Abstract
Background and Aim:
Babesia species are tick-borne protozoan parasites of apicomplexan type which infect the erythrocytes of dogs it ranges from subclinical to severe cases, depending on different factors such as immune status, age, and presence of other co-infections with the Babesia species. Hence, this study aimed to identify the protozoan parasites infecting police dogs of different breeds, ages, and both sexes in Egypt. Concerning molecular detection of Babesia vogeli using conventional polymerase chain reaction sequencing and phylogenetic analysis, followed by the assessment of immunological and biochemical status of infected dogs.
Materials and Methods:
The blood of 242 police K9 dogs was collected. The age, breed, sex, and health status with clinical signs of dogs were recorded. Hematological, biochemical, and oxidative stress analyses of the blood were performed together with gene expression analysis using two genes (gamma interferon [IFN-γ] and tumor necrosis factor-alpha [TNF-α]). The identification of the causative agent was performed using molecular analysis of the 18S ribosomal RNA (rRNA). The 18S rRNA region of canine Babesia spp. was successfully amplified, and sequencing data were deposited in GenBank (accession number: MT565474.1), which resembled those of B. vogeli.
Results:
The results of blood samples screening revealed that of the 242 blood samples, 62 were positive for B. vogeli infection. The infection rate in male dogs was higher than that in female dogs. The police dogs were classified into the following three groups of dogs: (1st group) healthy, (2nd infected with B. vogeli, and mixed infection of B. vogeli and Ehrlichia canis). The oxidative stress biomarkers levels in B. vogeli infected dogs were greater than that of healthy dogs. Likewise, IFN-γ and TNF-α level in B. vogeli infected dogs were elevated in infected dogs.
Conclusion:
Our findings demonstrated that B. vogeli had completely adverse effects on the health condition of the police dogs that may lead to death in some dogs.
Keywords: Babesia vogeli, Egypt, Ehrlichia canis, police dogs, tumor necrosis factor-alpha
Introduction
Dogs have several roles, for example, being a guard dog and in explosive and narcotic detection. These dogs may have several diseases such as bacterial, viral, and parasitic infections. Tick infection is one of the most common diseases in dogs; there are several protozoan parasites that infect these dogs, including Babesia canis, Hepatozoon canis, and Ehrlichia canis [1]. Babesiosis and ehrlichiosis are the most common, especially in summer. The infected dogs experience thrombocytopenia that characterizing babesiosis and hyperglobulinemia that characterizing ehrlichiosis [2,3]. The clinical signs of babesiosis in dogs include fever, hemolysis of red blood cells with anemia, hyperbilirubinemia, hemoglobinuria, jaundice, and organ failure [4,5] B. canis are tick-borne protozoan parasites of apicomplexan type that infect the erythrocytes of dogs [6-8]. Babesiosis ranges from subclinical to severe cases, depending on different factors such as immune status, age, and presence of other co-infections with the Babesia spp. [9,10]. The diagnosis of babesiosis depends on the microscopic detection of the intraerythrocytic piroplasms in peripheral blood smears [10,11].
The significance of this study as mentioned in a recent study by Badawi and Yousif [12] provides the molecular record and phylogenetic analysis of B. canis in dogs in Iraq, and it will be valuable to confirm clinical signs and study epidemiological risk factors of babesiosis in dogs. However, various species from the genus Babesia (B. canis, Babesia vogeli, and Babesia rossi) cannot be differentiated through microscopic examination of blood films, despite differences in their genetics, pathology, and vector associations [13].
Therefore, this study aimed to identify the protozoan parasites infecting police dogs of different breeds, ages, and both sexes in Egypt by interrupting the molecular detection of B. vogeli using conventional polymerase chain reaction (PCR) sequencing and phylogenetic analyses, followed by the assessment of immunological and biochemical status of infected dogs.
Materials and Methods
Ethical approval
Institutional and National guidelines for the care and use of animals were followed as directed by Faculty of Veterinary Medicine, Cairo University.
Study period and location
The study was conducted from August 2017 to December 2019. Samples were collected at Veterinary Health Care Unit at K9-Department of Police Academy and processed at Biotechnology Laboratory, Cairo University Research Park.
Sample collection
A total of 242 blood samples were collected from police dogs at (K9-department) in police Academy located in the first settlement in Cairo, Egypt. The survey was conducted along various seasons, from August (Summer), October (Autumn), December and February (Winter), as well as April and June (Spring). The ages of the dogs ranged between 2.5 and 12 years. General inspection indicated that 180 police dogs were healthy without visible clinical signs. However, 62 dogs exhibited clinical manifestations such as fever, emaciation, anemia, nostril and anal bleeding, edema, jaundice, and bloody urine. Many ways of control were used to eradicate and prevent further infection and infestation of ticks and protozoal parasites using tick repellents and antiprotozoal drugs. The detailed information, such as sex, breed, age, and health status of these dogs as well as season, is presented in Table-1. Across all samples, the percentage of female dogs was 16.12% (39 of 242), whereas the percentage of male dogs was 83.88% (203 of 242).
Table-1.
Prevalence of Babesia in Police dogs from Egypt (n=242).
