Abstract
In the period from 2015 to 2020, an entomological survey for the presence of West Nile virus (WNV) and Usutu virus (USUV) in mosquitoes was performed in northwestern Croatia. A total of 20,363 mosquitoes were sampled in the City of Zagreb and Međimurje county, grouped in 899 pools and tested by real-time RT-PCR for WNV and USUV RNA. All pools were negative for WNV while one pool each from 2016 (Aedes albopictus), 2017 (Culex pipiens complex), 2018 (Cx. pipiens complex), and 2019 (Cx. pipiens complex), respectively, was positive for USUV. The 2018 and 2019 positive pools shared 99.31% nucleotide homology within the USUV NS5 gene and both clustered within USUV Europe 2 lineage. The next-generation sequencing of one mosquito pool (Cx. pipiens complex) collected in 2018 in Zagreb confirmed the presence of USUV and revealed several dsDNA and ssRNA viruses of insect, bacterial and mammalian origin.
Keywords: Usutu virus, West Nile virus, mosquitoes, Croatia
1. Introduction
West Nile virus (WNV) and Usutu virus (USUV) are mosquito-borne arboviruses of the genus Flavivirus, family Flaviviridae. In Europe, both viruses have been detected in humans, horses, mosquitoes, and birds [1,2].
WNV is sustained in an enzootic cycle between birds and mosquitoes while humans and horses represent “dead-end” hosts. Numerous bird species are competent amplifier hosts for WNV, and mosquitoes of the genus Culex are the main vectors [3]. After the first isolation of WNV in Uganda (1937), sporadic WNV cases were recorded in the subsequent years. WNV has been responsible for sporadic outbreaks in Mediterranean countries since 1960s [4]. In the last decade, the occurrence of outbreaks in many European countries has significantly increased [1,5]. So far, the highest number of WNV infections in Europe was recorded in 2018. Number of cases were seven times higher than in 2017 (1605 versus 212) and exceeded all cases reported between 2011 and 2017 [6].
In Croatia, first human cases of WNV neuroinvasive disease (WNND) were reported in 2012 in eastern counties [7]. In the following years, small outbreaks as well as sporadic cases were continuously recorded in continental Croatian counties [5]. In addition to human cases, asymptomatic WNV infections and seropositivity in sentinel horses [8] as well as in poultry [9] were detected in the same geographic areas. In 2018, 54 cases of WNND and 7 cases of WNV fever were recorded in 11 Croatian continental counties. In the same year, WNV RNA was detected for the first time in two dead goshawks (Accipiter gentilis) from the same aviary in northwestern Croatia [10,11].
In Europe, the most important vector species of WNV are Culex pipiens, Cx. perexiguus, and Cx. modestus [12,13]. WNV has been isolated from several species in Europe: Cx. pipiens, Ochlerotatus caspius, and Cx. modestus in Italy [14,15,16,17]; Cx pipiens and Cx. modestus in Greece [18]; Cx. pipiens and Cx. perexiguus [19] in Spain; Cx. modestus and Cx. pipiens complex in Slovakia [20]; and Cx. pipiens, Aedes vexans, and Culiseta annnulata in Serbia [21].
USUV is a mosquito-borne arbovirus, genetically closely related to WNV. The natural cycle of USUV is similar to that of WNV and involves birds as the main amplifying hosts and Culex mosquitoes as the main vectors [22,23,24]. However, the virus has also been detected in mosquitoes from other genera within the family of Culicidae [22,25]. Humans and horses are incidental (dead-end) hosts of USUV as well. The first USUV isolation was in 1959 from Cx. neavei mosquito caught near the Usutu River in Swaziland [26]. Since then, the widespread circulation of the virus has been observed in many countries. In Europe, USUV emerged in 1996 in the Tuscany region (Italy) [27]. In the following years, until today, the virus has been detected in many European countries in birds, mosquitoes, horses, bats, and humans, indicating that USUV become endemic in Europe [22,25].
In Croatia, the first serologic evidence of USUV was reported in 2011 in two seropositive horses detected in northwestern Croatia [28]. In 2012, USUV neutralizing antibodies were found in a human sample from a resident of eastern Croatia. During the 2013 WNV outbreak, the first three cases of neuroinvasive USUV disease were detected in Zagreb and surrounding areas [29]. In 2018, during the largest WNV outbreak, three additional human cases of USUV neuroinvasive disease were detected in one northwestern and two eastern Croatian counties. Moreover, USUV RNA was detected for the first time in one dead blackbird (Turdus merula) in Zagreb County [5,25].
The first isolation of USUV in Europe was made in 2006 in Cx. pipiens mosquitoes from Catalonia, Spain [30]. Since then, the presence of USUV in mosquitoes was detected in several European countries in native and invasive mosquito species: Cx. perexiguus collected in southern Spain [19]; Cx. pipiens, Ae. albopictus, Cs. annulata, Oc. detritus, Anopheles maculipennis s.l., and Oc. caspius in Northern Italy [15,16,17,31,32,33]; Cx. pipiens in Serbia [34,35]; Cx. pipiens/Cx. torrentium and Ae. japonicus in Austria [36,37]; in overwintering Cx. pipiens pools in Belgium [38]; Cx. modestus in Czech Republic [39]; and Cx. pipiens in France [40] and Germany [41]. Although USUV has been found in several species, Cx. pipiens is the main vector for the virus [1,25].
Both viruses were most frequently detected in Cx. pipiens mosquitoes. Phylogenetic analysis of WNV strains from mosquitoes in different geographic areas in Europe showed circulation of WNV lineage 1 and 2 [2,5]. Analysis of USUV strains showed that the Europe 2 lineage is the most prevalent in mosquitoes; however, Europe 3 and 4 and Africa 2 and 3 lineages were also detected [25].
So far, 52 mosquito species have been detected in Croatia, of which two are invasive (Ae. albopictus and Ae. japonicus) [42]. In the Zagreb area, 32 mosquito species have been recorded so far [43,44], several species of them are potential vectors for arboviruses.
The first screening of mosquitoes for flaviviruses in Croatia was conducted during the 2012 WNV outbreak in three northeastern counties. Mosquitoes were sampled within the area where WNV human infections occurred and all tested Cx. pipiens complex pools were negative for WNV RNA [45]. The aim of this study was to detect the presence of WNV and USUV in mosquitoes in northwestern Croatian regions. Virus screening of mosquitoes started in 2015 and has been continuously carried out until the end of 2020.
