Abstract
Ovarian cancer (OC) accounts for the highest tumor-related mortality among the gynecologic malignancies. Most of the OC patients diagnosed with advanced-stage (III and IV) this situation creates panic and provokes an emergency to discover a new therapeutic strategy. Plants that possess medicinal properties are gaining attention as they are enriched with various chemical compounds that are potential to treat various diseases. It is a prolonged process to provide innovative and significant leads against a range of pharmacological targets for a human disease management system. Though challenges and difficulties are faced in the development of a new drug, the emergence of combinatorial chemistry is providing a new ray of hope and also, the executed effort in discovering the drug, and a chemical compound has been remarkably successful. This review discussed the role of medicinal plants that are native of South Africa in treating the Ovarian Cancer and in drug discovery.
Keywords: Ovarian cancer, south african medicinal plants, anti-ovarian cancer activity, drug development
Introduction
Plants with medicinal values have been a part of human culture and tradition [1]. It possesses significant nutrition and is prescribed for various therapeutic purposes [2]. World Health Organization (WHO) assessed that about 80% of the population primarily rely on plant medicines to stay healthy [3]. Also, 21,000 plant species are reported by WHO to possess to have therapeutic values to be used as medicines [4]. Plant chemicals are extracted and the compounds of interest are identified continuously till date and recently to standardize the herbal medicines (Table 1) and to elucidate analytical marker compounds drug discovery techniques are applied [5]. Discovering a drug is an interdisciplinary action where it includes various fields of science. Novel drug discovery is an extravagant, challenging process, it devours time as well. The important process (Figure 1) involved in identifying New Chemical Entities (NCEs) which possess characteristic features such as effective druggability and medicinal chemistry [6]. NCEs are synthesized synthetically using chemicals or obtained from a natural process by isolation, approximately it would take 12 years for a new drug to reach a clinic from its discovered stage, also the investment done for the drug discovery is 1 billion US$ [7]. Numerous examples have proven that natural sources (including semi-synthetic analogues) and its products have been the backbone of more than 80% of drug substances [8].
Table 1.
Some of the natural compounds from the medicinal plants
Figure 1.
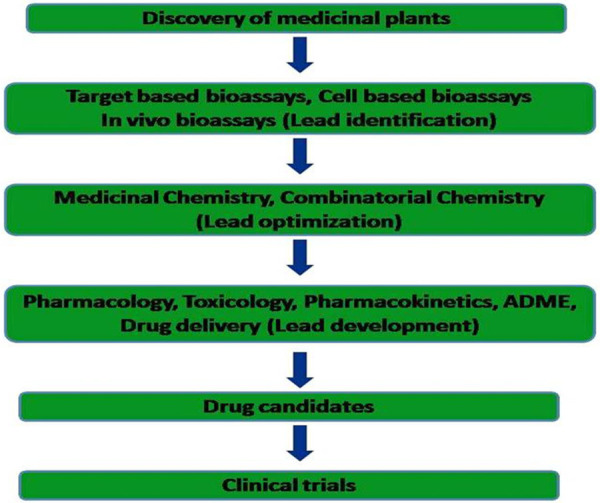
Modern drug discovery and development from the medicinal plant.
Cancer is referred to as the uncontrolled growth of abnormal cells anywhere in a body that can infiltrate normal body tissue and it is one of the leading fatal disease which leads to death worldwide [9]. The different types of cancer existence with histopathologies, genetic-epigenetic variations, and clinical outcomes are the challenges that persist in apprehending the mechanism of action of chemotherapeutics and in the development of innovative rehabilitations [10,11]. Ovarian cancer cruelly affects the human population when compared with other gynaecological malignancies in worldwide. There is an urgent need for novel therapies to treat and prevent this life-threatening disease. Innovative research interest is illustrating its attention towards naturally-derived compounds as they are considered to have less toxic side effects compared to current treatments such as chemotherapy, laser therapy, radiotherapy, gene therapy, hyperthermia and surgery. Plants produce secondary metabolites which are being investigated for their anti-ovarian cancer activities leading to the development of new clinical drugs. Development anti-ovarian cancer compounds from the medicinal plants have been utilized as staple drugs for treatment and prevention, the new technologies are emerging to expand the area further. Increasing demand for plant-derived drugs is putting pressure on high-value medicinal plants and risking their biodiversity. Plant-derived anti-ovarian cancer agents are effective inhibitors of cancer cells lines, making them in high demand [12].
Ovarian cancer and medicinal plants
Ovarian cancer (OC) stands seventh worldwide among the most commonly occurring types of cancer. Roughly it is estimated that twenty four hundred thousand females are diagnosed with the disease every year, this report depicts that 2% of all cancer cases around the world [13]. According to the World Cancer Research Fund International 2017, the highest incidence of OC occurs in Fiji (with estimated fifteen cases per hundred thousand), surprisingly very few cases are recorded in China and some parts of Africa (four cases among a hundred thousand female individuals) [14]. 1 in 70 women are prone to ovarian cancer risk. 150,000 deaths are recorded globally every year due to OC and this is considered as the fatal disease that leads to death when compared to other gynaecological malignancies. Due to poor diagnosis attributed to lack of symptoms at the early stages, as the symptoms appear at advanced stages (III and IV) [15]. The mortality is high comparatively. Chemotherapy, laser therapy, radiotherapy, gene therapy, hyperthermia, and surgery are few interventions practised or in trials to intervene in the growth of the cancer cells [16]. The medical aid includes a combined effort of incision, chemical treatments, and therapies which involves the action radiations [17]. Though there are pros in the procedures mentioned earlier the scientific community recognized that the interventions have disadvantages and limitations. Consequently, a development of drug that is capable of overcoming the obstacles and treating the disease effectively [18].
For centuries our ancestors were using plants to treat various human diseases and cancer is one among them [19-21]. Many medicinal plants have been reported to exhibit a variety of pharmacological and actions related to life functions namely antioxidant, antimicrobial, anticancer, antidiabetic properties and so on. Since plants acts as a store house of various phytochemicals they are capable of treating various ailments. these properties exhibited by plants [22-24]. The biologically important phytochemicals play a pivotal role in drug discovery [25-27] and the plant-derived biomolecules are recognized as an attractive and promising approach; possess high value in biomedical research for the development of drugs against cancer [28]. Interestingly in the recent past decades, plant that are medicinally important are been used to prepare drugs and the numbers are increasing comparatively. Inthe last twenty years a successful investigations are done on natural products especially to treat cancer much effectively in most parts of the world [29,30].
Anti-ovarian cancer activity of other medicinal plants
Several indigenous medicinal plants of Africa which include Aframomum arundinaceum, Aframomum. alboviolaceum, Aframomum kayserianum, Aframomum polyanthum [31], Echinops giganteus, Xylopia aethiopica, Piper capense, Imperata cylindrical [32,33]. Gladiolus quartinianus, Vepris soyauxii [34], Polygonum limbatum, Polycias fulva, Beilschmiedia acuta, Crinum zeylanicum, Dioscorea bulbifera, Elaoephorbia drupifera [35], Solanum aculeastrum, Albizia schimperiana, Zanthoxylum giletii and Strychnos usambarensis are used in the treatment and management of malignancies such as cancer, etc. these plants also showed significant cytotoxicity effect against the regions that have developed an immune against the drugs and that are endowed with sensation [36].
