Abstract
Background and Aim:
Trichuris trichiura and Hymenolepis diminuta are helminthic intestinal parasites that infect humans and other animals, including non-human primates. However, molecular detection of these parasites remains scarce in long-tailed macaques (Macaca fascicularis), which coexist with human communities in Thailand. Thus, this study aimed to molecularly confirm the occurrence of Trichuris spp. and Hymenolepis spp. infection and determine the species of both parasites that were found in long-tailed macaques.
Materials and Methods:
A total of 200 fecal samples were randomly collected from long-tailed macaques living in Lopburi, Thailand, and tested based on polymerase chain reaction (PCR) assays for Trichuris spp. and Hymenolepis spp. infections. The PCR products were submitted for DNA purification and sequencing. Phylogenetic analysis was performed using the maximum likelihood method.
Results:
Of 200 tested samples, three (1.5%) were positive for Trichuris spp. Sequence analysis of all positive samples revealed the presence of T. trichiura, while eight samples (8/200, 4%) positive for Hymenolepis spp. were classified as H. diminuta. No significant associations were found between parasite infection and sex of macaques.
Conclusion:
This study revealed that long-tailed macaques harbor T. trichiura and H. diminuta. These results suggested that local residents and tourists must pay attention to limiting contact with long-tailed macaques and take hygienic precautions to reduce the risk of zoonotic and anthroponotic transmission of these parasites between humans and long-tailed macaques.
Keywords: Hymenolepis diminuta, long-tailed macaque, Macaca fascicularis, Thailand, Trichuris trichiura
Introduction
Parasitic infections caused by intestinal helminths in humans are ubiquitous in distribution and constitute a major part of neglected tropical diseases [1]. Trichuris spp. worms or whipworms are soil-transmitted nematode parasites that can be an important cause of inflammation of the cecum and large intestine, bloody diarrhea, and chronic iron deficiency anemia in humans [2,3]. The infection develops by ingestion of embryonated eggs of the parasite, which contaminate the soil or food [2]. There are approximately 100 species in the genus Trichuris that infect a number of hosts, such as humans (Trichuris trichiura), pigs (Trichuris suis), dogs (Trichuris vulpis), and rodents (Trichuris muris). However, three species (T. trichiura, T. suis, and T. vulpis) are considered zoonotic parasites. Although T. suis and T. vulpis infections have been reported in humans, these parasites generally cause attenuated infections and rarely develop sexual maturity in humans [2,4]. Moreover, a study reported that non-human primates (NHPs) share whipworms with humans [3]. Trichuris spp. have been detected in several species of NHP in many countries, including Malaysia, Japan, China, Italy, Uganda, Gabon, and Thailand [5-12].
Hymenolepis diminuta (rat tapeworm) is a tapeworm of rodents that are present worldwide [13]. Human infections with this parasite have been reported in 80 countries from 1810 to 2018, including seven cases in Thailand [14]. Humans are infected by ingesting cysticercoid larvae that are harbored by beetles, fleas, caterpillars, and other insects [13]. Most reported cases are asymptomatic. Common symptoms are primarily associated with intestinal symptoms, such as abdominal pain and diarrhea. However, remittent fever, diffuse cutaneous itching, and arthromyalgia have been reported [15,16]. Hymenolepis spp. have been detected in NHP from Peru and white-headed capuchin monkeys from Ecuador [17,18].
The long-tailed macaque (Macaca fascicularis), also known as the crab-eating macaque or cynomolgus macaque, is the most commonly observed species of NHPs that inhabit many regions of Thailand [19]. Lopburi province is one of the most famous tourist attractions in Central Thailand, where these macaques live and share the environment with local residents and tourists. Macaques can carry numerous pathogens that are potentially zoonotic to humans, more specifically gastrointestinal parasites that cause the most common diseases found in NHPs [20]. Transmission of parasites from animals to humans can occur due to humans and NHPs sharing the same ecosystem [17].
The investigation of Trichuris spp. and Hymenolepis spp. infection in long-tailed macaques is necessary to determine their potential as a public health threat. However, there are still extremely few studies on both these parasites in long-tailed macaques, especially Hymenolepis spp. infection in Thailand. Furthermore, the conventional diagnosis of gastrointestinal helminth infections in NHP is based on the detection of parasitic eggs in feces, and consideration of only egg morphology may be insufficiently reliable for species identification [6].
