Abstract
Background: Antimicrobial resistance (AMR) is a growing global health problem. Staphylococcus aureus (SA) is a common bacterium associated with a variety of community and hospital infections. Methicillin-resistant Staphylococcus aureus (MRSA) accounts for most SA related morbidity and mortality. In this study, we determined the prevalence and factors associated with SA and MRSA in Myanmar. Methods: We collected the data retrospectively by reviewing an electronic register containing the results of bacterial culture and antibiotic susceptibility testing of biological specimens received from healthcare facilities during 2018–2019. Results: Of the 37,798 biological specimens with bacterial culture growth, 22% (8244) were Gram-positive. Among the Gram-positive bacteria, 42% (2801) were SA, of which 48% (1331) were judged as MRSA by phenotypic methods. The prevalence of MRSA was higher in the older age groups, in female patients, in urine specimens and specimens received from the intensive care unit and dermatology departments. One site (Site F) had the highest MRSA prevalence of the seven AMR sentinel sites. Most SA isolates were sensitive to vancomycin (90%) by phenotypic methods. Conclusions: The high prevalence of MRSA indicates a major public health threat. There is an urgent need to strengthen the AMR surveillance and hospital infection control program in Myanmar.
Keywords: antimicrobial resistance, hospital infections, MRSA, Gram-positive bacteria, SORT IT, operations research
1. Introduction
Antimicrobial resistance (AMR) is a serious emerging global health problem in this century. Staphylococcus aureus (SA) is an antibiotic-resistant pathogen of significant public health concern [1]. Humans can become infected with SA both in the community and in healthcare settings. SA causes a wide range of human infections like bacteremia, endocarditis, skin and soft tissue infections, bone and joint infections and hospital-acquired infections [2].
The inappropriate use of antibiotics contributes to antibiotic resistance in SA. MRSA (methicillin-resistant Staphylococcus aureus) is a subgroup of SA. As the name suggests, MRSA does not respond to common antibiotics, such as methicillin, amoxicillin, and penicillin. In the United States, the incidence of MRSA bloodstream infections declined from 74% to 40% during 2005–2016. Despite this decline, it is estimated that nearly 120,000 SA bloodstream infections and 20,000 SA-associated deaths occurred in 2017 [3]. The Asia Pacific Regional Resistance Surveillance program reported that 26% to 73% of SA isolates from healthcare settings in the region were resistant to methicillin [4].
Infection with MRSA remains associated with poorer clinical outcomes and increased healthcare costs. A multicenter study conducted in China between 2013 and 2015 showed that the MRSA infection was significantly associated with higher total hospital cost, longer length of hospital stay, and increased mortality rate as compared to Methicillin-sensitive Staphylococcus aureus (MSSA) infection, especially in patients with underlying diseases such as malignancy or chronic pulmonary diseases [5]. However, evidence from high-income countries proved that implementing an effective hospital infection control program significantly reduces the morbidity and mortality of MRSA-associated infections [6,7,8]. The improvement in hand hygiene compliance can significantly decrease MRSA rates in hospitals [9].
According to the World Health Organization (WHO) estimate, Myanmar has the highest MRSA proportion (26%) among the South East Asian countries that reported national data relating to antibiotic resistance. However, this estimate was based on approximately 30 isolates only [1]. To date, there is minimal information on the prevalence of MRSA, morbidity, and mortality in Myanmar from a few publications on microbiological and animal studies [10,11,12]. A recently published study conducted in a tertiary care hospital in Myanmar revealed that the molecular detection of MRSA accounted for 13.8% [13]. A retrospective study from one hospital examining blood culture results showed a decline in MRSA among SA isolates (38.7% to 18.8%) over eight years [14]. Establishing a proper surveillance system and an effective hospital infection control system is mandatory to minimize the emergence of MRSA and to reduce its spread. Although national guidelines on hospital infection control were developed in Myanmar in 2016, clinicians’ adherence to the guidelines is still low [15]. Besides, guidelines related to antibiotic prescription do not exist at the national level, although some tertiary hospitals have developed their own antibiotic guidelines. This study aimed to determine the prevalence of SA and the factors associated with MRSA in healthcare settings in Myanmar during 2018–2019. The objectives of the study were (1) to assess the number (and proportion) of samples with SA infection among the total biological samples received for bacterial culture and drug susceptibility testing at seven AMR sentinel sites between 2018 and 2019; (2) to describe the antibiotic susceptibility pattern of SA infection and assess the number and proportion with MRSA infection; (3) to describe the demographic and clinical profile of patients and determine their association with MRSA.
2. Material and Methods
2.1. Study Design
This was a retrospective descriptive study based on the electronic register record of seven AMR sentinel laboratories in Myanmar.
2.2. Setting
2.2.1. General Setting
The Union of the Republic of Myanmar is located in the South East Asian region and bordered by the Bay of Bengal, Andaman Sea, Gulf of Thailand, and the countries of Bangladesh, India, China, Laos, and Thailand. The country is administratively divided into 14 States/Regions and Nay Pyi Taw Union Territory. It has a population of 51.48 million. Healthcare is provided by both the public and private sectors. General practitioner clinics and drug shops are the initial points of healthcare seeking for most populations. Antibiotics are readily available over the counter.
2.2.2. Specific Setting
In Myanmar, the AMR surveillance system at the national level is being carried out through seven public hospitals and laboratories, which can cover AMR’s overall situation in Myanmar. Five of them (Site A, B, C, D and E) are located in Yangon Region, covering the Yangon Region population and some population from the lower part of Myanmar. Site F is located in the Mandalay Region, and it covers the population from upper Myanmar. Site G is located in Nay Pyi Taw Union Territory in the central part of Myanmar, and it covers the population in Nay Pyi Taw Union Territory and surrounding townships. The populations of Yangon Region and Mandalay Region are 7.3 million and 6.1 million, respectively. The distribution of the seven sentinel sites is shown in Figure 1.
