannabis cessation simultaneously (Becker et al., 2015; Hill et al., 2013; Lee et al., 2015; Adams et al., 2017). Most participants in these studies found the treatments acceptable, and there were some positive changes in tobacco use, but the efficacy of the interventions remains unclear. All four studies used non-randomized designs, had few participants achieve biochemically-verified tobacco abstinence (range = 0% - 28%), and none observed positive effects on cannabis use. The latter may have been due to withdrawal. Simultaneous cessation of tobacco cigarettes and cannabis produces more severe withdrawal symptoms than cessation of one substance alone (Vandrey et al., 2008), and greater withdrawal predicts worse cannabis cessation outcomes (Allsop et al., 2012; Budney et al., 2008; Haney et al., 2013a). While all these studies offered tobacco cessation pharmacotherapy, none utilized medication for cannabis withdrawal. Prior studies targeting other polysubstance-using populations demonstrate that combining medications shown to decrease self-administration, withdrawal, or craving for each substance independently are superior to monotherapies at reducing drug use (Kosten et al., 2003; Pettinati et al., 2008, Ray et al., 2014). To our knowledge, no prior studies have examined this approach among T+CUs.
Human laboratory studies are a fundamental component of addiction medication development research. Our group has been at the forefront in testing pharmacotherapies for CUD using a within-subjects, placebo-controlled, human laboratory model that examines medication effects on (1) cannabis withdrawal symptoms during a period of abstinence, and (2) a self-administration procedure that models cannabis relapse, by offering cannabis-abstinent participants repeated opportunities to purchase individual inhalations of cannabis using portions of their study earnings (see Balter et al., 2014 for review). Across all 10 drug classes examined using this model, oral synthetic cannabinoid agonists, administered alone, or in combination with other medications, have produced the strongest positive signal. Dronabinol (Haney et al., 2004), dronabinol+lofexidine (Haney et al., 2008), nabilone (Haney et al., 2013b), and nabilone+zolpidem (Herrmann et al., 2016) suppressed withdrawal-related disturbances in mood and sleep, and, in certain cases, reduced cannabis relapse. These findings provide strong support for testing cannabinoid agonists a component of combination pharmacotherapy approaches targeting T+CUs.
Despite substantial clinical comorbidity, relatively few controlled human laboratory studies have examined interactions between tobacco use and cannabis abstinence outcomes. Recently, our group performed a secondary analysis of data from five prior studies to examine predictors of cannabis relapse in our laboratory model (Haney et al., 2013a). Tobacco cigarette smoking status was the most robust predictor of cannabis relapse across all variables examined, with T+CUs relapsing on the first day of active cannabis availability at 4 times the rate of cannabis-only users (61% vs. 15%, respectively). These findings prompted a second study, summarized in the same report, that examined the effects of short-term tobacco abstinence on cannabis relapse among T+CUs. Cannabis relapse was assessed when participants were permitted to smoke tobacco cigarettes ad libitum, and when they were required to be tobacco/nicotine abstinent. The majority (≥87%) of participants relapsed to cannabis during both conditions, suggesting that neither acute nicotine exposure, nor cueing effects of tobacco cigarette smoking, increase cannabis relapse. Thus, further research is needed to develop strategies to improve treatment for T+CUs.
The aim of this double-blind, placebo-controlled, human laboratory study was to examine a combination pharmacotherapy approach for preventing cannabis relapse among T+CUs. Specifically, we tested whether (1) maintenance on the nicotinic receptor partial agonist varenicline (Chantix®) promotes tobacco cessation among T+CUs, and (2) if varenicline, administered alone, and in combination with nabilone (Cesamet®) reduces cannabis withdrawal and relapse to cannabis self-administration during combined tobacco and cannabis abstinence.
MATERIALS AND METHODS
Participant Recruitment, Screening, and Study Eligibility Requirements.
Otherwise healthy T+CUs co-users were recruited from the New York City area through media advertisements. Study eligibility was initially assessed via telephone screening, followed by in-person interviews and a medical examination. Inclusion criteria were: (1) 21–50 years of age, (2) smoking ≥ 4 tobacco cigarettes/day for the past 8 weeks, (3) using ≥ 2 cannabis cigarettes (or equivalent) per day, ≥ 6 days per week for the past 4 weeks, (4) having cannabis-positive urine drug tests, (5) being able to perform study procedures, and (6) non-pregnant, and practicing an effective form of birth control (women only). Primary exclusion criteria were: (1) meeting DSM-IV-TR criteria for an Axis I disorder requiring medical intervention, (2) use of other illicit drugs >1x/week, (3) problematic alcohol use, as determined using DSM-IV-TR criteria, and a subsequent clinical evaluation, (4) significant medical illness (e.g., diabetes, hypertension), (5) seeking treatment for cannabis or tobacco use, (6) using prescription medications daily, or (7) having allergies to study medications. All participants provided informed consent, and the New York State Psychiatric Institute (NYSPI) Institutional Review Board approved study procedures.
Laboratory Procedures
Outpatient Phase
Outpatient Visit Schedule.
Study flow is depicted in Fig. 1. Participants who provided consent were randomized, without stratification, to active- or placebo-varenicline. The NYSPI Research Pharmacy packaged medication in size 00 capsules, with riboflavin, and double-blind administration started on outpatient day 1. The purpose of the 15-day outpatient phase was for varenicline titration prior to inpatient measures. Participants completed 8 outpatient visits over these 15 days. At each laboratory visit, participants were administered that morning’s capsule, dispensed medication kits containing study capsules to be taken outside of the laboratory and monitored for medication adherence. Active-varenicline was titrated to 2 mg a day (1 mg, BID) over days 1–8, continued at this dose until the inpatient phase was completed, and then tapered during an outpatient follow-up period.
