Abstract
Background
Metabolic carts measure the carbon dioxide (CO2) produced and oxygen consumed by an individual when breathing to assess metabolic fuel usage (carbohydrates versus fats). However, these systems are expensive, time-consuming, and only available in health care laboratory settings. A small handheld device capable of determining metabolic fuel usage via CO2 from exhaled air has been developed.
Objective
The aim of this study is to evaluate the validity of a novel handheld device (Lumen) for measuring metabolic fuel utilization in healthy young adults.
Methods
Metabolic fuel usage was assessed in healthy participants (n=33; mean age 23.1 years, SD 3.9 years) via respiratory exchange ratio (RER) values obtained from a metabolic cart as well as % CO2 from the Lumen device. Measurements were performed at rest in two conditions: fasting, and after consuming 150 grams of glucose, in order to determine changes in metabolic fuel usage. Reduced major axis regression and simple linear regression were performed to test for agreement between RER and Lumen % CO2.
Results
Both RER and Lumen % CO2 significantly increased after glucose intake (P<.001 for both) compared with fasting conditions, by 0.089 and 0.28, respectively. Regression analyses revealed an agreement between the two measurements (F1,63=18.54; P<.001).
Conclusions
This study shows the validity of Lumen for detecting changes in metabolic fuel utilization in a comparable manner with a laboratory standard metabolic cart, providing the ability for real-time metabolic information for users under any circumstances.
Keywords: resting metabolic rate, Lumen, ParvoMedics TrueOne 2400, validation, respiratory exchange ratio, metabolism, fuel utilization, indirect calorimetry, breath, lung, respiratory, young adult, measurement, testing
Introduction
Indirect calorimetry (metabolic cart), which is currently the preferred method for determining metabolic fuel utilization, measures the carbon dioxide produced (VCO2) and oxygen consumed (VO2) when breathing. The ratio between VCO2 and VO2 is the respiratory exchange ratio (RER), which provides insight into the relative contribution of carbohydrates and lipids to overall energy expenditure [1,2]. Though indirect calorimetry is not invasive, this method is time-consuming (up to 40 minutes), only available in test laboratory settings, and requires technical and physiological expertise for handling the metabolic cart and interpretation of the metabolic data obtained.
Metaflow Ltd developed Lumen, a novel metabolic fuel utilization breathalyzer, which is a personalized handheld device that provides an individual’s metabolic state in real time by measuring CO2 from exhaled breath (Figure 1). The device indirectly measures metabolic fuel usage via a CO2 sensor and a flow sensor to determine the rate of CO2 production from a single breath maneuver. The % CO2 in the exhaled volume of air is determined from a specific breathing maneuver with a breath hold of 10 seconds. This concept is based on the fact that oxygen consumption is stable under resting conditions [3]; thus, a change in metabolic fuel use will generally be represented by changes in CO2 production. For carbohydrate oxidation, more carbon dioxide is produced relative to the consumption of oxygen. For fat oxidation, less carbon dioxide is produced [4]. The use of a smartphone app enables the user to track metabolic status outside of physiologic test laboratories.
Figure 1.
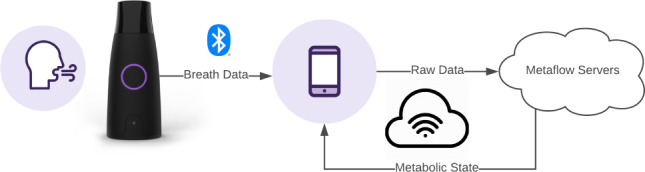
A schematic representation of the Lumen device and app.
Previous exploratory studies for algorithmic development of Lumen were performed to compare the Lumen measurement to the metabolic cart. In this study, we aim to evaluate agreement between the Lumen measurement and that of the metabolic cart in healthy participants before and after glucose ingestion under stable resting conditions.