Figure 1.
Distribution of seven AMR sentinel sites in Myanmar.
2.2.3. AMR Surveillance in Myanmar
The National Action Plan (NAP) to combat AMR has been developed in line with the Global Action Plan of AMR since 2017, with five strategic objectives (awareness, surveillance, infection prevention and control, antimicrobial usage, and research and innovation). National Multi-sectoral Steering Committee (NMSC) was organized to provide the necessary political commitments to fight against AMR. Five technical working groups (TWGs) were constituted under NMSC to implement the five strategic objectives of NAP AMR. The National Coordinating Centre (NCC) for AMR is responsible for coordinating between the NMSC and the five TWGs for combating AMR. Myanmar developed National AMR Surveillance Guidelines (draft) in 2020 for standardization of the AMR surveillance system.
2.2.4. Laboratory Procedure
Patients’ clinical specimens were collected and processed by using standard microbiological procedures. The first- and second-line drugs and antibiotic susceptibility testing of these drugs were conducted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [16]. MRSA was screened for phenotypically by using oxacillin MIC (≥4 µg/mL) in an automated system and cefoxitin disc (30 µg) for manual method. MIC determinations by broth or agar dilution or by E test using a 0.5 McFarland standard to prepare inoculum are the gold standard for determining vancomycin susceptibility. In this study, there was no standard method used for VRSA detection since the sentinel sites did not have this capacity. Vancomycin sensitivity testing was done by only automated Vitek 2 system for clinical purposes and vancomycin MIC ≥ 16 is interpreted as resistant. Antibiotic susceptibility pattern was detected by using the modified Kirby–Bauer method or Vitek 2 AST GP 67 according to the capacity of each sentinel sites. Due to the resource constraint, some sentinel sites (especially Site F) used manual method and some used automated Vitek 2 system. In this study 70% of samples from all sentinel sites were tested by Vitek 2 automated system and the rest were tested by manual method. The reference ranges of drug susceptibility pattern were set as shown in Table 1. The testing validity of all sentinel sites were evaluated by regular internal quality control and national external quality assessment scheme (EQAS) of national reference laboratory. The routine culture and sensitivity data were recorded both in the register book and electronic database WHONET software which is a free desktop Windows application for the management and analysis of microbiology laboratory data with a particular focus on antimicrobial resistance surveillance developed and supported by the WHO Collaborating Centre for Surveillance of Antimicrobial Resistance The AMR data from all sentinel sites were sent to the National Coordinating Centre (NCC) bi-annually. NCC combines all data and validates and then annually uploads it to the Global Antimicrobial Surveillance System (GLASS) IT platform. National Health Laboratory is responsible for quality control of laboratories throughout the country as an organizer and provider of national external quality assessment scheme (NEQAS) of culture and sensitivity testing bi-annually.
Table 1.
Zone diameter in millimeter and MIC breakpoints (µg/mL) for Staphylococcus aureus according to CLSI guidelines.
| Drug | Zone Diameter Breakpoints (mm) | MIC Breakpoints (µg/mL) | ||||
|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |
| First-Line Drugs | ||||||
| Cefoxitin | ≥22 | - | ≤21 | ≤4 | - | ≥8 |
| Oxacillin | - | - | - | ≤2 | - | ≥4 |
| Penicillin | ≥29 | - | ≤28 | ≤0.12 | - | ≥0.25 |
| Clindamycin | ≥21 | 15–20 | ≤14 | ≤0.5 | 1–2 | ≥4 |
| Erythromycin | ≥23 | 14–22 | ≤13 | ≤0.5 | 1–4 | ≥8 |
| Cotrimoxazole | ≥16 | 11–15 | ≤10 | <2/38 | - | ≥4/76 |
| Nitrofurantoin | ≥17 | 15–16 | ≤14 | ≤32 | 64 | ≥128 |
| Second-Line Drugs | ||||||
| Linezolid | ≥21 | - | ≤20 | ≤4 | - | ≥8 |
| Tetracycline | ≥19 | 15–18 | ≤14 | ≤4 | 8 | ≥16 |
| Vancomycin | - | - | - | ≤2 | 4–8 | ≥8 |
| Rifampicin | ≥20 | 17–19 | ≤16 | ≤1 | 2 | ≥4 |
| Gentamicin | ≥15 | 13–14 | ≤12 | ≤4 | 8 | ≥16 |
| Ciprofloxacin | ≥21 | 16–20 | ≤15 | ≤1 | 2 | ≥4 |
| Levofloxacin | ≥19 | 16–18 | ≤15 | ≤1 | 2 | ≥4 |
2.3. Study Population and Period
The study populations were all patients’ specimens sent for routine culture and antibiotic susceptibility testing in seven AMR sentinel sites during the study period of 1 January 2018 to 31 December 2019.
2.4. Data Variables and Sources of Data
Data variables included specimen ID, lab test registration date, sentinel sites, number of the specimen, demographic data, type and source (by ward) of the patient whose specimen was sent for culture and sensitivity, specimen type, culture result and antibiotic susceptibility pattern.
Routine laboratory register data at seven sentinel sites were maintained in an electronic database (WHONET software) starting from 2018. Data on different variables were entered routinely into the WHONET software from paper-based laboratory registers by technicians from each sentinel laboratory. We obtained data from the WHONET database of NCC and laboratory registers of seven sentinel sites.
2.5. Data Collection, Analysis and Statistics
Data from WHONET software was extracted into Microsoft Excel and imported into EpiData Analysis and Stata Statistical Software. We described the prevalence of SA, the antibiotic susceptibility pattern and MRSA in numbers and proportions.
