Abstract
Distribution of regional lymph nodes (LNs) is decisive for the lymphadenectomy boundary in radical resection of right-sided colon cancer (RCC). Currently, the data of LNs in central area remains ambiguous and scarce. Herein we aim to provide a more detailed anatomical research on LNs surrounding the superior mesenteric vessels for RCC and investigated the metastasis rate. In this study, Carbon Nanoparticles (CNs) and Indocyanine Green (ICG) were used for regional LNs mapping by preoperative colonoscopic tattooing (PCT) and we laparoscopically observed the stained LNs distribution pattern. Lastly, 143 RCC patients who received a “superior mesenteric artery (SMA)-oriented” hemicolectomy were included to calculate the probability of LNs metastasis in our target area. 27 patients diagnosed as RCC (mean age 58.04 years, 17 male) were included. 14 patients underwent CNs injection and 13 patients consented to the ICG, while 4 cases suffered from imaging failure. The unequal number of the regional LNs located between SMV and SMA was detected in 22 cases (81.48%), posterior to SMV area in 6 cases (22.22%), and anterior to SMA in 16 cases (59.26%), respectively. The presence of LNs posterior to SMV was associated with the crossing pattern of ileocolic artery (χ2 = 4.24, P = 0.039). The probability of LNs metastasis in the above areas (target areas) was 2.10% (3/143). In conclusion, right-hemi colon-draining lymphatic vessels anteriorly/posteriorly traversed the SMV and arrived at the surface of SMA near the middle colonic artery (MCA) level, which highlights the potential need of removing mesenteric tissue in our target area on lymphatic resection.
Keywords: Right-sided colon cancer, lymph nodes distribution, in-vivo, central area
Introduction
Since the concept of complete mesocolic excision (CME) was proposed in the last decade, it has been adopted to carry out more radical operations globally [1,2]. Regional LNs metastases are regarded as one of the most critical prognostic indicators for colorectal cancer (CRC), and the lymph node yield has been proved to be an independent risk factor in patients with CRC [3,4]. Therefore, the complete removal of the mesentery wrapped by visceral peritoneum and mesenteric fascia that contain the regional draining lymph nodes of the tumor area, become the main component of CME [5].
The awareness of regional LNs of RCC is undergoing continuous dynamics. Due to the complicated anatomical layers in the right colon, the extent of lymphadenectomy for RCC has not been defined clearly by NCCN or ESMO guidelines [6]. However, the central nodal metastasis rates (on the root of the artery) in RCC reported by different centers ranged from 0-5.8% [2,7], which suggested the necessity for refining research involving LNs distributed pattern in the central area.
Until now the internal border of the central area has remained fuzzy, and the data on regional LNs around superior mesenteric vessels are scanty [8,9]. Currently, most surgeons define SMV as the destination of RCC surgery, but this opinion is controversial. In 2013, Milan Spasojevic et al reported an anatomical postmortem study and established the presence of LNs posterior to SMV, which was associated with the cross-modal relationship between an ileocolic artery (ICA) and SMV [10]. Recent postmortem research developed the understanding that the long right colonic lymphovascular bundles were across the SMV and described the midline of SMA as the watershed between the small bowel and right colon lymphatics [8]. This research had shown the potential necessity to define the internal border of RCC resection to the midline of SMA. However, the perioperative detection of lymph nodes from RCC patients has not been systematically revealed, due to the limitation of visual observation to distinguish LNs from the adjacent connective tissue.
CNs and ICG were found to be reliable navigations in colonic surgery for lymph road mapping, guiding anatomical destination, precise positioning of the tumor, and reducing intraoperative and postoperative complications [11-14]. These tattooing agents are available tools that keep LNs visible during operation.
In this study, we aimed to investigate the regional LNs distribution pattern in the central area of the RCC patients and further discussed the subsequent need for the increased resection margins anterior to the SMA.
Material and methods
Patients
Patients were excluded if they had CNs or ICG hypersensitivity, PCT disagreement, or severe mental disease. 27 eligible patients from October 2019 to November 2020 at the Department of Colorectal Surgery in Guangdong Province Hospital of Traditional Chinese Medicine, with pathologically confirmed RCC, no history of abdominal surgery, were included in this research. Informed consent for research was obtained from patients and all the patients were scheduled for laparoscopic radical resection. The protocol for this study was approved by the ethical committee of Guangdong Provincial Hospital of Chinese Medicine. Besides, all ethical principles and applicable regulations to be followed in our research were certified. In addition, the clinical information of the 143 RCC patients from September 2016 to November 2020 who received the “SMA-orient” hemicolectomy was authorized to use.
Tattooing method
CNs and ICG were used for tattooing agents and all PCT was performed the day before the surgery. After bowel preparation, the patients were arranged for colonoscopy. Agents were colonoscopic injected at 2-3 sites in the proximal or distal portion approximately 1 cm away from the edge of the lesion. Before tattooing, the endoscopist lifted submucosa with a small deposit of normal saline, to determine the needle was in the submucosa and avoid intraperitoneal injection of the CNs and ICG. Then the premixed CNs (50 mg/1 ml) or diluted ICG (25 mg/10 ml) was injected in the same site using a 25-gauge needle. The tattooing method was performed by experienced endoscopist and the irritation of the lesion should be avoided.