| Variables | No. of tested dogs (%) (n=242) | Babesia infected dogs (n=62/242) | Mixed infection with Ehrlichia (n=28/62) | ||
|---|---|---|---|---|---|
| No. (%) | CI 95% | No. (%) | CI 95% | ||
| Breeds: | |||||
| German shepherd | 182 (75.21) | 51 (28.02) | 21.5-34.6 | 24 (47.06) | 33.4-60.8 |
| Malinois | 52 (21.49) | 10 (19.23) | 8.5-29.9 | 4 (40.00) | 9.6-70.4 |
| Dutch Shepherd | 7 (2.89) | 0.0 | 0.0 | 0.0 | 0.0 |
| Belgian shepherd | 1 (0.41) | 1 (100) | 100 | 0.0 | 0.0 |
| p-value | 0.071 | 0.858 | |||
| Gender: | |||||
| Female | 39 (16.12) | 8 (20.51) | 7.8-33.2 | 4 (50.00) | 15.4-84.7 |
| Male | 203 (83.88) | 54 (26.60) | 20.5-32.7 | 24 (44.44) | 31.2-57.7 |
| p-value | 0.425 | 1.000 | |||
| Intensity of tick infection: | |||||
| No | 108 (44.63) | 0.0 | 0.0 | 0.0 | 0.0 |
| Mild | 37 (15.29) | 0.0 | 0.0 | 0.0 | 0.0 |
| Moderate | 53 (21.90) | 24 (45.28)b | 31.9-58.7 | 10 (41.67) | 21.9-61.4 |
| Severe | 44 (18.18) | 38 (86.36)a | 76.2-96.5 | 18 (47.37) | 31.5-63.2 |
| p-value | < 0.0001 | 0.660 | |||
| Age (years): | |||||
| ≤3 | 62 (25.62) | 9 (14.52) | 0.0-32.2 | 1 (11.11)b | 0.0-31.6 |
| 4-6 | 86 (35.54) | 26 (30.23) | 20.5-39.9 | 12 (46.15)ab | 26.9-65.3 |
| 7-9 | 83 (34.30) | 25 (30.12) | 20.3-39.9 | 15 (60.00)a | 40.8-79.2 |
| ≥10 | 11 (4.55) | 2 (18.18) | 0.0-40.9 | 0.0 | 0.0 |
| p-value | 0.103 | 0.035 | |||
| Health status: | |||||
| Normal | 180 (66.12) | 0.0b | 0.0 | 0.0 | 0.0 |
| Clinical signs | 62 (33.88) | 62 (75.61)a | 66.3-84.9 | 27 (43.55) | 31.2-55.9 |
| P-value | <0.0001 | ||||
| Status: | |||||
| Live | 182 (75.21) | 9 (3.72)b | 1.8-8.1 | 1 (11.11) | 0.0-31.6 |
| Died | 60 (24.79) | 53 (88.33)a | 80.2-96.5 | 26 (49.06) | 35.6-62.5 |
| p-value | <0.0001 | 0.065 | |||
| Season: | |||||
| Autumn | 37 (15.29) | 21 (56.76)a | 40.8-72.7 | 11 (52.38) | 31.0-73.7 |
| Winter | 72 (29.75) | 26 (36.11)b | 25.0-47.2 | 14 (53.85) | 34.7-73.0 |
| Spring | 41 (16.94) | 6 (14.63)c | 3.8-25.5 | 1 (16.67) | 0.0-61.7 |
| Summer | 92 (38.02) | 9 (9.78) c | 3.7-15.9 | 1 (11.11) | 0.0-31.6 |
| p-value | <0.0001 | 0.060 | |||
a,b,c Different superscripts within the same column indicate significance at P<0.05; CI 95%=Confidence interval 95%
Collection of blood samples
Blood samples were collected from the cephalic vein of 242 police dogs; each sample was divided into two parts: One with ethylenediaminetetraacetic acid (EDTA) as an anticoagulant for preparing the examination of blood films and DNA extraction and the second one without an anticoagulant for serum separation for biochemical analysis.
Parasitological examination
The blood smears were placed on a clean slide, air dried, fixed in absolute methyl alcohol for 10 min, and Giemsa stained as per the manufacturer’s instruction. The smears were washed with tap water to remove extra stains and then air-dried. The stained blood smears were examined by light microscopy (×40 and ×100) (OLYMPUS CX41, Japan) for the detection of blood parasites.
Hematological analysis of blood infected with B. canis
A total of 180 whole blood samples with EDTA were subjected into hematological analysis to determine different blood parameters that could be changed during babesiosis infection in dogs. In addition, sera were subjected to biochemical analysis to determine aspartate aminotransferase (AST), alanine aminotransferase (ALT), and C-reactive protein (CRP) using specific test kits (Spectrum Diagnostics, Egypt) [14,15].
PCR analysis of the blood for identification of the B. canis subspecies
Molecular analysis of frozen blood from infested dogs with babesiosis was subjected to DNA extraction. The DNA was extracted from 200 μL of blood using the DNA blood kit (QIAGEN, Hilden, Germany) in the automatic extraction system QIAcube (QIAGEN). In each round of extraction, one sample of DNase/RNase-Free distilled water was included as a blind control for DNA extraction. To detect members of the genera Babesia, a fragment (~560 bp) of the 18S ribosomal RNA (rRNA) gene was amplified and sequenced using the forward primer PIRO-A (5′- AATACCCAATCCTGACACAGGG-3′) and PIRO-B (5′-TTAAATACGAAT GCCCCCAAC-3′) that amplify complete 18S rRNA gene under conditions described by Olmeda et al. [16]. The PCR cycling was: Initial denaturation for 2 min at 95°C for denaturation, annealing 30 s at 55°C, and 30 s at 72°C for extension and a finally 5 min at 72°C for extension. The resulting sequences were assembled using the SeqMan Pro software, edited with Edit Seq tools in Lasergene (DNASTAR, Inc., Madison, WI, USA) and compared with available sequences using Basic Local Alignment Search Tool (BLAST) in GenBank.
Measurement of oxidative stress markers
Different parameters of oxidative stress were evaluated in sera. The levels of malondialdehyde (MDA), superoxide dismutase (SOD), and catalase were evaluated using the positive and negative sera with the specific kit analysis [17,18].
Evaluation of tumor necrosis factor-alpha (TNF-α) and gamma interferon (IFN-γ) activity
Blood samples from the infected dogs had B. vogeli with blood films, and blood samples from five young reared uninfected puppies were collected in a similar manner and used as negative controls. All blood samples were aseptically preserved in −20°C.
RNA isolation
RNA isolation from 100 mg of blood was performed by a total RNA kit (Ambion, Applied Biosystems, USA) per the manufacturer’s instructions. The Thermo Scientific Nano-Drop was used to measure the RNA purity and quantity. Notably, 500 ng of RNA was made with DNase I amplification grade (Invitrogen, Germany) per the manufacturer’s instructions. The reverse transcription of treated RNA was performed by High-Capacity cDNA Archive Kit (Applied Biosystems) [19].
Quantitative real-time PCR protocol
PCR primer set specific for TNF-α and IFN-γ specific for dogs was designed and based on sequences deposited in the GenBank. β-actin was used as a reference gene and for sample normalization. The gene expression included in this study was tested on a separate pool of cDNA, generated from five uninfected dogs previously examined for the presence of any parasites [20].
Statistical analysis
The PASW Statistics version 18.0 software (SPSS Inc., Chicago, IL, USA) was used for data analysis. The Chi-square (χ2) test or Fisher’s exact test (FET) was used to determine the differences between Babesia infection rates under various parameters. Hematological, biochemical, oxidative stress, and gene expression parameters of healthy and diseased dogs were compared by one-way analysis of variance and independent sample t-test and expressed as mean±standard error. p<0.05 [21] was considered statistically significant.
Results
Morphological identification of the protozoan parasite
The infected dogs experienced tick infection from the genus Rhipicephalus sanguineus (Figure-1) which was identified by a light microscope. The examined stained blood smears revealed the presence of B. canis with a large form (3-5.5 μm in length). Each piroplasma was pyriform in shape, pointed at one end and round at the other end (Figure-1).
Figure-1.
