Abstract
Dysregulated cell division, which leads to aberrant cell proliferation, is one of the key hallmarks of cancer. Therefore, therapeutic targets that block cell division would be effective for cancer treatment. Cell division is mainly controlled by a complex composed of cyclin and cyclin dependent kinases (CDKs). To date, the CDK inhibitors (CDKIs), specifically the ones that block the enzyme activity of CDK4 and CDK6 (CDK4/6), have been approved by FDA for the treatment of metastatic hormone receptor positive breast cancer. However, due to the non-selectivity and significant toxicity, most of the first generation CDK inhibitors (so called pan-CDK inhibitors that target several CDKs), have not been approved for clinical application. Despite this, great efforts and progress have been made to enable pan-CDK inhibitors application in the clinical setting. Notably, the development of combination therapy strategies in recent years has made it possible to reduce the toxicity and side effects of pan-CDK inhibitors. Thus, as a combination therapy approach, pan-CDK inhibitors regain great potential in clinical application. In this review, we introduced the CDK family members and discussed their major functions in cell cycle controlling. Then, we summarized the research progress regarding CDK inhibitors, especially those other than CDK4/6 inhibitors. We reviewed first-generation pan-CDKIs Flavopiridol and Roscovitine, and second-generation CDKIs Dinaciclib, P276-00, AT7519, TG02, Roniciclib, RGB-286638 by focusing on their developing stages, clinical trials and targeting cancers. The specific CDKIs, which targets to increase specificity and decrease the side effects, were also discussed. These CDKIs include CDK4/6, CDK7, CDK9, and CDK12/13 inhibitors. Finally, the efficacy and discrepancy of combination therapy with CDK inhibitors and PD1/PDL1 antibodies were analyzed, which might give insights into the development of promising strategy for cancer treatment.
Keywords: CDK, pan-CDK inhibitors, specific CDK inhibitors, cancer, combination therapy
Introduction
Cell division is one of the fundamental biological activities, occurring in various physiological processes such as individual development, organ homeostasis, tissue regeneration, as well as in pathological process of tumorigenesis. The sequence of stages in cell division is known as the cell cycle, and is divided into a synthesis phase, a mitotic segregation phase and two intervenient phases G1 and G2 (Figure 1). Cell enlarges itself in the G1 phase to prepare for the DNA synthesis, which is regulated by a “restriction point” in mammals. Whether a cell can enter into the cell cycle is determined by both intrinsic factors (such as protein synthesis) and extrinsic factors (such as growth factors). The absence of these essential factors causes the cell to end its cell cycle and enter into a dormancy period, known as G0 phase. Cell cycle regulation involves three “checkpoints”: the G1/S, G2/M, and mitotic spindle checkpoints. Growing evidence has demonstrated that the eukaryotic cell cycle is driven by a conserved central mechanism, including cyclin-dependent kinases (CDKs), which promote DNA synthesis and chromosome segregation by phosphorylation of their substrate [1,2].
Figure 1.
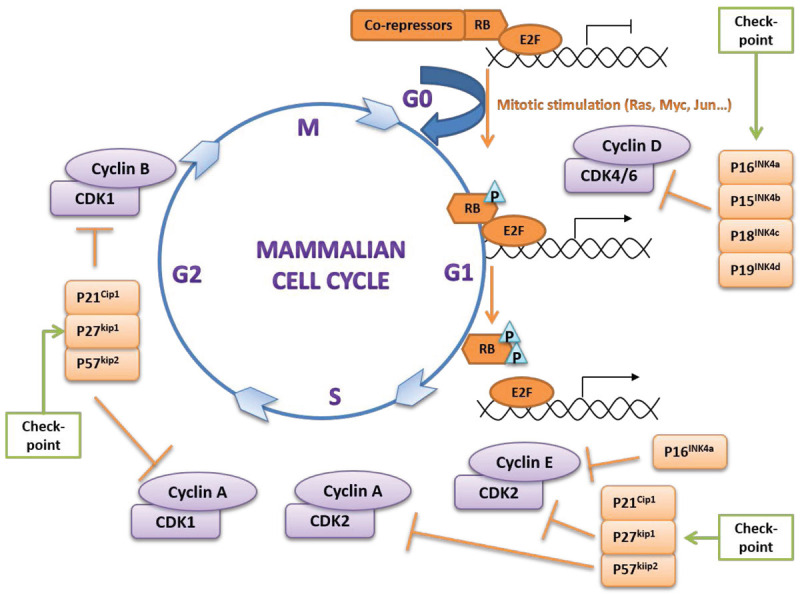
Major Functions of CDKs in cell cycle: Eukaryotic cell cycle is a precise process with order, which is regulated by CDKs, Cyclins and CDKIs. CDK-Cyclin complex can phosphorylate RB protein and regulate cell cycle positively, whereas CDKIs will inhibit part of the cell cycle process and play a negative regulatory role. As shown in this figure, after cell has been stimulated by mitotic signals, it then enters the G1 phase. Before it further enters the next phase, RB protein would be first phosphorylated by CDK4/6-CyclinD and CDK2-CyclinE complexes, thereby releasing E2F protein, promoting downstream cell cycle factors and transcription-related gene expression. P16INK4a, P21Cip1, P27Kip1 and P57Kip2 all belong to CDKIs, among which P16INK4a can inhibit the activity of CDK4, prevent the phosphorylation of RB protein and the release of E2F, thereby inhibit the cell life cycle in G1 phase. P21Cip1, P27Kip1 and P57Kip2, on the other hand, can inhibit the activity of CDKs and Cyclins more extensively and block cells at different stages.
CDKs are involved not only in the cell cycle but also in the other critical cellular processes, such as gene transcription, insulin secretion, glycogen synthesis and neuronal functions [3]. So far, 21 CDKs and 5 CDK-like genes have been identified in human genome based on their homologous sequences [4]. CDK1 emerges as a key determinant of mitotic progression, whereas CDK2 is more associated with DNA replication in higher eukaryotes. In metazoans, cell cycle entry is mostly elicited by CDK4 and CDK6, which are responsive to numerous growth-regulatory signals [5,6]. Besides cell cycle controlling, some other CDKs including CDK7, CDK8, CDK9 and CDK11, have been shown to participate in transcriptional regulation [4]. CDK7 can phosphorylate RNA polymerase II and contribute to the initiation of transcription. CDK8 is a part of the mediator complex which regulates a plethora of genes. CDK9 can phosphorylate RNA polymerase II and thereby promote elongation of transcription. CDK11 mainly acts on the splicing machinery. Accumulating evidence suggests that these transcription-regulating CDKs have potential to be efficient therapeutic targets for cancer treatment [7-9]. There is another type of CDKs, called atypical CDKs, which include CDK5, CDK14, CDK15, CDK16, CDK17, and CDK18 [4]. CDK5 is involved in postmitotic functions in specialized tissue settings [10,11]. CDK14 is reported to be involved in Wnt/β-catenin signaling pathway by combination with Cyclin Y [12,13].
Given the important function of CDKs in regulation of cell division, gene transcription and other critical biological processes, CDK inhibitors have been developed for the treatment of various diseases caused by CDK abnormalities. Over the past 20 years, numerous compounds targeting CDK enzyme activity have emerged and have been evaluated in the clinical trial. Here, we will perform a whole mount review of the history of research and progress of CDK inhibitors, particularly their involvement in the treatment of cancer.
Pan-CDK inhibitors
CDK inhibitors have been studied since the 1990s. The first generation of CDK inhibitors are pan-CDK inhibitors, including Flavopiridol and Roscovitine, etc. The main function of these inhibitors is to block cell cycle and inhibit cell proliferation by inhibiting the CDK enzyme activity. However, the first-generation of pan-CDK inhibitors have poor selectivity and high toxicity, leading to inevitable harmful effects on normal cells. As a result, most of the pan-CDK inhibitors failed in their clinical trials [14-16]. Subsequently, second-generation CDK inhibitors, including Dinaciclib, P276-00, AT7519, TG02, Roniciclib, RGB-286638 and so on, have been developed with better selectivity and less side effects. Most of the second generation CDK inhibitors have presented efficient anti-tumor activity in preclinical trials, although the safety and efficacy of these inhibitors need to be further verified in clinical studies. At present, there are approximately 40 pan-CDK inhibitors that are in various stages of their research and development. For instance, Dinaciclib, developed by Merck company, is in phase lll clinical study and has already presented a significant anti-tumor effect in the treatment of melanoma, breast cancer and leukemia. Besides, several pan-CDK inhibitors entered into phase I or phase II studies, while many other pan-CDK inhibitors have shown significant anti-tumor activity in preclinical studies. In order to minimize the side effects of pan-CDK inhibitors, numerous studies have been conducted on drug delivery strategies, especially in the area of combination therapy. Overall, pan-CDK inhibitors have shown promising clinical efficacy despite serious side effects and safety concerns. Here, we listed pan-CDK inhibitors currently under research and development, and summarized their structures, CDK targets, developmental stages and indications of target diseases or cancers (Table 1). Representative pan-CDK inhibitors are described in details as follows.
Table 1.
Pan-CDK inhibitors under development (updated to April, 2021)
Flavopiridol
Flavopiridol (Alvocidib) belongs to the first generation of pan-CDK inhibitors. It is the first pan-CDK inhibitor that was used in clinical trials and is also one of the most widely studied pan-CDK inhibitors. Flavopiridol mainly inhibits the activities of CDK1, CDK2, CDK4, CDK6, CDK7 and CDK9 with IC50 values at 30, 170, 100, 60, 300 and 10 nM, respectively [17]. Since 1997, 63 clinical trials have been carried out on Flavopiridol, which is mainly administrated for the treatment of ALL, AML, CLL, lymphoma, solid tumor, gastric cancer, mantle cell lymphoma, myeloid leukemia and so on. Results from a preclinical study indicated that Flavopiridol presented significant anti-tumor activity against prostate cancer, reducing tumor volume by 85% and extending survival by 30 days. In addition, Flavopiridol can induce apoptosis of primary and recurrent/refractory AML cells by 4.3 times in vitro [18]. It can also induce apoptosis in a large number of other hematopoietic cell lines [19]. In spite of these promising progress of preclinical study, Flavopiridol presented poor efficacy in clinical trials of solid tumors. Phase I clinical studies of AML showed that after three days treatment of Flavopiridol, the number of peripheral blood cells decreased by more than 50% in 44% of patients, suggesting that Flavopiridol can induce anti-leukemia cytotoxicity. Subsequently, 45 AML patients were studied in phase II clinical study, and 16% of the patients have cardiac dysfunction during treatment process. Phase I [20] and Phase II [21] clinical trials of the CLL patients have shown that Flavopiridol can alleviate the symptoms. Due to these side effects, clinical trials with Flavopiridol only made limited progress. In order to reduce side effects, researchers are trying to combine Flavopiridol with other drugs to treat cancer so as to improve the clinical efficacy of Flavopiridol [22,23].