In vitro studies were performed using Korean medicinal plants to study the anti-cancer activity, Ethyl Acetate fraction from the Lespedeza cuneata methanolic extract proved to possess a cell-poisoning effect on A2780 human ovarian carcinoma cells with the IC50 value of 77.25 ± 2.05 μg/mL. The lignanosides compound of (-)-9’-O-(α-l-rhamnopyranosyl) lyoniresinol from this plant possess in vitro antiproliferative activity on A2780 with an IC50 value of 77.24 ± 2.05 μM [37]. The seeds of tea (Camellia sinensis) which contains saponins exhibited cancer chemopreventive effects, this was identified and reported when an athymic mice study was performed for Anti-tumor efficacy in human SKOV3 ovarian cancer xenografts [38]. Curcumin is a polyphenol that occurs naturally in a plant species called Curcuma longa which also holds another compound called curcuminoid has a potency to inhibit IL 6 and IL 8 secretion that was induced by lysophosphatidic acid (LPA) and STAT 3 phosphorylation, where LPA is a bilipid which is found to stimulate the invasion of cancer cells, and cells that carry the infection from the infected part to various other parts of the body and STAT 3 phosphorylation that inhibits the motility of OC cells, as portrayed in PA and OVCAR3 [39]. In the past in Kwara and Lagos state, Pistia stratiotes was cited frequently to treat ovarian cancer. But a recent study has thrown light on another species called Securidaca longipedunculata which is now considered to be the commonly used botanical source in aiding ovarian cancer [40]. Notably, the above study focused only on the Ijebus, an ethnic Yoruba group. Similarly, different plants namely Kigelia africana, P. stratiotes, Chenopodium ambrosioides, Nymphaea lotus, Parquetina nigrescens, Nicotiana tabacum, Alstonia congensis, Elaeis guineensis, Piper guineense, Aframomum melegueta, Petiveria alliacea were been in practicein Ogun state by the natives, southwest Nigeria to treat cancer [41].
Anti-ovarian cancer activity of South African medicinal plants
Africa is enriched with flora, and the phytochemicals in plants exhibit structure that draws interest also along with the diverse biological activities they serve as a starting point for a development of a new drug [42]. South Africa holds rich biodiversity where 22,600 indigenous medicinal plants are present. The flora contributes about ten percent of the higher botanical species on Earth. The traditional medicinal and healthcare history of Africa is very long [43]. Although there is limited information about the anticancer activity of South African plants available in the literature, there are several pieces of evidence suggesting that some of these plants could be used for the development of new therapeutic drugs. The most relevant candidates are discussed below providing information or insights to their pharmacological potential [44].
Aspalathus linearis (Burm.f) R. Dahlgren: Aspalathus linearis is a bush that has legumes or pods (Rooibos) belongs to the family Fabaceae and the presence of legume is a characterstic feature of the family Fabaceae. It is native (Figure 2A) to the Cedarberg Mountains in the Western Cape region of South Africa. They are cultivated widely within the region as they are commercially useful from which herbal tea or tisane are produced [45,46]. This beverage has its History from South Africa and now it is profoundly known in other countries as well [47]. The compounds such as various types of polyphenols, flavonoids present in the plants are filled with medicinal properties also an added advantage is that caffeine and theaflavins are absent and hence it is used medicinally [48].
Figure 2.

Anti-ovarian cancer activity of South African medicinal plants.
Fantoukh et al. isolated 11 phytocompounds from methanolic extract of Aspalathus linearis such as Aspalathin (521 mg), Nothofagin (306 mg), Thermopsoside (23 mg), Isoorientin (90 mg), Vitexin (73 mg), Isovitexin (8 mg), Isoquercitrin (76 mg), Rutin (59 mg), Bioquercetin (136 mg), (R)/(S)-eriodictyol-6-C-β-D-glucopyranoside (68 mg) and Syringin (68 mg) which possess antioxidant, antimicrobial, anti-inflammatory, antidiabetic and anticancer activities [49,50]. Rooibos extracts targe the premalignant cells present in the skin and inhibits the cell proliferation and thus intervenes in the growth of cancerous cells in the skin, it also induces apoptosis of tumor cells [51]. The efficacy of the extract has been revealed through various studies and it had shown significant anti-cancer effects against other types of cancer as well. An oral dose of this extract suppresses the activity of methylbenzlnitrosamine which induces the oesophageal squamous cell carcinogenesis in male F344rats [52]. B1 rats were given a dose of the Rooibos and this repressed the development of the fumonisin induced hepatocellular carcinoma [53].
Brachylaena rotundata (S.Moore): Brachylaena rotundata (Asteraceae) is a shrub or a small tree that grows up to 8 meters (Figure 2B). It also occurs in most Southern nations, particularly eastern Botswana, Transvaal, Mozambique, Zambia, and Zimbabwe, with presence in open woodland, on rocky koppies, and slopes, and on stream banks [54]. The dichloromethane extract of Brachylaena rotundata leaves (Table 2) has been found to reveal anti-ovarian cancer activity in OVCAR-5 ovarian cancer cell line with an IC50 value of 19.95 μg/ml [55].
Table 2.
Reported South African medicinal plants with anti-ovarian activity
| S.No | Plant name | Type of Cell line | Extract with anti-ovarian action | References |
|---|---|---|---|---|
| 1 | Aspalathus linearis | Chinese hamster | Whole plant (Aqueous) | [50-52] |
| 2 | Brachylaena rotundata | OVCAR-5 | Leaf (Dichloromethane) | [55] |
| 3 | Catha edulis | Chinese hamster | Leaf (Aqueous) | [59,60] |
| 4 | Centella asiatica | SKOV3 and OVCAR-3 | Whole plant (Aqueous) | [66] |
| 5 | Dicoma anomala | Chinese hamster | Root (Ethyl acetate) | [71] |
| 6 | Dodonaea viscosa | A2780 human | Root (ethanol) | [75] |
| 7 | Drimia robusta | OVCAR-3 | Whole plant (methanol) | [55] |
| 8 | Gomphocarpus fruticosus | OVCAR-3 | Root (Ethanol) | [55] |
| 9 | Leyssera gnaphaloides | Chinese hamster | Whole plant (Hexane) | [83] |
| 10 | Parinari curatellifolia | SW626 human | 13-Methoxy-15-oxozoapatlin compound from the plant | [87] |
| 11 | Pelargonium acraeum | PA1 and OVCAR-3 | Whole plant (Aqueous and methanol) | [55] |
| 12 | Plumbago auriculata | PA1 | Whole plant ( methanol) | [92] |
| 13 | Solanum acanthoideum | IGROV1 | Root (methanol) | [55] |
| 14 | Solanum nigrum | ES-2, SKOV-3 and OVCAR-3 | Whole plant (Aqueous) | [99,100] |
| 15 | Xanthium strumarium | SKOV-3 | Leaf | [111,112] |
Catha edulis (Vahl.) Endl.: Catha edulis (Figure 2C) is the South African medicinal plant belonging to the Celastraceae family. Catha edulis leaves contain phytochemicals and an indicative amount of vitamin C [56]. The worth of the foliage depends on the presence of cathinone contents [57]. Getasetegn (2016) reported the chemical composition of khat which possess 81 phytocompounds are classified into 7 major classes such as Phenylalkyl-amines ((+)-Cathine, (-)-Cathinone, 3,6-Dimethyl-2,5-diphenyl pyrazine, Merucathine, Merucathinone, (-)-Norephedrine, (-)-Norephedrine N-formyl, 1-Phenylpropane-1,2-dione and Pseudomerucathine), Cathedulins (Cathedulin E1-E6, Cathedulin K1, 2, 6, 12, 15, 17, 19 and 20, Cathedulin Y7-Y10, Cathidine A, B, D and Euonyminol), Flavonoids (Dihydromyricetin, Dihydromyricetin-3-O-rhamnoside, Kaempferol, Myricetin, Myricetin-3-O-b-D-galactoside, Myricetin-3-O-rhamnoside, Quercetin and Quercetin-3-O-b-D-galactoside), Sterol and Triterpenes (Celastrol, Friedeline, Iguesterin, Pristimerin, b-Sitosterol Tingenin A, B and b-Sitosterol glycoside), Volatiles (Fenchone, Linalool, Nerol, Ocimene, β-Phellandrene, α-Pinene, β-pinene, α-Terpineol, Terpinolene, α-Thujone and b-thujone), Amino acids and Vitamins (Vitamin C, B3, B2 and B1.) which hold unique biological activities in human disease management system [58].
The fresh leaf extract of Catha edulis exhibited the anti-ovarian cancer activity in the Chinese hamster ovary cell line at the concentration of 50 µg/ml [59]. Alsanosy et al., (2020) examined the anti-ovarian cancer activity of Six different fractions from the extract of Catha edulis which exhibited the anti-ovarian cancer activity on A2780 with the significant IC50 values raging from 20.97 ± 5.03 to 53.78 ± 7.45 [60]. Elhag et al., (1999) isolated the phytocompound of 22b-hydroxytingenone from methanolic extract of Catha edulis and demonstrated their ovarian cancer activity in National Cancer Institute (USA) which showed the significant ED50 value of 2.35 µg/ml [61].