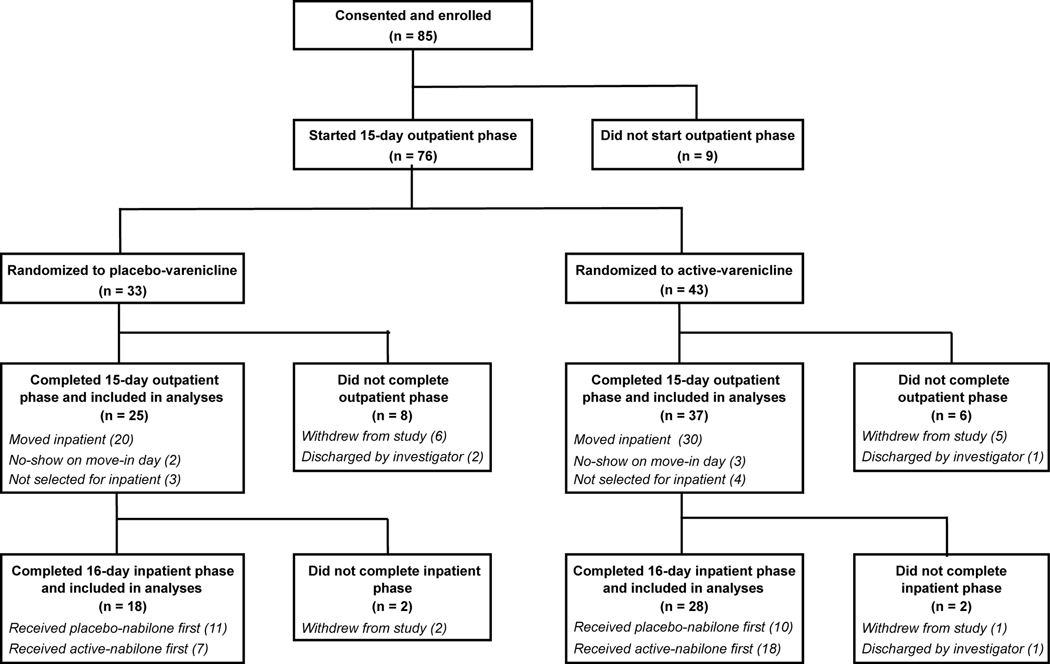
Figure 1.
Study design, participant randomization, and participant disposition. There were no significant differences in retention between active-varenicline vs. placebo-varenicline groups. Investigator-initiated study discharges were the result of participant noncompliance with study procedures. In instances when >4 participants in a cohort completed the outpatient phase, the investigators (blinded to medication assignment) selected n=4 for move in based on attendance reliability and adherence to outpatient study procedures, including tobacco cessation outcomes.
Tobacco Cessation and Abstinence Monitoring.
Participants in both groups were instructed to quit using tobacco on outpatient day 9, and to remain nicotine/tobacco abstinent for the remainder of the study. Breath carbon monoxide (CO) levels were tested approximately every other day during days 1–12 of the outpatient phase (6 CO tests in total). Urine cotinine was tested on days 14 and 15, and participants earned $50 cash incentives for each test indicating tobacco abstinence (cotinine ≤ 100 ng/ml). Participants were not instructed to change their cannabis use, per se, but were informed that smoking cannabis rolled in tobacco-containing wrappers (‘blunts’) may interfere with their ability to earn incentives for tobacco cessation. Participants also completed 3 sessions testing the subjective and cardiovascular effects of active (5.6% THC; ‘Dose A’) and 3 sessions of inactive (0% THC; ‘Dose B’) cannabis administration as a function of varenicline dose and tobacco smoking status (data to be reported separately). These sessions familiarized participants with the effects of each type of cannabis; self-administration testing was blind, i.e., participants were told whether ‘Dose A’ or ‘Dose B’ cannabis was available, but they were never told the THC content.
Inpatient Phase
Enrollment.
The inpatient phase was set in a residential laboratory with 4 individual bedrooms (see Haney et al., 1999 for detailed description). We intentionally over-enrolled during the outpatient phase to ensure each inpatient cohort would contain 3–4 participants. In instances where >4 participants completed the outpatient phase (see Fig. 1), the investigators (blinded to study medication assignment) selected 4 for move in based on factors that included attendance reliability and adherence to outpatient study procedures, including tobacco cessation outcomes.
The 16-day inpatient phase consisted of two 8-day medication periods. Participants were experimentally administered active cannabis on days 1–2 of each period, and received inpatient medications (active- and placebo-nabilone, in counterbalanced order) during days 3–8 of each period. Participants continued the outpatient medication dose (active or placebo varenicline) during the inpatient phase and were prohibited from using tobacco or nicotine-containing products. Compliance with inpatient abstinence requirements was ensured by staff search of participant’s belongings and 24-hour video monitoring. Participant vitals were measured daily.
Experimental Cannabis Administration.
During days 1–2 of each 8-day medication period, participants were administered 3 inhalations from active cannabis cigarettes (5.6% THC) 6 times each day (total = 18 inhalations per day) using a paced puffing procedure (Foltin et al., 1987). The purpose of experimental cannabis administration was to (1) standardize cannabis exposure prior to the onset of withdrawal measurements collected during active-nabilone and placebo-nabilone maintenance, and (2) to have a period of non-abstinence to use as a baseline for evaluating symptoms of cannabis withdrawal.
Inpatient Medication Administration.
During days 3–8 of each medication period, participants were administered either active-nabilone (4 mg, BID) or placebo-nabilone, along with their outpatient medication, at 0830 and 2230 each day.
Active Cannabis Abstinence.
During days 3–5, participants had the opportunity to self-administer individual 5-second inhalations from inactive cannabis cigarettes (0.0% THC; ‘Dose B’) by purchasing these inhalations using portions of their study earnings ($9 for the first inhalation of the day, and $2 for subsequent inhalations). Data collected during days 4–5 were used to evaluate medication effects on cannabis withdrawal; day 3 data were not used because mood-related cannabis withdrawal symptoms often take ~24 hours to onset (Haney et al., 2003).
‘Relapse’ to Cannabis Self-Administration.