2. Materials and Methods
2.1. Mosquito Collection and Identification
The research area was the northwestern part of Croatia. It included the City of Zagreb and Međimurje County. WNV and USUV were previously identified in that Croatian region, based on testing of human samples, horses, poultry and wild birds [10,29].
In the period from 2015 to 2020 a total of 618 mosquito sampling occasions were conducted using three methods (Table 1), 613 in the City of Zagreb, and 5 in Međimurje County.
Table 1.
Number of mosquito collecting using different methods at all sites.
| Method of Collecting | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
|---|---|---|---|---|---|---|---|
| CO2-baited trap | 7 | 75 | 96 | 96 | 274 | ||
| Aspirator | 116 | 23 | 57 | 41 | 43 | 51 | 331 |
| BG Sentinel | 13 | 13 | |||||
| Total | 129 | 23 | 64 | 116 | 139 | 147 | 618 |
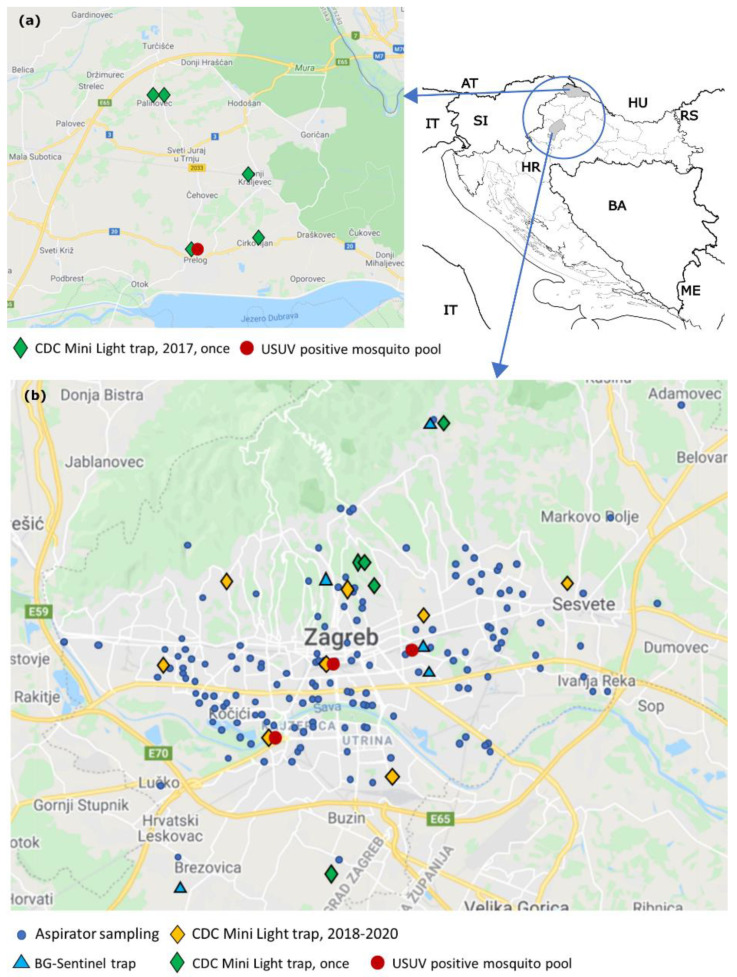
In the area of the City of Zagreb (641,355 square kilometers; 804,507 inhabitants), the mosquito sampling occasions were conducted over six years (2015−2020). Mosquitoes were collected by three different traps and methods, including CDC Mini Light traps (BioQuip, Products, Rancho Dominguez, CA, USA), BG-Sentinel traps (Biogents, Germany), and aspirator collection. CDC Mini Light traps were equipped with dry ice (CO2) as an attractant (CO2-baited traps) and used to collect adult mosquitoes of various species. They were placed approximately 1.5 m from the ground and set in the late afternoon, before sunset, left overnight, and removed after sunrise (07:00–10:00). Over the last three years (2018–2020), the CO2-baited traps were set at the same eight collection sites (Figure 1b, yellow marks), every 14 days, from May to October. A total of 264 sampling occasions were gathered (2018: 72; 2019: 96; 2020: 96) (Table 1). The following habitats were chosen for sampling: woods (three locations), a populated area close to the green belt (two locations), gardens in the urban part of the city (two locations), city center close to Zagreb botanical garden (one location). Additionally, during the research, the sampling occasions using CO2-baited trap were conducted once at five collection sites (Figure 1b, green marks). During all years, mosquito samples have been collected by aspirator as well as using the human landing collection method: during field surveys in the yards of citizens who complained of mosquitoes, on open area in public places (parks, cemeteries, green areas), from the walls in underground shelters, flats, and cellars (Figure 1b, blue dots). In the period between 2015 and 2017, most mosquito samples were collected using an aspirator. During six years of research, a total of 331 sampling occasions by an aspirator were gathered. In 2015, BG-Sentinel traps with BG Lure attractant were used for collecting Ae. albopictus. The sampling was performed during the afternoon at five sites (Figure 1b, light blue marks) two or three times, with a total of 13 occasions.
Figure 1.
Distribution of mosquito sampling locations and Usutu virus (USUV) positive mosquito pools in northwestern Croatia, (a) Međimurje County, 2017; (b) City of Zagreb, 2015–2020.
In Međimurje County (the northernmost Croatian county; 729.58 square kilometers; 113,804 inhabitants), mosquito samples were collected only once, between August 17 and 18 2017, from the late afternoon to the morning, at five different locations (Figure 1a, green marks) using traps with CO2. Four traps were placed next to chicken farms and next to a horse stable.
The mosquitoes sampled by traps with CO2 were transported to the laboratory in containers with dry ice, transferred to plastic tubes, and stored on dry ice until identification. Mosquitoes trapped by the aspirator were transported alive to the laboratory in aspirator, placed briefly in a freezer at −18 °C, then identified. Female mosquitoes were morphologically identified by species or species complex on a chilling surface under a stereomicroscope, using the determination keys by Becker et al. (2010) [46] and Schaffner et al. (2001) [47]. Specimens belonging to the same species/complex collected on the same day and at the same sampling site were pooled, with up to 60 individuals per pool, and stored at −80 °C until virological testing.