Centella asiatica (Linn.) Urb: Centella asiatica (Apiaceae), commonly called pennywort (Figure 2D) is a perennial creeper and the propagation is through stolons, they are commonly found in moist places. This plant has reported to have many medicinal values and the plant chemicals exhibit mitoprotective, antioxidant, anti-inflammatory, antioxidant, and anticancer properties [62-64]. It holds a very good healing property and this quality of the plant is assumed due to the presence of three active triterpenes such as Asiatic acid, madecassic acid and asiaticoside [65]. A triterpenoid compound of Asiatic acid was extracted from Centella asiatica and found to possess anti-ovarian cancer activity in SKOV3 and OVCAR-3 ovarian cancer cell lines (Table 2). At an intensity of 40 µg/mL of asiatic acid the practicability of the ovarian cancer cells was reduced to 50% and the colonization of the OC cells were also reduced by 25-30% at the concentration of 10 µg/mL of asiatic acid. Apoptosis of the tumor cells increased to 7-10 folds when the cells were treated with Asiatic acid and this also curbed the cell cycle at G0/G1 phase. Several molecular pathways were examined to study the asiatic-acid effect against ovarian cancer cells. The phosphorylation levels of P13, Akt, and mTOR were lowered in the asiatic-acid treated cells. The tumor cell cells are viable and constitutive overexpression of Akt reverses the cytotoxic effect of asiatic acid partially. The growth-suppressive activity of the acid was examined. The downregulation of Akt mimicked the activity of Asiatic acid in the repression of growth against tumor cells [66].
Dicoma anomala (Sond.): Dicoma anomala (Figure 2E) a member of Asteraceae Family and a resident of the South Africa known well for its medicinal values. It is an upright, partially bent, or an incumbent herb that bears a partially woody tuber which has a distinctive aroma at the base of a woody subterranean stem. This grassland species is widely distributed in sub-Saharan Africa [67]. There are many ethnomedical uses of Dicoma anomala. It is used in the treatment of cough and cold, fever, ulcer, dermatosis, venereal disease, labor pain, looseness of bowel, enternal parasite, abdominal pain, odontalgia and internal worm [68]. Its extracts also possess several pharmacological properties including anti-bacterial, anti-helminthic, anti-viral, anti-plasmodial, anti-spasmodic, wound healing, analgesic, anti-cancer, antioxidant, hepatoprotective, antidiabetic, cardioprotective, and anti-inflammatory activities [69]. Phytochemical investigations were done to identify various secondary metabolites and it revealed the presence of compounds such as acetylenic compounds, phenolic acids, flavonoids, sesquiterpene lactones, triterpenes, and phytosterols [70]. The compounds, Dehydrobrachylaenolide, and Chloroquine from Ethyl acetate extract of Dicoma anomala root have enormous cytotoxicity effects at the concentration (IC50 value) of 17.199 µM/ml and 35.800 µM/ml respectively on Chinese hamster ovary cell line [43].
Maroyi (2018) reported that different solvent extracts of (from non-polar to polar hexane, petroleum ether, chloroform, ethyl acetate, methanol and aqueous) leaf, root and twig of Dicoma anomala holds cytotoxicity activity on Chinese hamster ovary (CHO) cell line with moderate IC50 values from 0.44 µg/ml to 31.33 µg/ml. the isolated compound of (3aS,5aS,9aR,9bS)-5amethyl3,9-dimethylidene-4,5,9a,9b-tetrahydro-3aHnaphtho[7,8-d]furan-2,8-dione from methanolic extract of Dicoma anomala showed the cytotoxicity against CHO cell line using the MTT assay with potential IC50 value of 4.2 µg/ml. The phytocompound of 3-oxoeudesma-1,4(15),11(13)-triene-12,6a-lide from Dicoma anomala showed the significant cytotoxicity against (CHO cell line using the MTT assay with an IC50 value of 17.2 µM [71].
Dodonaea viscosa (Jacq.) var. augustifolia (L.f) Benth: Dodonaea viscosa (Sapindaceae) is a blossoming plant (Figure 2F) in the soapberry family and it has a cosmopolitan distribution in tropical, subtropical, and warm temperate regions of South Africa. This shrub is extensively grown around the world. The roots bind the soil and thus are useful in sustaining stability in dunes and also curb the soil from getting eroded [72]. A variety of phytocompounds have been recorded with Dodonaea viscosa, such as flavonoids, fatty acids, and cyanolipids. A decoction was prepared from the leaf tips emerged newly to treat fever by the Cape settlers. In rural areas, Dodonaea viscosa var. angustifolia is still commonly used to treat colds, influenza, stomach trouble, and measles. Patients with a strep throat and oral infections caused by fungus gargle the decoction prepared using the leaves. The Khoi-Khoi used a concoction prepared from the roots and is used to treat colds and influenza [73]. Moreover, in Namaqualand, the extract prepared by boiling the leaves, this is then filtered, this extract is used for treating influenza, colds, and it also induces sweating. It is also used to relieve coughs and congested feeling typical of influenza, croup, and diphtheria. The same extract is considered to alleviate stomach ailments and fever [74]. The ethanolic root extract of Dodonaea viscosa exhibited a reproducible cell-perniciousness to A2780 ovarian cancer cell line (Table 2) with an IC50 value of 6.0 µg/ml. Cao et al., (2009) secluded the phytocompounds of two novel triterpenoid saponins from the root of Dodonaea viscosa (ethanolic extract) namely Dodonaeaside A and Dodonaeaside B which possess considerable antiproliferative activity on the ovarian cancer cell line of A2780 with IC50 values of 0.79 and 0.70 µM correspondingly [75].
Drimia robusta (Hyacinthaceae) is an important medicinal plant (Figure 2G) in South Africa because of its extensive usage [76]. The hot water infusions prepared using the pounded bulbs and leaves are used as enema by the Zulus as the leaves are diuretic in action which helps in cleaning the bladder and treat uterus related disease. It also exhibits anticancer, antimicrobial and antimicrobial activities due to the presence of aromatic compounds such as 4-hydroxy-3-methoxybenzoic acid, 3,4-dihydroxybenzoic acid and trans-3-(40-hydroxyphenyl)-2-propenoic acid, these compounds were isolated using ethyl acetate extraction of macerated bulbs [77]. Dichloromethane-methanol extract of Drimia robusta (Whole plant) possesses anti-ovarian cancer activity at the IC50 value of 1.05 μg/ml in OVCAR-3 cell line (Table 2) [55].
Gomphocarpus fruticosus (Linn.) Aiton f. (Apocynaceae), they have other few common names such as swan plant (Figure 2H), milkweed, or white cotton, is a perennial herb, spindly shrub, often with watery or milky sap. It is native to South Africa with a wide distribution in most Provinces, including Free State, Gauteng, KwaZulu-Natal, Mpumalanga, Cape (Eastern, Western, Northern), and North West [78]. Gomphocarpus fruticosus is used medicinally to treat headaches, stomach pain, tuberculosis, and as an emetic [79]. The acetone extract of Gomphocarpus fruticosus root possessed antigonoccol activity [80]. Similarly, the ethanolic extract of the root is reported to have antiovarian cancer activity with an IC50 value of 3.72 μg/ml in OVCAR-3 cell line (Table 2) [55].
Leyssera gnaphaloides (Linn.) L. (Fabaceae) is one of the South African medicinal plants (Figure 2I). It is used as a folk medicine medicine to treat various ailments including bronchitis, cough, diarrhoea, fever, and even tuberculosis [81,82]. An extract was obtained using Hexane, bioactive fractions obtained from the extracts reveal that it possesses cytotoxic effects, however, more than 50% of the ovarian cells of Chinese hamster sustained even at a very high concentration. This result implies that the extract seems to be harmless to the Chinese hamster ovarian cells [83].