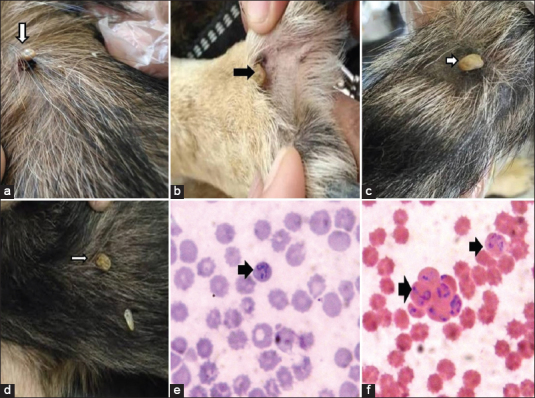
Ticks (Rhipicephalus sanguineus which identified by light microscope infection in Police dogs (A-D), E: F: stained blood smears revealed presence of Babesia vogeli with large form (3-5.5 μm in length). Each piroplasma was pyriform in shape, pointed at one end, and round at other end using 100×.
Molecular identification of B. canis infected police dogs
The 18S rRNA region of canine Babesia spp. was successfully amplified using the universal primers mentioned earlier in this study. Purified PCR products from this blood protozoan parasite was directly sequenced and yielded 554 bp of three specimens. These sequences were deposited in GenBank (accession number: MT565474.1). The comparison of these DNA sequences fragments with other nucleotide sequences and the divergence of B. canis canis and B. vogeli in GenBank indicated that this species was identified and confirmed as B. vogeli.
The BLAST analysis of B. vogeli (MT565474.1) of this study revealed 100.0% nucleotide identity with B. vogeli (AY371197.1 in Egypt and HQ662635.1 in Romania), 99.61% nucleotide similarity with B. vogeli (KT333456.1 in Brazil and KM199636.1 in Thailand), 96.41% nucleotide similarity with B. vogeli (MK674799.1 in France), 99.21% similarity (MT740272.1) in Kenya and HQ148663 in Taiwan, and 98.62% and 98.52% nucleotide similarity with B. vogeli (DQ439545.1 and AY150061.1 in Spain). In contrast, the present nucleotide similarity with B. canis canis was 96.08% nucleotide similarity (KX839230.1 in Italy, EU622793.1 in Poland, KF499115.1 in Turkey, HQ662634.1 in Romania, and FJ209025.1 in Croatia).
The derived phylogenetic tree based on the neighbor-joining model using the 18S rRNA region of B. vogeli revealed Two strong nodal supports of B. canis major clades. The first clade included B. canis grouped with B. vogeli and the second clade included B. canis canis to form two subclades. The first subclade of B. vogeli grouped Egypt and Romania together and separated from other groups of B. canis canis (Figure-2).
Figure-2.
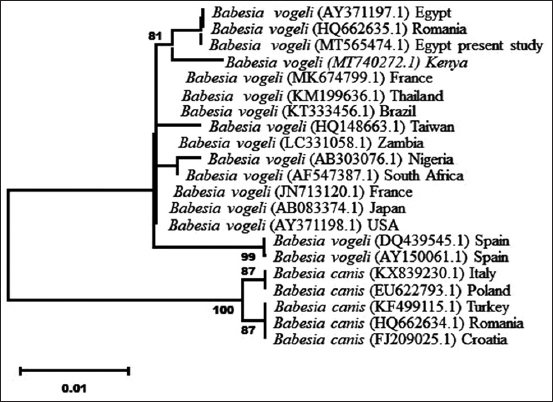
Phylogenetic tree of the 18S rDNA gene sequences created using the neighbor-joining method and bootstrap analysis of 500 replicates. Sequences with accession numbers have been taken from GenBank for comparison. The sample sequenced in the present study represents another identical sequence of the first genetic variant detected in this study (GA combination).
Prevalence study of B. vogeli in police dogs
The results of blood samples screening revealed that of the 242 blood samples, 62 were positive for B. vogeli infection (25.62% [62 of 242]; 95% confidence interval [CI], 20.12-31.12) (Table-1). No significant association was observed between the breed of dogs and B. vogeli infection rates (p=0.071; FET). The infection rate in male dogs (26.60% [54 of 203]; 95% CI, 20.52-32.68) was higher than in female dogs (20.51% [8 of 39]; 95% CI, 7.84-33.19); however, the difference was not significant (χ2 [1, N=242] = 0.636; p=0.425). Meanwhile, no significant association was observed among the various ages of dogs (χ2 [3, N=242] = 0.617; p=0.103). Notably, the infection rate in dogs that were severely infected with ticks was higher than those with lower rates of infection (χ2 [3, N=242] = 145.90; p<0.0001), and dogs showing clinical signs presented a significantly higher infection rate than the apparently normal dogs (χ2 [1, N=242] = 162.65; p<0.0001). Similarly, the detection rate of Babesia in dead dogs was significantly higher than in living dogs (χ2 [1, N=242] = 164.66; p<0.0001). Furthermore, the infection rates in autumn and winter were significantly higher than that in spring and summer (χ2 [3, N=242] = 37.69; p<0.0001).
Detection of mixed infection with B. vogeli and E. canis
The results of blood samples screening revealed that of the 62 positive Babesia blood samples, 28 were positive for E. canis infection (45.16% [28 of 62]; 95% CI, 32.77-57.55) (Table-1). No significant association was observed between the breed of dogs (p=0.858; FET), sex (p=0.530; FET), alive or dead status (p=0.065; FET), intensity of tick infection (χ2 [1, N=62] = 0.193; p=0.660), season of the year (p=0.060; FET), and rates of mixed Babesia and E. canis infection. Notably, the rates of mixed Babesia and E. canis infection in older dogs were significantly higher than in younger ones (p=0.035; FET).
Hematological and biochemical analysis
Hematological and biochemical results of sampled police dogs were classified into three groups, that is, healthy dogs, dogs infected with B. vogeli, and dogs that acquired mixed infection of Babesia and E. canis (Table-2). The hematology results of the sampled police dogs are presented in Table-2. Infected dogs exhibited lower hemoglobin concentration, packed cell volume (PCV), and total erythrocyte count than healthy dogs. Most of the dogs with low hematological values also clinically manifested anemia. Notably, low platelet count and leukopenia were observed in dogs with mixed Babesia and E. canis infection.
Table-2.
Hematological and biochemical parameters in healthy and infected dogs (n=180).
| Parameters(normal/reference range) | Mean±SE | F(2, 177) | p-value | ||
|---|---|---|---|---|---|
| Healthy dogs (n=127) | Babesiainfected dogs (n=27) | Mixed Babesiaand Ehrlichia infection (n=26) | |||
| Hematology: | |||||
| Total erythrocyte count(5.58.5×106μl) | 6.92±0.08a | 4.44±0.15b | 4.43±0.23b | 142.19 | <0.0001* |
| Hemoglobin concentration(1218g%) | 14.33±0.13a | 8.97±0.49b | 9.56±0.29b | 177.06 | <0.0001 |
| Platelet count(200900×102μl) | 264.21±6.40a | 240.44±14.75a | 58.12±14.24b | 86.56 | <0.0001 |
| Packed cell volume(Hematocrit)(3755%) | 40.44±0.52a | 35.04±0.27b | 34.44±0.47b | 24.26 | <0.0001 |
| Total leukocyte count(617×106μl) | 10.12±0.26a | 10.27±0.52a | 4.36±0.34b | 49.88 | <0.0001 |
| Biochemical: | |||||
| AST(848 IU/L) | 40.46±1.35b | 94.15±6.15a | 84.38±5.11a | 110.28 | <0.0001 |
| ALT(858 IU/L) | 51.39±1.62b | 120.74±5.46a | 117.42±4.86a | 193.93 | <0.0001 |
| Creatinine(0.51.6 mg%) | 0.91±0.17c | 1.89±0.08b | 2.20±0.08a | 323.65 | <0.0001 |
| Blood urea nitrogen (8.826 mg%) | 26.13±0.37c | 55.44±3.36b | 66.46±3.07a | 253.19 | <0.0001 |
a,b,cDifferent superscripts within the same row indicate significant difference at P<0.05and confidence level 95%; SE=Standard error, AST=Aspartate aminotransferase, ALT=Alanine aminotransferase. F (2, 177): F-value(degree of freedoms between groups, degree of freedoms within groups).