During days 6–8, participants had the opportunity to self-administer inhalations from active cannabis cigarettes (5.6% THC; ‘Dose A’; $9 for the first inhalation of the day, and $2 for subsequent inhalations). These data were used to examine cannabis ‘relapse’ using two sets of analyses: We (1) examined the effects of study medications on the number of cannabis inhalations purchased during these 3-day relapse periods, and (2) performed multivariate logistic regression to identify predictors of purchasing at least one inhalation on the first day of each relapse period.
Move-Out and Medication Taper.
Participants were discharged from the inpatient phase the morning after the second 8-day medication period, followed by a short outpatient medication taper for varenicline. Nabilone was not tapered.
Measures
Participant Characteristics.
Demographic and substance use characteristics were assessed via self-report questionnaires and in-person interviews completed during study screening (see Table 1).
Table 1.
Demographic and substance use characteristics of participants that completed the outpatient phase (n=62), and also completed the inpatient phase (n=46), sorted by medication group.
| Completed Outpatient | Completed Inpatient | |||
|---|---|---|---|---|
| Characteristic | Placebo Varenicline (n = 25) | Active Varenicline (n = 37) | Placebo Varenicline (n = 18) | Active Varenicline (n = 28) |
| Demographics | ||||
| Age, in years | 32.6 (9.3) | 32.2 (6.0) | 31.4 (8.9) | 32.7 (6.6) |
| Sex (% male) | 80 | 84 | 78 | 86 |
| Race (% black) | 84 | 76 | 83 | 75 |
| Ethnicity (% hispanic) | 24 | 32 | 22 | 32 |
| Education, in years1 | 12.2 (1.5) | 11.8 (1.8) | 12.1 (1.6) | 11.8 (2.0) |
| Tobacco Use | ||||
| Cigarettes per day | 7.4 (3.0) | 9.3 (5.7) | 8.3 (3.0) | 9.4 (5.9) |
| Age of first cigarette | 16.0 (4.5) | 15.6 (5.0) | 16.3 (4.9) | 16.1 (5.5) |
| Age started smoking daily | 18.2 (3.6) | 18.2 (6.6) | 18.5 (3.9) | 18.5 (6.8) |
| FTND score2 | 4.2 (2.2) | 4.1 (2.5) | 4.4 (2.2) | 3.9 (2.5) |
| Breath CO (ppm)3 | 10.78 (5.7) | 15.8 (8.3) | 11.7 (5.6) | 15.0 (8.4) |
| Cannabis Use | ||||
| Days used per week | 6.9 (0.4) | 7.0 (0.0) | 6.9 (0.4) | 7.0 (0.0) |
| Joints per day | 11.7 (7.0) | 12.2 (7.4) | 13.5 (6.8) | 11.0 (6.4) |
| Age of first use | 15.3 (3.5) | 15.3 (5.0) | 15.7 (4.0) | 16 (5.4) |
| Years using regularly | 13.6 (8.5) | 14.1 (5.7) | 13.5 (7.8) | 13.8 (6.3) |
| Other Substance Use | ||||
| Alcohol use (past month, %) | 60 | 76 | 61 | 71 |
| Drinks per week4 | 3.4 (2.7) | 3.8 (3.6) | 3.0 (2.0) | 4.1 (4.0) |
| Cocaine use (past month, %) | 0 | 8 | 0 | 7 |
Note. Values are mean (standard deviation) unless otherwise specified. Characteristics were compared between medication groups using independent-samples t -tests (continuous variables) and Fisher's exact tests (categorical variables), and examined as predictors of study retention using logistic regression.
There were no significant differences between medication groups, nor did any of these variables predict study retention (p > 0.05).
12 years of education = high school diploma or equivalent.
Fagerström Test for Nicotine Dependence
Breath Carbon Monoxide (in parts per million)
Among those who reported alcohol use during the past month.
Outpatient Phase
Tobacco and Other Substance Use.
Participants completed a Timeline Followback Interview (Sobel and Sobel, 1992) at each visit, reporting on use of tobacco, other nicotine-containing products, cannabis, alcohol, other illicit drugs, and over-the-counter medications. Breath CO measures were used to examine changes in smoking over the outpatient phase as a function of medication condition. Urine samples collected on days 14 and 15 were tested for cotinine/hydroxycotinine using NicAlert® test strips (Nymox Pharmaceutical Corporation). A result of Level 2 (≤ 100ng/ml) or below was considered evidence of tobacco abstinence.
Adverse Events.
Open-ended, self-report Timeline Followback interviews were conducted at each visit to collect data on any physical symptoms, changes in mood, and sleep disturbances experienced by participants during the outpatient phase.
Inpatient Phase
Mood and Craving.
Participants completed 44-item computerized questionnaires eight times each day. They viewed mood, physical symptom, drug effect, and drug craving descriptors, and used a 100-mm visual analog scale (VAS) to rate the extent each descriptor reflected how they felt at that moment, ranging from ‘not at all’ (0 mm) to ‘extremely’ (100 mm).
Sleep.
Objective data were obtained using the wrist-worn Actiwatch® Activity Monitoring System (Respironics Company, Bend OR). Participants also completed a seven-item VAS questionnaire each morning to collect subjective ratings of sleep quality (e.g., ‘Fell Asleep Easily,’ ‘Woke Often,’ ‘Number of Hours Slept’).
‘Relapse’ to Cannabis Self-Administration.
We recorded the number of inhalations purchased each day active cannabis was available, i. e., days 6–8 of the active-nabilone and placebo-nabilone periods.
Data Analysis
Demographic and substance use characteristics.
The active- and placebo-varenicline groups were compared using independent-samples t-tests for continuous variables and Fisher’s exact tests for categorical variables. These comparisons were performed for all outpatient phase completers (n=62), and again among the subset that also completed inpatient (n=46). These characteristics were also examined as predictors of study retention using logistic regression.
Outpatient Phase
Tobacco Use.