Dinaciclib
Dinaciclib (SCH727965), developed by Merck & Co. Ltd., has entered phase III clinical trials, and showed impressive anti-tumor activity in lung cancer, breast cancer, and chronic lymphocytic carcinoma. Dinaciclib mainly inhibits the activity of CDK9, thus preventing the phosphorylation of the carboxyl terminus of RNA polymerase II, which plays a transcriptional inhibitory role and induces cell apoptosis. Strikingly, it has been proved that Dinaciclib has the best therapeutic efficacy for leukemia. In acute lymphoblastic leukemia, Dinaciclib inhibited the growth of T-ALL cells and prolonged the survival time of mouse tumor xenograft models. In preclinical experiments, Dinaciclib combined with Panobinostat can induce MLL-AF9 tumor cell apoptosis. The number of leukocytes was significantly reduced in the mouse tumor model, showing a stronger survival advantage, with median survival increased from 33 days to 52 days [24]. Further studies demonstrated that Dinaciclib can eliminate many cytokines in the microenvironment, such as CD40L, BAFF, IL-4, etc., which are essential for the growth of CLL cells [25]. These studies indicated that Dinaciclib has great potential as a clinical treatment agent for CLL. Clinical trial results also showed that Dinaciclib was superior to Flavopiridol in the treatment of CLL. Recent studies have further demonstrated that Dinaciclib has a more remarkable anti-tumor effect when combined with PD1 monoclonal antibodies [26], making Dinaciclib a potential promising therapeutic target in clinical setting.
P276-00
P276-00 can effectively inhibit CDK1, CDK4 and CDK9 with IC50 values at 79 nM, 63 nM and 20 nM, respectively. P276-00 showed significant cytotoxicity against mantle cell lymphoma (MCL) cells in vitro [27]. In the Phase II clinical trial of MCL, 13 patients with relapsed and refractory MCL were treated with p276-00. Overall, both drug resistance and anti-tumor effects were significant. At present, the molecular mechanism of p276-00 in the treatment of MCL remains unclear [28]. Other studies have shown that p276-00 can arrest the cell cycle in the G1 phase, thereby inducing apoptosis of head and neck cancer cells [29]. The anti-tumor activity and safety of p276-00 was evaluated in a phase II clinical study in patients with recurrent and locally advanced head and neck cancer. The results suggested that P276-00 had good anti-tumor activity, while its safety needs to be further evaluated.
TG02
TG02 is a novel oral poly-kinase inhibitor that mainly inhibits CDK1, CDK2, CDK7 and CDK9 activities with IC50 values at 9 nM, 5 nM, 37 nM and 3 nM, respectively. Preclinical studies have shown that TG02 alone or in combination with TMZ can inhibit the proliferation of glioblastoma cells [30]. Phase I clinical studies have been conducted in China to determine the clinical dose and efficacy of TG02. The results showed that TG02 is effective in the treatment of hematological malignancies, and TG02 therapy has been found to promote tumor deposition and prolong survival in a variety of mouse models of leukemia. TG02 has broad-spectrum of anti-CDKs and anti-JAK2/Flt3 activity, which provides a theoretical basis for clinical treatment of patients with hematologic diseases [31]. Further studies have shown that Carfilzomib (the second-generation proteasome inhibitor) combined with TG02 improve the efficacy of relapsed/refractory multiple myeloma (MM) [32]. In conclusion, TG02 has shown promising therapeutic potentials in clinical trials, although further investigation is still needed in the future.
AT7519
AT7519 is a potent pan-CDK inhibitor that mainly inhibits CDK1, 2, 4, 6 and 9. Studies have shown that AT7519 not only has inhibitory activity against a variety of solid tumors, but also can inhibit hematologic malignancies. Preclinical trials have proved that AT7519 can induce apoptosis in various neuroblastoma cell lines [33]. In addition, AT7519 also induces neutrophils apoptosis and reduces inflammatory response in a pneumonia model. So, AT7519 has been evaluated as a potential agent for ARDS (acute respiratory distress syndrome with neutrophil dominant) in many studies [34]. The efficacy of AT7519 in patients with advanced refractory solid tumors or non-Hodgkin’s lymphoma has been evaluated in phase I clinical trials. Phase I clinical trials also provided guidance for dosages of AT7519 to be used in Phase II clinical trials, with a recommended dose of 27.0 mg/kg. AT7519 is in phase II clinical trials for the treatment of relapsed mantle cell lymphoma and recurrent refractory chronic lymphocytic leukemia. Furthermore, AT7519 in combination with Onalespib (HSP90 inhibitor) for the treatment of metastatic or unresectable solid tumors and AT7519 in combination with Bortezomib for the treatment of multiple myeloma are also in clinical trials [35]. Together, AT7519 exhibited great potential for clinical application.
Roniciclib
Roniciclib is an oral pan-CDK inhibitor. A study at the National University of Singapore Cancer Institute indicated that Roniciclib combined with cisplatin has a significant synergistic anti-tumor effect [36]. Another preclinical study showed that Roniciclib induced apoptosis of medullary thyroid cancer cells. The combination of Roniciclib and Soafenib further inhibited tumor growth in xenograft models compared to Roniciclib alone [37]. To date, the safety and tolerated dose of Roniciclib in patients with advanced malignancy have been evaluated in phase I clinical trials, and Roniciclib in combination with conventional chemotherapy agents for the treatment of extensive non-small cell lung cancer (ED-SCLC) has entered phase II clinical trials [38]. Unfortunately, the results showed that the combination treatment produced significant side effects and cytotoxicity, so the phase II clinical trial was terminated [39]. In addition to ED-SCLC, phase II trials in non-small cell lung cancer (NSCLC) and advanced breast cancer have also failed to meet expectations. Therefore, the future development of Roniciclib might need to be re-optimized in terms of the dosage and administration strategy.
RGB-286638
The main target of RGB-286638 is CDK9, so RGB-286638 is involved in controlling of cell cycle and regulation of gene transcription [40]. Preclinical experiments have shown that RGB-286638 can induce apoptosis of various human cancer cell lines. Intravenous injection of RGB-286638 for 5 consecutive days had the best inhibition effect on tumor growth in solid tumor and hematoma mouse models. Based on experience in preclinical trials, a phase I clinical trial of RGB-286638 is currently being conducted to evaluate safety and drug resistance in patients with recurrent or refractory blood cancer [41]. The clinical application of RGB-286638 still needs further investigation.
PHA-793887
The low concentration of PHA-793887 inhibits the phosphorylation of Rb protein, and thus prevents the progression of cell cycle [42]. In vitro experiments showed that PHA-793887 had a certain toxic effect on leukemia cells. Subcutaneous xenograft model and primary leukemia cell dissemination model were used to evaluate the therapeutic effect of PHA-793887 in vivo, and the results sound promising [43]. However, in a phase I clinical trial, there were 19 patients who showed severe hepatotoxicity. Therefore, the clinical application of PHA-793887 is still under development [44].
Specific CDK inhibitors
The biggest challenge in the clinical application of pan-CDK inhibitors is their low specificity and significant side effects on normal somatic cells. In order to solve this problem, researchers have successfully developed a variety of specific CDK inhibitors, including CDK4/6-, CDK7-, CDK9-, CDK12/13-inhibitors etc. Each type of tumor is associated with its own CDK expression landscape, selection of appropriate specific CDK inhibitors for relevant patients is therefore expected to assure the therapeutic effect, and to avoid toxic and side effects as well. At present, a variety of specific CDK inhibitors have shown significant anti-tumor effects in preclinical and clinical studies. Here, we briefly summarized the characteristics of some specific CDK inhibitors and their anti-tumor activity.
CDK4/6 inhibitors
CDK4/6 inhibitors are the first ones that were approved by FDA for clinical treatment. These inhibitors specifically inhibit CDK4/6 and show limited toxicity to normal cells. There are three FDA-approved CDK4/6 inhibitors and they are Palbociclib produced by Pfizer, Ribociclib produced by Novartis and Abemaciclib produced by Eli Lilly. Palbociclib is the first and most popular CDK4/6 inhibitor, which reached $2.135 billion of global sales in 2016, and is expected to reach $7 billion in 2022. Ribociclib is very similar to Palbociclib in structure, but Abemaciclib is quite different. In vitro studies indicated that Palbociclib has almost equivalent inhibition effect on CDK4 and CDK6, while Abemaciclib and Ribociclib are more potent against CDK4 than CDK6 [45-47].
All three CDK4/6 inhibitors can effectively arrest cell cycle from G1 to S phase by blocking the phosphorylation of Rb protein, and thus inhibit the proliferation of Rb-positive tumor cells. These inhibitors are currently approved for the first-line treatment of HR+ advanced breast cancer, which can effectively reduce resistance to mono-endocrine therapy and significantly extend survival in HR+/HER2- breast cancer patients. Recent studies have shown that, besides blocking of the cell cycle, CDK4/6 inhibitors also suppress tumor growth through multiple other mechanisms, including enhancing cytostasis caused by signaling pathway inhibitors, inducing senescence, regulation of cell metabolism, and even promoting anti-tumor immune responses [48]. These novel molecular mechanisms provide a theoretical basis for combination therapy with CDK4/6 inhibitors. For instance, CDK4/6 inhibitors combined with hormone receptor antagonist letrozole have been applied for breast cancer therapy. Many other combination therapies involving CDK4/6 inhibitors are currently under clinical trials for a variety of diseases including anti-cancer therapy.
CDK7 inhibitors
CDK7 has dual functions of cell cycle controlling and transcriptional regulation, which make CDK7 a potential target for cancer therapy. Several CDK7 specific inhibitors have been shown significant anti-tumor activity, including non-covalent inhibitors BS-181, ICEC0942, LDC4297, QS1189 and covalent inhibitors THZ1, THZ2, YKL-5-124. BS-181 is the first highly selective CDK7 inhibitor. Preclinical studies have shown that BS-181 inhibits cancer cell proliferation and xenograft tumor growth, but its bioavailability is poor and cell permeability is insufficient [49]. ICEC0942 (CT7001) is the first oral CDK7 inhibitor, which is developed from BS-181 and has higher drug-like properties compared with BS-181 [50,51]. Notably, ICEC0942 entered clinical trials in 2017 and is currently being investigated in phase I/II trials for a variety of therapies for advanced malignancies, including monotherapy or combination therapy for triple-negative breast cancer, castrate resistant prostate cancer (CRPC), and combination therapy with Fulvestrant for patients with HR+/HER2- breast cancer. (ClinicalTrials.gov identifier: NCT03363893).