2.2. Virological Testing
Viral RNA was extracted using a High Pure Viral Nucleic Acid Kit (Roche Applied Science). TaqMan real-time RT-PCR assays for detection of WNV and USUV RNA were performed according to the protocols of Tang et al. (2006) [48] and Nikolay et al. (2014) [49], respectively, using a Brilliant III Ultra-Fast QPCR Master Mix (Agilent Technologies) and a Rotor-Gene Q real-time PCR cycler (Hilden, Germany). Samples identified as positive using the real-time RT-PCR assays were tested by conventional RT-PCR using PrimeScript™ One Step RT-PCR Kit Ver.2 (Takara Bio Inc, Kusatsu, Japan) and panflavi primers targeting the NS5 gene (FP: 5′-TACAACATGATGGGVAARAGAGAGA-3′, RP: 5′-AGCATGTCTTCYGTBGTCATCCAYT-3′) to amplify 1085 bp (WNV) and 1084 bp (USUV) products according to the protocol of Weissenböck et al. (2002) [50]. After electrophoresis, DNA samples extracted from excised gel fragments were Sanger sequenced in both directions by Humanizing Genomics, Macrogen Inc. with the use of the same primers. Genotyping and phylogenetic grouping of obtained sequences were based on comparison with strains retrieved from GenBank and obtained using BlastN algorithm (http://www.ncbi.nlm.nih.gov (accessed on 28 February 2021)). Maximum likelihood phylogenetic analysis was conducted, and an evolutionary analysis was performed by using MEGA7 [51].
Ion Torrent high-throughput next-generation sequencing (NGS) technology was used to determine the virome in one selected Cx. pipiens pool. The RNA was extracted from sample suspensions with Trizol reagent (Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized from the extracted nucleic acids using the cDNA Synthesis System (Roche, Manheim, Germany) and Random Hexamer Primers (Roche, Manheim, Germany) and fragmented with a Covaris M220 targeting peak fragment lengths of 400 bp. The library was prepared with the GeneRead™ DNA Library L Prep Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions and sequenced on the Ion PGM platform using the Ion PGM™ Hi-Q™ View Sequencing Kit reagents (ThermoFisher Scientific–Ion Torrent, CA, USA). Sequenced reads were quality checked and trimmed using the Ion Torrent Suite v.5.6.0. Additionally, low quality bases were trimmed with Geneious software suite v.11.0.5 (Biomatters Ltd., Auckland, New Zealand). Clean reads were subjected to BlastX and BlastN search. The BlastX and BlastN results were analyzed with MEGAN6 [52] for the taxonomic assignment of the reads using the Lowest Common Ancestor (LCA) algorithm. To confirm the BlastX and BlastN results and to determine the exact number of reads belonging to the Blast identified RNA viruses, all clean reads were mapped against these RNA virus genomes with the Geneious reference mapper (Geneious software suite v.11.0.5, Biomatters Ltd., Auckland, New Zealand).
3. Results
During a six-year period (2015–2020) a total of 20,363 mosquitoes were collected and identified in northwestern Croatia, of which 20,291 from the City of Zagreb, and 72 mosquitoes from Međimurje County. The mosquitoes belong to 11 species. Of these, in the City of Zagreb, 31.1% of mosquitoes were identified as Oc. sticticus, followed by Ae. albopictus (23.5%), Ae. vexans (21,5%), and Cx. pipiens (20.7%). Other species were represented by less than 2%. Out of a total of 72 mosquitoes from Međimurje County, 52.8% belong to Cx. pipiens complex, followed by Ae. vexans (41.7%) and Oc. sticticus (5.5%) (Table 2).
Table 2.
Number of collected mosquitoes by species and sampling method.
| Mosquito Species | Sampling Method | Number of Collected Specimens per Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Subtotal | Total | % | ||
| City of Zagreb | ||||||||||
| Aedes albopictus | Aspirator | 1231 | 455 | 534 | 96 | 115 | 572 | 3003 | 4768 | 23.49 |
| CO2-baited trap | 380 | 619 | 537 | 1536 | ||||||
| BG Sentinel | 229 | 229 | ||||||||
| Aedes cinereus | Aspirator | 2 | 1 | 13 | 2 | 18 | 67 | 0.33 | ||
| CO2-baited trap | 49 | 49 | ||||||||
| Aedes rossicus | Aspirator | 18 | 18 | 338 | 1.67 | |||||
| CO2-baited trap | 320 | 320 | ||||||||
| Aedes vexans | Aspirator | 38 | 17 | 144 | 266 | 465 | 4359 | 21.48 | ||
| CO2-baited trap | 1 | 189 | 2290 | 1414 | 3894 | |||||
| Aedes japonicus | Aspirator | 2 | 2 | 2 | 0.01 | |||||
| Coquillettidia richiardii | CO2-baited trap | 8 | 8 | 8 | 0.04 | |||||
| Culex modestus | CO2-baited trap | 1 | 1 | 1 | 0.005 | |||||
| Culex pipiens complex | Aspirator | 475 | 53 | 345 | 2 | 257 | 1132 | 4210 | 20.75 | |
| CO2-baited trap | 20 | 1144 | 946 | 906 | 3016 | |||||
| BG Sentinel | 62 | 62 | ||||||||
| Ochlerotatus geniculatus | CO2-baited trap | 78 | 81 | 159 | 159 | 0.78 | ||||
| Ochlerotatus rusticus | Aspirator | 71 | 71 | 71 | 0.35 | |||||
| Ochlerotatus sticticus | Aspirator | 327 | 115 | 848 | 506 | 82 | 1878 | 6308 | 31.09 | |
| CO2-baited trap | 584 | 3541 | 305 | 4430 | ||||||
| Total | 2364 | 455 | 743 | 3670 | 8631 | 4428 | 20,291 | 20,291 | 100.00 | |
| Međimurje County | ||||||||||
| Culex pipiens complex | CO2-baited trap | 38 | 38 | 52.77 | ||||||
| Aedes vexans | CO2-baited trap | 30 | 30 | 41.66 | ||||||
| Ochlerotatus sticticus | CO2-baited trap | 4 | 4 | 5.55 | ||||||
| Total | 72 | 72 | 72 | 100.00 | ||||||
Mosquito specimens were sorted in 899 pools and tested for the presence of WNV and USUV, 893 from the City of Zagreb and 6 from Međimurje County (Table 3). All tested mosquito pools were negative for WNV. A total of three USUV-positive pools were detected in Zagreb (one Ae. albopictus pool in 2016, one Cx. pipiens complex pool in 2018 and one 2019, respectively) and one Cx. pipiens complex pool in 2017 from Međimurje County (Prelog). All USUV positive mosquitoes were trapped between July 19 and September 7. The USUV-positive Ae. albopictus pool contained 15 mosquitoes which were collected by human landing collection on September 2 and September 7, 2016, in two adjacent yards in Zagreb. The other three positive pools comprised Cx. pipiens complex mosquitoes collected by the CO2-baited trap. In 2017, one pool of mosquitoes collected on August 19 in Prelog (Međimurje County) next to a horse stable. In 2018, a positive pool was trapped on July 19 in Zagreb, in a wood near the Jarun lake. The last Cx. pipiens complex positive pool was collected on 1 August 2019, in the center of Zagreb, near the Botanical garden (Figure 1, red dots).