Methods
Participants
A total of 54 healthy volunteers reported to the Exercise Physiology Laboratory in the Department of Kinesiology at San Francisco State University to participate in this study. Inclusion criteria were being aged between 18-45 years with a BMI less than 30 kg/m2. Exclusion criteria were participation in high-intensity aerobic training or having a known cardiovascular, pulmonary, and/or metabolic disease. The study was approved by the university’s Institutional Review Board for Human Subjects, and written informed consent was obtained from each participant before testing.
Study Design
Participants were recruited and their height and weight were measured using a stadiometer and Seca scale (Seca). If they met the BMI criteria, they were provided their own Lumen device, which was labeled with their unique identification number. The Lumen device was paired and synchronized to the participant’s smartphone together with the Lumen app. Participants practiced the Lumen breathing technique while supervised and took the device home for a further familiarization period in order to show proficiency with the device and app. They were instructed to perform Lumen metabolic measurements for at least 30 sessions, with each session consisting of 3 breath maneuvers, and to complete 3 sessions at different time points each day. After the minimum amount of home breath sessions were collected, participants were scheduled for the study laboratory measurement day. All participants came to the test laboratory between 7 AM and 11 AM after a 12-hour fast and had abstained from any form of physical activity (other than walking).
On the laboratory testing day, blood glucose samples were taken by sterile finger prick blood sample and measured by a glucometer (OneTouch, LifeScan Inc). For the indirect calorimetry measurement, the participant had to lay down in supine position on a padded examination table, where a rigid clear plastic canopy with a comfortable, flexible seal was placed over the head and upper part of the torso. Once the metabolic cart measurement was completed, the participant was seated in a comfortable chair. After 5 minutes of rest, they were asked to perform two Lumen breath sessions (5-minute break between each session). The first Lumen session immediately after the metabolic cart measurement was used for data analysis. In case of an invalid first session (difference between breaths >0.2% CO2), the second session was used for analysis.
Once finished, participants were asked to drink 150 grams of a glucose solution (3 servings of 50 grams with 20-minute intervals between each serving). Subsequently, 45 minutes after the intake of the first drink (corresponding to 5 minutes after finishing the last serving), their glucose levels were reassessed, and the same assessment procedures as during the fasted state before the glucose intake were repeated. Participants were removed from the analysis if they were unable to finish all glucose drinks.
Metabolic Cart
RER was analyzed using a calibrated TrueOne 2400 metabolic cart (ParvoMedics), which was previously determined to provide a valid measurement for RER with 5% coefficient of variation [5]. This system uses a paramagnetic oxygen analyzer and infrared carbon dioxide analyzer with a Hans Rudolph heated pneumotach. The ParvoMedics system was warmed up for at least 60 minutes each day before testing to ensure accurate and stable readings. The gas analyzers and flow sensor were calibrated as per manufacturer’s recommendations: calibration of the analyzers was performed using a high-precision gas mixture (O2, CO2, remainder N2) and calibrated and accepted with a <0.1% error with the calibration gas. Flow and volume were calibrated using a calibrated 3 L syringe (Hans Rudolph, model 5530) to ≤1% error. In addition, verification of the calibration process was performed to ensure stability of the system. The ambient temperature was kept between 22 °C and 26 °C in the test laboratory. Relative humidity was maintained stable at roughly 60%. Once calibration was acceptable and complete, a ventilated hood with subject cover was placed over the participant’s head and positioned around the upper torso area to ensure no air could escape from the hood. The participants were required to stay awake during the measurement procedure. The hood ventilation was measured during the recording, and CO2 and O2 concentrations were measured from it. VCO2 and VO2 parameters were calculated and taken as 30-second averages. For this study, we defined the subject steady-state metabolic measurement based on observed variations in the VO2 and VCO2 of less than ≤5% coefficient of variation for a period of at least five consecutive minutes, with a subsequent RER stability of 2.5% in a fasted state and 3.7% after glucose consumption, in a similar manner to previous studies [6]. Inability to meet these criteria resulted in removal of the data from the analysis.