THZ1 is one of the most widely studied CDK7 covalent inhibitors. Preclinical studies have shown that THZ1 has strong anti-tumor activity in several cancer types [52-57]. Of note, THZ1 has been shown to inhibit not only CDK7 activity, but also CDK12 and CDK13 activity. In order to obtain a more specific inhibitor of CDK7, researchers developed the inhibitor YKL-5-124 by combining the covalent warhead of THZ1 with the pyrrolidinopyrazole core of PAK4 inhibitor PF-3758309 [58]. YKL-5-124 has a potent inhibition specificity against CDK7, but has no inhibitory activity against CDK12 and CDK13. Preclinical studies have shown that YKL-5-124 can potentiate genomic instability and trigger anti-tumor immune response in small cell lung cancer, which provides a theoretical basis for the combination therapy of CDK7 inhibitors and immunotherapy [59]. SY-1365, a THZ1 derived CDK7 inhibitor, entered phase I clinical trial in advanced solid tumors in May 2017 to evaluate its efficacy in the treatment of ovarian and breast cancer [60] (ClinicalTrials.gov identifier: NCT03134638). SY-5609 is another selective CDK7 inhibitor. Preclinical studies have shown that SY-5609 combined with Fulvestrant have potent anti-tumor activity against ovarian cancer, TNBC and ER+ breast cancer [61,62]. SY-5609 entered phase I clinical trials in January 2020 for the treatment of advanced solid tumors and in combination with Fulvestrant in patients with HR+/HER2- breast cancer (ClinicalTrials.gov identification: NCT04247126).
CDK9 inhibitors
CDK9 regulates cellular transcriptional elongation and mRNA maturation, and has become an attractive therapeutic target for many cancers, especially those caused by dysregulation of transcription [63,64]. Several CDK9 inhibitors have been developed, and their significant anti-tumor activity has been demonstrated in preclinical studies, such as Fadraciclib, AZD-4573, CDKI-73, MC180295, etc. In addition, Fadraciclib combined with temozolomide can effectively suppress MYCN-amplified neuroblastoma long-term [65]. AZD-4573 is a highly selective CDK9 inhibitor that can down-regulate the expression of oncogenic genes such as MCL-1. Preclinical studies have demonstrated that AZD-4573 has significant anti-cancer efficacy in hematologic malignancies [66,67]. CDKI-73 combined with Olaparib has a synergistic therapeutic effect in BRCA1-proficient ovarian cancer, which facilitates the use of CDK9 as a predictive biomarker for PARP inhibitors in clinical practice [68]. MC180295 can dephosphorylate SWI/SNF protein Brg1, thereby promoting gene activation and leading to the restoration of tumor suppressor gene expression. Additionally, CDK9 inhibition sensitizes to the immune checkpoint inhibitor α-PD-1 in vivo, making it an excellent target for epigenetic therapy on cancer [69]. The results of these preclinical studies have promoted the development of CDK9 inhibitors for clinical application.
A recent review summarized the progress of sixteen CDK9 inhibitors in various stages of clinical development for cancer therapy. Four of them, P276-00, ZK-304709, BAY-1000394 and SNS-032, have been terminated in clinical trials due to their poor selectivity and high toxicity. Other inhibitors, including Alvocidib, TP-1287, P-1446, BAY-1143572, BAY-1251152, TG-02, (R)-Roscovitine, Fadraciclib, Dinaciclib, AT7519, BTX-A51 and AZD-4573, are currently being evaluated in several clinical trials [70]. Actually, most of these compounds are not very specific, they also inhibit other CDKs, such as Dinaciclib which also targets CDK1, 2, 5, TG-02 which also targets CDK1, 2, 3, 5, AT7519 which also targets CDK1, 2, 4, 5, 6, etc. [71-73]. Thus, they are also considered as pan-CDK inhibitors. Due to the high homology of these CDKs in the catalytic domain, the development of specific CDK9 inhibitors remains a major challenge.
CDK12 inhibitors
CDK12 is another critical transcriptional regulator besides CDK7 and CDK9 among CDK family. It can bind to cyclin K to phosphorylate the CTD region of RNA polymerase II, thereby promote transcription elongation [74]. Several novel functions of CDK12 in cancer, especially breast cancer, have been revealed in recent studies. These novel functions are achieved by regulation of a variety of biological activities, including c-MYC expression, Wnt/β-catenin signaling, RNA splicing, ErbB-PI3K-AKT signaling, MAPK signaling as well as noncanonical NF-kB pathway, and DNA damage response (DDR) signaling [75-80]. In recent years, several CDK12 inhibitors have been developed. These inhibitors include SR-4835 and THZ531, which presented strong anti-tumor activity in preclinical studies. SR-4835 is a highly selective dual inhibitor of CDK12 and CDK13, which can suppress the expression of core DNA damage response proteins. This can provoke a “Brcaness” phenotype that leads to deficiencies in DNA damage repair, thereby promote the synergistic effect of DNA damage chemotherapy and PARP inhibitors in TNBC [81,82]. THZ531 is another covalent inhibitor of CDK12 and CDK13, which can significantly down-regulate the expression of DNA damage response genes and key super-enhancer-related transcription factors [83]. Recent studies indicated that THZ531 has a striking synergistic effect with sorafenib in the treatment of hepatocellular carcinoma [84]. To date, the inhibitors targeting CDK12 in clinical trials have all been pan-CDK inhibitors, including Dinaciclib. Therefore, development of CDK12 inhibitors with high specificity and drug properties is needed.
Combination therapy of CDK inhibitors and PD1-PDL1 antibodies
Through decades of research, cancer immunotherapy has emerged as a powerful and effective strategy for cancer treatment. In 1992, Dr. Honjo identified PD1 (programmed death receptor 1) and demonstrated PD1 expression in T cells. In 1999, Dr. Chen identified PDL1 (B7-H1) and demonstrated high PDL1 expression in immune and tumor cells. The interaction between PDL1 and PD1 induces T cell apoptosis and negatively regulates lymphocyte activation. Thus, blocking PD1-PDL1 immune checkpoints promotes T cell activation, which facilitates the cytotoxic effect of T cells on tumor cells. Although the blockade of the immune checkpoint PD1-PDL1 has achieved remarkable success in the clinical treatment of a variety of cancers, the majority of cancer patients still failed to respond to the immunotherapy. In addition, drug resistance may occur during the targeted therapy of PD1-PDL1. Therefore, many trials have been conducted to improve the responsiveness of cancer patients to immunotherapy through combination therapy strategies. Recent studies have shown that some CDK inhibitors can enhance the anti-tumor immune response. In preclinical and clinical trials, some CDK inhibitors have demonstrated potent anti-tumor activity when used in combination with PD1-PDL1 immunotherapy.
Dinaciclib enhances anti-PD1 mediated tumor suppression
Dinaciclib, a potent CDK inhibitor of CDK1, 2, 5, 9 and 12, can induce apoptosis in various tumor cells as described above. Hossain et al. reported that combination therapy with Dinaciclib and anti-PD1 antibody presented considerable anti-tumor activity. This combination therapy can induce T cell infiltration and DC activation, suggesting that combination therapy can improve anti-tumor immune response and lead to tumor regression. In addition, in combination with anti-PD1 antibodies, Dinaciclib can induce immunogenic cell death (ICD) to convert tumor cells into endogenous vaccines [26]. Together, this study has paved a new path in solving the toxic and side-effect issues of pan-CDK inhibitors, thus increases the application possibility of pan-CDK inhibitors.
CDK4/6 inhibitors augment the anti-tumor efficacy of PD1-PDL1 immune checkpoint blockade
CDK4 and CDK6 are fundamental drivers of the cell cycle and are required for the initiation and progression of various malignancies. Pharmacological inhibitors of CDK4/6 have displayed significant activity against several solid tumors. In a mouse tumor model study, Goel et al. found that CDK4/6 inhibitors not only induce tumor cell cycle arrest, but also promote anti-tumor immunity [85]. On one hand, CDK4/6 inhibitors activate expression of endogenous retroviral elements in tumor cells, thereby stimulates the production of type III interferons and simultaneously enhances tumor antigen presentation. On the other hand, CDK4/6 inhibitors markedly suppress the proliferation of regulatory T cells. Based on these two functions, clearance of tumor cells mediated by cytotoxic T cell is significantly promoted by treatment with CDK4/6 inhibitors. This study provided a theoretical basis for combination therapy using CDK4/6 inhibitors and PD1-PDL1 antibodies.
Abemaciclib is another CDK4/6 inhibitor, which has been clinically approved in the treatment of HR+ breast cancer. In a recent study, Schaer et al. reported that treatment with Abemaciclib can promote human T cell activation and can up-regulate expression of antigen presentation genes in breast cancer cells [86]. Further study indicated that Abemaciclib monotherapy can increase T cell inflammatory and delay tumor growth. Combination therapy with Abemaciclib and anti-PDL1 antibody can induce immunological memory and tumor elimination. These results suggested that combination therapy with Abemaciclib and anti-PDL1 antibody effectively stimulated both innate and adaptive immune response. Taken together, combination therapy with Abemaciclib and anti-PDL1 antibody have presented a great potential in clinical application.
Since the efficacy of PDL1 antibody therapy depends on the protein abundance of PDL1, Zhang et al. investigated the regulatory mechanism of PDL1 expression and stability [87]. They found that CDK4 is involved in the regulation of PDL1. Another study further proved that combination therapy with CDK4/6 inhibitors and anti-PDL1 antibody presented a remarkable anti-tumor activity [88]. Together, these findings reinforce the potential of combination therapy with CDK4/6 inhibitors and PD1-PDL1 antibody in clinical application. To date, in addition to the combination of CDK4/6 inhibitors and PDL1 antibody, other combination therapies are also being tried [89-92]. Combination therapies are expected to play an important role in future cancer therapy.
Other combination therapies
CDK12 mutant cases are known to be associated with elevated neoantigen burden and increased tumor T cell infiltration and clonal expansion [93]. CDK12 inactivation defines a distinct class of metastatic castration-resistant prostate cancer (mCRPC) that may benefit from immune checkpoint immunotherapy [93,94]. Indeed, a phase ll clinical trial was conducted in patients with metastatic prostate cancer harboring CDK12 deficiency (ClinicalTrials.gov Identifier: NCT03570619). These patients were administered with immune-checkpoint inhibitor in combination with nivolumab and ipilimumab followed by nivolumab monotherapy. A recent study also reported that SR-4835, a CDK12 and CDK13 specific inhibitor, induced immunogenic cell death, thereby enhanced the anti-tumor activity of PD1-PD-L1 immune checkpoint therapy in breast cancer [95]. Moreover, YKL-5-124 (CDK7 inhibitor) and MC18029 (CDK9 inhibitor) are also being studied in combination therapy with PD1/PD-L1 as mentioned above [59,69]. These findings suggested that the combination of CDK12 inhibitors and PD1-PDL1 immunotherapy will be a promising strategy for cancer treatment.