Table 3.
Number of mosquito pools tested for the presence of West Nile virus and Usutu virus RNA by real-time RT-PCR.
| Mosquito Species | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
|---|---|---|---|---|---|---|---|
| City of Zagreb | |||||||
| Aedes albopictus | 42 | 19 * | 27 | 32 | 49 | 54 | 223 |
| Aedes cinereus | 1 | 2 | 9 | 12 | |||
| Aedes rossicus | 17 | 17 | |||||
| Aedes vexans | 4 | 9 | 15 | 99 | 62 | 189 | |
| Aedes japonicus | 1 | 1 | |||||
| Coquillettidia richiardii | 2 | 2 | |||||
| Culex modestus | 1 | 1 | |||||
| Culex pipiens complex | 18 | 7 | 62 * | 55 * | 45 | 187 | |
| Ochlerotatus geniculatus | 6 | 7 | 13 | ||||
| Ochlerotatus rusticus | 9 | 9 | |||||
| Ochlerotatus sticticus | 14 | 9 | 57 | 136 | 23 | 239 | |
| Total | 79 | 19 | 52 | 177 | 372 | 193 | 893 |
| Međimurje County | |||||||
| Culex pipiens complex | 3 * | 3 | |||||
| Aedes vexans | 2 | 2 | |||||
| Ochlerotatus sticticus | 1 | 1 | |||||
| Total | 6 | 6 |
* Single pool tested positive for the presence of USUV RNA.
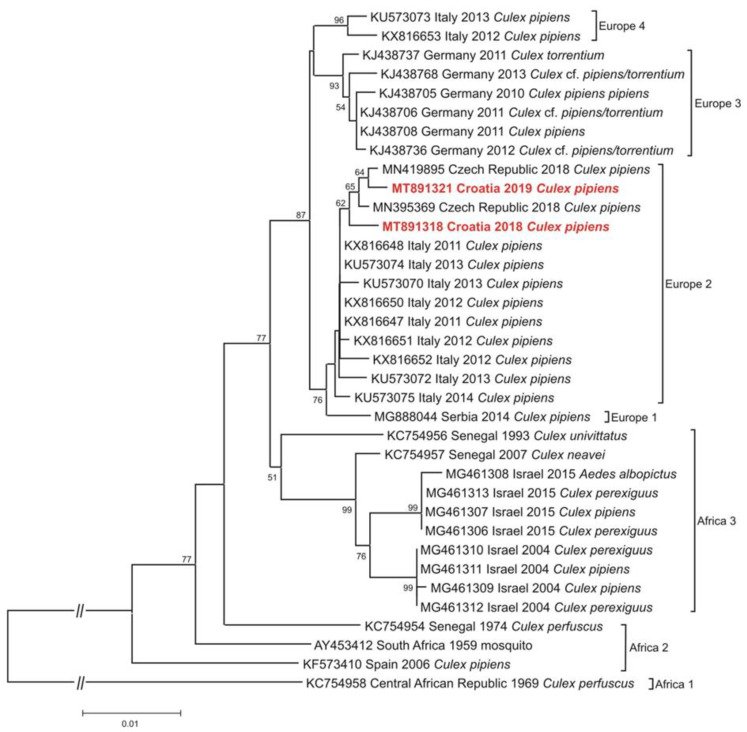
Conventional RT-PCR yielded a USUV positive result for Cx. pipiens pools collected in 2018 and 2019, and both PCR products were successfully sequenced. Both detected nucleotide sequences of the NS5 gene clustered within the USUV Europe 2 lineage (Figure 2) with 99.31% pairwise identity between each other. The 2018 USUV showed the highest similarity (99.71% nucleotide homology) with USUV detected in blackbirds (Turdus merula) in Austria in 2016 (accession number MF063042) and Czech Republic in 2017 (accession number MN419913), respectively. In contrast, the 2019 USUV detection shared the highest nucleotide homology (99.71%) with USUV detected in 2016 in Cx. modestus mosquito from Czech Republic.
Figure 2.
Phylogenetic neighbor-joining analysis of a 1015 nucleotide fragment of the USUV NS5 gene (corresponding nucleotide positions 9072–10,088 of the SAAR-1776 strain, GenBank accession numberAY453412) detected in Culex pipiens pool in Croatia and representative USUV strains from mosquitoes (n = 36). GenBank accession numbers, countries of origins, isolation/detection years and mosquito species are indicated at the branches. Viruses from Croatia are marked in bold and red color. USUV genetic lineages suggested by Cadar et al. (2017) [38] are indicated on the right. Supporting (≥50%) bootstrap values of 1000 replicates are displayed at the nodes. Horizontal distances are proportional to genetic distance. Scale bar indicates nucleotide substitutions per site. The interrupted branches, indicated by double slashes, were shortened by 80% for better graphic representation.
The previously determined RT-PCR USUV positive sample, which consisted of Cx. pipiens (collected near the Jarun lake Zagreb, 2018), was whole virome sequenced by using the NGS method and a total of 817,573 (mean length of 184 nucleotides) clean reads were obtained from the sample. The BlastX and BlastN search identified 16,888 reads (2.7% of total reads) belonging to viruses. Other reads belonged to cellular organisms, to other sequences and to unassigned sequences. Most of the virus sequences were assigned to viruses infecting mosquitoes. Virus sequences belonging to six different virus families and to unclassified viruses were identified, including dsDNA and ssRNA viruses of insect, bacterial and mammalian origin (Table 4). Bacteriophages belonging to family Myoviridae (39 reads) and insect viruses belonging to Nudiviridae (64 reads), which are dsDNA viruses, were detected in the sample. The ssRNA (+) virus sequences were predominantly from Culex picorna-like virus 1 (12,067 reads), sequenced in complete genome within Picornaviridae family. A closely related strain in Genbank was Culex picorna-like virus 1 strain 16-0168/ROK/2016 (MH703059) with 96.56% nucleotide identity to the determined virus. Other identified ssRNA (+) virus sequences revealed the complete genome (2409 reads) of Alphamesonivirus 1 with 99.36% nucleotide identity with Alphamesonivirus 1 strain 11/2008 (MF281710) from the Mesoniviridae family. The identified Culex mosquito virus 4 (1171 reads) which belongs to the family Nodaviridae and had 96.03% nucleotide identity with strain CMos/Santa Clara (MH188031). The presence of USUV was also confirmed (31 reads). Virus sequences with most homology to an unclassified ssRNA (−) virus (916 reads) and to an unclassified RNA virus (191 reads), both of mosquito origin, were also detected in the sample.