Therefore, the current study aimed to report the occurrence of Trichuris spp. and Hymenolepis spp. infection and determine the species of both parasites found in long-tailed macaques using molecular methods.
Materials and Methods
Ethical approval
This research was approved by the Animal Ethics Committee of Kasetsart University, Bangkok, Thailand (ACKU59-VTN-011).
Study period and location
The blood samples were collected in April 2014 from long-tailed macaques in Lopburi province, Thailand. Laboratory analysis was carried out in the Faculty of Veterinary Technology, Kasetsart University, Thailand.
Samples collection and study area
In April 2014, convenience sampling was used to collect fecal samples from 200 free-ranging long-tailed macaques (86 female and 114 male macaques) at the San Phra Kan Shrine, Lopburi province, Central Thailand. Sex was identified by observation of the external genital organ of each macaque. The feces were immediately collected from the ground after defecation of macaques and kept cool during transportation. All fecal specimens were washed with distilled water and sieved to eliminate the large sediment from the samples before storage of fecal suspensions at −20°C to perform freeze/thaw cycle of the samples before subjecting to DNA extraction [13].
Molecular analysis and DNA sequencing
DNA was extracted from 200 mL of fecal suspension using the E.Z.N.A.® stool DNA extraction kit (OMEGA Bio-tek Inc., USA), according to the manufacturer’s instructions. Extracted DNA samples were stored at −40°C until use in molecular analysis. The DNA samples were assayed for Trichuris spp. and Hymenolepis spp. For the confirmation of Trichuris spp., the genomic DNA was amplified, targeting the internal transcribed spacer region 1 (ITS-1) as per Ghai et al. [10]. The primary polymerase chain reaction (PCR) used the primers ExternalITS1_Trichuris-1417F (5’-AGGGACCAGCGACACTTTC-3’) and ExternalITS1_Trichuris-2505R (5’-GAGTGTC ACGT CGTTCTTCAAC-3’). The thermal cycling profile for primary PCR was as follows: 94°C for 5 min; 40 cycles of 94°C for 60 s, 61°C for 30 s, 72°C for 75 s, and a final extension at 72°C for 10 min. The secondary PCR used the primers InternalITS1_Trichuris-1567F (5’-GTTCTCGTGACTGGGAC-3’) and InternalITS1_Trichuris-2462R (5’-CTACGAGCCAAGTGATCC-3’). The nested PCR conditions consisted of the following: 94°C for 60 s, followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 75 s, and a final extension at 72°C for 10 min. The expected sizes of target amplicons were approximately 1088 and 895 bp for the primary and nested PCR, respectively.
For Hymenolepis spp., the amplicon of the partial 18S rRNA gene was amplified using the procedure of Olson et al. [21] with some modifications. The PCR used the primers Hn107F (5’-GGGAATGGGTGCACTTATTAGA-3’) and Hn312R (5’-GTTATCACCATGGTAGGCAGGT-3’), resulting in approximately 160 bp amplicons. The PCR cycling conditions were as follows: 94°C for 5 min; 35 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 75 s; and a final extension at 72°C for 10 min.
The PCR products were analyzed using 1.2% (w/v) agarose gel electrophoresis with TAE buffer and visualized under ultraviolet transillumination after staining the nucleic acid with GelStar® (Cambrex BioScience, USA). The positive samples of the correct size for the ITS-1 of Trichuris spp. and the partial 18S rDNA fragment of Hymenolepis spp. were submitted for DNA purification and sequencing.
Phylogenetic analysis
The ITS-1 sequences obtained from Trichuris spp. in this study were aligned with sequences available in GenBank, namely, AJ781762, GQ352553, GQ352555, GQ352558, KC877992, MH390365, and MN447320, and the 18S rRNA gene sequences obtained from Hymenolepis spp. were compared with published sequences, including AF124475, AF286983, AY193875, JX310720, and KX454312 using the BLAST program of the National Center for Biotechnology Information.
Phylogenetic analysis of the nucleotide sequences was performed using the maximum likelihood method based on the Kimura two-parameter model in the MEGA 7 software (The Biodesign Institute, Tempe, AZ, USA) (http://www.megasoftware.net). The reliability of the reconstructed phylogenies was assessed using the bootstrap method with 1000 replicates.