Lumen
Lumen is a device designed to be calibration-free, with a warm-up time of less than 10 seconds and the CO2 sensor taking into account the room CO2 concentration during every measurement. During the measurement day, participants completed 2 sessions of 3 Lumen breaths each after the metabolic cart measurement. The Lumen breathing maneuver consists of three phases, starting from the end of a normal expiration (functional residual capacity). The participant takes a deep breath in through the Lumen device, followed by a 10-second breath hold. Afterward, the subject exhales through the Lumen device, with a steady exhalation flow to at least the starting level of the maneuver. In order to confirm repeatability, breaths are taken in triplicate for each session. The Lumen smartphone app guides the participant through each phase of the Lumen maneuver. Each Lumen session was repeated after a 5-minute pause interval. Validity of breath maneuvers was systematically evaluated by the Lumen app. Inability to perform valid Lumen breath measures resulted in removal of the data from the analysis.
Statistical Analyses
All variables were tested and visualized for normal distribution before the tests.
To evaluate the changes after glucose intake, two-tailed paired parametric t tests were performed for blood glucose levels, RER levels, and Lumen % CO2 before and after glucose intake.
For agreement validation, major axis regression (Deming method) was performed to compare RER of the metabolic cart and % CO2 from the Lumen device [7]. As RER and % CO2 are in different units, the analysis is identical to ordinary least products regression (also known as reduced major axis regression), which is the most suitable analysis for comparison between two methods of measurement [8]. Moreover, a simple linear regression (ordinary least squares) was performed to determine the ability to predict Lumen values from the gold-standard value of RER.
Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software Inc). The threshold for significance was set at P<.05.
Ethics Statement
This study was approved by San Francisco State University’s Institutional Review Board for Human Subjects, and written informed consent was obtained from each participant before testing.
Results
From the original 54 participants recruited, 12 were excluded prior to laboratory testing and 9 had to be excluded during the testing day for failing to meet the inclusion criteria as detailed in the methods section: 1 participant was unable to consume all glucose drinks due to nausea, 3 participants did not achieve 5 minutes of stable metabolic cart measurement (coefficient of variation <5% in VO2 and VCO2), and 5 participants were unable to perform a valid Lumen measurement (Figure 2). Characteristics of the final 33 participants are presented in Table 1.
Figure 2.
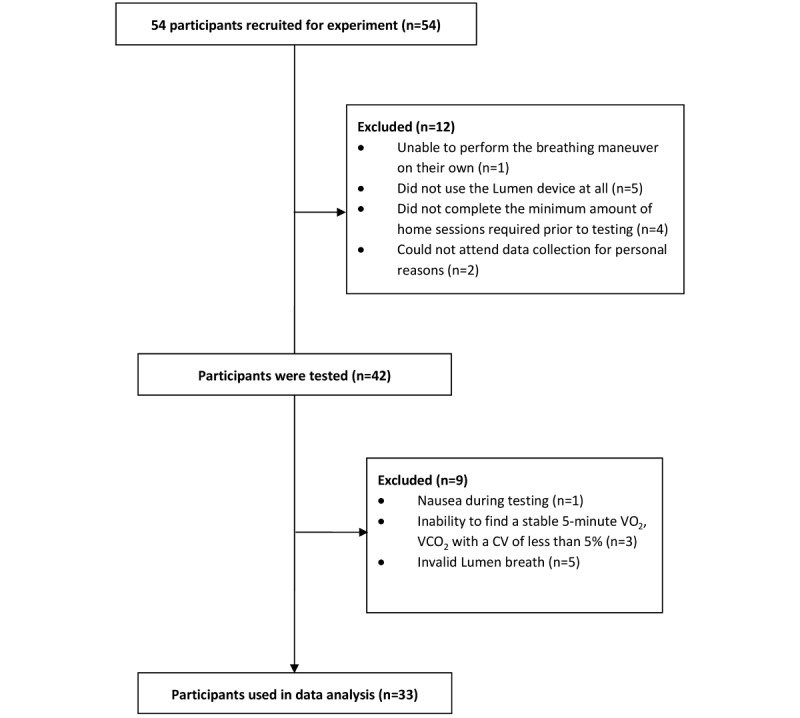
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. CV: coefficient of variation.
Table 1.