Procedure for laparoscopic surgery
To our center, an abdominal enhanced CT scan would be acquired for a preoperative evaluation. Once R0 resection was realized, the “artery first” technique with beyond D3 lymph node dissection on the midline of the SMA that we firstly proposed in 2019 was given priority [15]. Step 1, the operator mobilized the tri-junction of the ileocecal area and exposed the duodenum and pancreatic head. Step 2, ileocolic artery/vein (ICA/ICV), right colonic artery/vein (RCA/RCV), and middle colonic artery/vein (MCA/MCV) were bared successively along the midline of SMA from caudal to cranial side and ligated these vessels at the root. Step 3, nearly 2/3 proximal gastrocolic ligament was separated and we laterally dissected the anterior lobe of the transverse mesocolon to free the hepatic flexure. The key time-points during the operation along with stained LNs were as shown in the Figures S1, S2.
Outcome measures
Primary outcome measures were the detection of stained LNs posterior to SMV, between SMV and SMA, and anterior to SMA areas. In the course of laparoscopic operation, the carbon-containing LNs were visible, and ICG was activated with a near-infrared LED at a wavelength of 760 nm as the light source. The operators should expose the clear operation visual field and record surgical pictures. All the extracted LNs were preserved by formalin in different boxes according to location. Specimens were examined in accordance with standardized protocols. The agent concentration LNs were more easily detected by the pathologist and the agents would not change the histomorphology of LNs.
Statistical analysis
Descriptive statistics were used. The Row Mean and Std. Deviation (SD) calculations on the clinical characteristics in this study were performed by Graphpad Prism (Version 8.0). Student t-test was conducted for single comparisons and P<0.05 was considered significant.
Results
Patients’ background
A total of 27 patients (17 male, aged 58.04±13.43 years, BMI = 21.34±2.67 kg/m2 [mean ± SD]) accepted PCT. The levels of serum gastrointestinal tumor markers were preoperatively detected (CEA = 36.26±157.30 μg/L, CA199 = 14.58±12.47 U/ml, AFP = 3.22±1.94 ng/ml [mean ± SD]). The parameters including disease type, differentiation, tumor size, and the pathological stage were as shown in Table 1. No conversion to open surgery and severe postoperative complications occurred. One patient developed liver metastasis (case 6) and consented to radiofrequency ablation. All the patients were discharged uneventfully and with no re-hospitalization within 30 days.
Table 1.
Baseline clinical characteristics of 27 tattooed patients
| Case | Gender | Age | BMI | Disease type | Differentiation | Tumor size (cm) | Pathological stage | CEA (μg/L) | CA199 (U/ml) | AFP (ng/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 52 | 21.97 | Adenocarcinoma | Moderate | 9.5×6.5 | T3N0M0 | 4.27 | 2.73 | 3.28 |
| 2 | Male | 80 | 21.51 | Adenocarcinoma | Poor | 14.0×8.5 | T4N2M0 | 0.97 | 3.41 | 1.03 |
| 3 | Male | 61 | 18.45 | Adenocarcinoma | Moderate | 7.0×5.0 | T3N0M0 | 17.17 | 9.91 | 3.58 |
| 4 | Male | 53 | 20.20 | Adenocarcinoma | Moderate | 3.8×3.5 | T3N0M0 | 2.67 | 20.92 | 3.24 |
| 5 | Female | 56 | 20.70 | Adenocarcinoma | Moderate | 6.5×4.5 | T3N0M0 | 6.52 | 5.95 | 2.88 |
| 6 | Male | 32 | 21.55 | Adenocarcinoma | Moderate | 4.5×3.5 | T4N2M1 | 791.1 | 3.37 | |
| 7 | Female | 67 | 22.19 | Adenocarcinoma | Moderate | 6.5×6.0 | T4N0M0 | 10.8 | 22.60 | 1.78 |
| 8 | Male | 53 | 21.43 | Adenocarcinoma | Moderate | 6.5×4.2 | T3N0M0 | 3.63 | 19.24 | 3.33 |
| 9 | Female | 31 | 17.57 | Adenocarcinoma | Moderate | 3.5×1.5 | T4N2M0 | 0.73 | 35.39 | 3.96 |
| 10 | Female | 39 | 24.89 | Adenocarcinoma | Poor | 7.8×6.5 | T3N1M0 | |||
| 11 | Male | 42 | 23.31 | Adenocarcinoma | Poor | 4.5×3.0 | T4N2M0 | 12.35 | 34.63 | 1.49 |
| 12 | Male | 56 | 20.37 | Adenocarcinoma | Moderate | 3.0×2.0 | T4N1M0 | 0.36 | 2.62 | 3.44 |
| 13 | Female | 40 | 24.22 | Adenocarcinoma | Poor | 3.5×3.0 | T3N0M0 | 1.43 | 50.23 | 1.35 |
| 14 | Male | 51 | 25.16 | Adenocarcinoma | Moderate | 5.5×4.0 | T3N0M0 | 3.15 | 0.60 | 4.54 |
| 15 | Male | 59 | 21.59 | Adenocarcinoma | Moderate | T1N0M0 | ||||
| 16 | Female | 56 | 20.03 | Adenocarcinoma | Moderate | 2.2×2.4 | T3N1M0 | 1.02 | 6.81 | 8.41 |