Future perspective
CDK inhibitors developed in the early stage lack efficacy and selectivity in clinical practice, and the therapeutic effect is limited. Pan-CDK inhibitors have displayed remarkable anti-tumor efficacy. However, due to their low specificity and severe side effects, pan-CDK inhibitors have not been approved for clinical (cancer) treatment. Optimization of pan-CDK inhibitors together with combination therapy might hold some promise, and more relevant clinical trials are actually underway.
In contrast to pan-CDK inhibitors, great progress has been achieved in terms of selective Cdk4/6 inhibitors. Palbociclib has been licensed since 2015 for the treatment of hormone responsive, Rb positive breast cancer. Subsequently, Abemaciclib developed by Eli Lilly and Ribociclib developed by Novartis were also approved by the FDA. The biggest challenge in research and development of CDK inhibitors might be dealing with the adverse effects and potential drug tolerance. Further understanding of the behind mechanism and exploring ideal combination therapy might help overcome the selectivity and drug tolerance of CDK inhibitors.
Acknowledgements
This work is supported by grants from the Ministry of Science and Technology of China (National Program on Key Basic Research Project: 2019YFA0802804 to L. Y.), National Natural Science Foundation of China (NSFC 31871492 and 31671546 to C.C., NSFC 81672229 to Q.Z.), Dongguan Social Science and Technology Development (key) Project (2019507101163 to C.C.), the support from Jiangsu Provincial key research and development program (BE2020679 to Q. Z.), and the Shenzhen Science and Technology Program (Grant No. KQTD20170810154011370, Q.Z.).
Disclosure of conflict of interest
None.
References
- 1.Arellano M, Moreno S. Regulation of CDK/cyclin complexes during the cell cycle. Int J Biochem Cell Biol. 1997;29:559–573. doi: 10.1016/s1357-2725(96)00178-1. [DOI] [PubMed] [Google Scholar]
- 2.Swaffer MP, Jones AW, Flynn HR, Snijders AP, Nurse P. CDK substrate phosphorylation and ordering the cell cycle. Cell. 2016;167:1750–1761. e1716. doi: 10.1016/j.cell.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 4.Malumbres M, Harlow E, Hunt T, Hunter T, Lahti JM, Manning G, Morgan DO, Tsai LH, Wolgemuth DJ. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009;11:1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar MS, Heidemann M, Tietjen JR, Zhang DW, Chapman RD, Eick D, Ansari AZ. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol Cell. 2009;34:387–393. doi: 10.1016/j.molcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larochelle S, Amat R, Glover-Cutter K, Sansó M, Zhang C, Allen JJ, Shokat KM, Bentley DL, Fisher RP. Cyclin-dependent kinase control of the initiation-to-elongation switch of RNA polymerase II. Nat Struct Mol Biol. 2012;19:1108–1115. doi: 10.1038/nsmb.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q, Li T, Price DH. RNA polymerase II elongation control. Annu Rev Biochem. 2012;81:119–143. doi: 10.1146/annurev-biochem-052610-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhariwala FA, Rajadhyaksha MS. An unusual member of the Cdk family: Cdk5. Cell Mol Neurobiol. 2008;28:351–369. doi: 10.1007/s10571-007-9242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci U S A. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of wnt receptor activation. Dev Cell. 2009;17:788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Jiang M, Gao Y, Yang T, Zhu X, Chen J. Cyclin Y, a novel membrane-associated cyclin, interacts with PFTK1. FEBS Lett. 2009;583:2171–2178. doi: 10.1016/j.febslet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 15.Cicenas J, Kalyan K, Sorokinas A, Stankunas E, Levy J, Meskinyte I, Stankevicius V, Kaupinis A, Valius M. Roscovitine in cancer and other diseases. Ann Transl Med. 2015;3:135. doi: 10.3978/j.issn.2305-5839.2015.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whittaker SR, Mallinger A, Workman P, Clarke PA. Inhibitors of cyclin-dependent kinases as cancer therapeutics. Pharmacol Ther. 2017;173:83–105. doi: 10.1016/j.pharmthera.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeidner JF, Karp JE. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk Res. 2015;39:1312–1318. doi: 10.1016/j.leukres.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Parker BW, Kaur G, Nieves-Neira W, Taimi M, Kohlhagen G, Shimizu T, Losiewicz MD, Pommier Y, Sausville EA, Senderowicz AM. Early induction of apoptosis in hematopoietic cell lines after exposure to flavopiridol. Blood. 1998;91:458–465. [PubMed] [Google Scholar]
- 20.Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, Wu D, Blum KA, Fischer B, Mitchell SM, Moran ME, Brooker-McEldowney M, Heerema NA, Jarjoura D, Schaaf LJ, Byrd JC, Grever MR, Dalton JT. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, Blum KA, Flynn JM, Jones JA, Hu W, Moran ME, Mitchell SM, Smith LL, Wagner AJ, Raymond CA, Schaaf LJ, Phelps MA, Villalona-Calero MA, Grever MR, Byrd JC. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J. Clin. Oncol. 2009;27:6012–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bose P, Simmons GL, Grant S. Cyclin-dependent kinase inhibitor therapy for hematologic malignancies. Expert Opin Investig Drugs. 2013;22:723–738. doi: 10.1517/13543784.2013.789859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karp JE, Blackford A, Smith BD, Alino K, Seung AH, Bolaños-Meade J, Greer JM, Carraway HE, Gore SD, Jones RJ, Levis MJ, McDevitt MA, Doyle LA, Wright JJ. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2010;34:877–882. doi: 10.1016/j.leukres.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker A, Gregory GP, Verbrugge I, Kats L, Hilton JJ, Vidacs E, Lee EM, Lock RB, Zuber J, Shortt J, Johnstone RW. The CDK9 inhibitor dinaciclib exerts potent apoptotic and antitumor effects in preclinical models of MLL-rearranged acute myeloid leukemia. Cancer Res. 2016;76:1158. doi: 10.1158/0008-5472.CAN-15-1070. [DOI] [PubMed] [Google Scholar]
- 25.Johnson AJ, Yeh YY, Smith LL, Wagner AJ, Hessler J, Gupta S, Flynn J, Jones J, Zhang X, Bannerji R, Grever MR, Byrd JC. The novel cyclin-dependent kinase inhibitor dinaciclib (SCH727965) promotes apoptosis and abrogates microenvironmental cytokine protection in chronic lymphocytic leukemia cells. Leukemia. 2012;26:2554–2557. doi: 10.1038/leu.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossain DMS, Javaid S, Cai M, Zhang C, Sawant A, Hinton M, Sathe M, Grein J, Blumenschein W, Pinheiro EM, Chackerian A. Dinaciclib induces immunogenic cell death and enhances anti-PD1-mediated tumor suppression. J Clin Invest. 2018;128:644–654. doi: 10.1172/JCI94586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirsath NP, Manohar SM, Joshi KS. P276-00, a cyclin-dependent kinase inhibitor, modulates cell cycle and induces apoptosis in vitro and in vivo in mantle cell lymphoma cell lines. Mol Cancer. 2012;11:77. doi: 10.1186/1476-4598-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassaday RD, Goy A, Advani S, Chawla P, Nachankar R, Gandhi M, Gopal AK. A phase II, single-arm, open-label, multicenter study to evaluate the efficacy and safety of P276-00, a cyclin-dependent kinase inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2015;15:392–397. doi: 10.1016/j.clml.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra PB, Lobo AS, Joshi KS, Rathos MJ, Kumar GA, Padigaru M. Molecular mechanisms of anti-tumor properties of P276-00 in head and neck squamous cell carcinoma. J Transl Med. 2013;11:42. doi: 10.1186/1479-5876-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su YT, Chen R, Wang H, Song H, Zhang Q, Chen LY, Lappin H, Vasconcelos G, Lita A, Maric D, Li A, Celiku O, Zhang W, Meetze K, Estok T, Larion M, Abu-Asab M, Zhuang Z, Yang C, Gilbert MR, Wu J. Novel targeting of transcription and metabolism in glioblastoma. Clin Cancer Res. 2018;24:1124–1137. doi: 10.1158/1078-0432.CCR-17-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goh KC, Novotny-Diermayr V, Hart S, Ong LC, Loh YK, Cheong A, Tan YC, Hu C, Jayaraman R, William AD, Sun ET, Dymock BW, Ong KH, Ethirajulu K, Burrows F, Wood JM. TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia. 2012;26:236–243. doi: 10.1038/leu.2011.218. [DOI] [PubMed] [Google Scholar]
- 32.Ponder KG, Matulis SM, Hitosugi S, Gupta VA, Sharp C, Burrows F, Nooka AK, Kaufman JL, Lonial S, Boise LH. Dual inhibition of Mcl-1 by the combination of carfilzomib and TG02 in multiple myeloma. Cancer Biol Ther. 2016;17:769–777. [Google Scholar]