Descriptive statistics of study participants.
| Gender | Count | Age (years), mean (SD) | Weight (kg), mean (SD) | Height (cm), mean (SD) | BMI (kg/m2), mean (SD) |
| Male | 17 | 24.0 (3.0) | 73.7 (10.2) | 171.7 (7.8) | 24.9 (2.5) |
| Female | 16 | 22.3 (4.5) | 59.1 (6.4) | 160.9 (5.5) | 22.9 (2.6) |
| Total | 33 | 23.1 (3.9) | 66.2 (11.1) | 166.1 (8.6) | 23.9 (2.7) |
Blood glucose levels increased from 90.6 (SD 9.2) mg/dL to 145.2 (SD 25.3) mg/dL as a result of glucose intake (t32=11.04, P<.001; Figure 3A). RER levels increased from 0.787 (SD 0.043) to 0.876 (SD 0.053) in response to glucose intake (t32=10.84, P<.001; Figure 3B). Moreover, Lumen CO2 concentrations significantly rose from 4.20 (SD 0.4) to 4.48 (SD 0.34; t32=5.978, P<.001; Figure 3C). These analyses have confirmed the ability of both the metabolic cart and Lumen to detect changes in metabolic fuel utilization.
Figure 3.
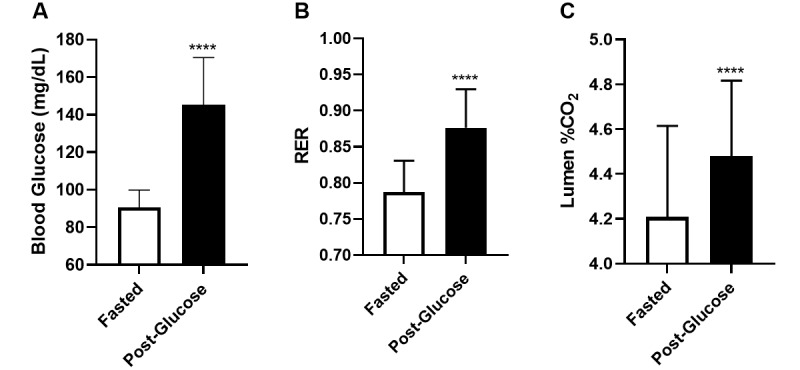
Changes in blood glucose as determined by (A) blood glucose test, (B) RER, and (C) Lumen % CO2. Data are presented as mean (SD). N=33 for each state. **** indicates P<.001. RER: respiratory exchange ratio.
To test for agreement between RER units from the metabolic cart and % CO2 from Lumen, reduced major axis regression was performed [9]. It revealed a significant relationship between RER and Lumen % CO2 (F1,63=18.54, P<.001, y=6.111x–0.7445, x-intercept=0.1218; Figure 4). This analysis confirmed the agreement between Lumen % CO2 and metabolic cart RER, with a systemic bias as a result of the nature of the different units.
Figure 4.
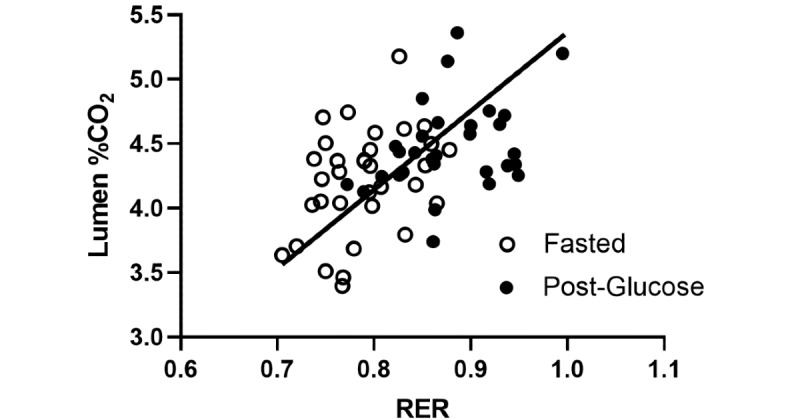
Reduced major axis regression of RER from the metabolic cart and Lumen % CO2 measurements for metabolic activity. N=33 for each state. RER: respiratory exchange ratio.