Parinari curatellifolia (Planch) ex. Benth. (Chrysobalanaceae) is a tree and is semi-circular in shape almost resembles a mushroom in its canopy and the hues are in blue-green and grey colour (Figure 3A). It is an evergreen, medium to large tree, that grows up to thirteen meters, but a height of twenty three-twenty six m also been recorded in certain areas [84]. The leaf extracts and bark are used for treating the symptoms of pneumonia, and eye/ear ailments. Many traditional healers incorporate the bark of Parinari curatellifolia in the formulation of their mixture or medicine [85]. The roots are soaked in water for about an hour or six in gelid water, it is used to aid cataract and earache respectively. This roots soaked water are used as eye and ear drops [86]. The bioactive compound, 13-Methoxy-15-oxozoapatlin from the plant, has significant cytotoxic activity against SW626 human ovarian adenocarcinoma cell line (Table 2) with the IC50 value of 0.6 µM/ml [87].
Figure 3.

Anti-ovarian cancer potential of South African medicinal plants.
Pelargonium acraeum R.A.Dyer (Geraniaceae) is a small shrub (Figure 3B) of South African origin that grows up to 1 m, or occasionally 2 m high. The whole plant extract of Pelargonium acraeum has been reported to have anticancer activity. The genus Pelargonium is endowed with varieties of flavonoids and alkaloids characterized by antioxidant, antimicrobial, anti-inflammatory, and anticancer activities [88]. The methanolic extract of Pelargonium acraeum showed minimum cytotoxic activity with the IC50 value of 10 μg/ml and 60 μg/ml in PA1 ovarian cancer cell line after 24 hr and 48 hr respectively of exposure. Additionally, the aqueous extract of this plant showed better anti-ovarian cancer activity with the lowest IC50 value of 6.92 μg/ml on OVCAR-3 cell line (Table 2) [55].
Plumbago auriculata (Lam.) (Plumbaginaceae) is a medicinal plant and an ornamental shrub with clusters of light blue flowers (Figure 3C). it is commonly found in South Africa [89]. It is a rich source of alkaloids such as plumagain (2-methyl-5-hydroxyl, 4-naphthoquinone which could be used as an anti-cancer agent while also exhibiting antibacterial, antioxidant, antifungal, anti-inflammatory and anticoagulant potentials against various diseases such as rheumatism, piles, diarrhoea and skin diseases [90,91]. The methanolic extract had a minimal cytotoxic activity at 10 μg/ml (24 hr) and 60 μg/ml (48 hr) on PA1 cell lines of human ovarian cancer. Consequently, of note is the significant morphological changes also observed in PA1 cancer cells (Table 2) by nuclear staining (4’,6-diamidino-2-phenylindole) method [92].
Plumbago zeylanica (Linn.) (Plumbaginaceae) is a perennial herb (Figure 3D) commonly distributed across South Africa [93]. The entire plant is used to prepare a variety of folk medicines in Africa, but the roots hold an effective bioactive compound called Plumbagin, it had shown to have anti-malarial, anti-obese, anti-ulcer, antimicrobial, anticancer, anti-inflammatory, antioxidant properties etc. [94]. The chemotherapeutic potential of Plumbagin acts as an anticancer agent in BRCA1-mutated ovarian cancer patients. The mitochondrial membrane is lost, the nucleus gets condensed, DNA gets fragmented and other morphological changes are induced by Plumbagin in ovarian cancer cells. Moreover, it binds to the active site of ER-α and inhibits the classical ER-α signaling pathway in ovarian cancer [95].
Solanum acanthoideum Drege ex Dunal (Solanaceae) is also a medicinal plant (Figure 3E) of South African origin traditionally used to treat fever, intestinal infections, asthma, and to heal sores. It is similarly used to stimulate milk production in cows and treat cattle that are affected by gall sickness [79]. The root extract is reported to have anticancer activity in cancer cell lines. Intriguingly, the methanolic root extract of the plant has been reported in a study to possess anti-ovarian cancer activity with the IC50 value at 18.62 μg/ml using IGROV1 ovarian cancer cell line (Table 2) [55].
Solanum nigrum (Linn.) (Solanaceae) is one of the prominent species (Figure 3F) in the genus Solanum regarded as a common, important and one of the largest genera, that comprises about 84 families and 3000 species [96]. South Africa, Eurasia are its native and introduced to America, Australia and Asia. It is also known as black nightshade [97]. The ripe berries are used as food by the natives, while other plant parts are used as traditional medicine. Traditionally, it is used as an analgesic, antispasmodic, antiseptic, antibacterial, antibiofilm, anti dysenteric, antinarcotic, emollient, diuretic, tonic, soporific, laxative, anticancer, antiulcer and also to treat the disarrays of the neuro-vegetative system, etc. All these curative dispositions exhibited by the plant attributed to the alkaloid contents in them [98]. Aqueous extract of Solanum nigrum possess an anti-ovarian cancer activity in ovarian cancer cell lines of ES-2, SKOV-3 and OVCAR-3 (Table 2) with the significant IC50 values of 1.052, 1.779 and 2.000 mg/ml respectively [99,100].
Sutherlandia frutescens (Linn.) Goldblath & J.C. Manning
Sutherlandia frutescens (Figure 3G) were newly referred to as Lessertia frutescens subsp. frutescens belong to the Fabaceae family, it is the third-largest family of flowering plants. These plants are the habitat of dry areas and are commonly found in South Western and Northern Provinces of Cape [101]. The plant can also be found in other areas of Southern Africa, especially Botswana, Zimbabwe, and Namibia [102]. Sutherlandia frutescens enhancing well-being, provide immune support for tuberculosis (TB), and acquired immune deficiency syndrome (AIDS) as well as in the treatment of cancer; hence, the name cancer bush [103]. Pharmacologically, it is established to have antioxidant, anti-inflammatory, anti-ovarian cancer, and anti-diabetic activities [104].
Withania somnifera (L.) Dunal (Solanaceae) is widespread but not common in all (Figure 3H) the Provinces of South Africa but also distributed in Namibia, Botswana, Swaziland, and Lesotho. It grows in a large number of vegetation types from dry areas to areas with reasonably high rainfall, such as coastal vegetation and or grassland [105]. It possesses chemical compounds that exceeds 80 notably alkaloids and steroids (withanolides) are present in W.somnifera. Many studies have been performed and the studies disclose the truth about the pivotal deeds namely antibiotic, anti-inflammatory, cytotoxic, anti-tumor, and cholesterol-lowering deeds of these compounds, which are predominantly acquired from leaves and roots [106]. In a study, the supplementation of plant extract reduces the progression of ovarian cancer in the animal model. Moreover, Withaferin A, is a bioactive compound that has been isolated from this plant inhibits the activity of ovarian epithelial cancer cell line (A2780) by 70-80%, also the tumor growth is reduced and metastasis inhibition is also a part of the function of the isolated compound when compared to untreated controls in nude mice [107,108].
Xanthium strumarium (Linn.) (Asteraceae) is a South African medicinal plant (Figure 3I) with global distribution found in abundance in Eurasia and America [109]. The entire plant has been used in the traditional medicine to treat the infections caused by bacteria, high-sugar, itching of the skin, and inflammatory diseases like coryza and rheumatoid arthritis. It has been used included in traditional Chinese medicine for anti-cancer treatment [110]. The fruit extract of the plant contains 3, 4-dihydroxybenzaldehyde investigated to inhibit malignant tumors in human. Two xanthanolide sesquiterpene lactones, 8-epi-xanthatin, and 8-epi-xanthatin-5b-epoxide have been isolated from leaves to inhibit ovarian cancer cell line of SK-OV-3 (Table 2) [111,112].
Conclusion
The role of Medicinal plants in treating Ovarian Cancer is inevitable. Only a very few numbers of plants were been explored and phytochemical studies have been performed among 270,000 plant species. Though there many synthetic medicines are involved to treat various diseases about 3/4th community of the population adhere to the traditional medicines for their primary healthcare needs; however, only a few indigenous medicinal plants of South African have been investigated to their full potential in terms of commercialization. The opportunity for bioprospecting of the medicinal plants and their compounds for novel pharmaceuticals remain largely untapped. This paper addressed the anti-ovarian cancer activity of some of these South African medicinal plants and their bioactive compounds with a view that these medicinal plants are the real sources with less or no side-effect in treating a disease, especially they would create a revolution in combating against the Disease Ovarian Cancer by developing novel leads. This would help an individual to sustain their life.