When the statistical test gives a P result=0.000, we express it as<0.0001
The biochemical results of the serum samples obtained from police dogs are presented in Table-2. High AST and ALT levels were observed in both dogs infected with Babesia and dogs that acquired mixed B. vogeli and E. canis infection, which indicated liver damage. Symptoms of liver damage clinically manifested as edema and jaundice. Increased levels of creatinine and blood urea nitrogen were observed in dogs infected with Babesia and dogs that acquired mixed B. vogeli and E. canis infection, which indicated renal failure. Symptoms of renal failure clinically manifested as emaciation and bloody urine.
Oxidative stress
On average, MDA levels in B. vogeli infected dogs (5.72±1.41) were greater than that of healthy dogs (1.50±0.01). This mean difference (4.22 nmol/mL [95% CI, ±4.49]) was not significant (t [df=6] = 2.25; p=0.065). Similarly, CAT levels in Babesia infected dogs (5326.80±1139.70) were greater than that in healthy dogs (2554.00±0.58). This mean difference (2772.80 nmol/mL [95% CI, ±3164.33]) was not significant (t [4] = 2.43; p=0.072]). However, SOD levels in Babesia infected dogs (7101.40±1439.28) were greater than that in healthy dogs (1997.67±1.33). This mean difference (5103.73 nmol/mL [95% CI, ±4695.71]) was significant (t [6] = 2.66; p=0.038]). Moreover, CRP levels in Babesia infected dogs (95.85±18.88) were greater than that in healthy dogs (3.50±0.38). This mean difference (92.35 [95% CI, ±42.71]) was significant (t [6] = 4.89; p=0.001]) (Table-3, Figure-3).
Table-3.
Oxidative stress and gene expression parameters in healthy and infected dogs.
| Oxidative stress and gene expression parameters | Healthy dogs | Babesiainfected dogs | t(df) | p-value |
|---|---|---|---|---|
| MDA(nmol/mL) | 1.50±0.00 | 5.72±1.41 | –2.25(6) | 0.065 |
| SOD(mg/L) | 1997.67±1.33b | 7101.40±1439.28a | –2.66(6) | 0.038 |
| CAT(mg/L) | 2554.00±0.58 | 5326.80±1139.70 | –2.43(4) | 0.072 |
| CRP(mg/L) | 3.50±0.38b | 95.85±18.88a | –4.89(9) | 0.001 |
| IFNγ(U/mL) | 3.33±0.17b | 8.20±1.16a | –3.14(6) | 0.02 |
| TNFα(U/mL) | 2.97±0.03b | 10.60±1.96a | –2.91(6) | 0.027 |
a,bDifferent superscripts within the same row indicate significant difference at p<0.05. MDA=Malondialdehyde, SOD=Superoxide dismutase, CAT=Catalase, CRP=C-reactive protein, IFN-γ=Gamma interferon, TNF-α=Tumor necrosis factor-alpha
Figure-3.
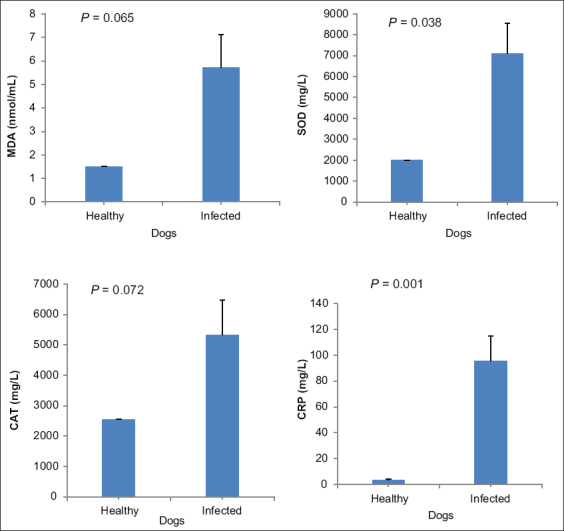
MDA=Malondialdehyde; SOD=Superoxide dismutase; CAT=Catalase and CRP (C-reactive protein). Significance was indicated at p<0.05.
Gene expression analysis
Likewise, IFN-γ levels in Babesia infected dogs (8.20±1.16) were greater than that in healthy dogs (3.33±0.17). This difference (4.87 [95% CI, −8.66 to −1.08]) was significant (t [6] = −3.14; p=0.020]). In addition, TNF-α levels in Babesia infected dogs (10.60±1.96) were greater than that in healthy dogs (2.97±0.03). This difference (7.63 U/mL [95% CI, −14.04 to −1.22]) was significant (t [6] = −2.91; p=0.027]) (Table-3, Figure-4).
Figure-4.
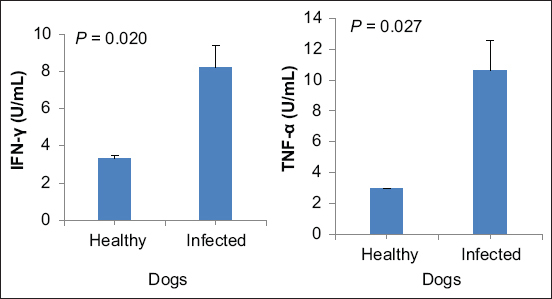
IFN-γ=Gamma-interferon; TNF-α=Tumor necrosis factor alpha. Significance was indicated at p<0.05.