The patient characteristics of those with SA infection are described in numbers and proportions. The associations between patient characteristics and the presence of MRSA among their specimens with SA infection are described by prevalence ratios and adjusted prevalence ratios. We used bi-variable and multivariable binomial log models for obtaining the prevalence and adjusted prevalence ratios. A p-value < 0.05 has been considered for statistical significance.
3. Results
3.1. Culture and Sensitivity of SA
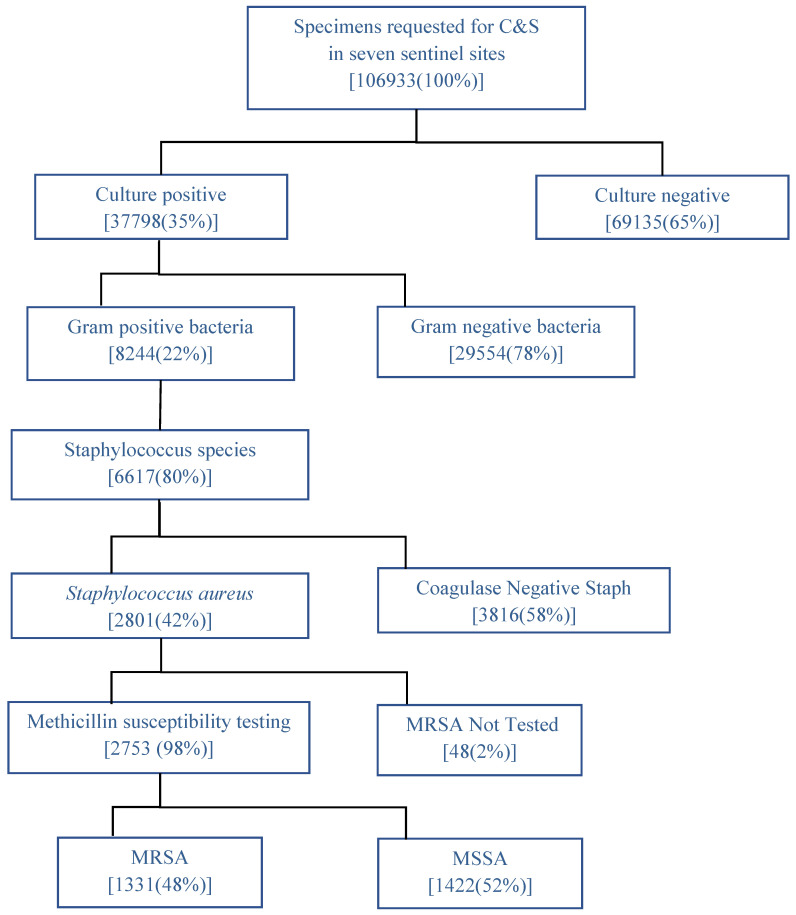
Figure 2 shows the results of culture and drug susceptibility testing of samples received at seven AMR sentinel sites in Myanmar in 2018–2019 and the prevalence of SA and MRSA. During 2018 and 2019, a total of 106,933 specimens were received for culture and sensitivity in the seven sentinel sites, of which Gram-positive bacterial growth was observed in 8244 samples. Nearly 80% of the Gram-positive bacteria belonged to the Staphylococcus species. Among the Staphylococcus species, 2801 were SA, of which 1331 (48%) were MRSA.
Figure 2.
Results of culture and drug susceptibility testing of samples received at seven AMR sentinel sites in Myanmar in 2018–2019 and the prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. C&S = culture and sensitivity. MRSA = Methicillin-resistant Staphylococcus aureus. MSSA = Methicillin sensitive Staphylococcus aureus. AMR = Antimicrobial Resistance.
3.2. Antibiotic Susceptibility Pattern of SA
The antibiotic susceptibility pattern of SA isolated in seven AMR Sentinel Sites is described in Table 2. Among 2753 SA isolates, 48% were found to be MRSA. The first-line and second-line drugs were grouped according to the Clinical Laboratory Standard Institute guideline. The most sensitive first-line drug was nitrofurantoin (97%), and the least sensitive first-line drug was penicillin (3%). Among the second-line drugs, high sensitivity was observed to Linezolid (91%), and high resistance was seen to tetracycline (58%).
Table 2.
Antibiotic susceptibility pattern of Staphylococcus aureus isolated in seven AMR Sentinel Sites, Myanmar, 2018–2019.
| Antibiotic Name (n) | Sensitive | Intermediate | Resistant | |||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| First-line drugs | ||||||
| Cefoxitin (n = 2753) | 1422 | (52) | 0 | (0) | 1331 | (48) |
| Penicillin (n = 1302) | 35 | (3) | 0 | (0) | 1267 | (97) |
| Clindamycin (n = 2755) | 1710 | (62) | 112 | (4) | 933 | (34) |
| Erythromycin (n = 2704) | 1211 | (45) | 206 | (8) | 1287 | (48) |
| Cotrimoxazole (n = 2678) | 1565 | (58) | 39 | (1) | 1074 | (40) |
| Nitrofurantoin (n = 1332) | 1287 | (97) | 12 | (1) | 33 | (2) |
| Second-line drugs | ||||||
| Linezolid (n = 2624) | 2375 | (91) | 0 | (0) | 249 | (9) |
| Tetracycline (n = 1426) | 833 | (58) | 6 | (0) | 587 | (41) |
| Vancomycin (n = 1249) | 1124 | (90) | 30 | (2) | 95 | (8) |
| Rifampicin (n = 1258) | 1032 | (82) | 54 | (4) | 172 | (14) |
| Gentamicin (n = 2754) | 1816 | (66) | 79 | (3) | 859 | (31) |
| Ciprofloxacin (n = 1409) | 1006 | (71) | 46 | (3) | 357 | (25) |
| Levofloxacin (n = 2765) | 1812 | (66) | 68 | (2) | 885 | (32) |
** organism which is resistant to cefoxitin disc (30 µg) or oxacillin MIC (≥4 µg/mL) were listed as MRSA.
3.3. Distribution of SA Infection among the Isolates
The demographic and clinical characteristics of patients whose isolates tested positive for SA infection in seven AMR Sentinel Sites are described in Table 3. The total number of isolates with SA infection among Gram-positive isolates in 2018 and 2019 were 1324 (35%) and 1477 (34%), respectively. In 2018 and 2019, SA infection was mainly found in the 15–44 years age group (about 38%). It was mainly found in the isolates of male patients (54%) and hospitalized patients (79% & 90% in respective years). The most common specimens associated with SA infection were wound/pus and blood.