Acknowledgements
The financial supports received from National Key Research & Development Program in China (Grant No. 2019YFD1002704), Shandong major projects of independent innovation (Grant No. 2019JZZY010722), the Key Research and Development Program of Shandong Province (Grant No. 2017YYSP024), Funds for Innovation Team of Jinan (Grant No. 2018GXRC004), and Special Funds for Taishan Scholars project are acknowledged. Authors also express our thankful to the Department of Plant Sciences, Faculty of Natural and Agricultural Sciences, University of the Free State, QwaQwa, South Africa for completion of this work in fine fulfillment.
Disclosure of conflict of interest
None.
References
- 1.Perumal PC, Sophia D, Raj CA, Ragavendran P, Starlin T, Gopalakrishnan VK. In vitro antioxidant activities and HPTLC analysis of ethanolic extract of Cayratia trifolia (L.) Asian Pac J Trop Dis. 2012;2:S952–S956. [Google Scholar]
- 2.Hassan BAR. Medicinal plants (Importance and uses) Pharmaceut Anal Acta. 2012;3:e139. [Google Scholar]
- 3.Ramaswamy L, Kanmani MG. Phytonutrient profile, health benefits and culinary applications of selected edible foliages. Int J Ayurvedic Herb Med. 2012;2:469–476. [Google Scholar]
- 4.Chakraborty P. Herbal genomics as tools for dissecting new metabolic pathways of unexplored medicinal plants and drug discovery. Biochim Open. 2018;6:9–16. doi: 10.1016/j.biopen.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perumal PC, Sowmya S, Pratibha P, Vidya B, Anusooriya P, Starlin T, Ravi S, Gopalakrishnan VK. Isolation, structural characterization and in silico drug-like properties prediction of a natural compound from the ethanolic extract of Cayratia trifolia (L.) Pharmacognosy Res. 2015;7:121–125. doi: 10.4103/0974-8490.147226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katiyar C, Gupta A, Kanjilal S, Katiyar S. Drug discovery from plant sources: an integrated approach. AYU (An Int Q J Res Ayurveda) 2012;33:10–19. doi: 10.4103/0974-8520.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey AL. Natural products in drug discovery. Drug Discov Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perumal PC, Sowmya S, Velmurugan D, Sivaraman T, Gopalakrishnan VK. Assessment of dual inhibitory activity of epifriedelanol isolated from Cayratia trifolia against ovarian cancer. Bangladesh J Pharmacol. 2016;11:545–551. [Google Scholar]
- 10.Ferreira D, Adega F, Chaves R. The Importance of cancer cell lines as in vitro models in cancer methylome analysis and anticancer drugs testing. Oncogenomics and Cancer Proteomics-Novel Approaches in Biomarkers Discovery and Therapeutic Targets in Cancer. 2013:139–166. [Google Scholar]
- 11.Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- 12.Greenwell M, Rahman PK. Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res. 2015;6:4103–4112. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coburn SB, Bray F, Sherman ME, Trabert B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int J Cancer. 2017;140:2451–2460. doi: 10.1002/ijc.30676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81:17–38. doi: 10.1007/s00280-017-3501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Ahmedin Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruibin J, Bo J, Danying W, Chihong Z, Jianguo F, Linhui G. Therapy effects of wogonin on ovarian cancer cells. Biomed Res Int. 2017;2017:9381513. doi: 10.1155/2017/9381513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandra A, Pius C, Nabeel M, Nair M, Vishwanatha JK, Ahmad S, Basha R. Ovarian cancer: current status and strategies for improving therapeutic outcomes. Cancer Med. 2019;8:7018–7031. doi: 10.1002/cam4.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palanisamy CP, Ashafa AOT. Analysis of novel C-X-C Chemokine receptor type 4 (CXCR4) inhibitors from hexane extract of Euclea crispa (Thunb.) Leaves by chemical fingerprint identification and molecular docking analysis. J Young Pharm. 2018;10:173–177. [Google Scholar]
- 20.Poornima K, Perumal PC, Gopalakrishnan VK. Protective effect of ethanolic extract of Tabernaemontana divaricata (L.) R. Br. against DEN and Fe NTA induced liver necrosis in Wistar Albino rats. Biomed Res Int. 2014;2014:240243. doi: 10.1155/2014/240243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guru D, Perumal PC, Kumar K, Gopalakrishnan VK. Dietary evaluation, antioxidant and cytotoxic activity of crude extract from chia seeds (Salvia hispanica L) against human prostate cancer cell line (PC-3) Int J Pharmacogn Phytochem Res. 2016;8:1358–1362. [Google Scholar]
- 22.Poornima K, Palanisamy CP, Sundaram S, Kanniappan GV. Chromatographic fingerprinting analysis of secondary metabolites present in ethanolic extract of Tabernaemontana divaricata (L.) R. Br. by HPTLC technique. Anal Chem Lett. 2017;7:20–29. [Google Scholar]
- 23.Sowmya S, Perumal PC, Anusooriya P, Vidya B, Pratibha P, Gopalakrishnan VK. In vitro antioxidant activity, in vivo skin irritation studies and HPTLC analysis of Cayratia trifolia (L.) Domin. Int J Toxicol Pharmacol Res. 2015;7:1–9. doi: 10.4103/0974-8490.147226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palanisamy CP, Kanakasabapathy D, Ashafa AOT. In vitro antioxidant potential of Euclea crispa (Thunb.) leaf extracts. Pharmacognosy Res. 2018;10:296–300. [Google Scholar]
- 25.Malarvizhi D, Anusooriya P, Meenakshi P, Sowmya S, Perumal PC, Oirere EK, Gopalakrishnan VK. Antioxidant properties and analysis of bioactive compounds present in n-hexane root extract of Zaleya decandra. Int J Pharm Sci Rev Res. 2015;34:118–123. [Google Scholar]
- 26.Palanisamy CP, Selvarajan R, Balogun FO, Kanakasabapathy D, Ashafa AOT. Antioxidant and antimicrobial activities of (6E, 10E)-2, 6, 24-trimethyl pentacosa-2, 6, 10-triene from Euclea crispa leaves. South African J Bot. 2019;124:311–319. [Google Scholar]
- 27.Balogun FO, Ashafa AOT, Sabiu S, Ajao AA, Perumal PC, Kazeem MI, Adedeji AA. Pharmacognosy: importance and drawbacks. Pharmacogn Med Plants. 2019:1–18. [Google Scholar]
- 28.Palanisamy CP, Ashafa AOT. Screening of potential phytocompounds from Euclea crispa (Thunb.) leaves targeting human epidermal growth factor receptor 2 (HER2) signaling pathway. J Pharm Bioallied Sci. 2019;11:155–161. doi: 10.4103/jpbs.JPBS_61_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palanirajan A, Raj CA, Perumal PC, Sundaram S, Balu V, Prabhakaran P, Gopalakrishnan VK. Screening of novel CXC chemokine receptor 4 inhibitors from ethyl acetate extract of alpinia purpurata using GC-MS analysis and its molecular docking studies. Int J Pharmacogn Phytochem Res. 2015;7:480–488. [Google Scholar]
- 30.Perumal PC, Pratibha P, Sowmya S, Oirere EK, Anusooriya P, Vidya B, Malarvizhi D, Poornima K, Ramkumar S, Gopalakrishnan VK. Discovery of novel inhibitors for HER2 from natural compounds present in Cayratia trifolia (l.): an in silico analysis. Int J Curr Pharm Rev Res. 2015;6:164–168. [Google Scholar]