Repeated Measures Analysis of Variance (RM-ANOVA) with planned contrasts, and a Generalized Estimating Equation (Ziegler and Vens, 2010) for repeated measures dichotomous data, were used to examine the effects of medication condition, time, and their interaction on breath CO and urine cotinine levels. Rates of self-reported tobacco abstinence on days 9–15 were universally high and had poor agreement with objective measures so were excluded from further analyses. Demographic and substance use characteristics were compared between participants who met vs. did not meet cotinine abstinence criteria using t-tests/Fisher’s exact tests.
Adverse Events.
Incidence rates of events reported by at least 5% of participants in each medication group were compared between groups using Fisher’s exact tests.
Inpatient Phase; Main Outcomes
Cannabis Intoxication and Withdrawal; Mood, Craving, and Sleep.
Based on cluster analyses conducted previously (Haney et al., 2010), we reduced 32 of the 44 mood scale items into six subscales: Miserable (‘miserable,’ ‘irritable’), Anxious (e.g., ‘anxious,’ ‘restless’), Bad effect (e.g., ‘depressed,’ ‘upset stomach’), Sedated (e.g., ‘tired,’ ‘sedated’), (5) Social (e.g., ‘friendly,’ ‘talkative’), and (6) High (‘high,’ ‘good effect’). Ratings of tobacco (‘I Want Cigarettes’) and cannabis (‘I Want Marijuana’) were examined separately, and daily peak ratings of mood and craving were used for analyses. Actiwatch® data were used to calculate total sleep time, sleep onset latency (time from lights out until sleep), and sleep efficiency (proportion of time asleep divided by total time in bed). These analyses were based on RM-ANOVA, with planned contrasts comparing (1) days 1–2 of placebo-nabilone between groups, to assess varenicline effects prior to cannabis abstinence, (2) days 1–2 vs. days 4–5 of placebo-nabilone, within each group separately, to assess cannabis withdrawal, and (3) between days 4–5 of placebo-nabilone vs. days 4–5 of active-nabilone, also within-groups, to assess the effects of nabilone on cannabis withdrawal.
‘Relapse’ to Cannabis Self-Administration.
The proportion of participants that purchased at least one inhalation of active cannabis was analyzed using Generalized Estimating Equations, based on negative binomial distributions, to model the effects of varenicline (between-groups), nabilone (within-subjects), and their interaction on total number of inhalations purchased on days 6–8.
Inpatient Phase; Predictor Analyses.
Our prior predictor analysis showed that tobacco smoking status was a robust predictor of cannabis relapse in this laboratory model (Haney et al., 2013a). Building directly on these findings, we used data from the present study to examine predictors of cannabis relapse among T+CUs. Since this study allocated participants to medication conditions (varenicline vs. placebo, nabilone dose order) that could influence relapse outcomes, preliminary analyses of these factors were conducted to inform variable definitions and model selection for predictor analyses.
Defining ‘relapse’ for predictor analyses.
Consistent with our prior findings (Haney et al., 2013a), the majority (~75%) of participants who relapsed during each medication period did so on the first day. Rates of first-day relapse did not differ between active vs. placebo-nabilone periods, and these outcomes were not strongly correlated (Phi Coefficient = 0.39). Thus, we defined relapse in the same manner as in our prior predictors analysis, i.e., purchasing at least one inhalation on the first day of active cannabis availability, and analyzed relapse outcomes independently.
Relations between study allocation and relapse.
Relapse outcomes did not differ as a function of varenicline group, nabilone dose order, or their interaction. Thus, they were not explicitly controlled for as covariates but were included among variables examined as potential predictors of relapse (see Table 2). These variables included: 1) baseline demographic, tobacco, and cannabis use characteristics, 2) measures of tobacco and cannabis use during the outpatient period, 3) ratings of positive drug effects (‘High’) during experimental cannabis administration (days 1–2), and 4) mood, craving, and sleep outcomes during the abstinence period (days 4–5) preceding each relapse assessment, respectively.
Table 2.
Variables included in regression analyses examining predictors of cannabis relapse during placebo-nabilone and active-nabilone medication periods.
| Demographics | Outpatient Phase |
| Age (years) | Varenicline condition |
| Sex (% male) | Mean cigarettes per day (days 1–8) |
| Race (% black) | Mean breath CO (days 9–15) |
| Ethnicity (% hispanic) | Urine cotinine ≤ 100ng/ml (day 15) |
| Education (years) | Mean joints per day (days 1–8, days 9–15) |
| Tobacco + Cannabis Use | Inpatient Phase |
| Tobacco cigarettes per day | Varenicline condition |
| Age of first cigarette use | Nabilone dose order |
| Age started smoking daily | Ratings of ‘High’ (days 1–2) |
| FTND score | Ratings of ‘Miserable,’ ‘Anxious’ (days 4–5) |
| Cannabis joints per day | ‘I Want Cigarettes,’…‘Marijuana’ (days 4–5) |
| Age of first cannabis use | Mean sleep latency (days 4–5) |
| Years using cannabis regularly | Mean sleep efficiency score (days 4–5) |
Note; For the outpatient phase, days 1–8 = varenicline titration period, and 9–15 = attempted tobacco abstinence. For the inpatient phase, days 1–2 = experimental cannabis adminstration, and days 4–5 = cannabis withdrawal (active-nabilone and placebo-nabilone periods, separately). Inpatient phase variables examined as relapse predictors were calculated using data from that period only.
Regression Model.
We used stepwise logistic regression with backward elimination to identify predictors for each relapse outcome. This method starts with all potential predictors in the model; one-by-one, the least significant predictor is removed, and the model refit, until only variables that significantly improve the model fit remain. This method allows us to identify the best set of predictors for each relapse outcome, while accounting for covariance among predictors (Solomon et al., 2007). Significance threshold for variable removal was set at α = 0.10, and derived models were compared to intercept-only models based on likelihood-ratio chi square tests and a significance level of α = .05.
RESULTS
Participant Characteristics.