Table 4.
Viruses detected by NGS in a Cx. pipiens complex mosquito pool sampled in Zagreb, 2018.
| Group | Order | Family/Genus (Species) | Number of Reads | Host |
|---|---|---|---|---|
| dsDNA | Caudovirales | Myoviridae | 39 | Bacteria |
| / | Nudiviridae | 64 | Insects | |
| ssRNA | Picornavirales | Picornaviridae (Culex picorna-like virus 1) | 12,067 | Mosquitoes |
| Nidovirales / |
Mesoniviridae/Alphamesonivirus (Alphamesonivirus 1) Nodaviridae (Culex mosquito virus 4) |
2409 1171 |
Mosquitoes Mosquitoes |
|
| / | Flaviviridae/Flavivirus (Usutu virus) | 31 | Mammals and birds | |
| / | Unclassified RNA virus (Hubei chryso-like virus 1) | 191 | Mosquitoes | |
| / | Unclassified RNA virus (Wuhan Mosquito Virus 8) | 916 | Mosquitoes |
4. Discussion
Human neuroinvasive USUV infections in Croatia were reported in 2013, and the first detection of USUV in mosquitoes was recorded in 2016. The USUV positive Ae. albopitus pool comprising 15 mosquitoes was collected in Zagreb, the same area where the first human cases were detected in 2013, i.e., two years before the start of the mosquito screening in Croatia. In 2017, there was a small WNV outbreak involving 8 human cases from five Croatian counties. Human USUV infections were not reported at that time; nevertheless, one pool of Cx. pipiens complex mosquitoes collected in north-west Croatia (Prelog, Međimurje County) tested positive for USUV RNA. In addition, one pool of Cx. pipiens complex mosquitoes collected in Zagreb in 2018 also tested positive for USUV RNA. In 2018, additional three cases of human neuroinvasive USUV infections were reported during the largest WNV outbreak in Croatia. One USUV infected patient was a resident of Zagreb County. Furthermore, USUV RNA was detected for the first time in one dead blackbird (Turdus merula) from the same geographic area (Zagreb County) [10]. USUV infections were not reported in humans and animals in Croatia since 2018. However, in 2019, USUV RNA was detected in one Cx. pipiens complex pool collected in Zagreb. Phylogenetic analysis of NS5 gene of USUV detected in the two Cx. pipiens pools collected in 2018 and 2019, respectively, showed their high nucleotide homology (99.31%), both of them clustering within USUV Europe 2 lineage. Detected sequences from a fatal human case (north-east Croatia, 2018) and a dead blackbird (north-west Croatia, 2018) also belonged to the USUV Europe 2 lineage [10]. This indicates USUV Europe 2 lineage endemization in Croatia. Further, each of the USUV sequences from the mosquito pools have shown high nucleotide homology (99.71%) with USUV strains from Czech Republic and Austria detected in 2016 and 2017. Unfortunately, the USUV positive mosquito pools detected in Croatia in 2016 and 2017 could not be successfully sequenced most probably due to the low amount of the virus in the samples (Ct value > 30, data not shown). Nevertheless, the USUV Europe 2 lineage endemization in Croatia should be observed in the context of the high prevalence of this lineage in other Central European countries [36,39].
Although outbreaks or sporadic WNV infections as well as seropositivity in humans and sentinel animals (horses and poultry) have been continuously recorded in continental Croatian counties [5,10,11], WNV RNA was not detected in any of the tested mosquito pools. This could be explained by targeted virological testing of symptomatic patients, sick or dead animals and particularly by serological testing of humans and sentinel animal in contrast to random virological testing of mosquitoes. Even though a large number of mosquitoes was tested, it was obviously underrepresented in terms of the WNV activity monitoring. This means that screening of mosquitoes for WNV has shown to be less sensitive than monitoring of WNV activity using sentinel animals.
In the Zagreb area, 32 species of mosquitoes have been recorded so far, two of them are invasive species, Ae. albopictus and Ae. japonicus [43,44]. Aedes albopictus is established in Zagreb and neighboring counties [53]. The presence of USUV in mosquitoes in Zagreb was first detected in the Ae. albopictus pool, whereas the other USUV positive pools were Cx. pipiens mosquitoes. Of all the tested pools, 25% were Ae. albopictus and 21% Cx. pipiens complex. Aedes albopictus is an invasive species that is rapidly adapting to temperate regions, already known as a vector of dengue, chikungunya, Zika and other arboviruses [54,55]. It is highly anthropophilic but also has a tendency to feed on different hosts, including birds, which significantly increases the potential role of the species in USUV transmission cycle [55]. Although Ae. albopictus seems to have low vector competence for USUV [56], numerous Ae. albopictus pools sampled through the surveillance program of the Emilia-Romagna region (Northern Italy) in the period 2009–2012 tested positive to this virus [16,31,32]. Taken together with the USUV positive Ae. albopicus pool in this study, it is necessary to monitor the development of this vector-pathogen association.
The Cx. pipiens complex includes two morphologically indistinguishable biotypes, Cx. pipiens biotype pipiens and Cx. pipiens biotype molestus. Members of the Cx. pipiens complex, as well as their hybrids, represent the most important vectors of arboviruses including WNV and USUV. In the city of Zagreb, the presence of both biotypes and their hybrids is confirmed by DNA barcoding (unpublished data, project “DNA Barcoding of Diversity of Croatian Fauna”). During the research in Zagreb and Međimurje area, Cx. pipiens complex individuals were sampled by CO2-baited trap and by aspirator from walls in apartments, underground shelters, and cellars. Approximately 20 pools were overwintering mosquitoes collected during November, December, and January in underground shelters and cellars. All three USUV positive Cx. pipiens pools were sampled in open area by CO2-baited trap and all overwintering mosquitoes were negative. Nevertheless, the high nucleotide homology (99.31%) between two USUV sequences found in Cx. pipiens in 2018 and 2019 in Zagreb raises question on USUV overwintering in mosquitoes in Croatia.