Discussion
In this study, we investigated the prevalence of 2 tick-borne pathogens in police working dogs in Egypt. Our results indicated the prevalence of B. vogeli (25.62%) and E. canis (11.57%). The previous studies reported that the prevalence of Babesia spp. in dogs ranged from 1.2% to 11.3% in China [22,23], 10.9% to 19.09% in India [24], 14.0% in Southern Italy [25], and 61.1% in Nigeria [26]. Results showed that breed, sex, and age did not significantly influence the incidence of B. vogeli, which agreed with. Adebayo et al. [26]. Canine babesiosis did not take enough consideration in research work in Egypt compared with other animal species [27]. Other studies investigated that the prevalence of E. canis in dogs was 10.3% in Korea [28], 1.3% in Southern China [29], 44.6% in North Trinidad [30], 29.26% in Mexico [31], and 40.6% in Colombia [32]. Although our study contradicts with reports on age difference regarding positivity to E. canis, there was a significant increase in E. canis infection rate in older dogs (7 years of age and older), which suggests that acquiring infection increases with age [30]. The diverse patterns for canine tick-borne pathogens among different countries may be attributed to the differences in the tick distribution [28].
The current study noted that the rate of Babesia infection increased by age (>3 years) and declined after the age of 9 years; however, the difference was not significant. This result is consistent with Salem and Farag [27] who reported that the highest infections were found in the age group of 3-5 years and explained that Babesia infection was known to increase by age, reaching its peak between the age of 3 and 5 years and then peter out [33]. Similarly, older years of age emerged as a risk factor for B. canis/B. vogeli sero-reactivity as suggested in the study by Veneziano et al. [25]. They stated that this finding is probably caused by the cumulative exposure to the relevant tick vectors rather than an increased susceptibility to the Babesia infection. However, other previous studies indicated that although canine babesiosis can occur in animals of all ages, young animals are more susceptible to Babesia infection and exhibited more severe clinical manifestations [23].
Furthermore, the current study remarked non-significant higher rates of infection in male dogs than females, which agrees with Mellanby et al. and Veneziano et al. [25,34] who stated that male dogs exhibited an increased risk of developing canine babesiosis, because they have a higher environmental exposure due to increased roaming behavior. Salem and Farag [27] stated that male dogs exhibited a higher infection rate than female ones; the aggressiveness and hormonal status of male dogs may be a contributory factor here. However, Martinod et al. [35] found no difference in sex susceptibility between males and females.
The study indicated that the rate of Babesia infection was not dependent on the breed of the surveyed dogs. However, earlier studies indicated a significantly higher seroprevalence in long-hair dogs, in which hard ticks can easily attach and avoid detection [25]. In addition, a breed predisposition has been suggested in Hungary, citing the vulnerability of the German shepherd breed to developing babesiosis caused by B. canis [33]. Salem and Farag [27] reported that German shepherd dogs seemed to be the breed with the highest infection rate, which was attributed to the prevalence of this breed in Egypt, which agreed with our findings.
Results reported that the rate of B. canis infection was significantly dependent on the intensity of tick infection in surveyed dogs. This finding comes in agreement with Farag [36] who reported that the tick population plays a crucial role in the incidence of the infection. Furthermore, the rate of Babesia infection was significantly dependent on the season of the year. The prevalence of Babesia infections was significantly higher in cold months (autumn and winter), which agrees with Farag [36] who stated that climatic condition represents a notable factor in the incidence of the infection. In addition, Veneziano et al. [25] stated that the tick vector favors cool and wet weather.
Babesia infections were significantly associated with the manifestation of clinical signs. The main clinical signs observed were fever, anemia, jaundice, edema, and bleeding. Schetters et al. [37] described the classical presentation of Babesia infections as febrile illness with apparent anemia. Most of the dead dog cases (88.33%) were positive for Babesia; 49.06% of them acquired co-infection with E. canis. Adebayo et al. [26] reported that babesiosis accounts for approximately 40% mortality in the canine cases presented in hospitals in Africa.
In the current study, we reported co-infection with E. canis in 28 of 62 dogs (45.16%) infected with Babesia. This study reported a significantly higher rate of B. vogeli and E. canis co-infection in old dogs than young ones. Co-infection with other pathogens, such as E. canis, is common in endemic areas, depending on the geographic location and the distribution of the arthropod vectors. In addition, co-infection is of major clinical relevance, because it complicates diagnoses, intensifies clinical symptoms, diminishes the effectiveness of the medication, and could worsen the prognosis [38,39].
Anemia was the most hematologic alterations observed in B. vogeli infected dogs. The mechanisms of inducing anemia have been poorly investigated. Salem and Farag [27] attributed the cause of anemia to the destruction of circulating red blood cells by autoantibodies. Leisewitz et al. [13] stated that the high incidence of hemoglobinuria is testimony to the very important role hemolysis plays in anemia. Thrombocytopenia, anemia, and leukopenia were the most hematologic alterations observed in the dogs that acquired co-infection with E. canis. Thrombocytopenia is the most consistent hematological finding detected in dogs infested with E. canis as reported by Frank and Breitschwerdt [40]. These findings agreed with the study by Asgarali et al. [30] who reported that 80.4% of E. canis seropositive dogs had low platelet counts. Moreover, Niwetpathomwat et al. [41] reported severe anemia in 18%, leukopenia in 19%, and thrombocytopenia in 93% of the dogs studied. Thrombocytopenia was attributed to multiple causes, such as increased platelet consumption, destruction and sequestration of platelets in the spleen, and co-infection with Babesia [42,43].
The significantly elevated levels of ALT and AST observed in B. vogeli and E. canis infected dogs were frequently reported in the previous studies and indicate the possibility of liver injury after primary infection [44,45]. However, Zygner et al. [44] noted that elevated AST levels can also be associated with kidney injury. All blood biochemical indicators of kidney function were significantly higher in B. canis infected dogs than healthy controls. Zygner et al. [46] in his study on dogs naturally infected with Babesia stated that creatinine was significantly correlated with urea concentration. However, they stated that high creatinine level is considered a poor prognostic indicator.
In our study of the canine babesiosis, there is a correlative association between TNF-α concentration, clinical severity, and parasitemia Zygner et al. [47]. TNF-α and IFN-γ play a significant role in the production of several harmful substances such as nitric oxide and free oxygen radicals, which could initiate the interaction between parasitized erythrocytes and the blood vessel wall [48]. Different metabolites of the nitric substance were considered as one of the mediators of multiple organ dysfunction syndromes that are present in canine babesiosis.
This study found a great difference in CRP between healthy and babesiosis-infested canine blood, whereas a study on B. rossi infections showed no differences in CRP levels between survivors and non-survivors [15]. However, an analysis showed that CRP was significantly associated with glucose levels. In dogs with Babesia gibsoni, CRP has a positive correlation with PCV recovery; hence, it could be used as a biomarker of disease condition and clearance [14]. This confirms the idea that the inflammatory mechanisms in canine babesiosis are important to be identified because tissue hypoxia and metabolic dysfunction play a great process in the disease process and condition.
Babesiosis infection was based on a diagnosis made by the morphology of stages of Babesia species within blood cells, but the similarity between species and subspecies was a restricting agent [49]. The PCR method represents a powerful tool for determining not only the genotype but also in which cases symptoms appear nonspecific and/or the blood smears do not present a particular thing the diagnosis. There are recent advances in molecular methodology (methods based on PCR and subsequent DNA sequencing). Genetic characterization was possible to detect and identify piroplasms with greater sensitivity and specificity than conventional ones [50]. Consequently, the knowledge on the prevalence of Babesia species and subspecies infecting police dogs has increased significantly.