Participant enrollment, medication condition allocation, and disposition are displayed in Fig. 1. Demographic and substance use characteristics are displayed in Table 1. Active- and placebo-varenicline groups did not differ on any of these characteristics, nor did any characteristics predict study retention. The difference in sample size between medication groups was the result of outpatient dropout or discontinuation.
Outpatient Phase
Tobacco Smoking Cessation Outcomes.
RM-ANOVA indicated breath CO levels decreased over time in the active-varenicline group (p < 0.001), but not in the placebo-varenicline group. The overall means (± standard error of the mean) of CO readings collected pre-quit (days 1–8) vs. post-quit date (days 9–15) were 10.7 (±0.8) vs. 6.2 (0.6) for the active-varenicline group, and 9.2 (± 1.0) vs. 7.6 (± 1.0) for the placebo-varenicline group. A Generalized Estimating Equation indicated that the percentage of participants providing urine samples indicative of tobacco abstinence increased from day 14 to day 15 in the active-varenicline group (from 24% to 46%; p = 0.001) but not in the placebo-varenicline group (from 32% to 24%). Tobacco abstinent vs. non-abstinent participants did not differ on any demographic or substance use history characteristics.
Adverse Events.
The most commonly reported events were gastrointestinal upset (47% in the active-varenicline group vs. 44% in the placebo-varenicline group), drowsiness (33% vs. 28%), sleep difficulty/strange dreams (19% vs. 28%), mood disturbances (8% vs. 36%), headache (17% vs. 8%), flatulence (8% vs. 16%), and appetite change (8% vs. 16%). The only significant difference was for mood disturbance, with a higher incidence in the placebo-varenicline group (p = 0.01).
Inpatient Phase; Main Outcomes
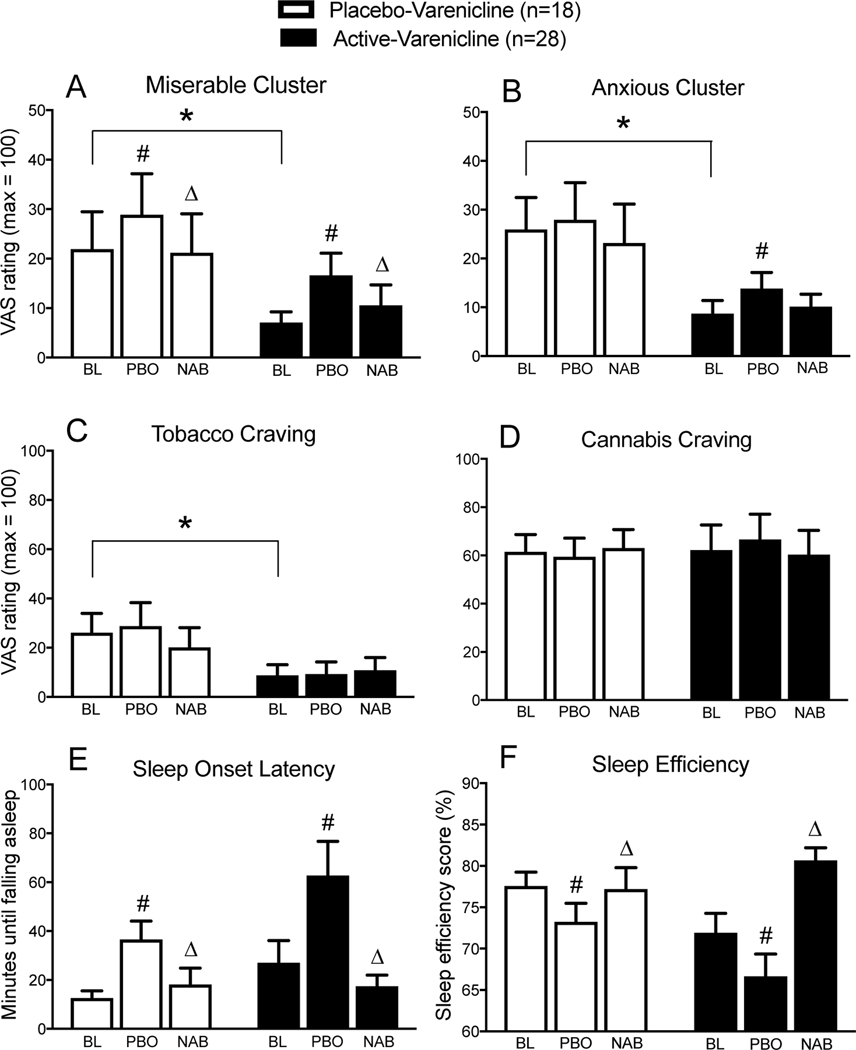
Mean peak ratings on ‘Miserable’ and ‘Anxious’ clusters, ‘I Want Cigarettes,’ ‘I Want Marijuana’ items, and objective measures of sleep onset latency and sleep efficiency are displayed in Fig. 2. Results for other variables are described, but not depicted.
Figure 2.
Mean (standard error of the mean) Visual Analog Scale (‘VAS’) ratings on ‘Anxious’ (A) and ‘Miserable’ (B) clusters, ratings of tobacco craving (‘I Want Cigarettes’; C), cannabis craving (‘I Want Marijuana,’; D), and mean (± standard error of the mean) sleep onset latency (E) and sleep efficiency (F) scores during baseline experimental cannabis administration (days 1–2; ‘BL’), during cannabis withdrawal on placebo-nabilone (days 4–5; ‘PBO’), and during cannabis withdrawal on active-nabilone (days 4–5; ‘NAB’) sorted as a function of varenicline group. ‘*’ indicate significant differences between placebo-varenicline vs. active-varenicline groups during days 1–2; ‘#’ indicate differences between days 1–2 vs. days 4–5 on placebo-nabilone, and ‘Δ’ indicate differences between placebo-nabilone vs. active-nabilone during days 4–5. Results are based on repeated-measures analysis of variance, with planned contrasts, and a significance threshold of p = 0.05
Experimental Cannabis Administration.