Statistical analysis
Statistical analysis was performed using Fisher’s exact test when the data did not comply with the central limit theorem and proportion test (Z test) when the data complied with the central limit theorem to determine the association between Trichuris spp. and Hymenolepis spp. infections and host sex. p < 0.05 indicated statistical significance.
Results
Of 200 fecal samples, 3 (1.5%) were positive for Trichuris spp. based on the nested PCR analysis of the ITS-1 of the ribosomal DNA complex. The amplicon size retrieved in positive samples was approximately 895 bp. The positive samples consisted of one male and two female macaques (Table-1). No significant association was observed between Trichuris spp. Infection and sex of macaques based on Fisher’s exact test (p=0.578).
Table-1.
Trichuris trichiura and Hymenolepis diminuta infection in long-tailed macaques (Macaca fascicularis).
| Gender | Number of animals | Positive samples (%) | |
|---|---|---|---|
| Trichuris trichiura | Hymenolepis diminuta | ||
| Male | 114 | 1 (0.87) | 5 (4.39) |
| Female | 86 | 2 (2.33) | 3 (3.49) |
| Total | 200 | 3 (1.50) | 8 (4.00) |
Sequence analysis of all positive samples revealed the presence of T. trichiura. All of these sequences shared 98-99% identity with the sequence of T. trichiura isolate TH1 obtained from a human in Thailand (accession number, GQ352553). The nucleotide sequences of the ITS-1 of T. trichiura were deposited in the GenBank™ databases under the following accession numbers: MK184210, MK192054, and MK192055. Moreover, the phylogenetic tree based on the ITS-1 of these positive samples revealed that the sequences of Trichuris spp. in this study were positioned in the same cluster as T. trichiura in humans (GQ352553) and Trichuris spp. in other NHPs (MN447320 and MH390365) (Figure-1).
Figure-1.
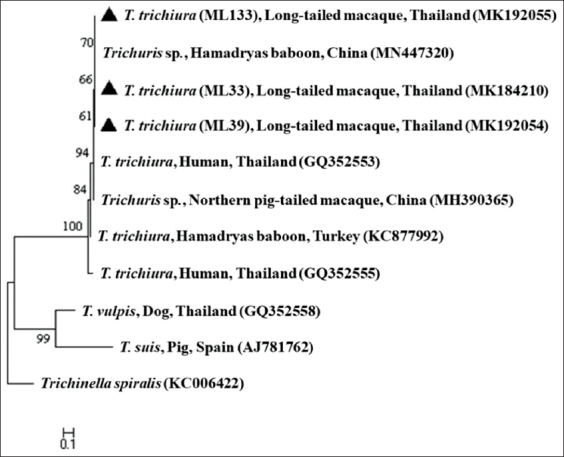
Phylogenetic tree based on internal transcribed spacer region 1 sequences of Trichuris trichiura based on the maximum likelihood method. Bootstrap percentages are shown at nodes. Trichinella spiralis is the outgroup. Isolates obtained in the current study are coded using triangular symbols.
Based on the PCR analysis of the partial 18S rRNA gene, amplicons (160 bp) consistent with Hymenolepis spp. were detected in 4% (8/200) of tested fecal samples. The infection rate in male macaques was 4.39% (5/114), which was higher than the infection rate in female macaques (3.49%, 3/86) (Table-1). However, no significant association was found between Hymenolepis spp. infection and sex of the host using the proportion test (p=0.748).
The sequence analysis of the positive samples of Hymenolepis spp. revealed that all of these samples were characterized as H. diminuta. All sequences had 97-100% similarity to H. diminuta isolated from Rattus norvegicus (brown rat) in Poland and Denmark (GenBank accession numbers, JX310720 and AF286983). The nucleotide sequences of the partial 18S rDNA of H. diminuta in the current study were also submitted to the GenBank™ databases under the following accession numbers: MK182764–MK182771. The 18S rRNA gene nucleotide sequences of H. diminuta detected in long-tailed macaques were most closely related to H. diminuta in R. norvegicus in the tree (Figure-2).
Figure-2.