Table 3.
Demographic and clinical characteristics of patients whose isolates tested positive for Staphylococcus aureus infection in seven AMR Sentinel Sites, Myanmar, 2018–2019.
| Variable | Patients Whose Isolates Were Tested Positive with SA Infection (2018, n = 1324) | Patients Whose Isolates Were Tested Positive with SA Infection (2019, n = 1477) | ||
|---|---|---|---|---|
| n | (%) | N | (%) | |
| Age (years) | ||||
| <15 | 274 | (21) | 284 | (19) |
| 15–44 | 502 | (38) | 545 | (37) |
| 45–64 | 363 | (27) | 448 | (30) |
| ≥65 | 173 | (13) | 186 | (13) |
| Unknown | 12 | (1) | 14 | (1) |
| Gender | ||||
| Male | 711 | (54) | 802 | (54) |
| Female | 609 | (46) | 672 | (46) |
| Unknown | 4 | (0) | 3 | (0) |
| Type of Patient | ||||
| Inpatient | 1046 | (79) | 1324 | (90) |
| Outpatient | 229 | (17) | 101 | (7) |
| Unknown | 49 | (4) | 52 | (4) |
| Type of Specimen | ||||
| Blood | 282 | (21) | 474 | (32) |
| Sputum/Respiratory | 149 | (11) | 172 | (12) |
| Urine | 107 | (8) | 105 | (7) |
| Wound (pus/swab) | 664 | (50) | 647 | (44) |
| Body fluid | 30 | (2) | 8 | (1) |
| Miscellaneous | 92 | (7) | 71 | (5) |
| Source of patient | ||||
| Medical | 370 | (28) | 472 | (32) |
| Surgical | 443 | (33) | 494 | (33) |
| Paediatric | 55 | (4) | 150 | (10) |
| ICU | 61 | (5) | 57 | (4) |
| Dermatology | 133 | (10) | 99 | (7) |
| Emergency/OPD | 94 | (7) | 81 | (5) |
| Unknown | 89 | (7) | 80 | (5) |
| Others | 79 | (6) | 44 | (3) |
3.4. Prevalence of SA and MRSA in Seven AMR Sentinel Sites
The prevalence of SA and MRSA in seven sentinel sites during 2018 and 2019 is shown in Table 4. The highest proportion of SA and MRSA were found in Site F. The lowest proportion of SA was found in Site B, while Site G had the lowest proportion of MRSA in both study years. There was a considerable decrease in the proportions of SA and MRSA in Site E in 2019.
Table 4.
Prevalence of Staphylococcus aureus and Methicillin-resistant Staphylococcus aureus isolated in seven AMR sentinel sites, Myanmar, 2018–2019.
| Sites | 2018 | 2019 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Isolates | Gram-Positive (n, %) |
SA (n, %) |
MRSA (n, %) |
Total Isolates | Gram-Positive (n, %) |
SA (n, %) |
MRSA (n, %) |
|||||||
| Site A | 1773 | 398 | (22) | 87 | (22) | 23 | (26) | 1388 | 287 | (21) | 56 | (20) | 18 | (32) |
| Site B | 5028 | 1570 | (31) | 298 | (19) | 114 | (38) | 5484 | 1877 | (34) | 348 | (19) | 124 | (36) |
| Site C | 1355 | 182 | (13) | 48 | (26) | 20 | (42) | 1279 | 228 | (18) | 71 | (31) | 17 | (24) |
| Site D | 4088 | 629 | (15) | 186 | (30) | 78 | (42) | 3378 | 625 | (21) | 187 | (27) | 89 | (48) |
| Site E | 2967 | 278 | (9) | 140 | (50) | 55 | (39) | 2880 | 391 | (14) | 106 | (27) | 23 | (22) |
| Site F | 3294 | 628 | (19) | 508 | (81) | 388 | (76) | 3371 | 796 | (24) | 607 | (76) | 359 | (59) |
| Site G | 480 | 110 | (23) | 57 | (52) | 10 | (18) | 1033 | 175 | (17) | 102 | (58) | 13 | (13) |
| Total | 18,985 | 3795 | 1324 | 688 | 18,813 | 4397 | 1477 | 643 | ||||||
3.5. Factors Associated with MRSA
Demographic and clinical factors associated with MRSA infection in seven AMR sentinel sites, Myanmar, are described in Table 5. The factors that were associated with a higher prevalence of MRSA were: year of sample collection (higher prevalence in 2018 when compared to 2019), age (higher prevalence in age groups 15 and above), gender (higher prevalence in females when compared to males), type of specimen (higher prevalence in urine when compared to blood), source of the patient (higher prevalence in those from ICU and dermatology when compared to medical wards), and the sentinel laboratory site (higher prevalence in Site F) when compared to all other sites.
Table 5.