- 33.Dolman ME, Poon E, Ebus ME, den Hartog IJ, van Noesel CJ, Jamin Y, Hallsworth A, Robinson SP, Petrie K, Sparidans RW, Kok RJ, Versteeg R, Caron HN, Chesler L, Molenaar JJ. Cyclin-dependent kinase inhibitor AT7519 as a potential drug for MYCN-dependent neuroblastoma. Clin Cancer Res. 2015;21:5100–9. doi: 10.1158/1078-0432.CCR-15-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorward DA, Felton JM, Robb CT, Craven T, Kipari T, Walsh TS, Haslett C, Kefala K, Rossi AG, Lucas CD. The cyclin-dependent kinase inhibitor AT7519 accelerates neutrophil apoptosis in sepsis-related acute respiratory distress syndrome. Thorax. 2017;72:182–185. doi: 10.1136/thoraxjnl-2016-209229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen EX, Hotte S, Hirte H, Siu LL, Lyons J, Squires M, Lovell S, Turner S, McIntosh L, Seymour L. A phase I study of cyclin-dependent kinase inhibitor, AT7519, in patients with advanced cancer: NCIC Clinical Trials Group IND 177. Br J Cancer. 2014;111:2262–2267. doi: 10.1038/bjc.2014.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syn NL, Lim PL, Kong LR, Wang L, Wong AL, Lim CM, Loh TKS, Siemeister G, Goh BC, Hsieh WS. Pan-CDK inhibition augments cisplatin lethality in nasopharyngeal carcinoma cell lines and xenograft models. Signal Transduct Target Ther. 2018;3:9. doi: 10.1038/s41392-018-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin SF, Lin JD, Hsueh C, Chou TC, Wong RJ. Activity of roniciclib in medullary thyroid cancer. Oncotarget. 2018;9:28030–28041. doi: 10.18632/oncotarget.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahleda R, Grilley-Olson JE, Govindan R, Barlesi F, Greillier L, Perol M, Ray-Coquard I, Strumberg D, Schultheis B, Dy GK, Zalcman G, Weiss GJ, Walter AO, Kornacker M, Rajagopalan P, Henderson D, Nogai H, Ocker M, Soria JC. Phase I dose-escalation studies of roniciclib, a pan-cyclin-dependent kinase inhibitor, in advanced malignancies. Br J Cancer. 2017;116:1505–1512. doi: 10.1038/bjc.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho BC, Dy GK, Govindan R, Kim DW, Pennell NA, Zalcman G, Besse B, Kim JH, Koca G, Rajagopalan P, Langer S, Ocker M, Nogai H, Barlesi F. Phase Ib/II study of the pan-cyclin-dependent kinase inhibitor roniciclib in combination with chemotherapy in patients with extensive-disease small-cell lung cancer. Lung Cancer. 2018;123:14–21. doi: 10.1016/j.lungcan.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Cirstea D, Hideshima T, Santo L, Eda H, Mishima Y, Nemani N, Hu Y, Mimura N, Cottini F, Gorgun G, Ohguchi H, Suzuki R, Loferer H, Munshi NC, Anderson KC, Raje N. Small-molecule multi-targeted kinase inhibitor RGB-286638 triggers P53-dependent and -independent anti-multiple myeloma activity through inhibition of transcriptional CDKs. Leukemia. 2013;27:2366–2375. doi: 10.1038/leu.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Biessen DA, Burger H, de Bruijn P, Lamers CH, Naus N, Loferer H, Wiemer EA, Mathijssen RH, de Jonge MJ. Phase I study of RGB-286638, a novel, multitargeted cyclin-dependent kinase inhibitor in patients with solid tumors. Clin Cancer Res. 2014;20:4776–83. doi: 10.1158/1078-0432.CCR-14-0325. [DOI] [PubMed] [Google Scholar]
- 42.Locatelli G, Bosotti R, Ciomei M, Brasca MG, Calogero R, Mercurio C, Fiorentini F, Bertolotti M, Scacheri E, Scaburri A, Galvani A, Pesenti E, De Baere T, Soria JC, Lazar V, Isacchi A. Transcriptional analysis of an E2F gene signature as a biomarker of activity of the cyclin-dependent kinase inhibitor PHA-793887 in tumor and skin biopsies from a phase I clinical study. Mol Cancer Ther. 2010;9:1265–73. doi: 10.1158/1535-7163.MCT-09-1163. [DOI] [PubMed] [Google Scholar]
- 43.Alzani R, Pedrini O, Albanese C, Ceruti R, Casolaro A, Patton V, Colotta F, Rambaldi A, Introna M, Pesenti E, Ciomei M, Golay J. Therapeutic efficacy of the pan-cdk inhibitor PHA-793887 in vitro and in vivo in engraftment and high-burden leukemia models. Exp Hematol. 2010;38:259–269. e252. doi: 10.1016/j.exphem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Massard C, Soria JC, Anthoney DA, Proctor A, Scaburri A, Pacciarini MA, Laffranchi B, Pellizzoni C, Kroemer G, Armand JP, Balheda R, Twelves CJ. A first in man, phase I dose-escalation study of PHA-793887, an inhibitor of multiple cyclin-dependent kinases (CDK2, 1 and 4) reveals unexpected hepatotoxicity in patients with solid tumors. Cell Cycle. 2011;10:963–970. doi: 10.4161/cc.10.6.15075. [DOI] [PubMed] [Google Scholar]
- 45.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- 46.Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, Torres R, Ajamie RT, Wishart GN, Flack RS, Neubauer BL, Young J, Chan EM, Iversen P, Cronier D, Kreklau E, de Dios A. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32:825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tripathy D, Bardia A, Sellers WR. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin Cancer Res. 2017;23:3251–3262. doi: 10.1158/1078-0432.CCR-16-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell. 2018;34:9–20. doi: 10.1016/j.ccell.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali S, Heathcote DA, Kroll SH, Jogalekar AS, Scheiper B, Patel H, Brackow J, Siwicka A, Fuchter MJ, Periyasamy M, Tolhurst RS, Kanneganti SK, Snyder JP, Liotta DC, Aboagye EO, Barrett AG, Coombes RC. The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity. Cancer Res. 2009;69:6208–6215. doi: 10.1158/0008-5472.CAN-09-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hazel P, Kroll SH, Bondke A, Barbazanges M, Patel H, Fuchter MJ, Coombes RC, Ali S, Barrett AG, Freemont PS. Inhibitor selectivity for cyclin-dependent kinase 7: a structural, thermodynamic, and modelling study. ChemMedChem. 2017;12:372–380. doi: 10.1002/cmdc.201600535. [DOI] [PubMed] [Google Scholar]
- 51.Patel H, Periyasamy M, Sava GP, Bondke A, Slafer BW, Kroll SHB, Barbazanges M, Starkey R, Ottaviani S, Harrod A, Aboagye EO, Buluwela L, Fuchter MJ, Barrett AGM, Coombes RC, Ali S. ICEC0942, an orally bioavailable selective inhibitor of CDK7 for cancer treatment. Mol Cancer Ther. 2018;17:1156–1166. doi: 10.1158/1535-7163.MCT-16-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chipumuro E, Marco E, Christensen CL, Kwiatkowski N, Zhang T, Hatheway CM, Abraham BJ, Sharma B, Yeung C, Altabef A, Perez-Atayde A, Wong KK, Yuan GC, Gray NS, Young RA, George RE. CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell. 2014;159:1126–1139. doi: 10.1016/j.cell.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christensen CL, Kwiatkowski N, Abraham BJ, Carretero J, Al-Shahrour F, Zhang T, Chipumuro E, Herter-Sprie GS, Akbay EA, Altabef A, Zhang J, Shimamura T, Capelletti M, Reibel JB, Cavanaugh JD, Gao P, Liu Y, Michaelsen SR, Poulsen HS, Aref AR, Barbie DA, Bradner JE, George RE, Gray NS, Young RA, Wong KK. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell. 2014;26:909–922. doi: 10.1016/j.ccell.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwiatkowski N, Zhang T, Rahl PB, Abraham BJ, Reddy J, Ficarro SB, Dastur A, Amzallag A, Ramaswamy S, Tesar B, Jenkins CE, Hannett NM, McMillin D, Sanda T, Sim T, Kim ND, Look T, Mitsiades CS, Weng AP, Brown JR, Benes CH, Marto JA, Young RA, Gray NS. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature. 2014;511:616–620. doi: 10.1038/nature13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagaraja S, Vitanza NA, Woo PJ, Taylor KR, Liu F, Zhang L, Li M, Meng W, Ponnuswami A, Sun W, Ma J, Hulleman E, Swigut T, Wysocka J, Tang Y, Monje M. Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell. 2017;31:635–652. e636. doi: 10.1016/j.ccell.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Zhang T, Kwiatkowski N, Abraham BJ, Lee TI, Xie S, Yuzugullu H, Von T, Li H, Lin Z, Stover DG, Lim E, Wang ZC, Iglehart JD, Young RA, Gray NS, Zhao JJ. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell. 2015;163:174–186. doi: 10.1016/j.cell.2015.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Zhou L, Bandyopadhyay D, Sharma K, Allen AJ, Kmieciak M, Grant S. The covalent CDK7 inhibitor THZ1 potently induces apoptosis in multiple myeloma cells in vitro and in vivo. Clin Cancer Res. 2019;25:6195–6205. doi: 10.1158/1078-0432.CCR-18-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olson CM, Liang Y, Leggett A, Park WD, Li L, Mills CE, Elsarrag SZ, Ficarro SB, Zhang T, Düster R, Geyer M, Sim T, Marto JA, Sorger PK, Westover KD, Lin CY, Kwiatkowski N, Gray NS. Development of a selective CDK7 covalent inhibitor reveals predominant cell-cycle phenotype. Cell Chem Biol. 2019;26:792–803. e710. doi: 10.1016/j.chembiol.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Christensen CL, Dries R, Oser MG, Deng J, Diskin B, Li F, Pan Y, Zhang X, Yin Y, Papadopoulos E, Pyon V, Thakurdin C, Kwiatkowski N, Jani K, Rabin AR, Castro DM, Chen T, Silver H, Huang Q, Bulatovic M, Dowling CM, Sundberg B, Leggett A, Ranieri M, Han H, Li S, Yang A, Labbe KE, Almonte C, Sviderskiy VO, Quinn M, Donaghue J, Wang ES, Zhang T, He Z, Velcheti V, Hammerman PS, Freeman GJ, Bonneau R, Kaelin WG Jr, Sutherland KD, Kersbergen A, Aguirre AJ, Yuan GC, Rothenberg E, Miller G, Gray NS, Wong KK. CDK7 inhibition potentiates genome instability triggering anti-tumor immunity in small cell lung cancer. Cancer Cell. 2020;37:37–54. e9. doi: 10.1016/j.ccell.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu S, Marineau JJ, Rajagopal N, Hamman KB, Choi YJ, Schmidt DR, Ke N, Johannessen L, Bradley MJ, Orlando DA, Alnemy SR, Ren Y, Ciblat S, Winter DK, Kabro A, Sprott KT, Hodgson JG, Fritz CC, Carulli JP, di Tomaso E, Olson ER. Discovery and Characterization of SY-1365, a selective, covalent inhibitor of CDK7. Cancer Res. 2019;79:3479–3491. doi: 10.1158/0008-5472.CAN-19-0119. [DOI] [PubMed] [Google Scholar]