- 31.Kuete V, Ango PY, Yeboah SO, Mbaveng AT, Mapitse R, Kapche GD, Ngadjui BT, Efferth T. Cytotoxicity of four Aframomum species (A. arundinaceum, A. alboviolaceum, A. kayserianum and A. polyanthum) towards multi-factorial drug resistant cancer cell lines. BMC Complement Altern Med. 2014;14:340. doi: 10.1186/1472-6882-14-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuete V, Sandjo LP, Wiench B, Efferth T. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat cancers: echinops giganteus, xylopia aethiopica, imperata cylindrica and piper capense. J Ethnopharmacol. 2013;149:245–253. doi: 10.1016/j.jep.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Kuete V, Efferth T. Pharmacogenomics of Cameroonian traditional herbal medicine for cancer therapy. J Ethnopharmacol. 2011;137:752–766. doi: 10.1016/j.jep.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 34.Kuete V, Fankam AG, Wiench B, Efferth T. Cytotoxicity and modes of action of the methanol extracts of six cameroonian medicinal plants against multidrug-resistant tumor cells. Evid Based Complement Alternat Med. 2013;2013:285903. doi: 10.1155/2013/285903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuete V, Voukeng IK, Tsobou R, Mbaveng AT, Wiench B, Beng VP, Efferth T. Cytotoxicity of Elaoephorbia drupifera and other Cameroonian medicinal plants against drug sensitive and multidrug resistant cancer cells. BMC Complement Altern Med. 2013;13:250. doi: 10.1186/1472-6882-13-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omosa LK, Midiwo JO, Masila VM, Gisacho BM, Munayi R, Francisca-Kamakama , Chemutai KP, Elhaboob G, Saeed ME, Hamdoun S, Kuete V, Efferth T. Cytotoxicity of 91 Kenyan indigenous medicinal plants towards human CCRF-CEM leukemia cells. J Ethnopharmacol. 2016;179:177–196. doi: 10.1016/j.jep.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 37.Baek J, Lee D, Lee TK, Song JH, Lee JS, Lee S, Yoo SW, Kang KS, Moon E, Lee S, Kim KH. (-)-9’-O-(α-L-Rhamnopyranosyl)lyoniresinol from Lespedeza cuneata suppresses ovarian cancer cell proliferation through induction of apoptosis. Bioorganic Med Chem Lett. 2018;28:122–128. doi: 10.1016/j.bmcl.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 38.Zhao W, Li N, Zhang X, Wang W, Li J, Si Y. Cancer chemopreventive theasaponin derivatives from the total tea seed saponin of Camellia sinensis. J Funct Foods. 2015;12:192–198. [Google Scholar]
- 39.Seo JH, Jeong KJ, Oh WJ, Sul HJ, Sohn JS, Kim YK, Cho DY, Kang JK, Park CG, Lee HY. Lysophosphatidic acid induces STAT3 phosphorylation and ovarian cancer cell motility: their inhibition by curcumin. Cancer Lett. 2010;288:50–56. doi: 10.1016/j.canlet.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 40.Segun PA, Ogbole OO, Ajaiyeoba EO. Medicinal plants used in the management of cancer among the ijebus of southwestern Nigeria. J Herb Med. 2018;14:68–75. [Google Scholar]
- 41.Olujimi OO, Bamgbose O, Arowolo T, Steiner O, Goessler W. Elemental profiles of herbal plants commonly used for cancer therapy in Ogun State, Nigeria. Part I. Microchem J. 2014;117:233–241. [Google Scholar]
- 42.Ntie-Kang F, Onguéné PA, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of anti-malarial compounds derived from African medicinal plants, part II: a hpharmacological evaluation of non-alkaloids and non-terpenoids. Malar J. 2014;13:81. doi: 10.1186/1475-2875-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker JV, Van Der Merwe MM, Van Brummelen AC, Pillay P, Crampton BG, Mmutlane EM, Parkinson C, Heerden FR, Crouch NR, Smith PJ, Mancama DT, Maharaj VJ. In vitro anti-plasmodial activity of dicoma anomala subsp. gerrardii (asteraceae): identification of its main active constituent, structure-activity relationship studies and gene expression profiling. Malar J. 2011;10:295. doi: 10.1186/1475-2875-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babiaka SB, Ntie-Kang F, Lifongo LL, Ndingkokhar B, Mbah JA, Yong JN. The chemistry and bioactivity of Southern African flora I: a bioactivity versus ethnobotanical survey of alkaloid and terpenoid classes. RSC Adv. 2015;5:43242–43267. [Google Scholar]
- 45.Khorombi T, Fouché G, Kolesnikova N, Maharaj V, Nthambeleni R, Merwe M. Investigation of South African plants for anti cancer properties. Pharmacologyonline. 2006;3:494–500. [Google Scholar]
- 46.McKay DL, Blumberg JB. A review of the bioactivity of South African herbal teas: Rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia) Phyther Res. 2007;21:1–16. doi: 10.1002/ptr.1992. [DOI] [PubMed] [Google Scholar]
- 47.Van-Wyk BE. The potential of South African plants in the development of new medicinal products. South African J Bot. 2011;77:812–829. [Google Scholar]
- 48.Joubert E, De-Beer D. Rooibos (Aspalathus linearis) beyond the farm gate: from herbal tea to potential phytopharmaceutical. South African J Bot. 2011;77:869–886. [Google Scholar]
- 49.Fantoukh OI, Dale OR, Parveen A, Hawwal MF, Ali Z, Manda VK, Khan SI, Chittiboyina AG, Viljoen A, Khan IA. Safety assessment of phytochemicals derived from the globalized South African rooibos tea (Aspalathus linearis) through interaction with CYP, PXR, and P-gp. J Agric Food Chem. 2019;67:4967–4975. doi: 10.1021/acs.jafc.9b00846. [DOI] [PubMed] [Google Scholar]
- 50.Johnson R, Shabalala S, Louw J, Kappo AP, Muller CJF. Aspalathin reverts doxorubicin-induced cardiotoxicity through increased autophagy and decreased expression of p53/mTOR/p62 signaling. Molecules. 2017;22:1589. doi: 10.3390/molecules22101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marnewick J, Joubert E, Joseph S, Swanevelder S, Swart P, Gelderblom W. Inhibition of tumour promotion in mouse skin by extracts of rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), unique South African herbal teas. Cancer Lett. 2005;224:193–202. doi: 10.1016/j.canlet.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Sissing L, Marnewick J, De Kock M, Swanevelder S, Joubert E, Gelderblom W. Modulating effects of rooibos and honeybush herbal teas on the development of esophageal papillomas in rats. Nutr Cancer. 2011;63:600–610. doi: 10.1080/01635581.2011.539313. [DOI] [PubMed] [Google Scholar]
- 53.Marnewick JL, van der Westhuizen FH, Joubert E, Swanevelder S, Swart P, Gelderblom WC. Chemoprotective properties of rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) herbal and green and black (Camellia sinensis) teas against cancer promotion induced by fumonisin B1 in rat liver. Food Chem Toxicol. 2009;47:220–229. doi: 10.1016/j.fct.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Thomas AD, Dougill AJ. Distribution and characteristics of cyanobacterial soil crusts in the Molopo Basin, South Africa. J Arid Environ. 2006;64:270–283. [Google Scholar]
- 55.Fouche G, Cragg GM, Pillay P, Kolesnikova N, Maharaj VJ, Senabe J. In vitro anticancer screening of South African plants. J Ethnopharmacol. 2008;119:455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Wabe NT. Chemistry, pharmacology, and toxicology of khat (catha edulis forsk): a review. Addict Heal. 2011;3:137–149. [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Alimi KR, Abdul Razak AA, Saub R, Alabsi AM. Tannins acid, ascorbic acid and fluoride from khat chewing plant. Int J Dent Oral Heal. 2017;3:4. [Google Scholar]
- 58.Getasetegn M. Chemical composition of Catha edulis (khat): a review. Phytochem Rev. 2016;15:907–920. [Google Scholar]
- 59.Al-Zubairi AS. Genotoxicity assessment of fresh Khat leaves extract in Chinese hamster ovary cell lines. J Med Sci. 2017;17:126–132. [Google Scholar]
- 60.Alsanosy R, Alhazmi HA, Sultana S, Abdalla AN, Ibrahim Y, Al Bratty M, Banji D, Khardali I, Khalid A. Phytochemical screening and cytotoxic properties of ethanolic extract of young and mature khat leaves. J Chem. 2020;2020:7897435. [Google Scholar]