The NGS results of a Cx. pipiens pool revealed, beside USUV, several dsDNA (Myoviridae, Nudiviridae) and ssRNA (Picornaviridae, Mesoniviridae, Nodaviridae) viruses as well as two unclassified RNA viruses, namely, Hubei chryso-like virus 1 and Wuhan Mosquito Virus 8. Although these viruses were not our primary research aim, this finding presents a contribution to the knowledge on a geographical variation of the viromes in medically important mosquito vectors.
5. Conclusions
Detection of USUV in mosquitoes during the four consecutive transmission seasons (2016–2019) indicates the virus has become endemic in northwestern Croatia. Although WNV infections in humans and sentinel animals have been continuously recorded, WNV has not been detected in mosquitoes in Croatia so far.
Acknowledgments
The authors thank Sandra Vrtaric, Tomislav Pismarovic, Danijel Poje, and Ivan Horvat for technical assistance.
Author Contributions
Conceptualization, A.K., T.V.-C. and V.S.; methodology, A.K., V.S., U.K. and I.T.; investigation, A.K., V.S., M.C.P., S.P., U.K., I.T. and J.M.; writing—original draft preparation, A.K., T.V.-C., V.S., M.C.P. and U.K.; writing—review and editing, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
The virological testing from 2017 to 2020 was supported by the Croatian Science Foundation, project No. IP-2016-06-7456: Prevalence and molecular epidemiology of emerging and re-emerging neuroinvasive arboviral infections in Croatia; CRONEUROARBO (to T.V.-C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nikolay B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R. Soc. Trop. Med. Hyg. 2015;109:609–618. doi: 10.1093/trstmh/trv066. [DOI] [PubMed] [Google Scholar]
- 2.Zannoli S., Sambri V. West Nile Virus and Usutu Virus Co-Circulation in Europe: Epidemiology and Implications. Microorganisms. 2019;7:184. doi: 10.3390/microorganisms7070184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz L.A., Flores F.S., Quaglia A., Contigiani M.S. Intertwined arbovirus transmission activity: Reassessing the transmission cycle paradigm. Front. Physiol. 2012;3:493. doi: 10.3389/fphys.2012.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubálek Z., Halouzka J. West Nile fever—A reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilibic-Cavlek T., Savic V., Petrovic T., Toplak I., Barbic L., Petric D., Tabain I., Hrnjakovic-Cvjetkovic I., Bogdanic M., Klobucar A., et al. Emerging Trends in the Epidemiology of West Nile and Usutu Virus Infections in Southern Europe. Front. Vet. Sci. 2019;6:437. doi: 10.3389/fvets.2019.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control (ECDC) Historical Data by Year—West Nile Fever Seasonal Surveillance. [(accessed on 27 February 2021)];2018 Available online: https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical.
- 7.Pem-Novosel I., Vilibic-Cavlek T., Gjenero-Margan I., Pandak N., Peric L., Barbic L., Listes E., Cvitkovic A., Stevanovic V., Savini G. First outbreak of West Nile virus neuroinvasive disease in humans, Croatia, 2012. Vector-Borne Zoonotic Dis. 2014;14:82–84. doi: 10.1089/vbz.2012.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbic L., Stevanovic V., Kovac S., Maltar L., Lohman Jankovic I., Vilibic-Cavlek T., Madic J. West Nile virus serosurveillance in horses in Croatia during the 2012 transmission season. Croatian Academy of Sciencies and Art. Med. Sci. 2013;39:95–104. [Google Scholar]
- 9.Savić V., Barbic L., Vilibic-Cavlek T., Balenović M., Stevanović V., Listes E., Savini G. Chickens and horses as sentinels for early warning system in prevention of human West Nile virus infections in Croatia. Slov. Vet. Res. 2016;53:292–294. [Google Scholar]
- 10.Vilibic-Cavlek T., Savic V., Sabadi D., Peric L., Barbic L., Klobucar A., Miklausic B., Tabain I., Santini M., Vucelja M., et al. Prevalence and molecular epidemiology of West Nile and Usutu virus infections in Croatia in the ‘One health’ context 2018. Transbound. Emerg. Dis. 2019;66:1946–1957. doi: 10.1111/tbed.13225. [DOI] [PubMed] [Google Scholar]
- 11.Vilibic-Cavlek T., Barbic L., Mrzljak A., Brnic D., Klobucar A., Ilic M., Janev-Holcer N., Bogdanic M., Jemersic L., Stevanovic V., et al. Emerging and Neglected Viruses of Zoonotic Importance in Croatia. Pathogens. 2021;10:73. doi: 10.3390/pathogens10010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balenghien T., Vazeille M., Grandadam M., Schaffner F., Zeller H., Reiter P., Sabatier P., Fouque F., Bicout D.J. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoonotic Dis. 2007;8:589–595. doi: 10.1089/vbz.2007.0266. [DOI] [PubMed] [Google Scholar]
- 13.Engler O., Savini G., Papa A., Figuerola J., Groschup M., Kampen H., Medlock J., Vaux A., Wilson A.J., Werner D., et al. European surveillance for West Nile virus in mosquito populations. Int. J. Environ. Res. Public Health. 2013;10:4869–4895. doi: 10.3390/ijerph10104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toma L., Cipriani M., Goffredo M., Romi R., Lelli R. First report on entomological field activities for the surveillance of West Nile disease in Italy. Vet. Ital. 2008;44:483–512. [PubMed] [Google Scholar]
- 15.Tamba M., Bonilauri P., Bellini R., Calzolari M., Albieri A., Sambri V., Dottori M., Angelini P. Detection of Usutu Virus Within a West Nile Virus Surveillance Program in Northern Italy. Vector Borne Zoonotic Dis. 2011;11:551–557. doi: 10.1089/vbz.2010.0055. [DOI] [PubMed] [Google Scholar]