- 61.Hu S, Marineau J, Hamman K, Bradley M, Savinainen A, Alnemy S, Rajagopal N, Orlando D, Chuaqui C, Olson E. Abstract 4421: SY-5609, an orally available selective CDK7 inhibitor demonstrates broad anti-tumor activity in vivo . Cancer Res. 2019;79:4421–4421. [Google Scholar]
- 62.Johannessen LH, Hu S, Ke N, D’Ippolito A, Rajagopal N, Marineau J, Savinainen A, Zamboni W, Hodgson G. Abstract C091: preclinical evaluation of PK, PD, and antitumor activity of the oral, non-covalent, potent and highly selective CDK7 inhibitor, SY-5609, provides rationale for clinical development in multiple solid tumor indications. Mol Cancer Ther. 2019;18:C091–C091. [Google Scholar]
- 63.Bacon CW, D’Orso I. CDK9: a signaling hub for transcriptional control. Transcription. 2019;10:57–75. doi: 10.1080/21541264.2018.1523668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franco LC, Morales F, Boffo S, Giordano A. CDK9: a key player in cancer and other diseases. J Cell Biochem. 2018;119:1273–1284. doi: 10.1002/jcb.26293. [DOI] [PubMed] [Google Scholar]
- 65.Poon E, Liang T, Jamin Y, Walz S, Kwok C, Hakkert A, Barker K, Urban Z, Thway K, Zeid R, Hallsworth A, Box G, Ebus ME, Licciardello MP, Sbirkov Y, Lazaro G, Calton E, Costa BM, Valenti M, De Haven Brandon A, Webber H, Tardif N, Almeida GS, Christova R, Boysen G, Richards MW, Barone G, Ford A, Bayliss R, Clarke PA, De Bono J, Gray NS, Blagg J, Robinson SP, Eccles SA, Zheleva D, Bradner JE, Molenaar J, Vivanco I, Eilers M, Workman P, Lin CY, Chesler L. Orally bioavailable CDK9/2 inhibitor shows mechanism-based therapeutic potential in MYCN-driven neuroblastoma. J Clin Invest. 2020;130:5875–5892. doi: 10.1172/JCI134132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cidado J, Boiko S, Proia T, Ferguson D, Criscione SW, San Martin M, Pop-Damkov P, Su N, Roamio Franklin VN, Sekhar Reddy Chilamakuri C, D’Santos CS, Shao W, Saeh JC, Koch R, Weinstock DM, Zinda M, Fawell SE, Drew L. AZD4573 is a highly selective CDK9 inhibitor that suppresses MCL-1 and induces apoptosis in hematologic cancer cells. Clin Cancer Res. 2020;26:922–934. doi: 10.1158/1078-0432.CCR-19-1853. [DOI] [PubMed] [Google Scholar]
- 67.McLaughlin RP, He J, van der Noord VE, Redel J, Foekens JA, Martens JWM, Smid M, Zhang Y, van de Water B. A kinase inhibitor screen identifies a dual cdc7/CDK9 inhibitor to sensitise triple-negative breast cancer to EGFR-targeted therapy. Breast Cancer Res. 2019;21:77. doi: 10.1186/s13058-019-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Zhi X, Chen S, Shen X, Chen C, Yuan L, Guo J, Meng D, Chen M, Yao L. CDK9 inhibitor CDKI-73 is synergetic lethal with PARP inhibitor olaparib in BRCA1 wide-type ovarian cancer. Am J Cancer Res. 2020;10:1140–1155. [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Pandey S, Travers M, Sun H, Morton G, Madzo J, Chung W, Khowsathit J, Perez-Leal O, Barrero CA, Merali C, Okamoto Y, Sato T, Pan J, Garriga J, Bhanu NV, Simithy J, Patel B, Huang J, Raynal NJ, Garcia BA, Jacobson MA, Kadoch C, Merali S, Zhang Y, Childers W, Abou-Gharbia M, Karanicolas J, Baylin SB, Zahnow CA, Jelinek J, Graña X, Issa JJ. Targeting CDK9 reactivates epigenetically silenced genes in cancer. Cell. 2018;175:1244–1258. e1226. doi: 10.1016/j.cell.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu T, Qin Z, Tian Y, Wang J, Xu C, Li Z, Bian J. Recent developments in the biology and medicinal chemistry of CDK9 inhibitors: an update. J Med Chem. 2020;63:13228–13257. doi: 10.1021/acs.jmedchem.0c00744. [DOI] [PubMed] [Google Scholar]
- 71.Anda S, Rothe C, Boye E, Grallert B. Consequences of abnormal CDK activity in S phase. Cell Cycle. 2016;15:963–973. doi: 10.1080/15384101.2016.1152423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cherukupalli S, Chandrasekaran B, Aleti RR, Sayyad N, Hampannavar GA, Merugu SR, Rachamalla HR, Banerjee R, Karpoormath R. Synthesis of 4,6-disubstituted pyrazolo[3,4-d] pyrimidine analogues: cyclin-dependent kinase 2 (CDK2) inhibition, molecular docking and anticancer evaluation. Bioorg Chem. 2018;79:46–59. doi: 10.1016/j.bioorg.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 73.Galbraith MD, Bender H, Espinosa JM. Therapeutic targeting of transcriptional cyclin-dependent kinases. Transcription. 2019;10:118–136. doi: 10.1080/21541264.2018.1539615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng SW, Kuzyk MA, Moradian A, Ichu TA, Chang VC, Tien JF, Vollett SE, Griffith M, Marra MA, Morin GB. Interaction of cyclin-dependent kinase 12/CrkRS with cyclin K1 is required for the phosphorylation of the C-terminal domain of RNA polymerase II. Mol Cell Biol. 2012;32:4691–4704. doi: 10.1128/MCB.06267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi HJ, Jin S, Cho H, Won HY, An HW, Jeong GY, Park YU, Kim HY, Park MK, Son T, Min KW, Jang KS, Oh YH, Lee JY, Kong G. CDK12 drives breast tumor initiation and trastuzumab resistance via WNT and IRS1-ErbB-PI3K signaling. EMBO Rep. 2019;20:e48058. doi: 10.15252/embr.201948058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henry KL, Kellner D, Bajrami B, Anderson JE, Beyna M, Bhisetti G, Cameron T, Capacci AG, Bertolotti-Ciarlet A, Feng J, Gao B, Hopkins B, Jenkins T, Li K, May-Dracka T, Murugan P, Wei R, Zeng W, Allaire N, Buckler A, Loh C, Juhasz P, Lucas B, Ennis KA, Vollman E, Cahir-McFarland E, Hett EC, Ols ML. CDK12-mediated transcriptional regulation of noncanonical NF-κB components is essential for signaling. Sci Signal. 2018;11:eaam8216. doi: 10.1126/scisignal.aam8216. [DOI] [PubMed] [Google Scholar]
- 77.Liu H, Shin SH, Chen H, Liu T, Li Z, Hu Y, Liu F, Zhang C, Kim DJ, Liu K, Dong Z. CDK12 and PAK2 as novel therapeutic targets for human gastric cancer. Theranostics. 2020;10:6201–6215. doi: 10.7150/thno.46137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naidoo K, Wai PT, Maguire SL, Daley F, Haider S, Kriplani D, Campbell J, Mirza H, Grigoriadis A, Tutt A, Moseley PM, Abdel-Fatah TMA, Chan SYT, Madhusudan S, Rhaka EA, Ellis IO, Lord CJ, Yuan Y, Green AR, Natrajan R. Evaluation of CDK12 protein expression as a potential novel biomarker for DNA damage response-targeted therapies in breast cancer. Mol Cancer Ther. 2018;17:306–315. doi: 10.1158/1535-7163.MCT-17-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng F, Yang C, Kong Y, Huang X, Chen Y, Zhou Y, Xie X, Liu P. CDK12 promotes breast cancer progression and maintains stemness by activating c-myc/β-catenin signaling. Curr Cancer Drug Targets. 2020;20:156–165. doi: 10.2174/1568009619666191118113220. [DOI] [PubMed] [Google Scholar]
- 80.Tien JF, Mazloomian A, Cheng SG, Hughes CS, Chow CCT, Canapi LT, Oloumi A, Trigo-Gonzalez G, Bashashati A, Xu J, Chang VC, Shah SP, Aparicio S, Morin GB. CDK12 regulates alternative last exon mRNA splicing and promotes breast cancer cell invasion. Nucleic Acids Res. 2017;45:6698–6716. doi: 10.1093/nar/gkx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hopkins JL, Zou L. Induction of BRCAness in triple-negative breast cancer by a CDK12/13 inhibitor improves chemotherapy. Cancer Cell. 2019;36:461–463. doi: 10.1016/j.ccell.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Quereda V, Bayle S, Vena F, Frydman SM, Monastyrskyi A, Roush WR, Duckett DR. Therapeutic targeting of CDK12/CDK13 in triple-negative breast cancer. Cancer Cell. 2019;36:545–558. e547. doi: 10.1016/j.ccell.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Zhang T, Kwiatkowski N, Olson CM, Dixon-Clarke SE, Abraham BJ, Greifenberg AK, Ficarro SB, Elkins JM, Liang Y, Hannett NM, Manz T, Hao M, Bartkowiak B, Greenleaf AL, Marto JA, Geyer M, Bullock AN, Young RA, Gray NS. Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors. Nat Chem Biol. 2016;12:876–884. doi: 10.1038/nchembio.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C, Wang H, Lieftink C, du Chatinier A, Gao D, Jin G, Jin H, Beijersbergen RL, Qin W, Bernards R. CDK12 inhibition mediates DNA damage and is synergistic with sorafenib treatment in hepatocellular carcinoma. Gut. 2020;69:727–736. doi: 10.1136/gutjnl-2019-318506. [DOI] [PubMed] [Google Scholar]