| 17 | Male | 56 | 24.81 | Adenocarcinoma | Moderate | 9.5×8.0 | T3N0M0 | 2.5 | 3.63 | 2.05 |
| 18 | Male | 70 | 23.03 | Adenocarcinoma | Moderate | 6.5×6.5 | T3N0M0 | 2.47 | 19.28 | 1.61 |
| 19 | Female | 81 | 17.1 | Adenocarcinoma | Moderate | 4.5×4.0 | T4N1M0 | 4.64 | 1.14 | 5.72 |
| 20 | Female | 76 | 24.7 | Adenocarcinoma | Moderate | 3.5×3.0 | T3N2M0 | 5.01 | 4.87 | 7.83 |
| 21 | Female | 57 | 17.22 | Adenocarcinoma | Moderate | 3.5×2.5 | T3N0M0 | 2.22 | 20.03 | 2.59 |
| 22 | Male | 59 | 17.01 | Adenocarcinoma | Moderate | 3.7×3.0 | T4N2M0 | 1.89 | 15.35 | 1.27 |
| 23 | Male | 69 | 18.73 | Adenocarcinoma | Moderate | 4.4×4.5 | T3N0M0 | 6.98 | 22.71 | 5.41 |
| 24 | Male | 69 | 24.61 | Adenocarcinoma | Well | 6.8×6.0 | T2N0M0 | 2.18 | 7.56 | 2.90 |
| 25 | Male | 76 | 23.43 | Adenocarcinoma | Moderate | 5.0×3.0 | T4N0M0 | 3.35 | 11.04 | 1.85 |
| 26 | Male | 63 | 17.58 | Adenocarcinoma | Moderate | 3.5×3.5 | T3N0M0 | 13.14 | 16.93 | 1.28 |
| 27 | Female | 63 | 22.94 | Adenocarcinoma | Moderate | 6.0×4.8 | T3N0M0 | 5.90 | 12.23 | 2.37 |
LNs posterior to SMV
As for LNs posterior to SMV, we could observe fluorescence aggregation or local carbon-containing in 4 cases, and it had been confirmed by pathological examination in 4 cases (Total 6 cases) (Figure 1 and Table S1). Under this circumstance, we bared tissues posterior to SMV that helped avoid the incomplete dissection of potential positive LNs (Figure 1A-C). Milan Spasojevic et al had reported that LNs anterior or posterior to SMV related to the crossing pattern of ICA and SMV [10]. In the above 6 cases, ICA crossed posterior to SMV in 5 of 6 cases while ICA crossed anteriorly to SMV in another (χ2 = 4.24, P = 0.039).
Figure 1.
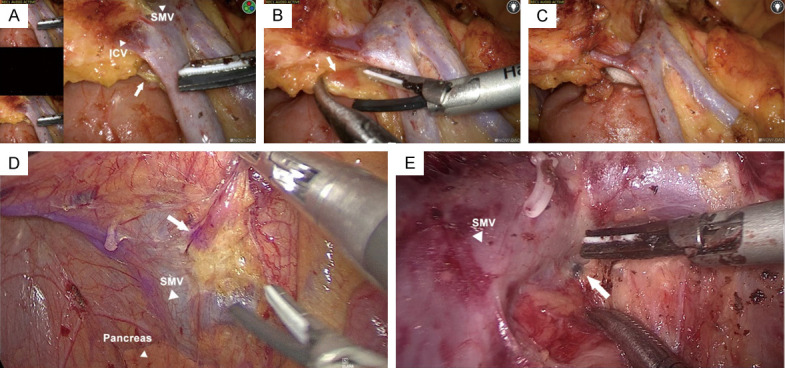
LNs posterior to SMV. Representative photos of ICG imaging (A-D) or carbon-containing (E) LNs posterior to SMV. (B, C) Removal of the tissues posterior to SMV if staining LNs were observed during operation. The white triangle indicated corresponding vessels and the arrows indicated LNs posterior to SMV.
LNs between SMV and SMA
The clear presence of LNs between SMV and SMA was realized in 22/27 cases (Figures 2, S3). However, no positive node in this area was detected. We noted that these nodes were presented within the area from ICA to MCA level, and did not affect the crossing pattern of the ICA/MCA to the SMV.
Figure 2.

LNs between SMV and SMA. A, B. The carbon-containing LNs between SMV/SMA. The white triangle indicated corresponding vessels and the arrows indicated target LNs.
LNs anterior to SMA
Hardly any date indicated that regional lymphatic drainage of the right-sided colon could across the SMA, while this phenomenon was observed in 16/27 cases (Figures 3, S3) and no metastatic node was detected from this area. However, the level of gastrointestinal tumor markers and tumor parameters lacked enough discriminative power to predict the appearance of the regional LNs anterior to SMA.
Figure 3.

LNs anterior to SMA. The carbon-containing (A-C) or ICG imaging (D) LNs anterior to SMA. The white arrows indicated LNs anterior to SMA and the red dashed line represented SMA.
It is noteworthy that all these nodes were anterior to the SMA plane, none of them was detected in the opposite direction. The dyed LNs appeared more frequently near the MCA level.
Intrathecal lymph tube of SMA
In our previous research on the vascular sheath of SMA, we had confirmed that nerve fiber was one of the integral component [16]. Meanwhile, a detailed lymphangiology put forward the concepts of the lymphovascular bundle to generalize the collecting lymph vessels attach to blood vessels [17]. However, when we exposed the vascular sheath, ICG aggregation or black-stained could not be observed (Figure 4). By comprehensive consideration, we currently favor the theory that intrathecal lymph tubes of SMA most probably deliver lymph from intrathecal tissues, like the nerve and vascular wall, instead of the mesentery.
Figure 4.

Intrathecal Lymph Tube of SMA. A-D. ICG tattooing LNs could not be observed in the vascular sheath of SMA, which indicated that intrathecal lymph tubes might not deliver lymph from right colon mesentery. The white triangle indicated SMV and black triangle indicated the sheath of SMA. Dashed box denoted the sheath of SMA.