RM-ANOVA planned contrasts indicated that participants in the active-varenicline group had significantly lower ratings of ‘Miserable,’ Anxious,’ and ‘I Want Cigarettes’ vs. the placebo-varenicline group during days 1–2 of the placebo-nabilone period (p < 0.05). Ratings reflective of cannabis drug effect strength (e.g., ‘High’) did not differ between groups.
Cannabis Abstinence on Placebo-Nabilone.
Relative to baseline, cannabis abstinence under placebo-nabilone conditions was associated with increased ratings of ‘Miserable,’ and ‘Irritable,’ in both groups (p < 0.05), and increases in ‘Anxious’ and ‘Bad’ in the active-varenicline group (p < 0.05). Ratings of ‘High’ decreased in both groups (p < 0.05). Abstinence was also associated with increased sleep latency, decreased sleep efficiency, and decreased ratings of ‘Fell Asleep Easily,’ in both groups (p < 0.05); ratings of ‘Woke Often’ also increased in the active-varenicline group only (p < 0.05).
Nabilone Attenuation of Cannabis Withdrawal.
Active-nabilone significantly attenuated all withdrawal-related changes in mood and sleep observed during the placebo-nabilone period in both groups (p < 0.05), except for increases in ratings of ‘Anxious’ in the active-varenicline group. Active-nabilone also produced minor, but statistically significant increases in ratings of ‘High’ in both groups, relative to placebo (p < 0.05), and increased ratings of ‘Sedated’ in the active-varenicline group (p < 0.05).
Cannabis ‘Relapse’.
There were no significant effects of varenicline or nabilone on the number of active cannabis inhalations purchased. Across active- and placebo-varenicline groups, 54% of participants purchased ≥ 1 inhalation of active cannabis during the three-day relapse period while on placebo-nabilone, and 51% did so while on active-nabilone. Participants who relapsed in the placebo-nabilone condition purchased an average of 5.4 (± 3.7) inhalations/day; those that relapsed on active-nabilone purchased 4.0 (± 3.2) inhalations/day.
Inpatient Phase: Examining predictors of Cannabis ‘Relapse’
Overall Rates of First-Day Relapse.
Data on several variables of interest (e.g., Age of First Tobacco Cigarette) were not collected from the first five inpatient completers, resulting in n = 41 for predictor analyses. Overall, 39% of participants relapsed on the first day of active cannabis availability during both the placebo-nabilone and active-nabilone periods. Relapse rates did not differ between active-varenicline vs. placebo-varenicline groups during either placebo-nabilone (38% vs. 40%) or active-nabilone (44% vs. 36%) periods. Relapse rates also did not differ between first vs. second inpatient periods, overall (44% vs. 34%, respectively), or as a function of dose order during placebo-nabilone (44% vs. 35%) or active-nabilone (43% vs. 33%) maintenance. These factors were treated as like other candidate predictor variables, allowing us to test for effects on relapse that are only apparent when other variables are in the model (i.e., suppressor effects).
Regression Results.
Relapse on Placebo-Nabilone.
Regression analysis indicated that Age of First Tobacco Cigarette was the only variable that accounted for independent variance in relapse during the placebo-nabilone period. For every one-year increase in Age of First Tobacco Cigarette , there was a 19% decrease in the odds of relapse (OR = 0.81, C.I = 0.68–0.98, p = 0.03). Splitting the sample according to the median Age of First Tobacco Cigarette (<16 vs. ≥16 years old) revealed a robust difference in the odds of relapse between these two groups (54% vs. 21%, respectively).
Relapse on Active-Nabilone.
Stepwise logistic regression identified two-factor predictive model for relapse during the active-nabilone period; Age of First Tobacco Cigarette , and visual analog scale ratings of ‘I Want Cigarettes’ during the preceding withdrawal phase predicted independent variance in the odds of cannabis relapse χ2 = 10.41, p = 0.005). Nearly identical to placebo-nabilone, every one-year increase in the Age of First Tobacco Cigarette reduced odds of relapse by 18% (OR = 0.82, C.I = 0.68–1.00, p = 0.05). For every 1 mm increase in VAS ratings of ‘I Want Cigarettes’ during the preceding withdrawal phase, the odds of relapse increased by ~3% (OR = 1.028, CI = 1.002–1.054, p = 0.03).
DISCUSSION
This report summarizes the first study examining the effects of varenicline, alone, and in combination with nabilone, on tobacco cessation, tobacco and cannabis withdrawal, and a laboratory measure of cannabis relapse among daily T+CUs. There are three major sets of findings. First, varenicline was well-tolerated, increased outpatient tobacco cessation, and reduced negative mood and cigarette craving during controlled, inpatient tobacco abstinence. Second, nabilone attenuated cannabis withdrawal in both active- and placebo-varenicline groups, but did not alter cannabis relapse in either group. Third, results of predictor analyses indicated that behavioral markers of tobacco use severity were independent predictors of cannabis relapse among T+CUs, extending our prior findings on this topic (Haney et al., 2013a).
The present results demonstrate that varenicline (1 mg BID), combined with financial incentives ($50 per cotinine-negative urine sample) can produce high rates of experimental tobacco abstinence among T+CUs. Participants on active-varenicline had significant reductions in breath CO after the outpatient quit date, and almost half provided urine samples indicative of tobacco abstinence. The active-varenicline group also reported lower levels of negative mood and cigarette craving during the inpatient phase. These differences were likely a function of varenicline’s effects on nicotine withdrawal. Varenicline was well-tolerated, with no self-reported adverse effects exceeding placebo. Overall, these data indicate that varenicline may be effective at promoting tobacco cessation among T+CUs, and suggest further research examining varenicline as a smoking cessation treatment among T+CUs is warranted.