- 61.Elhag H, Mossa JS, El-olemy MM. Antimicrobial and cytotoxic activity of the extracts of khat callus cultures. Perspect new crop new uses. 1999;(Mic):463–466. [Google Scholar]
- 62.Oyedeji OA, Afolayan AJ. Chemical composition and antibacterial activity of the essential oil of Centella asiatica growing in South Africa. Pharm Biol. 2005;43:249–252. [Google Scholar]
- 63.Yao CH, Yeh JY, Chen YS, Li MH, Huang CH. Wound-healing effect of electrospun gelatin nanofibres containing Centella asiatica extract in a rat model. J Tissue Eng Regen Med. 2017;11:905–915. doi: 10.1002/term.1992. [DOI] [PubMed] [Google Scholar]
- 64.Naidoo DB, Phulukdaree A, Anand K, Sewram V, Chuturgoon AA. Centella asiatica fraction-3 suppresses the nuclear factor erythroid 2-related factor 2 anti-oxidant pathway and enhances reactive oxygen species-mediated cell death in cancerous lung A549 cells. J Med Food. 2017;20:959–968. doi: 10.1089/jmf.2017.0005. [DOI] [PubMed] [Google Scholar]
- 65.Somboonwong J, Kankaisre M, Tantisira B, Tantisira MH. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: an experimental animal study. BMC Complement Altern Med. 2012;12:103. doi: 10.1186/1472-6882-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ren L, Cao QX, Zhai FR, Yang SQ, Zhang HX. Asiatic acid exerts anticancer potential in human ovarian cancer cells via suppression of PI3K/Akt/mTOR signalling. Pharm Biol. 2016;54:2377–2382. doi: 10.3109/13880209.2016.1156709. [DOI] [PubMed] [Google Scholar]
- 67.Balogun FO, Tshabalala NT, Ashafa AOT. Antidiabetic medicinal plants used by the basotho tribe of eastern free state: a review. J Diabetes Res. 2016;2016:4602820. doi: 10.1155/2016/4602820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asfaw N, Demissew S. Handbook of African medicinal plants. Econ Bot. 1994:1–508. [Google Scholar]
- 69.Balogun FO, Ashafa AOT. Aqueous root extracts of Dicoma anomala (Sond.) extenuates postprandial hyperglycaemia in vitro and its modulation on the activities of carbohydrate-metabolizing enzymes in streptozotocin-induced diabetic Wistar rats. South African J Bot. 2017;112:102–111. [Google Scholar]
- 70.Bohlmann F, Singh P, Jakupovic J. New germacranolides and other sesquiterpene lactones from Dicoma species. Phytochemistry. 1982;21:2029–2033. [Google Scholar]
- 71.Maroyi A. Dicoma anomala sond.: a review of its botany, ethnomedicine, phytochemistry and pharmacology. Asian J Pharm Clin Res. 2018;11:70–77. [Google Scholar]
- 72.Lawal D, Yunusa I. Dodonea viscosa linn: its medicinal, pharmacological and phytochemical properties. Int J Innov Appl Stud. 2013;2:477–483. [Google Scholar]
- 73.Getie M, Gebre-Mariam T, Rietz R, Neubert RH. Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae) Pharmazie. 2002;57:320–322. [PubMed] [Google Scholar]
- 74.Colodel EM, Traverso SD, Seitz AL, Correa A, Oliveira FN, Driemeier D, Gava A. Spontaneous poisoning by Dodonea viscosa (Sapindaceae) in cattle. Vet Hum Toxicol. 2003;45:147–148. [PubMed] [Google Scholar]
- 75.Cao S, Brodie P, Callmander M, Randrianaivo R, Razafitsalama J, Rakotobe E, Rasamison VE, Dyke KT, Shen Y, Suh EM, Kingston DG. Antiproliferative triterpenoid saponins of Dodonaea wiscosa from the madagascar dry forest. J Nat Prod. 2009;72:1705–1707. doi: 10.1021/np900293x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ngugi GW, Jäger AK, Van-Staden J. In vitro propagation of Drimia robusta Bak. South African J Bot. 1998;64:266–268. [Google Scholar]
- 77.Xego S, Kambizi L, Nchu F. Threatened medicinal plants of South Africa: case of the family hyacinthaceae. African J Tradit Complement Altern Med. 2016;13:169–180. [Google Scholar]
- 78.van-Wyk BE. A broad review of commercially important southern African medicinal plants. J Ethnopharmacol. 2008;119:342–355. doi: 10.1016/j.jep.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 79.Scott LC. The medicinal and poisonous plants of Southern Africa. Am J Trop Med Hyg. 1933;2:112. [Google Scholar]
- 80.Goyder DJ, Nicholas A. A revision of Gomphocarpus R. Br. (Apocynaceae: Asclepiadeae) Kew Bull. 2001;56:769–836. [Google Scholar]
- 81.Green E, Samie A, Obi CL, Bessong PO, Ndip RN. Inhibitory properties of selected South African medicinal plants against Mycobacterium tuberculosis. J Ethnopharmacol. 2010;130:151–157. doi: 10.1016/j.jep.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 82.Tsichritzis F, Jakupovic J. Diterpenes from Leyssera gnaphaloides . Phytochemistry. 1991;30:211–213. [Google Scholar]
- 83.Bamuamba K, Gammon DW, Meyers P, Dijoux-Franca MG, Scott G. Anti-mycobacterial activity of five plant species used as traditional medicines in the Western Cape Province (South Africa) J Ethnopharmacol. 2008;117:385–390. doi: 10.1016/j.jep.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Ogbonnia SO, Adekunle A, Olagbende-Dada SO, Anyika EN, Enwuru VN, Orolepe M. Assessing plasma glucose and lipid levels, body weight and acute toxicity following oral administration of an aqueous ethanolic extract of Parinari curatellifolia Planch, (Chrysobalanaceae) seeds in alloxan-induced diabetes in rats. African J Biotechnol. 2008;7:2998–3003. [Google Scholar]
- 85.Lee IS, Shamon LA, Chai HB, Chagwedera TE, Besterman JM, Farnsworth NR, Gordell GA, Pezzuto JM, Kinghorn AD. Cell-cycle specific cytotoxicity mediated by rearranged ent-kaurene diterpenoids isolated from Parinari curatellifolia . Chem Biol Interact. 1996;99:193–204. doi: 10.1016/0009-2797(95)03669-5. [DOI] [PubMed] [Google Scholar]
- 86.Olaleye MT, Amobonye AE, Komolafe K, Akinmoladun AC. Protective effects of Parinari curatellifolia flavonoids against acetaminophen-induced hepatic necrosis in rats. Saudi J Biol Sci. 2014;21:486–492. doi: 10.1016/j.sjbs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mi Q, Lantvit D, Reyes-Lim E, Chai H, Zhao W, Lee IS, Peraza-Sánchez S, Ngassapa O, Kardono LBS, Riswan S, Hollingshead GM, Mayo JG, Farnsworth NR, Cordell GA, Kinghorn AD, Pezzuto JM. Evaluation of the potential cancer chemotherapeutic efficacy of natural product isolates employing in vivo hollow fiber tests. J Nat Prod. 2002;65:842–850. doi: 10.1021/np010322w. [DOI] [PubMed] [Google Scholar]
- 88.Williams CA, Harborne JB, Newman M, Greenham J, Eagles J. Chrysin and other leaf exudate flavonoids in the genus Pelargonium. Phytochemistry. 1997;46:1349–1353. doi: 10.1016/s0031-9422(97)00514-1. [DOI] [PubMed] [Google Scholar]
- 89.Karishma S, Yougasphree N, Baijnath H. A comprehensive review on the genus plumbago with focus on plumbago (Plumbaginaceae) Afr J Tradit Complement Altern Med. 2018;15:199–215. [Google Scholar]
- 90.Min Y, Wang J, Yang J, Liu W. Chemical constituents of Plumbago zeylanica L. Advanced Materials Research. 2011;310:1662–1664. [Google Scholar]
- 91.Dorni AIC, Vidyalakshmi KS, Vasanthi HR, Rajamanickam GV. HPTLC Method for the quantification of plumbagin in three plumbago species. Res J Phytochem. 2007;1:46–51. [Google Scholar]
- 92.Lakshmanan G, Bupesh G, Vignesh A, Sathiyaseelan A, Murugesan K. Micropropagation and anticancer activity of methanolic extract of Plumbago auriculata Lam. Int J Adv Biotechnol Res. 2016;7:2001–2011. [Google Scholar]
- 93.Chauhan M. A review on Morphology, Phytochemistry and Pharmacological activities of medicinal herb Plumbago Zeylanica Linn. J Pharmacogn Phytochem. 2014;3:95–118. [Google Scholar]