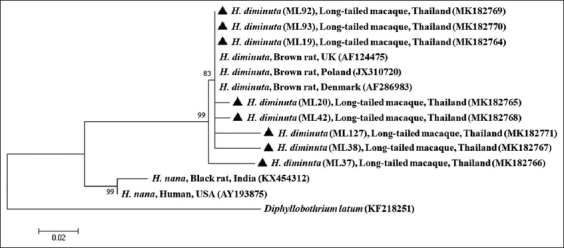
Phylogenetic tree based on partial 18S rRNA gene sequences of Hymenolepis diminuta based on the maximum likelihood method. Bootstrap percentages are shown at nodes. Diphyllobothrium latum is the outgroup. The isolates obtained in the current study are coded using triangular symbols.
Discussion
The prevalence of Trichuris spp. in this investigation was identified at a low rate (1.5%) based on nested PCR assay of the fecal sample of long-tailed macaques in Thailand. Compared with the current findings, higher infection rates in different species of NHP have been reported in several studies from Africa, including 6.1% (9/147) in wild galagos in Gabon [11], 16.4% (23/140) in baboons in Uganda [9], and 13.3% (4/30), 14.3% (6/42), and 88.9% (24/27) in chimpanzees, gray-cheeked mangabeys, and olive baboons, respectively, in Uganda [10]. Moreover, some studies from Asia have also described higher prevalence rates, including 20.5% (686/3349) in 34 NHP species in China [7], 23.1% (71/308) in 12 NHP species in Malaysia [5], and 82.1% in proboscis monkeys in Malaysia [20]. In Thailand, a higher prevalence rate (20/102, 19.6%) of Trichuris spp. infection has been previously reported in long-tailed macaques in Maha Sarakham province, Northeast Thailand, using the formalin-ethyl acetate sedimentation technique [12]. The low infection rate of T. trichiura in the current study might have been caused by collection of a single fecal sample from each animal, since parasitic eggs of soil-transmitted helminths are only intermittently shed in the feces of the host, particularly in mild infection [22].
Trichuris spp. found in the current study were identified as T. trichiura based on sequence and phylogenetic analyses, as were observed in wild Japanese macaques (Macaca fuscata) from Japan [6] and ring-tailed lemurs from Italy [8].
The results of the current study suggested that proper sanitation and personal hygiene should be encouraged for local residents and tourists to reduce the risk of zoonotic transmission of T. trichiura from NHPs, since there has been a report of the circulation of Trichuris species between humans and NHPs [3]. Furthermore, this parasite is a major zoonotic intestinal helminth in Southeast Asia, and the highest prevalence of human trichuriasis has been reported in this region [1].
In the current study, H. diminuta was identified at a relatively low rate in long-tailed macaques, which was similar to that in a previous study on NHPs with 2.3% (9/382) in Aotus nancymaae, 1.8% (4/221) in Aotus vociferans, and 1% (1/90) in Saguinus mystax in Peru [17]. Conversely, a high prevalence of Hymenolepis spp. was reported in white-headed capuchin monkeys (38.46%, 10/26) from Ecuador [18].
The molecular characterization of both parasites is recommended since microscopic examination is insufficient for species identification. Precise diagnosis of zoonotic parasites is necessary for disease surveillance, control, and elimination programs [1]. Although the prevalence of these parasites in the current study was low, the identification of both helminths might indicate the potential of zoonotic transmission in this area.
Further studies should include surveys of Trichuris spp. and Hymenolepis spp. in various NHPs in other locations in Thailand. Characterization of these parasites from humans and NPHs living in a shared area is needed to quantify the transmission between humans and NHPs. Moreover, investigations of H. diminuta in rodents in the same location as long-tailed macaques seem necessary because rodents are the main definitive host of this parasite [1], and studies have found this parasite in rodents in Mexico and Malaysia [14,23].
Conclusion
The current study identified a low infection rate of T. trichiura and H. diminuta in long-tailed macaques in Lopburi province, Thailand. However, both parasites are pathogenic to humans. Therefore, the results from this investigation suggested that contact should be reduced between long-tailed macaques and humans. Moreover, appropriate personal hygiene should be maintained by local residents and tourists during the handling of these macaques to reduce the zoonotic and anthroponotic transmission of these parasites.