To determine the ability of metabolic cart RER to predict Lumen % CO2, ordinary least squares regression was performed to estimate Lumen values from RER measures, with the assumption that RER is an accurate independent measure, to predict Lumen % CO2. A significant model effect was present (F1,63=18.54, P<.001, R2=0.2274; Figure 5). The RER parameter estimate indicated that for every 1-unit increase in RER, a 2.914-unit increase (SE 0.6767) in Lumen % CO2 is expected. Since a full unit increase in RER is not a plausible outcome, this parameter estimate can be interpreted similarly by a 0.1-unit increase in RER (eg, 0.7 to 0.8) to produce a 0.2914-unit increase in Lumen % CO2.
Figure 5.
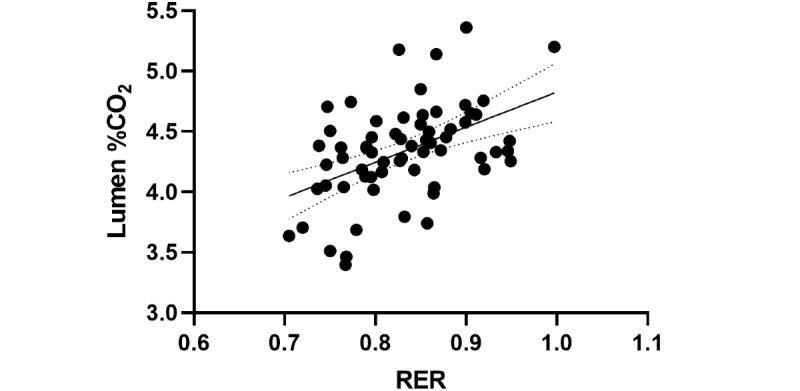
Ordinary least squares regression of RER and Lumen % CO2. N=33 for each state. RER: respiratory exchange ratio.
Discussion
Principal Findings
This study evaluated the ability of the Lumen device to assess changes in the body’s metabolic fuel utilization in healthy young adults compared to the indirect calorimetry metabolic cart measurement. Our results show that Lumen CO2 levels are in agreement with RER values from the metabolic cart, which correspond to relative changes in metabolic fuel utilization.
Both Lumen CO2 levels and metabolic cart RER showed significant increases in metabolic levels as a result of glucose intake in healthy individuals in resting conditions (Figure 3). These results can be expected, as cells using more carbohydrates as fuel produce more CO2 relative to O2 consumption compared to cells metabolizing fat. The ratio between CO2 production and O2 consumption in this process is known as the respiratory quotient (RQ) or RER. RQ and RER vary depending on the energy source of the cell (carbohydrate versus fat), and the acronyms are commonly used interchangeably [2,10,11]. In resting conditions, oxygen consumption is fairly stable [12,13], meaning that participants’ changes in RQ are due to changes in CO2 production. This is the underlying concept of the Lumen device, enabling it to track changes in metabolic fuel utilization. For that reason, it was important to ensure that participants in this study were at rest before and during their measurements.
Reduced major axis regression revealed an agreement between RER and Lumen CO2 levels (Figure 4). This analysis enables us to test for agreement between methods with different units and verify the validity of the Lumen device with a metabolic cart. It demonstrates the ability of the Lumen device to provide equivalent results to the metabolic cart in assessing metabolic fuel utilization.
Furthermore, the results from the simple linear regression predicting Lumen % CO2 using RER values suggest that, while there is measurement agreement between the Lumen % CO2 and RER, the proportion of variance remains low (Figure 5). Thus, Lumen can be seen to be an effective instrument for monitoring individual changes in metabolic responses (within-subject consistency), rather than a substitute for the metabolic cart (between-subject precision).