- 16.Calzolari M., Gaibani P., Bellini R., Defilippo F., Pierro A., Albieri A., Maioli G., Luppi A., Rossini G., Balzani A., et al. Mosquito, bird and human surveillance of West Nile and Usutu viruses in Emilia Romagna Region (Italy) in 2010. PLoS ONE. 2012;7:e38058. doi: 10.1371/journal.pone.0038058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calzolari M., Chiapponi C., Bonilauri P., Lelli D., Baioni L., Barbieri I., Lavazza A., Pongolini S., Dottori M., Moreno A. Co-circulation of two Usutu virus strains in Northern Italy between 2009 and 2014. Infect. Genet. Evol. 2017;51:255–262. doi: 10.1016/j.meegid.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Mavridis K., Fotakis A.E., Kioulos I., Mpellou S., Konstantas S., Varela E., Gewehr S., Diamantopoulos V., Vontas J. Detection of West Nile Virus–Lineage 2 in Culex pipiens mosquitoes, associated with disease outbreak in Greece, 2017. Acta Trop. 2018;182:64–68. doi: 10.1016/j.actatropica.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Vázquez A., Ruiz S., Herrero L., Moreno J., Molero F., Magallanes A., Sánchez-Seco M.P., Figuerola J., Tenorio A. West Nile and Usutu Viruses in Mosquitoes in Spain, 2008–2009. Am. J. Trop. Med. Hyg. 2011;85:178–181. doi: 10.4269/ajtmh.2011.11-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Čabanová V., Šikutová S., Straková P., Šebesta O., Vichová B., Zubríkova D., Miterpáková M., Mendel J., Hurníkova Z., Hubálek Z., et al. Co-Circulation of West Nile and Usutu Flaviviruses in Mosquitoes in Slovakia, 2018. Viruses. 2019;11:639. doi: 10.3390/v11070639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrić D., Petrović T., Cvjetković I.H., Zgomba M., Milošević V., Lazić G., Ćupina A.I., Lupulović D., Lazić S., Dondur D., et al. West Nile virus ‘circulation’ in Vojvodina, Serbia: Mosquito, bird, horse and human surveillance. Mol. Cell. Probes. 2017;31:28–36. doi: 10.1016/j.mcp.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Gaibani P., Rossini G. An overview of Usutu virus. Microbes Infect. 2017;19:382–387. doi: 10.1016/j.micinf.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Barzon L. Ongoing and emerging arbovirus threats in Europe. J. Clin. Virol. 2018;107:38–47. doi: 10.1016/j.jcv.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Fros J.J., Miesen P., Vogels B.C., Gaibani P., Sambri V., Martina B.E., Koenraadt C.J., van Rij R.P., Vlak J.M., Takken W., et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health. 2015;1:31–36. doi: 10.1016/j.onehlt.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilibic-Cavlek T., Petrovic T., Savic V., Barbic L.J., Tabain I., Stevanovic V., Klobucar A., Mrzljak A., Ilic M., Bogdanic M., et al. Epidemiology of Usutu Virus: The European Scenario. Pathogens. 2020;9:699. doi: 10.3390/pathogens9090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams M.C., Simpson D.I., Haddow A.J., Knight E.M. The isolation of West Nile Virus from man and of Usutu virus from the bird-biting mosquito Mansonia aurites (Theobald) in the Entebbe area of Uganda. Ann. Trop. Med. Parasitol. 1964;58:367–374. doi: 10.1080/00034983.1964.11686258. [DOI] [PubMed] [Google Scholar]
- 27.Weissenböck H., Bakonyi T., Rossi G., Mani P., Nowotny N. Usutu virus, Italy, 1996. Emerg. Infect. Dis. 2013;19:274–277. doi: 10.3201/eid1902.121191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbic L., Vilibic-Cavlek T., Listes E., Stevanovic V., Gjenero-Margan I., Ljubin-Sternak S., Pem-Novosel I., Listes I., Mlinaric-Galinovic G., Di Gennaro A., et al. Demonstration of Usutu virus antibodies in horses, Croatia. Vector Borne Zoonotic Dis. 2013;13:772–774. doi: 10.1089/vbz.2012.1236. [DOI] [PubMed] [Google Scholar]
- 29.Vilibic-Cavlek T., Kaic B., Barbic L., Pem-Novosel I., Slavic-Vrzic V., Lesnikar V., Kurecic-Filipovic S., Babic-Erceg A., Listes E., Stevanovic V., et al. First evidence of simultaneous occurrence of West Nile virus and Usutu virus neuroinvasive disease in humans in Croatia during the 2013 outbreak. Infection. 2014;42:689–695. doi: 10.1007/s15010-014-0625-1. [DOI] [PubMed] [Google Scholar]
- 30.Busquets N., Alba A., Allepuz A., Aranda C., Núñez J.I. Usutu Virus Sequences in Culex pipiens (Diptera:Culicidae), Spain. Emerg. Infect. Dis. 2008;14:861–863. doi: 10.3201/eid1405.071577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calzolari M., Bonilauri P., Bellini R., Albieri A., Defilippo F., Galletti G., Barbieri I., Tamba M., Lelli D., Carra E., et al. Evidence of Simultaneous Circulation of West Nile and Usutu Viruses in Mosquitoes Sampled in Emilia-Romagna Region (Italy) in 2009. PLoS ONE. 2010;5:e14324. doi: 10.1371/journal.pone.0014324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calzolari M., Bonilauri P., Bellini R., Albieri A., Defilippo F., Tamba M., Tassinari M., Gelati A., Cordioli P., Angelini P., et al. Usutu virus persistence and West Nile virus inactivity in the Emilia-Romagna region (Italy) in 2011. PLoS ONE. 2013;8:e63978. doi: 10.1371/journal.pone.0063978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pautasso A., Radaelli M.C., Ballardini M., Francese D.R., Verna F., Modesto P., Grattarola C., Desiato R., Bertolini S., Vitale N., et al. Detection of West Nile and Usutu Viruses in Italian Free Areas: Entomological Surveillance in Piemonte and Liguria Regions, 2014. Vector-Borne Zoonotic Dis. 2016;16:292–294. doi: 10.1089/vbz.2015.1851. [DOI] [PubMed] [Google Scholar]