Authors’ Contributions
WS designed and conducted the study, interpreted the results, and drafted the manuscript. TI revised and finalized the manuscript for submission. SK and NI supervised the molecular analyses. TJ interpreted the results. All authors read and approved the final manuscript.
Acknowledgments
This research was funded by the Kasetsart University Research Development Institute, Thailand (Grant no. 49.60), the Center for Agricultural Biotechnology, Kasetsart University, and the Center of Excellence on Agricultural Biotechnology, Science and Technology Postgraduate Education and Research Development Office, Office of Higher Education Commission, Ministry of Education, Science, Research Innovation, Thailand (AG-BIO/PERDO-CHE) (PERDO/004/56-002).
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Betson M, Alonte A.J.I, Ancog R.C, Aquino A.M.O, Belizario V.Y, Bordado A.M.D, Clark J, Corales M.C.G, Dacuma M.G, Divina B.P, Dixon M.A, Gourley S.A, Jimenez J.R.D, Jones B.P, Manalo S.M.P, Prada J.M, van Vliet A.H.M, Whatley K.C.L, Paller V.G.V. Rollinson D, Stothard R, editors. Chapter two zoonotic transmission of intestinal helminths in Southeast Asia:Implications for Control and Elimination. Advances in Parasitology. Academic Press, Cambridge, Massachusetts. 2020:47–131. doi: 10.1016/bs.apar.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Else K.J, Keiser J, Holland C.V, Grencis R.K, Sattelle D.B, Fujiwara R.T, Bueno L.L, Asaolu S.O, Sowemimo O.A, Cooper P.J. Whipworm and roundworm infections. Nat. Rev. Dis. Primers. 2020;6(1):44. doi: 10.1038/s41572-020-0171-3. [DOI] [PubMed] [Google Scholar]
- 3.Betson M, Søe M.J, Nejsum P. Human trichuriasis:Whipworm genetics, phylogeny, transmission and future research directions. Curr. Trop. Med. Rep. 2015;2(4):209–217. [Google Scholar]
- 4.Cavallero S, De Liberato C, Friedrich K.G, Di Cave D, Masella V, D'Amelio S, Berrilli F. Genetic heterogeneity and phylogeny of Trichuris spp 2015 from captive non-human primates based on ribosomal DNA sequence data. Infect. Genet. Evol. 34:450–456. doi: 10.1016/j.meegid.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Adrus M, Zainudin R, Ahamad M, Jayasilan M.A, Abdullah M.T. Gastrointestinal parasites of zoonotic importance observed in the wild, urban, and captive populations of non-human primates in Malaysia. J. Med. Primatol. 2019;48(1):22–31. doi: 10.1111/jmp.12389. [DOI] [PubMed] [Google Scholar]
- 6.Arizono N, Yamada M, Tegoshi T, Onishi K. Molecular identification of Oesophagostomum and Trichuris eggs isolated from wild Japanese macaques. Korean J. Parasitol. 2012;50(3):253–257. doi: 10.3347/kjp.2012.50.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Dong H, Wang R, Yu F, Wu Y, Chang Y, Wang C, Qi M, Zhang L. An investigation of parasitic infections and review of molecular characterization of the intestinal protozoa in non-human primates in China from 2009 to 2015. Int. J. Parasitol. Parasites Wildl. 2017;6(1):8–15. doi: 10.1016/j.ijppaw.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Filippo M.M, Berrilli F, De Liberato C, Di Giovanni V, D'Amelio S, Friedrich K.G, Cavallero S. Molecular characterization of Trichuris spp 2020 from captive animals based on mitochondrial markers. Parasitol. Int. 75:102043. doi: 10.1016/j.parint.2019.102043. [DOI] [PubMed] [Google Scholar]