Evidence suggests that the assessment of RER can be beneficial for multiple applications, such as nutrition, diabetes prevention, or weight management [14]. It has previously been shown that RER could be a prognostic marker of weight loss and a predictor of weight gain [15,16]. Moreover, minute-to-minute RER measured in a respiratory chamber calorimeter showed that the slopes of RER were different in response to different dietary interventions [17]. However, although RER is currently the preferred method for determining metabolic fuel, it is a time-consuming, uncomfortable, and costly and impractical tool for real-time day-to-day assessments of metabolic activity. In contrast, the Lumen device is small, mobile, user-specific, and relatively cheap, and delivers the outcome immediately to the user and enables real-time decisions.
Limitations
This study is the first to show agreement between Lumen % CO2 and RER. However, it is important to note that participants in this study were young (mean age 22.4 years) and healthy individuals. With increasing age, metabolism changes, as can be seen in various metabolic cart studies [18-20]. Future studies will need to examine whether RER metabolic cart levels correspond to Lumen CO2 levels in older subjects and those with metabolic conditions.
Unlike the metabolic cart, the Lumen device does not measure oxygen consumption. Accordingly, the Lumen measurement should be performed under resting conditions with stable VO2, allowing the correct interpretation of changes of % CO2 as changes in metabolic state.
In addition, results from this study showed a high peak of blood glucose levels 45 minutes after glucose intake (5 minutes after the third drink), whereas both RER and Lumen % CO2 showed a more moderate increase in levels. It is possible that the metabolic cart and Lumen measurements were performed too early, as it may be that in some of our participants, the peak glucose levels occurred more than 45 minutes after ingestion; thus, it was not yet fully metabolized [21].
Conclusions
In summary, Lumen can provide valid information regarding an individual's metabolic state, and in agreement with results from the metabolic cart. Unlike the metabolic cart, Lumen measurement can be performed anywhere, anytime, without the need for a specialized laboratory, equipment, and technical staff. The Lumen device is able to detect changes in metabolism due to dietary intake, similarly to the metabolic cart. The capability of taking metabolic measurements continuously outside of laboratory settings can provide new insights about the metabolic state of an individual so as to obtain further knowledge and understanding about metabolism and nutrition.
Acknowledgments
This work was supported by Metaflow Ltd. We would like to thank the participants for their time in taking part in this study, and the Lumen team for their support. We would like to acknowledge Casey Curl for his work in setting up the protocols and procedures during preliminary testing.
Abbreviations
- RER
respiratory exchange ratio
- RQ
respiratory quotient
- VCO2
carbon dioxide production
- VO2
oxygen consumption
Footnotes
Authors' Contributions: KAL and SY analyzed the data and prepared the manuscript. RA and JO coordinated the project and collected the data. JRB reviewed and edited the manuscript. MM and MK conceived, designed, and supervised the study as well as reviewed and edited the manuscript. All authors approved the manuscript before submission.
Conflicts of Interest: SY and MM are employees of Metaflow Ltd, and contributed to the design and analysis of the study as well as the preparation of the manuscript. The other authors declare no conflicts of interest.