- 34.Kemenesi G., Buzás D., Zana B., Kurucz K., Krtinić B., Kepner A., Földes F., Jakab F. First genetic characterization of Usutu virus from Culex pipiens mosquitoes Serbia, 2014. Infect. Genet. Evol. 2018;63:58–61. doi: 10.1016/j.meegid.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Cvjetković I.H., Petrović T., Petrić D., Milošević U., Radovanov J., Kovačević G., Galović A.J., Patić A., Nikolić N., Cvjetković D., et al. Usutu Virus: An Emerging Flavivirus In Europe. Arch. Vet. Med. 2017;10:25–35. doi: 10.46784/e-avm.v10i1.79. [DOI] [Google Scholar]
- 36.Weidinger P., Kolodziejek J., Bakonyi T., Brunthaler R., Erdélyi K., Weissenböck H., Nowotny N. Different dynamics of Usutu virus infections in austria and Hungary, 2017–2018. Transbound. Emerg. Dis. 2020;67:298–307. doi: 10.1111/tbed.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camp J.V., Kolodziejek J., Nowotny N. Targeted surveillance reveals native and invasive mosquito species infected with Usutu virus. Parasites Vectors. 2019;12:46. doi: 10.1186/s13071-019-3316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cadar D., Lühken R., van der Jeugd H., Garigliany M., Ziegler U., Keller M., Lahoreau J., Lachmann J., Becker N., Kik M., et al. Widespread activity of multiple lineages of Usutu virus, Western Europe, 2016. Eurosurveillance. 2016;22:30452. doi: 10.2807/1560-7917.ES.2017.22.4.30452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudolf I., Bakonyi T., Šebesta O., Mendel J., Peško J., Betášová L., Blažejová H., Venclíková K., Strakova P., Nowotny N., et al. Co-circulation of Usutu virus and West Nile virus in a reed bed ecosystem. Parasites Vectors. 2015;8:520. doi: 10.1186/s13071-015-1139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eiden M., Gil P., Ziegler U., Rakotoarivony I., Marie A., Francés B., L’Ambert G., Simonin Y., Foulongne V., Groschup M.H., et al. Emergence of two Usutu virus lineages in Culex pipiens mosquitoes in the Camargue, France, 2015. Infect. Genet. Evol. 2018;61:151–154. doi: 10.1016/j.meegid.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Sieg M., Schmidt V., Ziegler U., Keller M., Höper D., Heenemann K., Rückner A., Nieper H., Muluneh A., Groschup M.H., et al. Outbreak and Cocirculation of Three Different Usutu Virus Strains in Eastern Germany. Vector Borne Zoonotic Dis. 2017;17:662–664. doi: 10.1089/vbz.2016.2096. [DOI] [PubMed] [Google Scholar]
- 42.Merdić E., Klobučar A., Žitko T., Sudarić Bogojević M., Vrućina I., Turić N., Vignjević G. Update checklist of the mosquitoes (Diptera: Culicidae) of Croatia. J. Vector Ecol. 2020;45:135–139. doi: 10.1111/jvec.12381. [DOI] [PubMed] [Google Scholar]
- 43.Klobucar A., Benic N., Krajcar D., Kosanovic-Licina M.L., Tesic V., Merdic E., Vrucina I., Savic V., Barbic L., Stevanovic V., et al. An overview of mosquitoes and emerging arboviral infections in the Zagreb area, Croatia. J. Infect. Dev. Ctries. 2016;10:1286–1293. doi: 10.3855/jidc.7988. [DOI] [PubMed] [Google Scholar]
- 44.Klobučar A., Lipovac I., Žagar N., Mitrović-Hamzić S., Tešić V., Vilibić-Čavlek T., Merdić E. First record and spreading of the invasive mosquito Aedes japonicus japonicus (Theobald, 1901) in Croatia. Med. Vet. Entomol. 2019;33:171–176. doi: 10.1111/mve.12337. [DOI] [PubMed] [Google Scholar]
- 45.Merdić E., Vignjević G., Turić N., Bogojević M.S., Milas J., Vrućina I., Zahirović Z. Mosquito survey during West Nile virus outbreak 2012 in northeast Croatia. Coll. Antropol. 2014;38:423–428. [PubMed] [Google Scholar]
- 46.Becker N., Petric D., Zgomba M., Boase C., Dahl C., Madon M., Kaiser A. Mosquito and Their Control. 2nd ed. Springer; Berlin/Heidelberg, Germany: 2010. 577p [Google Scholar]
- 47.Schaffner F., Angel G., Geoffroy B., Hervy J.P., Rhaiem A., Brunhes J. The Mosquitoes of Europe—An Identification and Training Programme. CD Rom, IRD Editions & EID Méditerranée; Montpellier, France: 2001. [Google Scholar]
- 48.Tang Y., Hapip C.A., Liu B., Fang C.T. Highly sensitive TaqMan RT-PCR assay for detection and quantification of both lineages of West Nile virus RNA. J. Clin. Virol. 2006;36:177–182. doi: 10.1016/j.jcv.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Nikolay B., Weidmann M., Dupressoir A., Faye O., Boye C.S., Diallo M., Sall A.A. Development of a Usutu virus specific real-time reverse transcription PCR assay based on sequenced strains from Africa and Europe. J. Virol. Methods. 2014;197:51–54. doi: 10.1016/j.jviromet.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 50.Weissenböck H., Kolodziejek J., Url A., Lussy H., Rebel-Bauder B., Nowotny N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002;8:652–656. doi: 10.3201/eid0807.020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huson D.H., Auch A.F., Qi J., Schuster S.C. MEGAN Analysis of Metagenomic Data. Genome Res. 2007;17:377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klobučar A. Ph.D. Thesis. University of Zagreb; Zagreb, Croatia: 2017. Spreading and Vector Role of Invasive Mosquitoes Aedes albopictus and Aedes japonicus in the Northwestern Croatia. [Google Scholar]
- 54.Urbanelli S., Bellini R., Carrieri M., Sallicandro P., Celli G. Population structure of Aedes albopictus (Skuse): The mosquito which is colonizing Mediterranean countries. Heredity. 2000;84:331–337. doi: 10.1046/j.1365-2540.2000.00676.x. [DOI] [PubMed] [Google Scholar]
- 55.Paupy C., Delatte H., Bagny L., Corbel V., Fontenille D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Puggiolia A., Bonilaurib P., Calzolari M., Lellib D., Carrieria M., Urbanellid S., Pudare D., Bellini R. Does Aedes albopictus (Diptera: Culicidae) play any role in Usutu in Northern Italy? Experimental oral infection and field evidences. Acta Trop. 2017;172:192–196. doi: 10.1016/j.actatropica.2017.05.006. [DOI] [PubMed] [Google Scholar]