The metastasis rate of LNs in target area
From September 2016 to December 2020, total 143 patients diagnosed as RCC were treated surgically with “artery first” laparoscopic right hemicolectomy, the demographic and clinical baseline characteristics were as shown in Table S2. There were 2 cases of lymph node metastasis to the area between SMV and SMA, and 1 case posterior to SMV, proven by the pathological findings. Therefore, as a rough estimation, the probability of lymph node metastasis in the target area was 2.10% (3/143).
Furthermore, to raise the awareness of metastatic potential of LNs in our target area, we would like to present a case of isolated lymph node metastasis and recurrence after 6 years of right hemicolectomy. A 51-year-old male was diagnosed as RCC (moderately differentiated adenocarcinoma, pT3N0M0) in 2014, and received SMV-oriented radical resection. When we reviewed the abdominal CT scan, an abnormally enlarged lymph node located between SMV and SMA was clearly observed but had not been removed during the surgery (Figure 5A). Unfortunately, in the reexamination via abdominal enhanced CT scan in May 2020, the size of the node was increased to 23 mm × 27 mm (Figure 5B-D). We preformed radical tumor excision (Figure 5E) and postoperative pathology prompted that the primary disease responsible for this lymph node metastasis was colon cancer.
Figure 5.

A case of lymph node metastasis in target area. A. An abnormally enlarged lymph node was observed between SMV and SMA on preprocedure CT image. B-D. The assessment of tumor progression in a follow up CT 6 years after surgery. E. Intraoperative photo during reoperation. Dashed box denoted the tumor region. LN; lymph node.
Evaluation of dyes
The application of CNs and ICG allowed the operator to distinguish tumor-draining LNs from peripheral adipose tissue or normal nodes (Figure 6A, 6B). The carbon-concentration was sustainably visualized while ICG needed to be activated by 760 nm as the light source. However, there were still some limitations in real-time LNs navigation. The fluorescence intensity was too weak in case 1 (Figure 6D) whereas too strong in another (Case 7) (Figure 6E), and the CNs failed to enter the regional lymphatic system in the other 2 cases (Figure 6F). This made it impossible to play an indicative role during laparoscopic surgery. However, taking the above 143 RCC patients into account, the number total harvested LNs in 27 tattooed patients was significantly more than 116 non-tattooed patients (21.81±12.55 vs. 40.74±21.93, P<0.01, Figure 6G).
Figure 6.
Advantage and disadvantage of tattooing agents application. (A, B) It is easy to distinguish regional LNs (the white arrows) from adipose tissue. ICG imaging (C) or CNs staining (D) was no observed after PCT was performed. Intraperitoneal injection of the ICG (E) and CNs (F). (G) Box plots of total harvested lymph nodes in tattooed patients and non-tattooed patients. ***; P<0.001.
Discussion
The extent of regional LNs for RCC has undergone a long developing process. In the early 20th century, Jamison et al firstly demonstrated that lymphatics of the colon ran with blood-vessels, and proposed metastases to central LNs surrounding superior mesenteric vessels [18]. However, the LNs drainage area had not been precisely defined for many years [19]. Decades later, the traditional tumor drainage area that followed the order of pericolic, intermediate, and main lymphoid groups, was finally accepted [20,21]. However, when it comes to the distribution pattern of LNs in the D3 area, the descriptions remained vague for a long time. In 2013, an anatomical study began to explore the nodes anterior or posterior to the superior mesenteric vessels and found out a significant correlation to the crossing manners between ICA and SMV [10]. Another post-mortems study described the number, size, and density of LNs in the ascending mesocolon through serial histological sectioning [3]. Later research declaimed that long lymph vessels delivered lymph from the right-side colon were able to traverse SMV to the midline of SMA [8]. To our knowledge, there is not data involving LNs around the central area in vivo, especially for LNs on the medial side of SMA.
The tireless efforts to meet the demands toward the understanding of regional LNs can pave the way for precisely defining the extent of lymphadenectomy for RCC. Since Dr. Hohenberger proposed the technique on CME in colon cancer surgery, surgeons and guidelines have increasingly adopted a radical approach to satisfy the requirement [5,22]. According to some points, dissection close to SMA increasing risk for damage to blood vessels and splanchnic nerves, and a Japanese investigation showed that lymph flowed towards central vessels hardly arrived at the left side of the anterior surface of the SMV [23]. Thus many surgeons insist that SMV serve as a landmark is concisely and safely, and ligated up feeding arteries 1 cm away from their origin is enough [24,25]. However, it cannot be overlooked that the central nodal metastases rate in colon cancer was considered nearly 3% [21,26-28]. Some researchers demonstrated that patients diagnosed as stage II colon cancer, with or without LNs metastases could benefit from enlarged lymphadenectomy [29,30], which might be a possible hint that the above strategies could not completely consistent with CME, and may not have been optimal.
The anatomic view holds that lymphatic watershed between the right-side colon and small bowel is often located in the midline of SMA, and the position of watershed most probably anterior to SMA [8,17]. It means that lymphatic vessels collecting from the right colon were capable of traversing SMV then approaching SMA. Therefore Japanese guidelines emphasized that D3 dissection required to remove the highest draining nodes, which might contain potential metastases [22]. Starting from 2016, our center has begun to develop an ‘artery-first’ approach to extend the internal boundary line of D3 cleaning from SMV to the right side wall of SMA [31]. With the deepening of understanding of the lymphatically draining rule, we have further performed resection along the midline of SMA in the caudal to cranial direction, which proved to be safe and feasible with the advantage of increasing lymph node yield. Performing the “artery-first” plus “caudal-to-cranial” technique could easy to expose the proper plane and reduce the manipulation of the tumor-bearing area so that the surgeon could better identify the retroperitoneal structures and more adopted the “no-touch” isolation principle [15,31].