Demographic and clinical factors of patients whose isolates were associated with MRSA infection in seven AMR sentinel sites, Myanmar, 2018–2019.
| Variable | Patients with SA Infection (N = 2801) |
Patients with MRSA Infection (N = 1331) |
Prevalence Ratio (95% CI) |
Adjusted PR (95% CI) |
p-Value | |||
|---|---|---|---|---|---|---|---|---|
| n | n | (%) | ||||||
| Year | ||||||||
| 2018 | 1324 | 688 | (52) | Ref | Ref | |||
| 2019 | 1477 | 643 | (44) | 0.84 | (0.78–0.91) | 0.86 | (0.80–0.92) | <0.001 |
| Age (years) | ||||||||
| <15 | 558 | 256 | (46) | Ref | Ref | |||
| 15–44 | 1047 | 482 | (46) | 0.99 | (0.89–1.11) | 1.15 | (1.02–1.31) | 0.021 |
| 45–64 | 811 | 399 | (49) | 1.06 | (0.94–1.19) | 1.19 | (1.04–1.35) | <0.001 |
| ≥65 | 359 | 185 | (52) | 1.11 | (0.97–1.27) | 1.27 | (1.09–1.47) | 0.001 |
| Unknown | 26 | 9 | (35) | 0.73 | (0.43–1.25) | 0.80 | (0.46–1.40) | 0.451 |
| Gender | ||||||||
| Male | 1513 | 692 | (46) | 0.92 | (0.85–1.00) | 0.92 | (0.86–0.99) | 0.034 |
| Female | 1281 | 634 | (49) | Ref | ||||
| Unknown | 7 | 5 | (71) | 1.42 | (0.88–2.27) | 1.86 | (0.91–3.80) | 0.144 |
| Type of Patient | ||||||||
| Inpatient | 2370 | 1105 | (47) | Ref | Ref | |||
| Outpatient | 330 | 185 | (56) | 1.22 | (1.10–1.36) | 1.07 | (0.93–1.24) | 0.296 |
| Unknown | 101 | 41 | (41) | 0.85 | (0.67–1.08) | 0.86 | (0.66–1.11) | 0.265 |
| Type of Specimen | ||||||||
| Blood | 756 | 390 | (52) | Ref | Ref | |||
| Sputum/Respiratory | 321 | 172 | (54) | 1.03 | (0.91–1.16) | 1.00 | (0.89–1.13) | 0.870 |
| Urine | 212 | 143 | (68) | 1.35 | (1.20–1.50) | 1.21 | (1.08–1.36) | 0.001 |
| Wound (pus/swab) | 1311 | 524 | (40) | 0.78 | (0.71–0.86) | 0.98 | (0.88–1.09) | 0.754 |
| Body fluid | 38 | 17 | (45) | 0.86 | (0.60–1.23) | 0.77 | (0.53–1.11) | 0.164 |
| Miscellaneous | 163 | 85 | (52) | 1.02 | (0.87–1.20) | 1.15 | (0.97–1.35) | 0.088 |
| Source of Patient | ||||||||
| Medical | 842 | 413 | (49) | Ref | Ref | |||
| Surgical | 937 | 353 | (38) | 0.77 | (0.70–0.86) | 0.83 | (0.75–0.94) | 0.003 |
| Paediatric | 205 | 103 | (50) | 1.02 | (0.88–1.19) | 0.87 | (0.73–1.05) | 0.154 |
| ICU | 118 | 87 | (74) | 1.49 | (1.31–1.69) | 1.16 | (1.03–1.30) | 0.013 |
| Dermatology | 232 | 153 | (66) | 1.34 | (1.19–1.50) | 2.04 | (1.73–2.40) | <0.001 |
| OPD/Emergency | 175 | 90 | (51) | 1.06 | (0.90–1.24) | 0.91 | (0.74–1.11) | 0.388 |
| Unknown | 169 | 54 | (32) | 0.67 | (0.53–0.84) | 1.13 | (0.79–1.61) | 0.487 |
| Others | 123 | 78 | (63) | 1.32 | (0.14–1.53) | 1.17 | (1.01–1.36) | 0.037 |
| Laboratory Sites | ||||||||
| Site F | 1115 | 747 | (67) | Ref | Ref | |||
| Site A | 143 | 41 | (29) | 0.44 | (0.34–0.57) | 0.38 | (0.25–0.59) | <0.001 |
| Site B | 646 | 238 | (37) | 0.55 | (0.49–0.61) | 0.41 | (0.36–0.47) | <0.001 |
| Site C | 119 | 37 | (31) | 0.46 | (0.35–0.60) | 0.47 | (0.35–0.62) | <0.001 |
| Site D | 373 | 167 | (45) | 0.66 | (0.58–0.74) | 0.56 | (0.49–0.65) | <0.001 |
| Site E | 246 | 78 | (32) | 0.47 | (0.39–0.57) | 0.49 | (0.41–0.60) | <0.001 |
| Site G | 159 | 23 | (15) | 0.24 | (0.16–0.35) | 0.28 | (0.19–0.41) | <0.001 |
4. Discussion
This study revealed three important findings on the prevalence of SA, MRSA and their associated factors. First, there was no significant difference in SA prevalence among isolates between 2018 and 2019; however, there was a decrease in MRSA prevalence in 2019. Second, the antibiotic susceptibility pattern showed SA isolates were highly resistant to a variety of first-line antibiotics (penicillin, erythromycin) and second-line antibiotics (tetracycline, gentamicin, ciprofloxacin, levofloxacin). Almost all samples of SA were resistant to penicillin but were highly sensitive to nitrofurantoin. Third, MRSA infection was significantly associated with gender, age, specimen types, source of patients and sentinel sites.
The prevalence of SA infection among biological specimens sent for culture and drug susceptibility testing in this study were five times higher than the results reported in the previous two studies from Myanmar, both of which were conducted in a single hospital and only focused on blood specimens [17,18]. Our study also showed a higher SA prevalence than the studies undertaken in India and Nepal [19,20]. The reasons for the high prevalence need to be addressed. The national guidelines on infection prevention and control were launched in 2016. The Ministry of Health and Sports Myanmar allocated the required budget for hospitals to implement the infection control activities and provided the necessary training. Nevertheless, our study results indicate that infection control efforts should go beyond establishing guidelines, budget allocation, and training. On the other hand, limited human resources and poor hospital infrastructure may have led to the lax implementation of infection prevention and control measures.