Characterization by molecular methods confirmed the presence of three distinct genotypes of B. vogeli. Among these, B. vogeli was recognized as the most common agent of canine babesiosis in temperate regions of Europe [51]. In this study, the detected B. vogeli agreed with the results recorded by Elsafey [52], Farag [36], and Abdel-Rhman et al. [53] who reported that B. vogeli was found to naturally infect dogs in Egypt.
Compared with the 18S rRNA sequence obtained through the present BLAST analysis of B. vogeli (GenBank accession number: MT565474.1) with other Babesia species, these sequences were completely identical to a B. vogeli sequence from Egypt and Romania (AY371197.1 [54] and HQ662635.1 [55], respectively).
The present results indicate a very close genetic relation with 99.61% nucleotide similarity and the affiliation of the canine Babesia from Brazil (KT333456.1) [56] to B. vogeli. When the present sequences were compared with the sequence of the canine Babesia from B. vogeli, 96.41% nucleotide similarity was found in France (MK674799.1) [57], whereas 99.21% similarity was found in Kenya (MT740272.1) [58] and in Taiwan (HQ148663.1) [59]. In contrast, the present nucleotide similarity of B. vogeli was compared with B. canis canis; 96.08% nucleotide similarity was found in Italy (KX839230.1) [60], in Poland (EU622793.1) [61], in Turkey (KF499115.1) [62], in Romania (HQ662634.1) [55], and in Croatia (FJ209025.1).
Conclusion
The causative agent of tick-borne illness in police dogs was B. vogeli. Therefore, from this study, the dogs must be treated against the babesiosis and tick infection together with spraying of the area where the dogs live (ground, dog houses, and walls). Breed disposition, sex, and age did not significantly influence the incidence of B. vogeli. The potential contribution of tick exposure, sex, or breed predisposition requires further epidemiological studies.
Authors’ Contributions
AAZ: Collection of the samples. OAM and MMA identified the parasites and did the serological analysis EI: Did the statistical analysis. All authors sharing in writing this manuscript and revised it. All authors have read and approved the final manuscript.
Acknowledgments
The authors acknowledge the Police Academy for their support in sampling. The authors did not receive any funds for this study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Filipović-Kovačević M, Beletić A, Božović-Ilić A, Milanović Z, Tyrrell P, Buch J, Breitschwerdt E.B, Birkenheuer A.J, Chandrashekaret R. Molecular and serological prevalence of Anaplasma phagocytophilum, A platys, Ehrlichia canis, E. chaffeenses, E. ewingii, Borrelia burgdorferi, Babesia canis, B. gibsoni and B. vogeli among clinically healthy outdoor dogs in Serbia. Vet. Parasitol. Reg. Stud. Reports. 2018;14(4):117–122. doi: 10.1016/j.vprsr.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Matthewman L.A, Kelly P.J, Bobade P.A, Tagwira M, Mason P.R, Majok A, Brouqui P, Raoult D. Infections with Babesia canis and Ehrlichia canis in dogs in Zimbabwe. Vet. Rec. 1993;133(14):344–346. doi: 10.1136/vr.133.14.344. [DOI] [PubMed] [Google Scholar]
- 3.Assarasakorn S, Niwetpathomwat A. A complicated case of concurrent canine babesiosis and canine ehrlichiosis. Comp. Clin. Pathol. 2007;16:281–284. [Google Scholar]
- 4.Köster L.S, Lobetti R.G, Kelly P. Canine babesiosis:A perspective on clinical complications, biomarkers, and treatment. Vet. Med. (Auckl) 2015;6:119–128. doi: 10.2147/VMRR.S60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urquhart G.M, Dunn A.M, Jennings F.W. Veterinary Parasitology. 2nd ed. Hoboken, New Jersey: Blackwell Publishers; 1996. pp. 234–235. [Google Scholar]
- 6.Cardoso L, Tuna J, Vieira L, Yisaschar-Mekuzas Y, Baneth G. Molecular detection of Anaplasma platys and Ehrlichia canis in dogs from the North of Portugal. Vet. J. 2008;183(2):232–233. doi: 10.1016/j.tvjl.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Solano-Gallego L, Trotta M, Carli E, Carcy B, Caldin M, Furlanello T. Babesia caniscanis and B vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Vet. Parasitol. 2008;157(3-4):211–221. doi: 10.1016/j.vetpar.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi M.G, Ferrer L, Bourdeau P, Oliva G, Baneth G. Leish Vet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors. 2011;4:86–10. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boozer A.L, Macintire D.K. Canine babesiosis. Vet. Clin. North Am. Small Anim. Pract. 2003;33(4):885–904, viii. doi: 10.1016/s0195-5616(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 10.Irwin P.J. Canine babesiosis:From molecular taxonomy to control. Parasit. Vectors. 2009;2(Suppl 1):S4. doi: 10.1186/1756-3305-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solano-Gallego L, Baneth G. Babesiosis in dogs and cats expanding parasitological and clinical spectra. Vet. Parasitol. 2011;181(1):48–60. doi: 10.1016/j.vetpar.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Badawi N.M, Yousif A.A. Babesia canis spp. in dogs in Baghdad Province, Iraq:First molecular identification and clinical and epidemiological study. Vet. World. 2020;13(3):579–585. doi: 10.14202/vetworld.2020.579-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leisewitz A.L, Goddard A, Clift S, Thompson P.N, De Gier J, Van Engelshoven J.M, Schoeman J.P. A clinical and pathological description of 320 cases of naturally acquired Babesia rossi infection in dogs. Vet. Parasitol. 2019;271(8):22–30. doi: 10.1016/j.vetpar.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Wakabayashi H, Takahashi M, Fukushima K, Yabuki A, Endo Y. A possible treatment strategy and clinical factors to estimate the treatment response in Babesia gibsoni infection. J. Vet. Med. Sci. 2007;69(5):563–568. doi: 10.1292/jvms.69.563. [DOI] [PubMed] [Google Scholar]
- 15.Koster L.S, Van Schoor M, Goddard A, Thompson P.N, Matjila P.T, Kjelgaard-Hansen M. C-reactive protein in canine babesiosis caused by Babesia rossi and its association with outcome. J. S. Afr. Vet. Assoc. 2009;80(2):87–91. doi: 10.4102/jsava.v80i2.177. [DOI] [PubMed] [Google Scholar]