In this study, LNs distributed posterior to SMV, between SMV to SMA, and anterior to SMA could be observed during the laparoscopic radical colectomy for RCC. An important find in this article is the presence of regional LNs of RCC on the left side of SMA, especially under close to a physiological condition, which has not yet been reported. The above observation corresponds with the regional LNs distribution reported in the anatomical pieces of literature [8,10]. In combination, lymphatic vessels collected right-hemi colon anteriorly/posteriorly across SMV, passing through arteriovenous compartments, pooling at the MCA level, then part of vessels traversing the surface of SMA and finally arriving at the left side of the artery.
However, the serum level of gastrointestinal tumor markers (CEA, CA199, and AFP) and tumor parameters (volume, differentiation, and pathological type) might weakly predict the appearance of regional LNs in the above area, or the clear trend would be seen if more cases included in the future. By analyzing the clinical data of 143 RCC patients who accepted “SMA-oriented” right hemicolectomy in our center for nearly a year, the probability of LNs metastasis in our target area was 2.10%. Taking together, these observations explained the potential necessity for expanding the lymphadenectomy scope to some extent.
Except for keeping the mesenteric envelope more intact, locate the internal boundary to SMA can increase the lymph nodes yield in the surgical specimen. It’s currently believed that higher lymph nodes yield suggest a contribution of survival improvement regardless of the stage or LNs metastases [3,32]. The growing number of LNs harvested resulting in an increased likelihood of identifying LNs harboring metastases, which is known as the “Will Rogers phenomenon”. The World Congress of Gastroenterology recommended that at least 12 LNs should be pathologically assessed from surgical specimens to prevent under staging of colorectal cancer [33]. Although more emphasis should be placed on lymph nodes yield, fatty replacement is one of the major obstacles. This difficulty lied in the recognition of LNs in adipose tissue, and it had been reported that fatty infiltration was occurred in about 30% of LNs detected from specimen [4,34]. Another obstacle is that more than 90% of LNs in mesocolons were smaller than 5 mm and almost 60% were less than 2 mm in maximum length [4]. Furthermore, RCC has been considered to have higher lymph nodes yield because of the longer colon needed to be respected, a greater proportion of tumors with microsatellite instability, and better antitumor immune response [35-38]. Thus some pathologists are encouraged to identify more than 12 LNs through other technology [39].
Several pieces of research had suggested that intraoperative application of dyes could depict the extent of mesenterectomy more successfully and safely, and allowed higher adequate lymph nodes harvest [40-42]. In 1975, Ponsky et al firstly proposed preoperative colonoscopic tattooing for surgeons to localize tumors at operation [43]. Later, widely using tattooing agents including ICG, CNs, methylene blue, and indigo carmine, it had been accepted that an important advantage of real-time regional LNs mapping without radiation expose might heighten the sensitivity of LNs metastasis detection [42,44,45]. The proper molecular weight and hydrodynamic diameter of ICG and CNs render them the promising lymphatic contrast tools to when they enter the regional lymphatic system by peritumoral injection [46,47]. In our research, the mean number of LNs harvested from resected species was 40.74±21.93 (mean ± SD), greatly exceed the lymph nodes yield benchmark of 12 [10]. Furthermore, the application of ICG and CNs could efficiently identify LNs in the D3 area, thus might help to precisely positioning the internal boundary of right hemicolectomy. However, there were still 4 case with imaging failure, which was possible that the dosage of dye was too low, the blockage of lymph vessels, or the intraperitoneal injection of agents. This suggested a high operational skills requirement of endoscopists when PCT was performed.
Further than increasing lymph nodes yield, however, the complications after bared the SMA including gastrointestinal dysfunction, severe post-operative diarrhea, and lymphatic leakage, should also be taken into account [2]. In the early days of exploration, we often open the vascular sheath of SMA for intrathecal cleaning, and we found the incidence of lymphatic leakage and diarrhea was increased after surgery. With the deepening of research, we had confirmed that a tiny fascial that contained autonomic nerve fibers was surrounding the SMA. A postmortem study had reported the long lymph vessels within the lymphovascular bundle were crossing the SMV then cranial and caudal along the SMA [8]. In our study, neither ICG aggregation nor CNs-stained occurred when we exposed the SMA sheath. Therefore, we prefer the viewpoint that the lymph vessels which attach to the sheath of SMA were delivering lymph from intrathecal tissues, like the nerve and vascular wall, instead of from mesentery. Taken together, it was reasonable to protect the autonomic nerve fibers by preserving the sheath of SMA, and consistent with the principle of standardized D3 cleaning.
However, this study had several limitations. First of all, our data was based on small sample size, hence the potential for a type II error was ineluctable, and the observation of 27 cases was not enough to come to the final conclusion. Therefore, a more consistent and comprehensive interpretation of LNs distributed in central areas should be defined in further exploration involving a larger cohort. Second, it would be more interesting to see the 5-year survival curve of RCC patients with or without lymph node metastasis surrounding the superior mesenteric vessels. But the relevant research has conducted just for one year and the patients are being followed up closely, so the long-term survival data in these patients are still lacking. Third, this study was retrospective, in the early stage of the LNs pathologic examination, the lymph node stations were simply separated into N1 (pericolic), N2 (intermediate), and N3 (central) stations. Thus the anatomic location between LNs and vessels in the D3 area did not been a specific description in the pathological report, and the report could not reflect the intraoperative observation well. Finally, because of the small sample size, no metastasis node was identified in the target anatomic area of 27 patients, the metastasis rate needs to be substantiated in a larger sample size.