Studies across Asia showed a wide range of methicillin resistance among SA isolates with higher rates in hospital settings (0.7% to 75%) and lower rates in community settings (0% to 48%) [21,22,23,24]. This is perhaps due to the differences in the study population and geographic locations where the studies were conducted. Previous studies have shown that the prevalence of MRSA among SA is highly variable. According to the Annual NHL report, there was a rise in MRSA prevalence in Myanmar from 30% in 2016 to 44% in 2019; however, our study observed a significant decline in 2019 (0.78–0.91; p < 0.001) compared to 2018 [25,26]. This reduction may be likely due to raised AMR awareness among healthcare providers, improved testing capacity of laboratories and diagnostic stewardship activities. The lower percentage in MRSA was found in two studies performed in Myanmar in which the MRSA prevalence was 8% and 13.8%, respectively [10,13]. The reason is due to the differences in detection methodology which use mecA gene PCR for MRSA detection and the focus on only one hospital.
Resistance to the penicillin group of antibiotics, erythromycin and gentamycin, among MRSA isolates has been reported in two previous studies in Myanmar, one of which reviewed hospital records since 2005 [10,14]. However, Myanmar-based studies determining drug sensitivity for second-line antibiotics among MRSA are scant, and our study is the first one to report on this. Our study indicates high levels of resistance to second-line antibiotics. Over the counter availability and widespread use of penicillin group of antibiotics among the general practitioners and community may be responsible for high levels of resistance to these drugs. A similar antibiotic susceptibility pattern for second-line antibiotics was found in a study in Pakistan [27] but it was quite different from a study undertaken in India [28]. When reviewing the global prevalence of VRSA in a meta-analysis study, the overall percentage of VRSA increased from 1.2% to 2.4% over a 10 year duration [29]. In this study, the percentage of vancomycin-resistant SA (VRSA) is quite high (8%). This is an important indication that vancomycin testing in Myanmar should be standardized and required confirmation to get more reliable and correct data. Further focused research on VRSA prevalence in Myanmar is recommended.
Our study found that SA infection was common among male patients, while MRSA infection was significantly associated with the female gender. The effect of gender on SA infection is unknown. It is speculated that female sex hormones may modify the immune response and impact contracting infection [30].
Our study observed a positive association between MRSA infection and age [31], and a systematic literature review from India also showed similar evidence [32]. Older age groups are more prone to get infected due to decreased host resistance and increased exposure to healthcare settings. In contrast to other studies, we found the highest proportion of MRSA in urine specimens [23,33,34]. This may be due to urinary catheterization practice and the colonization by MRSA of indwelling urinary catheters.
We identified that certain units of the hospital (ICU and dermatological wards) and in particular the laboratory site (Site F) were significantly associated with a higher rate of MRSA infection. The reason for the significantly higher rate of MRSA needs to be addressed. As a first opinion, there may really be a higher MRSA prevalence in the population, especially at site F. The second probable reason is the methods used for MRSA detection that are the phenotypic method (automated Vitek 2 system or manual method by modified Kirby–Bauer method) by cefoxitin disc (30 µg); site F used only manual methods. Detailed evaluation of the situation related to site F will be considered for future research. The phenotypic method could be influenced by various factors, e.g., temperature and period of incubation, salt concentration in the media and the potency of the antibiotic disc. In such a case, the results of susceptibility testing by phenotypic methods may show generally higher resistance rate. Therefore, the quality of the antibiotic disc and methodology need to be validated. Moreover, the infrastructure of wards and hospitals, the effective implementation of infection control activities, and having patients with a high proportion of community-acquired MRSA are the possible factors influencing such an association [31]. Additionally, the lack of screening among healthcare providers for MRSA infection could also lead to higher MRSA prevalence in hospitals [35].
The strengths of this study are: (a) the use of nationwide data from all sentinel sites has increased the representativeness of the study results; (b) this is the first study assessing antibiotic susceptibility pattern of SA and MRSA in both first- and second-line antibiotics. The gold standard to identify MRSA is detection of mecA by PCR, but this was not used in this study and that is a limitation. Therefore, as described above, the detection rate of MRSA in this study might be higher than actual real prevalence of MRSA. Besides, the different methods used for MRSA screening might vary across the sites. Another limitation of the study is that we observed suboptimal recording and reporting systems in some sentinel sites. The first author tried to minimize these errors by validating all the data acquired from WHONET with the registers and records of all sentinel sites. Despite this effort, we are unable to rule out data errors.
5. Conclusions
Myanmar has a high prevalence of MRSA infection in healthcare settings, which poses a major public health threat. Appropriate action is needed to enhance the infection control programs in healthcare settings and to focus more on the appropriate use of antibiotics. The study results offer a baseline for the future surveillance of MRSA in Myanmar and have the potential to contribute to AMR policy and stewardship. There is also a higher rate of vancomycin resistance reported in this study among SA isolates and this finding is alarming in the context of AMR and the accuracy of the testing needs to be validated with precise methods for confirmation.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership coordinated by TDR, the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (TDR). The specific SORT IT program that led to these publications included a partnership of TDR with the WHO Country offices of Nepal and Myanmar and was implemented along with The Tuberculosis Research and Prevention Center Non-Governmental Organization, Armenia; International Union Against Tuberculosis and Lung Diseases, Paris and South East Asia offices; Institute of Tropical Medicine, Antwerp, Belgium; Sustainable Health Systems, Freetown, Sierra Leone; Department of Medical Research, Ministry of Health and Sports, Myanmar; School of Public Health, Kathmandu, Nepal; BahirDar University BahirDar, Ethiopia; Centre National de Formation et de Recherche en Santé Rurale de Maferinyah, Guinea; Department of Antibiotics and Infection Control of the Public Health Agency oy Sweden; the University of Toronto, Canada and the University of Washington.