- 16.Olmeda A.S, Armstrong P.M, Rosenthal B.M, Valladares B, del Castillo A, de Armas F, Miguelez M, Gonzalez A, Rodriguez J.A, Spielman A, Telford S.R., 3rd A subtropical case of human babesiosis. Acta Trop. 1997;67(3):229–234. doi: 10.1016/s0001-706x(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 17.Crnogaj M, Cerón J.J, Šmit I, Kiš I, Gotić J, Brkljačić M, Matijatko V, Rubio C.P, Kučer N, Mrljak V. Relation of antioxidant status at admission and disease severity and outcome in dogs naturally infected with Babesia caniscanis. BMC Vet. Res. 2017;13(1):114. doi: 10.1186/s12917-017-1020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AbdElKader N.A, Sheta E, AbuBakr H.O, El-Shamy O.A.A, Oryan A, Attia M.M. Effects of chitosan nanoparticles, ivermectin and their combination in the treatment of Gasterophilus intestinalis (Diptera:Gasterophilidae) larvae in donkeys (Equus asinus) Int. J. Trop. Insect Sci. 2020;41(2):43–54. [Google Scholar]
- 19.Attia M.M, El-Gameel S.M, Ismael E. Evaluation of tumor necrosis factor-alpha (TNF-α);gamma interferon (IFN-c) genes and oxidative stress in sheep:Immunological responses induced by Oestrus ovis (Diptera:Oestridae) infestation. J. Parasit. Dis. 2020;44(2):332–337. doi: 10.1007/s12639-020-01220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younis N.A, Laban S.E, Al-Mokaddem A.K, Attia M.M. Immunological status and histopathological appraisal of farmed Oreochromis niloticus exposed to parasitic infections and heavy metal toxicity. Aquac. Int. 2020;28(6):2247–2262. [Google Scholar]
- 21.Snedecor G.W, Cochran W.G. 8th ed. Ames: Iowa State Press; 1989. Statistical Methods. [Google Scholar]
- 22.Xu D, Zhang J, Shi Z, Song C, Zheng X, Zhang Y, Kelly P. Molecular detection of vector-borne agents in dogs from ten provinces of China. Parasit. Vectors. 2015;8(1):501. doi: 10.1186/s13071-015-1120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X.W, Zhang X.L, Huang H.L, Li W.J, Wang S.J, Huang S.J, Shao J.W. Prevalence and molecular characterization of Babesia in pet dogs in Shenzhen, China. Comp. Immunol. Microbiol. Infect. Dis. 2020;70(3):101452. doi: 10.1016/j.cimid.2020.101452. [DOI] [PubMed] [Google Scholar]
- 24.Mittal M, Kundu K, Chakravarti S, Mohapatra J.K, Singh V.K, Kumar B.R, Kumar A. Canine babesiosis among working dogs of organized kennels in India:A comprehensive hematological, biochemical, clinic pathological and molecular epidemiological multiregional study. Prevent. Vet. Med. 2019;169(8):104696. doi: 10.1016/j.prevetmed.2019.104696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veneziano V, Piantedosi D, Ferrari N, Neola B, Santoro M, Pacifico L, Tyrrell P. Distribution and risk factors associated with Babesia spp infection in hunting dogs from Southern Italy. Ticks Tick Borne Dis. 2018;9(6):1459–1463. doi: 10.1016/j.ttbdis.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Adebayo O.O, Ajadi R.A, Omobowale T.O, Omotainse S.O, Dipeolu M.A, Nottidge H.O, Otesile E.B. Reliability of clinical monitoring for the diagnosis of babesiosis in dogs in Nigeria. Vet. Med. (Auckl) 2016;7:85–90. doi: 10.2147/VMRR.S104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salem N.Y, Farag H.S. Clinical, hematologic, and molecular findings in naturally occurring Babesia canisvogeli in Egyptian dogs. Vet. Med. Int. 2014;2014:270345. doi: 10.1155/2014/270345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Lee H, Park J.W, Yoon S.S, Seo H.J, Noh J, So B.J. Prevalence of antibodies against Anaplasma spp, Borrelia burgdorferi Sensu lato, Babesia gibsoni, and Ehrlichia spp. in dogs in the Republic of Korea. Ticks Tick Borne Dis. 2020;11(4):101412. doi: 10.1016/j.ttbdis.2020.101412. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Liu Q, Wang D, Li W, Beugnet F, Zhou J. Epidemiological survey of ticks and tick-borne pathogens in pet dogs in South-Eastern China. Parasite. 2017;24:35. doi: 10.1051/parasite/2017036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asgarali Z, Pargass I, Adam J, Mutani A, Ezeokoli C. Haematological parameters in stray dogs seropositive and seronegative to Ehrlichia canis in North Trinidad. Ticks Tick Borne Dis. 2012;3(4):207–211. doi: 10.1016/j.ttbdis.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Ojeda-Chi M.M, Rodriguez-Vivas R.I, Esteve-Gasent M.D, de León A.A.P, Modarelli J.J, Villegas-Perez S.L. Ehrlichia canis in dogs of Mexico:Prevalence, incidence, co-infection and factors associated. Comp. Immunol. Microbiol. Infect. Dis. 2019;67(6):101351. doi: 10.1016/j.cimid.2019.101351. [DOI] [PubMed] [Google Scholar]
- 32.Vargas-Hernández G, André M. R, Faria J. L. M, Munhoz T. D, Hernandez-Rodriguez M, Machado R. Z, Tinucci-Costa M. Molecular and serological detection of Ehrlichiacanis and Babesia vogeli in dogs in Colombia. Vet. Parasitol. 2012;186(3-4):254–260. doi: 10.1016/j.vetpar.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Hornok S, Edelhofer R, Farkas R. Seroprevalence of canine babesiosis in Hungary suggesting breed predisposition. Parasitol. Res. 2006;99(6):638–642. doi: 10.1007/s00436-006-0218-8. [DOI] [PubMed] [Google Scholar]
- 34.Mellanby R.J, Handel I.G, Clements D.N, de Bronsvoort B.M.C, Lengeling A, Schoeman J.P. Breed and sex risk factors for canine babesiosis in South Africa. J. Vet. Intern. Med. 2011;25(5):1186–1189. doi: 10.1111/j.1939-1676.2011.00779.x. [DOI] [PubMed] [Google Scholar]
- 35.Martinod S, Laurent N, Moreau Y. Resistance and immunity of dogs against Babesia canis in an endemic area. Vet. Parasitol. 1986;19(3-4):245–254. doi: 10.1016/0304-4017(86)90072-5. [DOI] [PubMed] [Google Scholar]
- 36.Farag H.S. Epidemiological, Diagnostic and Therapeutic Studies on Canine Babesiosis [MS. Thesis] Faculty of Veterinary Medicine, Cairo University, Giza, Egypt. 2012 [Google Scholar]