Both active- and placebo-varenicline groups showed disruptions in mood and sleep during cannabis abstinence. Nabilone attenuated almost all of these disruptions in both groups, making this the third consecutive study supporting nabilone’s efficacy in this regard (Haney et al., 2013b; Herrmann et al. 2016). However, contrary to our prior studies, nabilone did not reduce relapse to cannabis self-administration. This may represent a floor effect; the proportion of participants relapsing on placebo-nabilone, and the mean number of inhalations purchased among those that relapsed, were both ~30% lower than those observed among T+CUs in our prior studies (Haney et al., 2013a). Although not tested experimentally, we hypothesize that the longer durations of tobacco abstinence observed here may have produced reductions in cannabis self-administration that interfered with our ability to detect effects of nabilone on relapse.
In a prior report, we examined predictors of cannabis relapse in the human laboratory using data from five prior inpatient laboratory studies. The analyses revealed that cigarette smoking status was the most robust predictor of cannabis relapse among all variables examined (Haney et al., 2013a). The present study extended these findings by conducting predictor analyses among a sample composed entirely of T+CUs and examining tobacco-related variables not assessed previously. These analyses demonstrated that Age of First Tobacco Cigarette predicted cannabis relapse during placebo-nabilone maintenance, and that Age of First Tobacco Cigarette and peak ratings of ‘I Want Cigarettes’ during cannabis withdrawal were independent predictors of relapse during active-nabilone maintenance. These results extend our prior findings by suggesting that the association between tobacco use and cannabis relapse extend beyond what is captured by a dichotomous measure of tobacco smoking status. Interestingly, while tobacco use severity variables predicted cannabis relapse across varenicline and nabilone treatment conditions, withdrawal-related variables did not relate to either cannabis relapse outcome. This suggests that factors other than withdrawal are mediating relations between tobacco smoking and cannabis relapse.
The results of our predictor analyses closely mirror findings from the tobacco cessation literature, as prior studies have demonstrated that younger age of 1st tobacco cigarette (Breslau and Peterson, 1996; Khuder et al., 1999) and greater cigarette craving during early abstinence (Allen et al., 2008; Killen and Fortmann, 1997) are predictive of poor tobacco cessation outcomes. We hypothesize two potential explanations for our findings. First, age of 1st tobacco cigarette and craving levels during abstinence are proxy variables indicating underlying vulnerability to poor cessation outcomes across both substances. Second, tobacco abstinence may reduce cannabis relapse in a matter moderated by these variables. The differences in cannabis relapse between early vs. late-onset cigarette smokers in the present study (21% for ≥16 years old vs. 55% for <16 years old) resemble those observed among non-cigarette smokers vs. non-abstinent cigarette smokers with our prior analyses (15% vs. 61%; Haney et al., 2013a). We hypothesize that the durations of tobacco abstinence tested here may have been sufficient to reduce cannabis relapse in later-onset, but not earlier-onset, tobacco smokers, but this hypothesis needs to be tested empirically.
The results of this study should be considered in the context of three important limitations. First, participants were non-treatment-seekers, and data were collected in a controlled residential environment, using procedures developed to model (not mimic) cannabis relapse. We are unable to evaluate the model’s validity in predicting relapse in a clinical context in lieu of clinically-efficacious pharmacotherapies for CUD. However, several outpatient treatment studies evaluated medications that were also tested using this model, and results were largely consistent across laboratory and clinic, so the laboratory mirrors negative effects seen in outpatient clinical trials (see Brezing and Levin, 2018). Second, the utility of using breath CO for monitoring initial tobacco abstinence among T+CUs has not been previously established. Since both tobacco and cannabis smoking elevate CO levels, interpretation of CO results in the context of ongoing cannabis use may be problematic. Third, predictor analyses were not components of the initial study design; the results should be interpreted as preliminary. That said, the observed relations between Age of First Tobacco Cigarette and early relapse to cannabis self-administration were robust in magnitude and uniform across medication conditions. Considering these results in the context of their consistency with our prior findings (Haney et al., 2013a), and findings in the tobacco cessation literature, suggests that these results are unlikely to be spurious.
In summary, these data indicate that both varenicline and nabilone hold promise as pharmacotherapies for T+CU. Varenicline was well-tolerated, improved outpatient tobacco cessation rates, and reduced negative mood and cigarette craving inpatient. Taken together with findings from other recent studies demonstrating that varenicline is a highly effective among other co-morbid subgroups of smokers (Anthenelli et al., 2016; O’Malley et al., 2018), these results demonstrate that varenicline is a promising medication for tobacco cessation among T+CUs. Nabilone robustly attenuated cannabis withdrawal-related changes in mood and sleep, was well-tolerated, produced few side effects, and appears to have lower abuse liability than smoked cannabis. Although neither medication reduced cannabis relapse, per se, the orderly relations between behavioral markers of tobacco use severity and cannabis relapse outcomes observed here are compelling. Overall, results provide strong rationale for additional research on the effects of extended tobacco cessation on CUD; This avenue may lead to the development of effective cessation treatments for T+CU, a large population with poor health outcomes.
ACKNOWLEDGEMENTS
Funding and conflict of interest disclosure: This project was funded by NIDA R01DA031005 and Dr. Herrmann received salary support from NIDA T32DA007294. Dr. Haney’s research is funded by NIDA. She and Dr. Cooper have received partial salary support for an investigator-initiated study from Insys Therapeutics Inc., which did not conflict with this study. Dr. Comer has received partial salary support from studies supported by Braeburn Pharmaceuticals, Cerecor, Indivior, MediciNova, and Reckitt-Benckiser Pharmaceuticals. In addition, Dr. Comer has served (or will serve) as a consultant to the following companies/organizations over the past 3 years: Advances in Pain Management, AstraZeneca, Clinilabs, Collegium Pharmaceutical, Daiichi Sankyo, Depomed, Egalet, Endo, the Food and Drug Administration, Guidepoint Global, Heptares Therapeutics Limited, Inspirion Delivery Sciences, IntelliPharmaCeutics, Janssen, KemPharm, Mallinckrodt, National Institutes of Health, Neuromed, Opiant, Orexo, Pfizer, Shire, and the World Health Organization. None of these responsibilities conflicted with this study. The other authors have no potential conflicts of interest.