Author Contributions
Conceptualization, P.E.S., W.W.H. and K.D.S.; methodology, P.E.S., W.W.H., K.D.S. and S.S.; software, S.S.; data collection and validation, P.E.S.; formal analysis, P.E.S. and S.S.; data curation, P.E.S.; writing—original draft preparation, P.E.S.; writing—review and editing, W.W.H., K.D.S., P.S. and S.S.; visualization, W.W.H., K.D.S. and S.S.; supervision, T.T.H. and H.H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This SORT IT AMR program was funded by the National Institute of Health Research, Department of Health & Social Care of the United Kingdom and supported by implementing partners. The cost of data collection was funded by the WHO Headquarters.
Institutional Review Board Statement
The permission for using the routine laboratory data from all sentinel sites was obtained from the chairperson of NCC for AMR, Myanmar. Ethics approval was obtained from the ethics advisory group of the International Union against Tuberculosis and Lung Disease, Paris, France (approval No. 67/19 of 19 August 2019) and the institutional review board of Department of Medical Research, Myanmar (approval No. Ethics/DMR/2019/126 of 6 November 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner N.A., Sharma-Kuinkel B.K., Maskarinec S.A., Eichenberger E.M., Shah P.P., Carugati M., Holland T.L., Fowler V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019;17:203–218. doi: 10.1038/s41579-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kourtis A.P., Hatfield K., Baggs J., Mu Y., See I., Epson E., Nadle J., Kainer M.A., Dumyati G., Petit S., et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019 doi: 10.15585/mmwr.mm6809e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendes R.E., Mendoza M., Banga Singh K.K., Castanheira M., Bell J.M., Turnidge J.D., Lin S.S.F., Jones R.N. Regional resistance surveillance program results for 12 Asia-Pacific nations (2011) Antimicrob. Agents Chemother. 2013;57:5721–5726. doi: 10.1128/AAC.01121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhen X., Lundborg C.S., Zhang M., Sun X., Li Y., Hu X., Gu S., Gu Y., Wei J., Dong H. Clinical and economic impact of methicillin-resistant Staphylococcus aureus: A multicentre study in China. Sci. Rep. 2020 doi: 10.1038/s41598-020-60825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatokoro M., Kihara K., Masuda H., Ito M., Yoshida S., Kijima T., Yokoyama M., Saito K., Koga F., Kawakami S., et al. Successful reduction of hospital-acquired methicillin-resistant Staphylococcus aureus in a urology ward: A 10-year study. BMC Urol. 2013;13:35. doi: 10.1186/1471-2490-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duerden B., Fry C., Johnson A.P., Wilcox M.H. The Control of Methicillin-Resistant Staphylococcus aureus Blood Stream Infections in England. Open Forum Infect. Dis. 2015;2 doi: 10.1093/ofid/ofv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui A.H., Koirala J. Methicillin Resistant Staphylococcus Aureus (MRSA) StatPearls Publishing; Treasure Island, FL, USA: 2019. [PubMed] [Google Scholar]
- 9.Marimuthu K., Pittet D., Harbarth S. The effect of improved hand hygiene on nosocomial MRSA control. Antimicrob. Resist. Infect. Control. 2014;3:1–6. doi: 10.1186/2047-2994-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aung M.S., Zi H., Nwe K.M., Maw W.W., Aung M.T., Min W.W., Nyein N., Kawaguchiya M., Urushibara N., Sumi A., et al. Drug resistance and genetic characteristics of clinical isolates of staphylococci in Myanmar: High prevalence of PVL among methicillin-susceptible Staphylococcus aureus belonging to various sequence types. New Microbes New Infect. 2016;10:58–65. doi: 10.1016/j.nmni.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aung M.S., San T., Aye M.M., Mya S., Maw W.W., Zan K.N., Htut W.H.W., Kawaguchiya M., Urushibara N., Kobayashi N. Prevalence and Genetic Characteristics of Staphylococcus aureus and Staphylococcus argenteus Isolates Harboring Panton-Valentine Leukocidin, Enterotoxins, and TSST-1 Genes from Food Handlers in Myanmar. Toxins. 2017;9:241. doi: 10.3390/toxins9080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myaing T.T., Thaw K.K., Htun L.L., Mhon M.M., Bawm S., Linn K.S., Wai S.S. Antimicrobial resistant pattern of methicillin-resistant Staphylococcus aureus isolated from stray dogs’ nasal swabs to fifteen antimicrobials in Myanmar. Int. J. Infect. Dis. 2016;53:48. doi: 10.1016/j.ijid.2016.11.125. [DOI] [Google Scholar]
- 13.Aung M.S., San T., Urushibara N., San N., Oo W.M., Soe P.E., Kyaw Y., Ko P.M., Thu P.P., Hlaing M.S. Molecular Characterization of Methicillin-Susceptible and-Resistant Staphylococcus aureus Harboring Panton-Valentine Leukocidin-Encoding Bacteriophages in a Tertiary Care Hospital in Myanmar. Microb. Drug Resist. 2020;26:360–367. doi: 10.1089/mdr.2019.0208. [DOI] [PubMed] [Google Scholar]
- 14.Myat T.O., Prasad N., Thinn K.K., Win K.K., Htike W.W., Zin K.N., Murdoch D.R., Crump J.A. Bloodstream infections at a tertiary referral hospital in Yangon, Myanmar. Trans. R. Soc. Trop. Med. Hyg. 2014;108:692–698. doi: 10.1093/trstmh/tru151. [DOI] [PubMed] [Google Scholar]
- 15.Ministry of Health and Sports . Hospital Infection Control Guidelines. Ministry of Health and Sports; Naypyitaw, Myanmar: 2016. [Google Scholar]