- 94.Sharma A, Singh N. A multifarious potent herb: plumbago zeylanica - a mini review. Int J Recent Sci Res. 2015;6:4825–4829. [Google Scholar]
- 95.Thasni KA, Rakesh S, Rojini G, Ratheeshkumar T, Srinivas G, Priya S. Estrogen-dependent cell signaling and apoptosis in BRCA1-blocked BG1 ovarian cancer cells in response to plumbagin and other chemotherapeutic agents. Ann Oncol. 2008;19:696–705. doi: 10.1093/annonc/mdm557. [DOI] [PubMed] [Google Scholar]
- 96.Huang HC, Syu KY, Lin JK. Chemical composition of Solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in AU565 breast cancer cells. J Agric Food Chem. 2010;58:8699–8708. doi: 10.1021/jf101003v. [DOI] [PubMed] [Google Scholar]
- 97.Shokrzadeh M, Azadbakht M, Ahangar N, Hashemi A, Saravi SS. Cytotoxicity of hydro-alcoholic extracts of Cucurbita pepo and Solanum nigrum on HepG2 and CT26 cancer cell lines. Pharmacogn Mag. 2010;6:176–179. doi: 10.4103/0973-1296.66931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malaikozhundan B, Vijayakumar S, Vaseeharan B, Jenifer AA, Chitra P, Prabhu NM, Kannapiran E. Two potential uses for silver nanoparticles coated with Solanum nigrum unripe fruit extract: biofilm inhibition and photodegradation of dye effluent. Microb Pathog. 2017;111:316–324. doi: 10.1016/j.micpath.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 99.Tai CJ, Wang CW, Chen CL, Wang CK, Chang YJ, Jian JY, Lin CS, Tai CJ, Tai CJ. Cisplatin-, doxorubicin-, and docetaxel-induced cell death promoted by the aqueous extract of solanum nigrum in human ovarian carcinoma cells. Integr Cancer Ther. 2015;14:546–555. doi: 10.1177/1534735415588826. [DOI] [PubMed] [Google Scholar]
- 100.Lee SJ, Oh PS, Ko JH, Lim K, Lim KT. A 150-kDa glycoprotein isolated from Solanum nigrum L. has cytotoxic and apoptotic effects by inhibiting the effects of protein kinase C alpha, nuclear factor-kappa B and inducible nitric oxide in HCT-116 cells. Cancer Chemother Pharmacol. 2004;54:562–572. doi: 10.1007/s00280-004-0850-x. [DOI] [PubMed] [Google Scholar]
- 101.Aboyade OM, Styger G, Gibson D, Hughes G. Sutherlandia frutescens: the meeting of science and traditional knowledge. J Altern Complement Med. 2014;20:71–76. doi: 10.1089/acm.2012.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vuuren SF. Antimicrobial activity of South African medicinal plants. J Ethnopharmacol. 2008;119:462–472. doi: 10.1016/j.jep.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 103.Tai J, Cheung S, Chan E, Hasman D. In vitro culture studies of Sutherlandia frutescens on human tumor cell lines. J Ethnopharmacol. 2004;93:9–19. doi: 10.1016/j.jep.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 104.Chinkwo KA. Sutherlandia frutescens extracts can induce apoptosis in cultured carcinoma cells. J Ethnopharmacol. 2005;98:163–170. doi: 10.1016/j.jep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 106.Rodríguez-Burford C, Barnes MN, Berry W, Partridge EE, Grizzle WE. Immunohistochemical expression of molecular markers in an avian model: a potential model for preclinical evaluation of agents for ovarian cancer chemoprevention. Gynecol Oncol. 2001;81:373–379. doi: 10.1006/gyno.2001.6191. [DOI] [PubMed] [Google Scholar]
- 107.Barua A, Bradaric MJ, Bitterman P, Abramowicz JS, Sharma S, Basu S, Lopez H, Bahr JM. Dietary supplementation of ashwagandha (Withania somnifera, Dunal) enhances NK cell function in ovarian tumors in the laying hen model of spontaneous ovarian cancer. Am J Reprod Immunol. 2013;70:538–550. doi: 10.1111/aji.12172. [DOI] [PubMed] [Google Scholar]
- 108.Kakar SS, Ratajczak MZ, Powell KS, Moghadamfalahi M, Miller DM, Batra SK, Singh SK. Withaferin a alone and in combination with cisplatin suppresses growth and metastasis of ovarian cancer by targeting putative cancer stem cells. PLoS One. 2014;9:e107596. doi: 10.1371/journal.pone.0107596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McMillan C, Chavez PI, Plettman SG, Mabry TJ. Systematic implications of the sesquiterpene lactones in the “strumarium” morphological complex (Xanthium strumarium, Asteraceae) of Europe, Asia and Africa. Biochem Syst Ecol. 1975;2:181–184. [Google Scholar]
- 110.Panda SK, Luyten W. Antiparasitic activity in Asteraceae with special attention to ethnobotanical use by the tribes of Odisha, India. Parasite. 2018;25:10. doi: 10.1051/parasite/2018008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Mekhlafi FA, Abutaha N, Mashaly AMA, Nasr FA, Ibrahim KE, Wadaan MA. Biological activity of Xanthium strumarium seed extracts on different cancer cell lines and Aedes caspius, Culex pipiens (Diptera: Culicidae) Saudi J Biol Sci. 2017;24:817–821. doi: 10.1016/j.sjbs.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sánchez-Lamar A, Piloto-Ferrer J, Fiore M, Stano P, Cozzi R, Tofani D, Cundari E, Francisco M, Romero A, Gonzalez ML, Degrassi F. Xanthium strumarium extract inhibits mammalian cell proliferation through mitotic spindle disruption mediated by xanthatin. J Ethnopharmacol. 2016;194:781–788. doi: 10.1016/j.jep.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 113.Gallelli L. Escin: a review of its anti-edematous, antiinflammatory, and venotonic properties. Drug Des Devel Ther. 2019;13:3425–3437. doi: 10.2147/DDDT.S207720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.León F, Habib E, Adkins JE, Furr EB, McCurdy CR, Cutler SJ. Phytochemical characterization of the leaves of Mitragyna speciosa grown in USA. Nat Prod Commun. 2009;4:907–910. [PMC free article] [PubMed] [Google Scholar]
- 115.Neag MA, Mocan A, Echeverría J, Pop RM, Bocsan CI, Crisan G, Buzoianu AD. Berberine: Botanical Occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front Pharmacol. 2018;9:557. doi: 10.3389/fphar.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumar A, Sharma PR, Mondhe DM. Potential anticancer role of colchicine-based derivatives: an overview. Anticancer Drugs. 2017;28:250–262. doi: 10.1097/CAD.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 117.Kaliyadasa E, Samarasinghe BA. A review on golden species of Zingiberaceae family around the world: genus Curcuma. Afr J Agric Res. 2019;14:519–531. [Google Scholar]
- 118.Akinboye ES, Bakare O. Biological Activities of Emetine. Open Nat Prod J. 2011;4:8–15. [Google Scholar]
- 119.Mahmoud AM, Bautista RJ, Sandhu MA, Hussein OE. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev. 2019;2019:5484138. doi: 10.1155/2019/5484138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pires TC, Dias MI, Calhelha RC, Carvalho AM, Queiroz MJ, Barros L, Ferreira IC. Bioactive properties of Tabebuia impetiginosa-based phytopreparations and phytoformulations: a comparison between extracts and dietary supplements. Molecules. 2015;20:22863–22871. doi: 10.3390/molecules201219885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rahman S, Ansari RA, Rehman H, Parvez S, Raisuddin S. Nordihydroguaiaretic acid from creosote bush (Larrea tridentata) mitigates 12-O-tetradecanoylphorbol-13-acetate-induced inflammatory and oxidative stress responses of tumor promotion cascade in mouse skin. Evid Based Complement Alternat Med. 2011;2011:734785. doi: 10.1093/ecam/nep076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, Rosenthal PJ, Alessandro UD. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]