- 37.Schetters T.P, Kleuskens J.A.G, Van De Crommert J, De Leeuw P.W.J, Finizio A.L, Gorenflot A. Systemic inflammatory responses in dogs experimentally infected with Babesia canis;a hematological study. Vet. Parasitol. 2009;162(1-2):7–15. doi: 10.1016/j.vetpar.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Miró G, Montoya A, Roura X. Seropositivity rates for agents of canine vector-borne diseases in Spain:A multicenter study. Parasit. Vectors. 2013;6(1):117. doi: 10.1186/1756-3305-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis:The European perspective. Parasit. Vectors. 2016;9(1):336. doi: 10.1186/s13071-016-1596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank J.R, Breitschwerdt E.B. A retrospective study of ehrlichiosis in 62 dogs from North Carolina and Virginia. J. Vet. Intern. Med. 1999;13(3):194–201. doi: 10.1892/0891-6640(1999)013<0194:arsoei>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Niwetpathomwat A, Techangamsuwan S, Suvarnavibhaja S. A retrospective study of the clinical hematology and biochemistry of canine ehrlichiosis in an animal hospital population in Bangkok, Thailand. Comp. Clin. Pathol. 2006;14(4):217–220. [Google Scholar]
- 42.Harrus S, Waner T, Avidar Y, Bogin E, Peh H, Bark H. Serum protein alterations in canine ehrlichiosis. Vet. Parasitol. 1996;66(3-4):241–249. doi: 10.1016/s0304-4017(96)01013-8. [DOI] [PubMed] [Google Scholar]
- 43.Foglia M.V, Cappiello S, Oliva G. Tick-transmitted diseases in dogs:Clinicopathological findings. Parasitologia. 2006;48(1-2):135–136. [PubMed] [Google Scholar]
- 44.Zygner W, Gójska-Zygner O, Norbury L.J, Wedrychowicz H. Increased AST/ALT ratio in azotaemic dogs infected with Babesia canis. Pol. J. Vet. Sci. 2012;15(3):483–486. doi: 10.2478/v10181-012-0074-7. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Zhang J, Kelly P, Zheng X, Li M, You J, Li J. First description of the pathogenicity of Babesia vogeli in experimentally infected dogs. Vet. Parasitol. 2018;253(5):1–7. doi: 10.1016/j.vetpar.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 46.Zygner W, Gójska-Zygner O. Increased serum urea to creatinine ratio and its negative correlation with arterial pressure in canine babesiosis. Acta Parasitol. 2014;59(3):548–551. doi: 10.2478/s11686-014-0273-8. [DOI] [PubMed] [Google Scholar]
- 47.Zygner W, Gójska-Zygner O, Bąska P, Długosz E. Serum concentrations of tumor necrosis factor in dogs naturally infected with Babesia canis and its relation to severity of disease. Parasitol. Res. 2014;113(4):1499–1503. doi: 10.1007/s00436-014-3792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobson L.S, Lobetti R, Becker P, Reyers F, Scott T.V. Nitric oxide metabolites in naturally occurring canine babesiosis. Vet. Parasitol. 2002;104(1):27–41. doi: 10.1016/s0304-4017(01)00606-9. [DOI] [PubMed] [Google Scholar]
- 49.Jefferies R, Ryan U.M, Muhlnickel C.J, Irwin P.J. Two species of canine Babesia in Australia:Detection and characterization by PCR. J. Parasitol. 2003;89(2):409–412. doi: 10.1645/0022-3395(2003)089[0409:TSOCBI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 50.Ionita M, Mitrea I.L, Pfister K, Hamel D, Buzatu C.M, Silaghi C. Canine babesiosis in Romania due to Babesia canis and Babesia vogeli:A molecular approach. Parasitol. Res. 2012;110(5):1659–1664. doi: 10.1007/s00436-011-2683-y. [DOI] [PubMed] [Google Scholar]
- 51.Uilenberg G, Franssen F.F.J, Perie N.M, Spanjer A.A. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet. Q. 1989;11(1):33–40. doi: 10.1080/01652176.1989.9694194. [DOI] [PubMed] [Google Scholar]
- 52.Elsafey A.A. Studies on Some Blood Parasites in Dogs. M.V.Sc. Thesis, Faculty of Veterinary Medicine, Cairo University Giza, Egypt. 2009 [Google Scholar]
- 53.Abdel-Rhman A.A, Hegazy Y.M, Sultan K, Al-Gaabary M.H. Clinical, hematological and parasitological:Study on canine babesiosis. Kafrelsheikh Vet. Med. J. 2015;13(2):37–47. [Google Scholar]
- 54.Passos L.M, Geiger S.M, Ribeiro M.F, Pfister K, Zahler-Rinder M. First molecular detection of Babesia vogeli in dogs from Brazil. Vet. Parasitol. 2005;127(1):81–85. doi: 10.1016/j.vetpar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 55.Codreanu M.D, Turcitu M.A, Tudor P, Tudor N, Codreanu I, Fernoaga C, Cornila M. Genetic characterization of canine babesiosis in Romania Accession No.in GenBank (HQ662635.1) 2011 [Google Scholar]
- 56.Moraes P.H, Rufino C.P, Baraúna A.R, Reis T, Agnol L.T, Meneses A.M, Aguiar D.C, Nunes M.R, Gonçalves E.C. Molecular characterization of Babesia vogeli in dogs from Belém, Northern Brazil. Genet. Mol. Res. 2015;14(4):16364–16371. doi: 10.4238/2015.December.9.4. [DOI] [PubMed] [Google Scholar]
- 57.Medkour H, Laidoudi Y, Marié J.L, Fenollar F, Davoust B, Mediannikov O. Molecular investigation of vector-borne pathogens in red foxes (Vulpes vulpes) from Southern France. J. Wildl. Dis. 2020;56(4):837–850. doi: 10.7589/2019-09-234. [DOI] [PubMed] [Google Scholar]
- 58.Ngoka I.T, Pelle R, Kyallo M, Mbogo K, Juma P. Molecular Detection of Babesia Species Infecting Dogs from Select Regions in Kenya and their Phylogenetic Relationship. Accession No. in GenBank (MT740272.1) 2020 [Google Scholar]
- 59.Hsieh Y.C, Lee C.C, Tsang C.L, Chung Y.T. Identification and molecular characterization of a new strain of B. vogeli from naturally infected dogs in Taiwan. Exp. Appl. Acarol. 2010;68(3):539–551. [Google Scholar]
- 60.Zanet S, Bassano M, Trisciuoglio A, Taricco I, Ferroglio E. Horses infected by piroplasms different from Babesia caballi and Theileria equi:Species identification and risk factors analysis in Italy. Vet. Parasitol. 2017;236(4):238–241. doi: 10.1016/j.vetpar.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Adaszek L, Winiarczyk S. Molecular characterization of Babesia caniscanis isolates from naturally infected dogs in Poland. Vet. Parasitol. 2008;152(3-4):235–241. doi: 10.1016/j.vetpar.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 62.Vatansever Z, Gunduz N, Tasci G.T, Gokce E, Uzlu E, Kirmizigil A.H. Molecular Characterization of Autochthonous Cases of Babesia caniscanis in Turkey. Accession No. in GenBank (KF499115.1) 2013 [Google Scholar]