- 16.CLSI CLSI M100-ED29: 2019 Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. Clinical and Laboratory: Standards Institute; Wayne, PA, USA: 2019. [Google Scholar]
- 17.Thi K.S., Moe Z.W., Thein K.N. GP242 Early onset sepsis in extramural hospital of myanmar(burma) BMJ. 2019;104 doi: 10.1136/archdischild-2019-epa.301. [DOI] [Google Scholar]
- 18.Myat T.O., Oo K.M., Mone H.K., Htike W.W., Biswas A., Hannaway R.F., Murdoch D.R., Ussher J.E., Crump J.A. A prospective study of bloodstream infections among febrile adolescents and adults attending Yangon general hospital, Yangon, Myanmar. PLoS Negl. Trop. Dis. 2020 doi: 10.1371/journal.pntd.0008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sapkota J., Sharma M., Jha B., Bhatt C.P. Prevalence of staphylococcus aureus isolated from clinical samples in a tertiary care hospital: A descriptive cross-sectional study. J. Nepal Med. Assoc. 2019 doi: 10.31729/jnma.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelghafar A.A., Yousef N., Askora M. Prevalence and antimicrobial susceptibility patterns of Staphylococcus aureus isolated from different clinical sources. Zagazig J. Pharm. Sci. 2020;29:1–8. doi: 10.21608/zjps.2020.21790.1006. [DOI] [Google Scholar]
- 21.Wong J.W.H., Ip M., Tang A., Wei V.W.I., Wong S.Y.S., Riley S., Read J.M., Kwok K.O. Prevalence and risk factors of community-associated methicillin-resistant staphylococcus aureus carriage in asia-pacific region from 2000 to 2016: A systematic review and meta-analysis. Clin. Epidemiol. 2018;10:1489. doi: 10.2147/CLEP.S160595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wi Y.M., Rhee J.Y., Kang C.I., Chung D.R., Song J.H., Peck K.R. Clinical predictors of methicillin-resistance and their impact on mortality associated with Staphylococcus aureus bacteraemia. Epidemiol. Infect. 2018;146:1326–1336. doi: 10.1017/S0950268818001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanal L.K., Adhikari R.P., Guragain A. Prevalence of Methicillin Resistant Staphylococcus aureus and Antibiotic Susceptibility Pattern in a Tertiary Hospital in Nepal. J. Nepal Health Res. Counc. 2018 doi: 10.3126/jnhrc.v16i2.20305. [DOI] [PubMed] [Google Scholar]
- 24.Kaur Heyar A., Kaur Gill A., Mahajan A., Kaur K. Prevalence and Antibiotic Sensitivity Pattern of Staphylococcus Aureus From All Clinical Samples With Emphasis on Mrsa in a Tertiary Care Hospital. J. Evol. Med. Dent. Sci. 2017;6:5857–5860. doi: 10.14260/jemds/2017/1272. [DOI] [Google Scholar]
- 25.National Health Laboratory Analysis Report on Hospital Antimicrobial Resistance in Myanmar 2016. National Health Laboratory; Yangon, Myanmar: 2018. [Google Scholar]
- 26.National Health Laboratory Analysis Report on Hospital Antimicrobial Resistance in Myanmar 2017. National Health Laboratory; Yangon, Myanmar: 2019. [Google Scholar]
- 27.Hanif E., Hassan S.A. Evaluation of antibiotic resistance pattern in clinical isolates of Staphylococcus aureus. Pak. J. Pharm. Sci. 2019;32:1749–1753. [PubMed] [Google Scholar]
- 28.Vamsi Muni Krishna P., Sreenivasulu Reddy V., Praveen Kumar V., Suresh P. Antibiotic suscepyibility pattern of staphylococcus aureus and methicillin–resistant staphylococcus aureus isolated from various clinical specimens in a tertiary care teaching hospital, Pondicherry. Indian J. Public Heal. Res. Dev. 2019;10:266–271. doi: 10.37506/v10/i12/2019/ijphrd/191956. [DOI] [Google Scholar]
- 29.Shariati A., Dadashi M., Moghadam M.T., van Belkum A., Yaslianifard S., Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: A systematic review and meta-analysis. Sci. Rep. 2020 doi: 10.1038/s41598-020-69058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennell L.M., Galligan C.L., Fish E.N. Sex affects immunity. J. Autoimmun. 2012;38:J282–J291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Zhou L., Wu H., Soe M., Pollock D., Edwards J. Risk Factors Associated with Hospital-Onset MRSA Proportion—National Healthcare Safety Network, 2017–2018. Infect. Control Hosp. Epidemiol. 2020;41:s375–s376. doi: 10.1017/ice.2020.1007. [DOI] [Google Scholar]
- 32.Ghia C.J., Waghela S., Rambhad G. A Systemic Literature Review and Meta-Analysis Reporting the Prevalence and Impact of Methicillin-Resistant Staphylococcus aureus Infection in India. Infect. Dis. Res. Treat. 2020;13:1178633720970569. doi: 10.1177/1178633720970569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abubakar U., Sulaiman S.A.S. Prevalence, trend and antimicrobial susceptibility of Methicillin Resistant Staphylococcus aureus in Nigeria: A systematic review. J. Infect. Public Health. 2018;11:763–770. doi: 10.1016/j.jiph.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Nsofor C.A. Prevalence and Antibiotic Susceptibility Pattern of Staphylococcus Aureus Isolated from Various Clinical Specimens in South East Nigeria. MOJ Cell Sci. Rep. 2016 doi: 10.15406/mojcsr.2016.03.00054. [DOI] [Google Scholar]
- 35.Hawkins G., Stewart S., Blatchford O., Reilly J. Should healthcare workers be screened routinely for meticillin-resistant Staphylococcus aureus? A review of the evidence. J. Hosp. Infect. 2011;77:285–289. doi: 10.1016/j.jhin.2010.09.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon request.