- 9.Hope K, Goldsmith M, Graczyk T. Parasitic health of olive baboons in Bwindi Impenetrable National Park, Uganda. Vet. Parasitol. 2004;122(2):165–170. doi: 10.1016/j.vetpar.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Ghai R.R, Simons N.D, Chapman C.A, Omeja P.A, Davies T.J, Ting N, Goldberg T.L. Hidden population structure and cross-species transmission of whipworms (Trichuris spp 2014) in humans and non-human primates in Uganda. PLoS. Negl. Trop. Dis. 8(10):e3256–e3256. doi: 10.1371/journal.pntd.0003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boundenga L, Moussadji C, Mombo I.M, Ngoubangoye B, Lekana-Douki J.B, Hugot J.P. Diversity and prevalence of gastrointestinal parasites in two wild Galago species in Gabon. Infect. Genet. Evol. 2018;63:249–256. doi: 10.1016/j.meegid.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Schurer J.M, Ramirez V, Kyes P, Tanee T, Patarapadungkit N, Thamsenanupap P, Trufan S, Grant E.T, Garland-Lewis G, Kelley S, Nueaitong H, Kyes R.C, Rabinowitz P. Long-tailed macaques (Macaca fascicularis) in Urban landscapes:Gastrointestinal parasitism and barriers for healthy coexistence in northeast Thailand. Am. J. Trop. Med. Hyg. 2019;100(2):357–364. doi: 10.4269/ajtmh.18-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panti-May J.A, Servían A, Ferrari W, Zonta M.L, Hernández-Mena D.I, Hernández-Betancourt S.F, Robles M.D.R, Machain-Williams C. Morphological and molecular identification of hymenolepidid cestodes in children and synanthropic rodents from rural Mexico. Parasitol. Int. 2020;75:102042. doi: 10.1016/j.parint.2019.102042. [DOI] [PubMed] [Google Scholar]
- 14.Panti-May J.A, Rodríguez-Vivas R.I, García-Prieto L, Servián A, Costa F. Worldwide overview of human infections with Hymenolepis diminuta. Parasitol. Res. 2020;119(7):1997–2004. doi: 10.1007/s00436-020-06663-x. [DOI] [PubMed] [Google Scholar]
- 15.Tiwari S, Karuna T, Rautaraya B. Hymenolepis diminuta infection in a child from a rural area:A rare case report. J. Lab. Physicians. 2014;6(1):58–59. doi: 10.4103/0974-2727.129096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patamia I, Cappello E, Castellano-Chiodo D, Greco F, Nigro L, Cacopardo B. A human case of Hymenolepis diminuta in a child from eastern Sicily. Korean J. Parasitol. 2010;48(2):167–169. doi: 10.3347/kjp.2010.48.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michaud C, Tantalean M, Ique C, Montoya E, Gozalo A. A survey for helminth parasites in feral new world non-human primate populations and its comparison with parasitological data from man in the region. J. Med. Primatol. 2003;32(6):341–345. doi: 10.1046/j.1600-0684.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Solano S, Carrillo-Bilbao G.A, Ramirez W, Celi-Erazo M, Huynen M.-C, Levecke B, Benitez-Ortiz W, Losson B. Gastrointestinal parasites in captive and free-ranging Cebus albifrons in the Western Amazon, Ecuador. Int. J. Parasitol. Parasites Wildl. 2017;6(3):209–218. doi: 10.1016/j.ijppaw.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malaivijitnond S, Hamada Y. Current situation and status of long-tailed macaques (Macaca fascicularis) in Thailand. Nat. Hist. J. Chulalongkorn Univ. 2008;8(2):185–204. [Google Scholar]
- 20.Klaus A, Zimmermann E, Röper K.M, Radespiel U, Nathan S, Goossens B, Strube C. Co-infection patterns of intestinal parasites in arboreal primates (proboscis monkeys, Nasalis larvatus) in Borneo. Int. J. Parasitol. Parasites Wildl. 2017;6(3):320–329. doi: 10.1016/j.ijppaw.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson P.D, Yoder K, Fajardo L.G.L, Marty A.M, van de Pas S, Olivier C, Relman D.A. Lethal invasive cestodiasis in immunosuppressed patients. J. Infect. Dis. 2003;187(12):1962–1966. doi: 10.1086/375357. [DOI] [PubMed] [Google Scholar]
- 22.Khurana S, Sethi S. Laboratory diagnosis of soil-transmitted helminthiasis. Trop. Parasitol. 2017;7(2):86–91. doi: 10.4103/tp.TP_29_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tijjani M, Majid R.A, Abdullahi S.A, Unyah N.Z. Detection of rodent-borne parasitic pathogens of wild rats in Serdang, Selangor, Malaysia:A potential threat to human health. Int. J. Parasitol. Parasites Wildl. 2020;11:174–182. doi: 10.1016/j.ijppaw.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