REFERENCES
- Adams TR, Arnsten JH, Ning Y, Nahvi S (2017) Feasibility and Preliminary Effectiveness of Varenicline for Treating Co-Occurring Cannabis and Tobacco Use. J Psychoactive Drugs, 50: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J et al. (2012). Quantifying the clinical significance of cannabis withdrawal. PloS one 7: e44864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D et al. (2016). Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. The Lancet 387: 2507–2520. [DOI] [PubMed] [Google Scholar]
- Badiani A, Boden JM., De Pirro S, Fergusson DM, Horwood LJ, Harold GT (2015). Tobacco smoking and cannabis use in a longitudinal birth cohort: evidence of reciprocal causal relationships. Drug Alcohol Depend 150, 69–76. [DOI] [PubMed] [Google Scholar]
- Balter RE, Cooper ZD, Haney M (2014): Novel pharmacologic approaches to treating cannabis use disorder. Cur Addict Rep 1: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Haug S, Kraemer T, Schaub MP (2015). Feasibility of a group cessation program for co-smokers of cannabis and tobacco. Drug Alcohol Rev 34: 418–426 [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL (1996). Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health 86: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezing CA, Levin FR (2018). The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology 43: 173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Lee JY, Finch SJ, Brown EN (2010). Course of comorbidity of tobacco and marijuana use: psychosocial risk factors. Nicotine Tob Res 12: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z (2008). Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat 35: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS (2008). Cannabinoid receptor 1 gene association with nicotine dependence. Arch Gen Psychiatry 65: 816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L, Braymiller J, Rose J, Goodwin R, Selya A. (2018). Nicotine dependence predicts cannabis use disorder symptoms among adolescents and young adults. Drug Alcohol Depend, 187, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD (1987). Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav 28: 459–464. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Pacek LR, Copeland J, Moeller SJ, Dierker L, Weinberger A. et al. (2018). Trends in Daily Cannabis Use Among Cigarette Smokers: United States, 2002–2014. Am J Public Health 108: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Sonne SC, McClure EA, Ghitza UE, Matthews AG, McRae-Clark AL et al. (2017). A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend, 177: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, Foltin RW. (2013a). Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol Psychiatry 73: 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW (2013b). Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology 38: 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW (2008). Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology 197: 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW (2004). Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology, 29: 158–170. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Ward AS, Foltin RW. (2003). Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology, 165(2), 157–165. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW (1999). Abstinence symptoms following oral THC administration to humans. Psychopharmacology 141: 385–394 [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD et al. (2016). Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology, 233: 2469–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Xia JX, Shaban NC, Curran HV (2017). Acute memory and psychotomimetic effects of cannabis and tobacco both ‘joint’ and individually: a placebo-controlled trial. Psychological Med, 47: 2708–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Shaban ND, Freeman TP, Das RK, Gale G, Schafer G et al. (2015). Associations between cigarette smoking and cannabis dependence: a longitudinal study of young cannabis users in the United Kingdom. Drug Alcohol Depend, 148, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KP, Toto LH, Lukas SE, Weiss RD, Trksak GH, Rodolico JM, Greenfield SA (2013). Cognitive Behavioral Therapy and the Nicotine Transdermal Patch for Dual Nicotine and Cannabis Dependence: A Pilot Study. Am J Addict 22: 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuder SA, Dayal HH, Mutgi AB (1999). Age at smoking onset and its effect on smoking cessation. Addict Behav 24: 673–677. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP (1997). Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol 5: 137–142. [DOI] [PubMed] [Google Scholar]
- Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E et al. (2003). Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend 70: 315–325. [DOI] [PubMed] [Google Scholar]
- Lee DC, Budney AJ, Brunette MF, Hughes JR, Etter JF, Stanger C (2015). Outcomes from a computer-assisted intervention simultaneously targeting cannabis and tobacco use. Drug Alcohol Depend 155: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Budney AJ (2001). Tobacco smoking in marijuana-dependent outpatients. J Subst Abuse 13: 583–596. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Zweben A, Fucito LM, Wu R, Piepmeier ME, Ockert DM et al. (2018). Effect of varenicline combined with medical management on alcohol use disorder with comorbid cigarette smoking: a randomized clinical trial. JAMA Psychiatry 75: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Copeland J, Dierker L, Cunningham CO, Martins SS, Goodwin RD. (2018) Among whom is cigarette smoking declining in the United States? The impact of cannabis use status, 2002–2015. Drug Alcohol Depend. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Zanettini C, Barnes C, Solinas M, Goldberg SR (2013). Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacology, 38: 1198–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, Lukas SA (2005). Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend 79: 211–223. [DOI] [PubMed] [Google Scholar]
- Peters EN, Schwartz RP, Wang S, O’Grady KE, Blanco C (2014). Psychiatric, psychosocial, and physical health correlates of co-occurring cannabis use disorders and nicotine dependence. Drug Alcohol Depend 134: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Xie H, Dackis C, Rabinowitz AR, Charles PO (2008). A double blind, placebo-controlled trial that combines disulfiram and naltrexone for treating co-occurring cocaine and alcohol dependence. Addict Behav 33: 651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED (2014). Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: human laboratory findings. Psychopharmacology 231: 3843–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992). Timeline follow-back: a technique for assessing self-reported alcohol consumption. Measuring Alcohol Consumption Eds.: Litten R. and Allen J. 01992 The Humane Press Inc. [Google Scholar]
- Solomon LJ, Higgins ST, Heil SH, Badger GJ, Thomas CS, Bernstein IM (2007). Predictors of postpartum relapse to smoking. Drug Alcohol Depend 90: 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A (2008). A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend 92: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A, Vens M (2010). Generalized estimating equations. Methods Inf Med 49: 421–425. [DOI] [PubMed] [Google Scholar]