Conclusion
In summary, our research described the regional LNs distribution pattern in the central area of the right-sided colon cancer. Lymphatic vessels collected right-hemi colon anteriorly/posteriorly across SMV, passing through arteriovenous compartments, pooling anterior to SMA at the MCA level (Figure 7). This phenomenon illustrated the potential necessity for expanding the scope of the tumor-draining area of RCC. Meanwhile, we recommended preserving the vascular sheath of SMA when SMA-oriented procedure was performed. This study was supporting a more radical anatomic destination in the lymphadenectomy for RCC and may form a basis for a real-time navigation laparoscopic surgery.
Figure 7.

Schematic depiction of the regional LNs distributed pattern in our research. Lymphatic vessels collected right-hemi colon anteriorly/posteriorly across SMV, pooling near the MCA level, and then approaching the surface of SMA and part of vessels finally arriving at the left side of the artery.
Acknowledgements
Our authors deeply appreciate the editors and reviewers for their kindly help with the manuscript. Supported by Science and Technology Planning Project of Guangzhou, China (202002030436) and Medical Scientific Research Foundation of Guangdong province, China (A2019569).
Informed consent for research was obtained from patients and all the patients were scheduled for laparoscopic radical resection. The protocol for this study was approved by the ethical committee of Guangdong Provincial Hospital of Chinese Medicine.
Disclosure of conflict of interest
None.
Abbreviations
- LNs
Regional lymph nodes
- RCC
Right-sided colon cancer
- CRC
Colorectal cancer
- PCT
Preoperative colonoscopic tattooing
- CNs
Carbon Nanoparticles
- ICG
Indocyanine Green
- SMV
Superior mesenteric vein
- SMA
Superior mesenteric artery
- ICA/ICV
Ileocolic artery/vein
- RCA/RCV
Right colonic artery/vein
- MCA/MCV
Middle colonic artery/vein
Supporting Information
References
- 1.Zurleni T, Cassiano A, Gjoni E, Ballabio A, Serio G, Marzoli L, Zurleni F. Surgical and oncological outcomes after complete mesocolic excision in right-sided colon cancer compared with conventional surgery: a retrospective, single-institution study. Int J Colorectal Dis. 2018;33:1–8. doi: 10.1007/s00384-017-2917-2. [DOI] [PubMed] [Google Scholar]
- 2.Sondenaa K, Quirke P, Hohenberger W, Sugihara K, Kobayashi H, Kessler H, Brown G, Tudyka V, D’Hoore A, Kennedy RH, West NP, Kim SH, Heald R, Storli KE, Nesbakken A, Moran B. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery: proceedings of a consensus conference. Int J Colorectal Dis. 2014;29:419–428. doi: 10.1007/s00384-013-1818-2. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadi O, Stringer MD, Black MA, McCall JL. Clinico-pathological factors influencing lymph node yield in colorectal cancer and impact on survival: analysis of New Zealand Cancer Registry data. J Surg Oncol. 2015;111:451–458. doi: 10.1002/jso.23848. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadi O, McCall JL, Stringer MD. Mesocolic lymph node number, size, and density: an anatomical study. Dis Colon Rectum. 2015;58:726–735. doi: 10.1097/DCR.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 5.Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354–364. doi: 10.1111/j.1463-1318.2008.01735.x. discussion 364-355. [DOI] [PubMed] [Google Scholar]
- 6.Lu JY, Xu L, Xue HD, Zhou WX, Xu T, Qiu HZ, Wu B, Lin GL, Xiao Y. The radical extent of lymphadenectomy - D2 dissection versus complete mesocolic excision of LAparoscopic Right colectomy for right-sided colon cancer (RELARC) trial: study protocol for a randomized controlled trial. Trials. 2016;17:582. doi: 10.1186/s13063-016-1710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyota S, Ohta H, Anazawa S. Rationale for extent of lymph node dissection for right colon cancer. Dis Colon Rectum. 1995;38:705–711. doi: 10.1007/BF02048026. [DOI] [PubMed] [Google Scholar]
- 8.Nesgaard JM, Stimec BV, Soulie P, Edwin B, Bakka A, Ignjatovic D. Defining minimal clearances for adequate lymphatic resection relevant to right colectomy for cancer: a post-mortem study. Surg Endosc. 2018;32:3806–3812. doi: 10.1007/s00464-018-6106-3. [DOI] [PubMed] [Google Scholar]
- 9.Spasojevic M, Stimec BV, Fasel JF, Terraz S, Ignjatovic D. 3D relations between right colon arteries and the superior mesenteric vein: a preliminary study with multidetector computed tomography. Surgical Endoscopy. 2010;25:1883–1886. doi: 10.1007/s00464-010-1480-5. [DOI] [PubMed] [Google Scholar]
- 10.Spasojevic M, Stimec BV, Dyrbekk AP, Tepavcevic Z, Edwin B, Bakka A, Ignjatovic D. Lymph node distribution in the d3 area of the right mesocolon: implications for an anatomically correct cancer resection. A postmortem study. Dis Colon Rectum. 2013;56:1381–1387. doi: 10.1097/01.dcr.0000436279.18577.d3. [DOI] [PubMed] [Google Scholar]
- 11.Hamabe A, Ogino T, Tanida T, Noura S, Morita S, Dono K. Indocyanine green fluorescence-guided laparoscopic surgery, with omental appendices as fluorescent markers for colorectal cancer resection: a pilot study. Surg Endosc. 2019;33:669–678. doi: 10.1007/s00464-018-6504-6. [DOI] [PubMed] [Google Scholar]