- 85.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, Hoog J, Ellis MJ, Ma CX, Ramm S, Krop IE, Winer EP, Roberts TM, Kim HJ, McAllister SS, Zhao JJ. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schaer DA, Beckmann RP, Dempsey JA, Huber L, Forest A, Amaladas N, Li Y, Wang YC, Rasmussen ER, Chin D, Capen A, Carpenito C, Staschke KA, Chung LA, Litchfield LM, Merzoug FF, Gong X, Iversen PW, Buchanan S, de Dios A, Novosiadly RD, Kalos M. The CDK4/6 inhibitor abemaciclib induces a T Cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22:2978–2994. doi: 10.1016/j.celrep.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 87.Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, Tan Y, Ci Y, Wu F, Dai X, Guo J, Huang YH, Fan C, Ren S, Sun Y, Freeman GJ, Sicinski P, Wei W. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–95. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, Ivanova E, Paweletz CP, Bowden M, Zhou CW, Herter-Sprie GS, Sorrentino JA, Bisi JE, Lizotte PH, Merlino AA, Quinn MM, Bufe LE, Yang A, Zhang Y, Zhang H, Gao P, Chen T, Cavanaugh ME, Rode AJ, Haines E, Roberts PJ, Strum JC, Richards WG, Lorch JH, Parangi S, Gunda V, Boland GM, Bueno R, Palakurthi S, Freeman GJ, Ritz J, Haining WN, Sharpless NE, Arthanari H, Shapiro GI, Barbie DA, Gray NS, Wong KK. CDK4/6 inhibition augments antitumor immunity by Enhancing T-cell activation. Cancer Discov. 2018;8:216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kruse U, Pallasch CP, Bantscheff M, Eberhard D, Frenzel L, Ghidelli S, Maier SK, Werner T, Wendtner CM, Drewes G. Chemoproteomics-based kinome profiling and target deconvolution of clinical multi-kinase inhibitors in primary chronic lymphocytic leukemia cells. Leukemia. 2011;25:89–100. doi: 10.1038/leu.2010.233. [DOI] [PubMed] [Google Scholar]
- 90.Gao N, Dai Y, Rahmani M, Dent P, Grant S. Contribution of disruption of the nuclear factor-κB pathway to induction of apoptosis in human leukemia cells by histone deacetylase Inhibitors and flavopiridol. Mol Pharmacol. 2004;66:956–63. doi: 10.1124/mol.104.002014. [DOI] [PubMed] [Google Scholar]
- 91.Dai Y, Rahmani M, Grant S. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-κB-dependent process. Oncogene. 2003;22:7108–7122. doi: 10.1038/sj.onc.1206863. [DOI] [PubMed] [Google Scholar]
- 92.Lin TS, Blum KA, Fischer DB, Mitchell SM, Ruppert AS, Porcu P, Kraut EH, Baiocchi RA, Moran ME, Johnson AJ, Schaaf LJ, Grever MR, Byrd JC. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J. Clin. Oncol. 2010;28:418–423. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu YM, Cieślik M, Lonigro RJ, Vats P, Reimers MA, Cao X, Ning Y, Wang L, Kunju LP, de Sarkar N, Heath EI, Chou J, Feng FY, Nelson PS, de Bono JS, Zou W, Montgomery B, Alva A, Robinson DR, Chinnaiyan AM. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018;173:1770–1782. e1714. doi: 10.1016/j.cell.2018.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Antonarakis ES. Cyclin-dependent kinase 12, immunity, and prostate cancer. N Engl J Med. 2018;379:1087–1089. doi: 10.1056/NEJMcibr1808772. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Zhang H, Li Q, Zou P, Huang X, Wu C, Tan L. CDK12/13 inhibition induces immunogenic cell death and enhances anti-PD-1 anticancer activity in breast cancer. Cancer Lett. 2020;495:12–21. doi: 10.1016/j.canlet.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 96.Siemeister G, Luecking U, Wagner C, Detjen K, Mc Coy C, Bosslet K. Molecular and pharmacodynamic characteristics of the novel multi-target tumor growth inhibitor ZK 304709. Biomed Pharmacother. 2006;60:269–272. doi: 10.1016/j.biopha.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Cho SJ, Lee SS, Kim YJ, Park BD, Choi JS, Liu L, Ham YM, Moon Kim B, Lee SK. Xylocydine, a novel Cdk inhibitor, is an effective inducer of apoptosis in hepatocellular carcinoma cells in vitro and in vivo. Cancer Lett. 2010;287:196–206. doi: 10.1016/j.canlet.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 98.Ham YM, Choi KJ, Song SY, Jin YH, Chun MW, Lee SK. Xylocydine, a novel inhibitor of cyclin-dependent kinases, prevents the tumor necrosis factor-related apoptosis-inducing ligand-induced apoptotic cell death of SK-HEP-1 cells. J Pharmacol Exp Ther. 2004;308:814–819. doi: 10.1124/jpet.103.059568. [DOI] [PubMed] [Google Scholar]
- 99.Wu Y, Chen C, Sun X, Shi X, Jin B, Ding K, Yeung SC, Pan J. Cyclin-dependent kinase 7/9 inhibitor SNS-032 abrogates FIP1-like-1 platelet-derived growth factor receptor alpha and bcr-abl oncogene addiction in malignant hematologic cells. Clin Cancer Res. 2012;18:1966–1978. doi: 10.1158/1078-0432.CCR-11-1971. [DOI] [PubMed] [Google Scholar]
- 100.Misra RN, Xiao HY, Kim KS, Lu S, Han WC, Barbosa SA, Hunt JT, Rawlins DB, Shan W, Ahmed SZ, Qian L, Chen BC, Zhao R, Bednarz MS, Kellar KA, Mulheron JG, Batorsky R, Roongta U, Kamath A, Marathe P, Ranadive SA, Sack JS, Tokarski JS, Pavletich NP, Lee FY, Webster KR, Kimball SD. Correction to N-(Cycloalkylamino)acyl-2-aminothiazole inhibitors of cyclin-dependent kinase 2. N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl] methyl] thio] -2-thiazolyl] -4-piperidineca rboxamide (BMS-387032), a highly efficacious and selective antitumor agent. J Med Chem. 2015;58:7609. doi: 10.1021/acs.jmedchem.5b01294. [DOI] [PubMed] [Google Scholar]
- 101.Kamath AV, Chong S, Chang M, Marathe PH. P-glycoprotein plays a role in the oral absorption of BMS-387032, a potent cyclin-dependent kinase 2 inhibitor, in rats. Cancer Chemother Pharmacol. 2005;55:110–116. doi: 10.1007/s00280-004-0873-3. [DOI] [PubMed] [Google Scholar]
- 102.Misra RN, Xiao HY, Kim KS, Lu S, Han WC, Barbosa SA, Hunt JT, Rawlins DB, Shan W, Ahmed SZ, Qian L, Chen BC, Zhao R, Bednarz MS, Kellar KA, Mulheron JG, Batorsky R, Roongta U, Kamath A, Marathe P, Ranadive SA, Sack JS, Tokarski JS, Pavletich NP, Lee FY, Webster KR, Kimball SD. N-(cycloalkylamino)acyl-2-aminothiazole inhibitors of cyclin-dependent kinase 2. N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide (BMS-387032), a highly efficacious and selective antitumor agent. J Med Chem. 2004;47:1719–1728. doi: 10.1021/jm0305568. [DOI] [PubMed] [Google Scholar]
- 103.Sorf A, Novotna E, Hofman J, Morell A, Staud F, Wsol V, Ceckova M. Cyclin-dependent kinase inhibitors AZD5438 and R547 show potential for enhancing efficacy of daunorubicin-based anticancer therapy: interaction with carbonyl-reducing enzymes and ABC transporters. Biochem Pharmacol. 2019;163:290–298. doi: 10.1016/j.bcp.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 104.DePinto W, Chu XJ, Yin X, Smith M, Packman K, Goelzer P, Lovey A, Chen Y, Qian H, Hamid R, Xiang Q, Tovar C, Blain R, Nevins T, Higgins B, Luistro L, Kolinsky K, Felix B, Hussain S, Heimbrook D. In vitro and in vivo activity of R547: a potent and selective cyclin-dependent kinase inhibitor currently in phase I clinical trials. Mol Cancer Ther. 2006;5:2644–2658. doi: 10.1158/1535-7163.MCT-06-0355. [DOI] [PubMed] [Google Scholar]
- 105.Chu XJ, DePinto W, Bartkovitz D, So SS, Vu BT, Packman K, Lukacs C, Ding Q, Jiang N, Wang K, Goelzer P, Yin X, Smith MA, Higgins BX, Chen Y, Xiang Q, Moliterni J, Kaplan G, Graves B, Lovey A, Fotouhi N. Discovery of [4-Amino-2-(1-methanesulfonylpiperidin-4-ylamino)pyrimidin-5-yl](2,3-difluoro-6-methoxyphenyl)methanone (R547), a potent and selective cyclin-dependent kinase inhibitor with significant in vivo antitumor activity. J Med Chem. 2006;49:6549–6560. doi: 10.1021/jm0606138. [DOI] [PubMed] [Google Scholar]
- 106.Caligiuri M, Becker F, Murthi K, Kaplan F, Dedier S, Kaufmann C, Machl A, Zybarth G, Richard J, Bockovich N, Kluge A, Kley N. A proteome-wide CDK/CRK-specific kinase inhibitor promotes tumor cell death in the absence of cell cycle progression. Chem Biol. 2005;12:1103–1115. doi: 10.1016/j.chembiol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 107.Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, LeClerc S, Meijer L, Kim SH, Lockhart DJ, Schultz PG. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science. 1998;281:533–538. doi: 10.1126/science.281.5376.533. [DOI] [PubMed] [Google Scholar]
- 108.Villerbu N, Gaben AM, Redeuilh G, Mester J. Cellular effects of purvalanol A: a specific inhibitor of cyclin-dependent kinase activities. Int J Cancer. 2002;97:761–769. doi: 10.1002/ijc.10125. [DOI] [PubMed] [Google Scholar]
- 109.Echalier A, Cot E, Camasses A, Hodimont E, Hoh F, Jay P, Sheinerman F, Krasinska L, Fisher D. An integrated chemical biology approach provides insight into Cdk2 functional redundancy and inhibitor sensitivity. Chem Biol. 2012;19:1028–1040. doi: 10.1016/j.chembiol.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 110.Raynaud FI, Whittaker SR, Fischer PM, McClue S, Walton MI, Barrie SE, Garrett MD, Rogers P, Clarke SJ, Kelland LR, Valenti M, Brunton L, Eccles S, Lane DP, Workman P. In vitro and in vivo pharmacokinetic-pharmacodynamic relationships for the trisubstituted aminopurine cyclin-dependent kinase inhibitors olomoucine, bohemine and CYC202. Clin Cancer Res. 2005;11:4875–4887. doi: 10.1158/1078-0432.CCR-04-2264. [DOI] [PubMed] [Google Scholar]
- 111.McMillin DW, Delmore J, Negri J, Buon L, Jacobs HM, Laubach J, Jakubikova J, Ooi M, Hayden P, Schlossman R, Munshi NC, Lengauer C, Richardson PG, Anderson KC, Mitsiades CS. Molecular and cellular effects of multi-targeted cyclin-dependent kinase inhibition in myeloma: biological and clinical implications. Br J Haematol. 2011;152:420–432. doi: 10.1111/j.1365-2141.2010.08427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bettayeb K, Tirado OM, Marionneau-Lambot S, Ferandin Y, Lozach O, Morris JC, Mateo-Lozano S, Drueckes P, Schachtele C, Kubbutat MH, Liger F, Marquet B, Joseph B, Echalier A, Endicott JA, Notario V, Meijer L. Meriolins, a new class of cell death inducing kinase inhibitors with enhanced selectivity for cyclin-dependent kinases. Cancer Res. 2007;67:8325–8334. doi: 10.1158/0008-5472.CAN-07-1826. [DOI] [PubMed] [Google Scholar]