References
- 1.Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr. 1988 Apr;47(4):608–28. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- 2.McClave SA, Lowen CC, Kleber MJ, McConnell JW, Jung LY, Goldsmith LJ. Clinical use of the respiratory quotient obtained from indirect calorimetry. JPEN J Parenter Enteral Nutr. 2003;27(1):21–6. doi: 10.1177/014860710302700121. [DOI] [PubMed] [Google Scholar]
- 3.Moon JK, Butte NF. Combined heart rate and activity improve estimates of oxygen consumption and carbon dioxide production rates. J Appl Physiol (1985) 1996 Oct 01;81(4):1754–61. doi: 10.1152/jappl.1996.81.4.1754. https://tinyurl.com/yhz6uuup. [DOI] [PubMed] [Google Scholar]
- 4.Elia M, Livesey G. Theory and validity of indirect calorimetry during net lipid synthesis. Am J Clin Nutr. 1988 Apr;47(4):591–607. doi: 10.1093/ajcn/47.4.591. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JA, Watras AC, O'Brien MJ, Luke A, Dobratz JR, Earthman CP, Schoeller DA. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J Am Diet Assoc. 2009 Jan;109(1):128–32. doi: 10.1016/j.jada.2008.10.004. http://europepmc.org/abstract/MED/19103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbin K, Krajmalnik-Brown R, Carnero E, Bock C, Emerson R, Rittmann B, Marcus A, Davis T, Dirks B, Ilhan Z, Champagne C, Smith S. Integrative and quantitative bioenergetics: Design of a study to assess the impact of the gut microbiome on host energy balance. Contemp Clin Trials Commun. 2020 Sep;19:100646. doi: 10.1016/j.conctc.2020.100646. https://linkinghub.elsevier.com/retrieve/pii/S2451-8654(20)30130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brace RA. Fitting straight lines to experimental data. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1977 Sep 01;233(3):R94–R99. doi: 10.1152/ajpregu.1977.233.3.r94. [DOI] [PubMed] [Google Scholar]
- 8.Ludbrook J. Linear regression analysis for comparing two measurers or methods of measurement: but which regression? Clin Exp Pharmacol Physiol. 2010 Jul;37(7):692–9. doi: 10.1111/j.1440-1681.2010.05376.x. [DOI] [PubMed] [Google Scholar]
- 9.Ludbrook J. Comparing methods of measurements. Clin Exp Pharmacol Physiol. 1997 Feb;24(2):193–203. doi: 10.1111/j.1440-1681.1997.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 10.Benedict F, Cathcart E. Muscular Work, a Metabolic Study with Special Reference to the Efficiency of the Human Body as a Machine. Washington, DC: Carnegie Institution of Washington; 1913. [Google Scholar]
- 11.Brooks GA, Fahey TD, Baldwin KM. Exercise Physiology: Human Bioenergetics and Its Applications. New York, NY: McGraw-Hill Education; 2004. [Google Scholar]
- 12.Spurr GB, Prentice AM, Murgatroyd PR, Goldberg GR, Reina JC, Christman NT. Energy expenditure from minute-by-minute heart-rate recording: comparison with indirect calorimetry. Am J Clin Nutr. 1988 Sep;48(3):552–9. doi: 10.1093/ajcn/48.3.552. [DOI] [PubMed] [Google Scholar]
- 13.Green J. The heart rate method for estimating metabolic rate: review and recommendations. Comp Biochem Physiol A Mol Integr Physiol. 2011 Mar;158(3):287–304. doi: 10.1016/j.cbpa.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Ferrannini E. The theoretical bases of indirect calorimetry: A review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 15.Valtueña S, Salas-Salvadó J, Lorda P. The respiratory quotient as a prognostic factor in weight-loss rebound. Int J Obes Relat Metab Disord. 1997 Sep 18;21(9):811–7. doi: 10.1038/sj.ijo.0800480. [DOI] [PubMed] [Google Scholar]
- 16.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990 Nov;259(5 Pt 1):E650–7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 17.Gribok A, Leger JL, Stevens M, Hoyt R, Buller M, Rumpler W. Measuring the short-term substrate utilization response to high-carbohydrate and high-fat meals in the whole-body indirect calorimeter. Physiol Rep. 2016 Jun 28;4(12):e12835. doi: 10.14814/phy2.12835. doi: 10.14814/phy2.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riera CE, Dillin A. Tipping the metabolic scales towards increased longevity in mammals. Nat Cell Biol. 2015 Mar 27;17(3):196–203. doi: 10.1038/ncb3107. [DOI] [PubMed] [Google Scholar]
- 19.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004 Jan;286(1):E92–101. doi: 10.1152/ajpendo.00366.2003. https://tinyurl.com/rmm6t568. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R, Ramachandran R, Venkatesan P, Anoop S, Joseph M, Thomas N. Indirect calorimetry: From bench to bedside. Indian J Endocr Metab. 2017;21(4):594. doi: 10.4103/ijem.ijem_484_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyth E, Basit H, Smith CJ. Glucose Tolerance Test. Treasure Island, FL: StatPearls Publishing; 2021. [PubMed] [Google Scholar]