- 12.Lin C, Zhang Z, Wang L, Lin N, Yang W, Wu W, Wang W, Wang R, Wang Y. Effect of nano carbon tattooing on the lesion localization in the early colon cancer for additional surgical procedure after endoscopic resection. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:910–913. [PubMed] [Google Scholar]
- 13.Watanabe J, Ota M, Suwa Y, Suzuki S, Suwa H, Momiyama M, Ishibe A, Watanabe K, Masui H, Nagahori K, Ichikawa Y, Endo I. Evaluation of the intestinal blood flow near the rectosigmoid junction using the indocyanine green fluorescence method in a colorectal cancer surgery. Int J Colorectal Dis. 2015;30:329–335. doi: 10.1007/s00384-015-2129-6. [DOI] [PubMed] [Google Scholar]
- 14.Kang J, Park HS, Kim IK, Song Y, Baik SH, Sohn SK, Lee KY. Effect of preoperative colonoscopic tattooing on lymph node harvest in T1 colorectal cancer. Int J Colorectal Dis. 2015;30:1349–1355. doi: 10.1007/s00384-015-2308-5. [DOI] [PubMed] [Google Scholar]
- 15.Yi X, Li H, Lu X, Wan J, Diao D. “Caudal-to-cranial” plus “artery first” technique with beyond D3 lymph node dissection on the right midline of the superior mesenteric artery for the treatment of right colon cancer: is it more in line with the principle of oncology? Surg Endosc. 2020;34:4089–4100. doi: 10.1007/s00464-019-07171-5. [DOI] [PubMed] [Google Scholar]
- 16.Diao D, Wan J, Yi X, Lu X, Wang W, Li H, Xiong W, He Y. Feasibility and application value of autonomic nerve-preserving D3 radical resection for right-sided colon cancer under laparoscope. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:908–912. [PubMed] [Google Scholar]
- 17.Culligan K, Sehgal R, Mulligan D, Dunne C, Walsh S, Quondamatteo F, Dockery P, Coffey JC. A detailed appraisal of mesocolic lymphangiology--an immunohistochemical and stereological analysis. J Anat. 2014;225:463–472. doi: 10.1111/joa.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson JK, Dobson JF. Lymphatics of the colon: with special reference to the operative treatment of cancer of the colon. Ann Surg. 1909;50:1077–1090. doi: 10.1097/00000658-190912000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouffet F, Hay JM, Vacher B, Fingerhut A, Elhadad A, Flamant Y, Mathon C, Gainant A. Curative resection for left colonic carcinoma: hemicolectomy vs. segmental colectomy. A prospective, controlled, multicenter trial. French association for surgical research. Dis Colon Rectum. 1994;37:651–659. doi: 10.1007/BF02054407. [DOI] [PubMed] [Google Scholar]
- 20.General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Part II. Histopathological classification. Japanese Research Society for Cancer of the Colon and Rectum. Jpn J Surg. 1983;13:574–598. doi: 10.1007/BF02469506. [DOI] [PubMed] [Google Scholar]
- 21.Toyota S, Ohta H, Anazawa S. Rationale for extent of lymph node dissection for right colon cancer. Dis Colon Rectum. 1995;38:705–711. doi: 10.1007/BF02048026. [DOI] [PubMed] [Google Scholar]
- 22.Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42. doi: 10.1007/s10147-019-01485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hida J, Okuno K, Yasutomi M, Yoshifuji T, Uchida T, Tokoro T, Shiozaki H. Optimal ligation level of the primary feeding artery and bowel resection margin in colon cancer surgery: the influence of the site of the primary feeding artery. Dis Colon Rectum. 2005;48:2232–2237. doi: 10.1007/s10350-005-0161-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhao LY, Liu H, Wang YN, Deng HJ, Xue Q, Li GX. Techniques and feasibility of laparoscopic extended right hemicolectomy with D3 lymphadenectomy. World J Gastroenterol. 2014;20:10531–10536. doi: 10.3748/wjg.v20.i30.10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Culligan K, Walsh S, Dunne C, Walsh M, Ryan S, Quondamatteo F, Dockery P, Coffey JC. The mesocolon: a histological and electron microscopic characterization of the mesenteric attachment of the colon prior to and after surgical mobilization. Ann Surg. 2014;260:1048–1056. doi: 10.1097/SLA.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 26.Hashiguchi Y, Hase K, Ueno H, Mochizuki H, Shinto E, Yamamoto J. Optimal margins and lymphadenectomy in colonic cancer surgery. Br J Surg. 2011;98:1171–1178. doi: 10.1002/bjs.7518. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi H, Ueno H, Hashiguchi Y, Mochizuki H. Distribution of lymph node metastasis is a prognostic index in patients with stage III colon cancer. Surgery. 2006;139:516–522. doi: 10.1016/j.surg.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Kotake K, Honjo S, Sugihara K, Hashiguchi Y, Kato T, Kodaira S, Muto T, Koyama Y. Number of lymph nodes retrieved is an important determinant of survival of patients with stage II and stage III colorectal cancer. Jpn J Clin Oncol. 2012;42:29–35. doi: 10.1093/jjco/hyr164. [DOI] [PubMed] [Google Scholar]