- 113.Krystof V, Chamrad I, Jorda R, Kohoutek J. Pharmacological targeting of CDK9 in cardiac hypertrophy. Med Res Rev. 2010;30:646–666. doi: 10.1002/med.20172. [DOI] [PubMed] [Google Scholar]
- 114.Zaharevitz DW, Gussio R, Leost M, Senderowicz AM, Lahusen T, Kunick C, Meijer L, Sausville EA. Discovery and initial characterization of the paullones, a novel class of small-molecule inhibitors of cyclin-dependent kinases. Cancer Res. 1999;59:2566–2569. [PubMed] [Google Scholar]
- 115.Lyssiotis CA, Foreman RK, Staerk J, Garcia M, Mathur D, Markoulaki S, Hanna J, Lairson LL, Charette BD, Bouchez LC, Bollong M, Kunick C, Brinker A, Cho CY, Schultz PG, Jaenisch R. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci U S A. 2009;106:8912–8917. doi: 10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodland GE, Melhus K, Generalov R, Gilani S, Bertoni F, Dahle J, Syljuasen RG, Patzke S. The dual cell cycle kinase inhibitor JNJ-7706621 reverses resistance to CD37-targeted radioimmunotherapy in activated B cell like diffuse Large B cell lymphoma cell lines. Front Oncol. 2019;9:1301. doi: 10.3389/fonc.2019.01301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhong S, Wu B, Dong X, Han Y, Jiang S, Zhang Y, Bai Y, Luo SX, Chen Y, Zhang H, Zhao G. Identification of driver genes and key pathways of glioblastoma shows JNJ-7706621 as a novel antiglioblastoma drug. World Neurosurg. 2018;109:e329–e342. doi: 10.1016/j.wneu.2017.09.176. [DOI] [PubMed] [Google Scholar]
- 118.Guo Q, Jin L, Zhu HY, Xing XX, Xuan MF, Luo QR, Zhang GL, Luo ZB, Wang JX, Yin XJ, Kang JD. The cyclin-dependent kinase inhibitor, JNJ-7706621, improves in vitro developmental competence of porcine parthenogenetic activation and somatic cell nuclear transfer embryos. Reprod Fertil Dev. 2018;30:1002–1010. doi: 10.1071/RD17194. [DOI] [PubMed] [Google Scholar]
- 119.Matsuhashi A, Ohno T, Kimura M, Hara A, Saio M, Nagano A, Kawai G, Saitou M, Takigami I, Yamada K, Okano Y, Shimizu K. Growth suppression and mitotic defect induced by JNJ-7706621, an inhibitor of cyclin-dependent kinases and aurora kinases. Curr Cancer Drug Targets. 2012;12:625–639. doi: 10.2174/156800912801784839. [DOI] [PubMed] [Google Scholar]
- 120.Danhier F, Ucakar B, Magotteaux N, Brewster ME, Preat V. Active and passive tumor targeting of a novel poorly soluble cyclin dependent kinase inhibitor, JNJ-7706621. Int J Pharm. 2010;392:20–28. doi: 10.1016/j.ijpharm.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 121.Emanuel S, Rugg CA, Gruninger RH, Lin R, Fuentes-Pesquera A, Connolly PJ, Wetter SK, Hollister B, Kruger WW, Napier C, Jolliffe L, Middleton SA. The in vitro and in vivo effects of JNJ-7706621: a dual inhibitor of cyclin-dependent kinases and aurora kinases. Cancer Res. 2005;65:9038–9046. doi: 10.1158/0008-5472.CAN-05-0882. [DOI] [PubMed] [Google Scholar]
- 122.Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, Niederberger E, Tang W, Eisenbrand G, Meijer L. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 123.Heredia A, Davis C, Bamba D, Le N, Gwarzo MY, Sadowska M, Gallo RC, Redfield RR. Indirubin-3’-monoxime, a derivative of a Chinese antileukemia medicine, inhibits P-TEFb function and HIV-1 replication. AIDS. 2005;19:2087–2095. doi: 10.1097/01.aids.0000194805.74293.11. [DOI] [PubMed] [Google Scholar]
- 124.Leclerc S, Garnier M, Hoessel R, Marko D, Bibb JA, Snyder GL, Greengard P, Biernat J, Wu YZ, Mandelkow EM, Eisenbrand G, Meijer L. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 125.Byth KF, Thomas A, Hughes G, Forder C, McGregor A, Geh C, Oakes S, Green C, Walker M, Newcombe N, Green S, Growcott J, Barker A, Wilkinson RW. AZD5438, a potent oral inhibitor of cyclin-dependent kinases 1, 2, and 9, leads to pharmacodynamic changes and potent antitumor effects in human tumor xenografts. Mol Cancer Ther. 2009;8:1856–1866. doi: 10.1158/1535-7163.MCT-08-0836. [DOI] [PubMed] [Google Scholar]
- 126.Jones CD, Andrews DM, Barker AJ, Blades K, Daunt P, East S, Geh C, Graham MA, Johnson KM, Loddick SA, McFarland HM, McGregor A, Moss L, Rudge DA, Simpson PB, Swain ML, Tam KY, Tucker JA, Walker M. The discovery of AZD5597, a potent imidazole pyrimidine amide CDK inhibitor suitable for intravenous dosing. Bioorg Med Chem Lett. 2008;18:6369–6373. doi: 10.1016/j.bmcl.2008.10.102. [DOI] [PubMed] [Google Scholar]
- 127.Vermeulen K, Strnad M, Krystof V, Havlicek L, Van der Aa A, Lenjou M, Nijs G, Rodrigus I, Stockman B, van Onckelen H, Van Bockstaele DR, Berneman ZN. Antiproliferative effect of plant cytokinin analogues with an inhibitory activity on cyclin-dependent kinases. Leukemia. 2002;16:299–305. doi: 10.1038/sj.leu.2402378. [DOI] [PubMed] [Google Scholar]
- 128.Kitagawa M, Higashi H, Takahashi IS, Okabe T, Ogino H, Taya Y, Hishimura S, Okuyama A. A cyclin-dependent kinase inhibitor, butyrolactone I, inhibits phosphorylation of RB protein and cell cycle progression. Oncogene. 1994;9:2549–2557. [PubMed] [Google Scholar]
- 129.Meijer L, Thunnissen AM, White AW, Garnier M, Nikolic M, Tsai LH, Walter J, Cleverley KE, Salinas PC, Wu YZ, Biernat J, Mandelkow EM, Kim SH, Pettit GR. Inhibition of cyclin-dependent kinases, GSK-3beta and CK1 by hymenialdisine, a marine sponge constituent. Chem Biol. 2000;7:51–63. doi: 10.1016/s1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 130.Jessen BA, Lee L, Koudriakova T, Haines M, Lundgren K, Price S, Nonomiya J, Lewis C, Stevens GJ. Peripheral white blood cell toxicity induced by broad spectrum cyclin-dependent kinase inhibitors. J Appl Toxicol. 2007;27:133–142. doi: 10.1002/jat.1177. [DOI] [PubMed] [Google Scholar]
- 131.Chen P, Lee NV, Hu W, Xu M, Ferre RA, Lam H, Bergqvist S, Solowiej J, Diehl W, He YA, Yu X, Nagata A, VanArsdale T, Murray BW. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther. 2016;15:2273–2281. doi: 10.1158/1535-7163.MCT-16-0300. [DOI] [PubMed] [Google Scholar]
- 132.Mettey Y, Gompel M, Thomas V, Garnier M, Leost M, Ceballos-Picot I, Noble M, Endicott J, Vierfond JM, Meijer L. Aloisines, a new family of CDK/GSK-3 inhibitors. SAR study, crystal structure in complex with CDK2, enzyme selectivity, and cellular effects. J Med Chem. 2003;46:222–236. doi: 10.1021/jm020319p. [DOI] [PubMed] [Google Scholar]
- 133.Lahusen T, De Siervi A, Kunick C, Senderowicz AM. Alsterpaullone, a novel cyclin-dependent kinase inhibitor, induces apoptosis by activation of caspase-9 due to perturbation in mitochondrial membrane potential. Mol Carcinog. 2003;36:183–194. doi: 10.1002/mc.10114. [DOI] [PubMed] [Google Scholar]
- 134.Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, Gussio R, Senderowicz AM, Sausville EA, Kunick C, Meijer L. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur J Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- 135.Chang YT, Gray NS, Rosania GR, Sutherlin DP, Kwon S, Norman TC, Sarohia R, Leost M, Meijer L, Schultz PG. Synthesis and application of functionally diverse 2,6,9-trisubstituted purine libraries as CDK inhibitors. Chem Biol. 1999;6:361–375. doi: 10.1016/S1074-5521(99)80048-9. [DOI] [PubMed] [Google Scholar]
- 136.Kabadi SV, Stoica BA, Loane DJ, Luo T, Faden AI. CR8, a novel inhibitor of CDK, limits microglial activation, astrocytosis, neuronal loss, and neurologic dysfunction after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2014;34:502–513. doi: 10.1038/jcbfm.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bettayeb K, Oumata N, Echalier A, Ferandin Y, Endicott JA, Galons H, Meijer L. CR8, a potent and selective, roscovitine-derived inhibitor of cyclin-dependent kinases. Oncogene. 2008;27:5797–5807. doi: 10.1038/onc.2008.191. [DOI] [PubMed] [Google Scholar]
- 138.Dey J, Deckwerth TL, Kerwin WS, Casalini JR, Merrell AJ, Grenley MO, Burns C, Ditzler SH, Dixon CP, Beirne E, Gillespie KC, Kleinman EF, Klinghoffer RA. Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk Diffuse Large B-cell Lymphoma to BCL2 inhibition. Sci Rep. 2017;7:18007. doi: 10.1038/s41598-017-18368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gupta S, Jain MM, Maru A, Nag SM, Somani N, Mehta AO, Kulkarni S, Acharya SA, Dhobe P, Jadhav N. A phase I study of selective cyclin dependent kinase inhibitor P1446A-05 administered on an intermittent schedule in patients with advanced refractory tumors. J. Clin. Oncol. 2012;30:3011–3011. [Google Scholar]
- 140.Hao D, Chu Q, Welch S, Yau CYF, Spratlin JL, Tang P, MacKenzie MJ, Townsley CA, Arndt D, Johnson L, Trapsa D, Degendorfer P, Kulkarni S, Jawlekar G, Dhobe P, Oza AM. A phase I and pharmacokinetic (PK) study of continuous daily administration of P1446A-05, a potent and specific oral Cdk4 inhibitor. J. Clin. Oncol. 2012;30:3013–3013. [Google Scholar]
- 141.Paiva C, Godbersen JC, Soderquist RS, Rowland T, Kilmarx S, Spurgeon SE, Brown JR, Srinivasa SP, Danilov AV. Cyclin-dependent kinase inhibitor P1446A induces apoptosis in a JNK/p38 MAPK-dependent manner in chronic lymphocytic leukemia B-Cells. PLoS One. 2015;10:e0143685. doi: 10.1371/journal.pone.0143685. [DOI] [PMC free article] [PubMed] [Google Scholar]