- 29.Tsai HL, Lu CY, Hsieh JS, Wu DC, Jan CM, Chai CY, Chu KS, Chan HM, Wang JY. The prognostic significance of total lymph node harvest in patients with T2-4N0M0 colorectal cancer. J Gastrointest Surg. 2007;11:660–665. doi: 10.1007/s11605-007-0119-x. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, Kopp R, Putterich E, Ruppert R, Schuster T, Friess H, Holzel D. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. 2010;251:1070–1078. doi: 10.1097/SLA.0b013e3181d7789d. [DOI] [PubMed] [Google Scholar]
- 31.Yi XJ, Lu XQ, Li HM, Wang W, Xiong WJ, Wan J, Diao DC. Feasibility and efficacy of laparoscopic radical right hemicolectomy with complete mesocolic excision using an ‘artery-first’ approach. Gastroenterol Rep (Oxf) 2019;7:199–204. doi: 10.1093/gastro/goy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J. Clin. Oncol. 2006;24:3570–3575. doi: 10.1200/JCO.2006.06.8866. [DOI] [PubMed] [Google Scholar]
- 33.Fielding LP, Arsenault PA, Chapuis PH, Dent O, Gathright B, Hardcastle JD, Hermanek P, Jass JR, Newland RC. Clinicopathological staging for colorectal cancer: an international documentation system (IDS) and an international comprehensive anatomical terminology (ICAT) J Gastroenterol Hepatol. 1991;6:325–344. doi: 10.1111/j.1440-1746.1991.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 34.Hadamitzky C, Spohr H, Debertin AS, Guddat S, Tsokos M, Pabst R. Age-dependent histoarchitectural changes in human lymph nodes: an underestimated process with clinical relevance? J Anat. 2010;216:556–562. doi: 10.1111/j.1469-7580.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ovrebo K, Rokke O. Extended lymph node dissection in colorectal cancer surgery. Reliability and reproducibility in assessments of operative reports. Int J Colorectal Dis. 2010;25:213–222. doi: 10.1007/s00384-009-0829-5. [DOI] [PubMed] [Google Scholar]
- 36.West NP, Hohenberger W, Finan PJ, Quirke P. Mesocolic plane surgery: an old but forgotten technique? Colorectal Dis. 2009;11:988–989. doi: 10.1111/j.1463-1318.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 37.Turnbull RB Jr. Current concepts in cancer. Cancer of the GI tract: colon, rectum, anus. The no-touch isolation technique of resection. JAMA. 1975;231:1181–1182. doi: 10.1001/jama.231.11.1181. [DOI] [PubMed] [Google Scholar]
- 38.Sloothaak DA, Grewal S, Doornewaard H, van Duijvendijk P, Tanis PJ, Bemelman WA, van der Zaag ES, Buskens CJ. Lymph node size as a predictor of lymphatic staging in colonic cancer. Br J Surg. 2014;101:701–706. doi: 10.1002/bjs.9451. [DOI] [PubMed] [Google Scholar]
- 39.Resch A, Langner C. Lymph node staging in colorectal cancer: old controversies and recent advances. World J Gastroenterol. 2013;19:8515–8526. doi: 10.3748/wjg.v19.i46.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goo JJ, Ryu DG, Kim HW, Park SB, Kang DH, Choi CW, Kim SJ, Nam HS, Kim HS, Son GM, Park BS. Efficacy of preoperative colonoscopic tattooing with indocyanine green on lymph node harvest and factors associated with inadequate lymph node harvest in colorectal cancer. Scand J Gastroenterol. 2019;54:666–672. doi: 10.1080/00365521.2019.1612940. [DOI] [PubMed] [Google Scholar]
- 41.Liberale G, Bohlok A, Bormans A, Bouazza F, Galdon MG, El Nakadi I, Bourgeois P, Donckier V. Indocyanine green fluorescence imaging for sentinel lymph node detection in colorectal cancer: a systematic review. Eur J Surg Oncol. 2018;44:1301–1306. doi: 10.1016/j.ejso.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 42.Hirche C, Mohr Z, Kneif S, Doniga S, Murawa D, Strik M, Hunerbein M. Ultrastaging of colon cancer by sentinel node biopsy using fluorescence navigation with indocyanine green. Int J Colorectal Dis. 2012;27:319–324. doi: 10.1007/s00384-011-1306-5. [DOI] [PubMed] [Google Scholar]
- 43.Ponsky JL, King JF. Endoscopic marking of colonic lesions. Gastrointest Endosc. 1975;22:42–43. doi: 10.1016/s0016-5107(75)73687-8. [DOI] [PubMed] [Google Scholar]
- 44.Bartels SA, van der Zaag ES, Dekker E, Buskens CJ, Bemelman WA. The effect of colonoscopic tattooing on lymph node retrieval and sentinel lymph node mapping. Gastrointest Endosc. 2012;76:793–800. doi: 10.1016/j.gie.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Markl B, Arnholdt HM, Jahnig H, Spatz H, Anthuber M, Oruzio DV, Kerwel TG. A new concept for the role of ex vivo sentinel lymph nodes in node-negative colorectal cancer. Ann Surg Oncol. 2010;17:2647–2655. doi: 10.1245/s10434-010-1030-3. [DOI] [PubMed] [Google Scholar]
- 46.Groenlund JH, Telinius N, Skov SN, Hjortdal V. A validation study of near-infrared fluorescence imaging of lymphatic vessels in humans. Lymphat Res Biol. 2017;15:227–234. doi: 10.1089/lrb.2016.0061. [DOI] [PubMed] [Google Scholar]
- 47.Aldecoa I, Montironi C, Planell N, Pellise M, Fernandez-Esparrach G, Gines A, Delgado S, Momblan D, Moreira L, Lopez-Ceron M, Rakislova N, Martinez-Palli G, Balust J, Bombi JA, de Lacy A, Castells A, Balaguer F, Cuatrecasas M. Endoscopic tattooing of early colon carcinoma enhances detection of lymph nodes most prone to harbor tumor burden. Surg Endosc. 2017;31:723–733. doi: 10.1007/s00464-016-5026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



