Abstract
Nivolumab monotherapy has a modest objective response rate (ORR) in hepatocellular carcinoma (HCC). To overcome the lack of biomarkers that predict delayed alpha-fetoprotein (AFP) response beyond 4 weeks, we applied a novel 50-10 rule of AFP response for unresectable HCC patients under nivolumab monotherapy and proposed an algorithm based on on-treatment AFP reduction at different time-points. Ninety unresectable HCC patients who underwent nivolumab monotherapy in 2015-2019 were retrospectively recruited and divided into four classes: rapid AFP decrease of ≥ 50% of baseline at week 4 (class I), AFP changes within ± 50% of baseline at week 4 that later decreased to ≥ 10% of baseline (class II) or not (class III) at week 12, and rapid AFP increase of ≥ 50% of baseline at week 4 (class IV). ORR was 47.4%, 36.0%, 7.7%, and 5.0% in class I-IV patients, respectively. Rapid (class I) and delayed (class II) AFP responders had significantly higher ORR, overall survival (OS) and progression-free survival (PFS) than non-responders (class III and IV) (ORR: 40.9% vs. 6.5%, P<0.001; median OS: not reached vs. 9.6 months, log-rank P<0.001; median PFS: 9.6 vs. 2.8 months, log-rank P<0.001). In multivariate analysis, AFP response was an independent factor associated with good OS (hazard ratio [HR]=0.301, P=0.001) and PFS (HR=0.332, P<0.001). Moreover, AFP responders had higher ORR and better OS as well as PFS than non-responders, regardless of nivolumab as a first- or more than a second-line therapy (all P<0.05). In conclusion, the novel 50-10 rule of AFP response provides practical guidance for nivolumab monotherapy in unresectable HCC patients. However, this algorithm remains to be verified in a large prospective cohort.
Keywords: Immunotherapy, AFP response, overall survival, progression-free survival
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related deaths worldwide [1]. However, patients are often diagnosed with HCC at an advanced stage, where only systemic treatment can be offered [2,3]. Sorafenib was the first effective drug available for this unresectable liver cancer [4,5], showing survival benefits in two pivotal phase III clinical trials [6,7]. Currently, there are multiple options of first- [7-9] and second-line systemic therapies [10-14] for patients with unresectable HCC. Nivolumab is an anti-programmed cell death-1 (PD-1) antibody and an immune checkpoint inhibitor (ICI) [13]. This drug is recommended by current guidelines as an option of second-line systemic therapy [2,15,16], and has a marginal benefit as a first-line systemic therapy [17]. Although the objective response rate (ORR) of nivolumab is only 15-20%, it shows durable clinical benefits. Unfortunately, there is currently no useful biomarker to identify patients who will respond to nivolumab therapy before treatment initiation [2].
Alpha-fetoprotein (AFP) is a glycoprotein expressed and secreted by HCC cells in approximately 70% of HCC patients [15]. In patients receiving various systemic therapies, a decline in serum AFP level after treatment is associated with tumor response [18-20]. Previous studies showed that AFP response, defined as a ≥ 20% decline in serum AFP level within the first 4 weeks of treatment initiation relative to pretreatment levels, can be associated with objective tumor response in patients who either received antiangiogenic therapy [21], sorafenib [22] or ICI [23]. Lee et al [24] further suggested a 10-10 rule, in which an AFP reduction ≥ of 10% within 4 weeks represents a better indicator of tumor response than AFP reduction >20%. These studies provided insight for developing a method to identify potential responders early after the initiation of ICI treatment. However, it remains unclear whether this method applies to unresectable HCC patients with nivolumab monotherapy or whether it can predict the prognosis of patients with delayed AFP response beyond the first 4 weeks. In this retrospective observational study, we not only verified AFP response by using AFP decline ≥ 10% from baseline at week 4 and 12 as a criterion but also further applied a novel 50-10 rule of AFP response for patients with unresectable HCC under nivolumab monotherapy. We proposed a novel treatment algorithm based on AFP responses at week 4 and 12 to guide the initiation of nivolumab monotherapy for patients with HCC.
Patients and methods
Patient recruitment
A total of 122 patients who received nivolumab monotherapy for unresectable HCC at our hospital, from 2015 to 2019 were retrospectively reviewed. We included patients who received nivolumab monotherapy for HCC as first or more than a second-line systemic therapy because their liver tumor was in Barcelona Clinic Liver Cancer (BCLC) stage C or was not amenable to locoregional therapy in BCLC stage B. None of these patients were enrolled in previous or ongoing ICI clinical trials. Patients who were liver preserved function as Child-Pugh class C, lost to follow-up or had no radiological evaluation, had no available AFP data, had ICI as an adjuvant therapy after curative ablation, had a malignancy other than HCC, undergone liver transplantation, and human immunodeficiency virus infection were excluded. Finally, 90 patients with complete medical records were included in the analysis. Administration of nivolumab was based on the recommended dosing and safety information (2-3 mg/kg every 2 weeks until tumor progression or intolerance) [13]. Safety assessment and grading were performed based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE; version 4.03). The Institutional Review Board at our hospital approved this study and the review board waived signed informed consent owing to the retrospective nature of the study.
Diagnosis of hepatocellular carcinoma and follow-up protocol
Diagnosis of HCC was based on the European Association for the Study of the Liver/European Organization for Research and Treatment of Cancer (EASL/EORTC) diagnostic guidelines [2]. We monitored HCC status by dynamic CT or MRI every 8-12 weeks, and measured the serum AFP levels at week 4, 12, and post nivolumab monotherapy. AFP level before treatment was measured within 14 days before treatment initiation. We defined a novel 50-10 rule, which consisted of four classes: a rapid decrease in AFP response of ≥ 50% of baseline at week 4 (class I), an AFP change within ± 50% of baseline at week 4 that later declined to ≥ 10% of baseline (class II) or not (class III) at week 12, and a rapid increase in AFP of ≥ 50% of baseline at week 4 (class IV). Class I and II were AFP responders, whereas classes III and IV were AFP non-responders (Figure 1).
Figure 1.
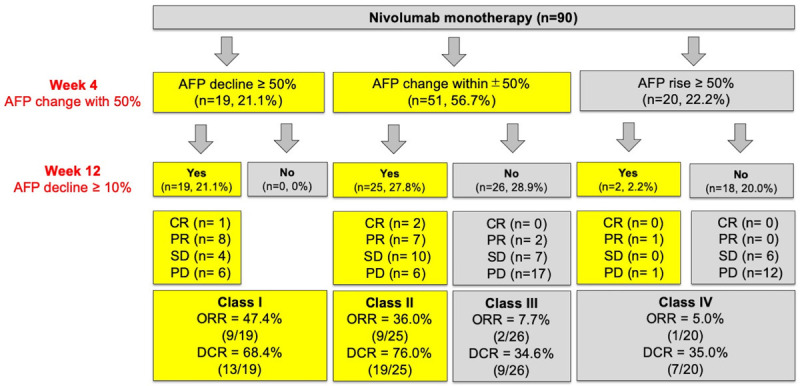
Four classifications by the 50-10 rule. Class I was defined as AFP response rapidly declined ≥ 50% of baseline at week 4. Class II and III were defined as AFP changes within the range of ± 50% at week 4, followed by AFP decline ≥ 10% of baseline (class II) or not (class III) at week 12. Class IV was defined as AFP rapidly rising by ≥ 50% of baseline at week 4.
Tumor response was assessed according to the revised Response Evaluation Criteria in Solid Tumors version 1.1 (RECISTv1.1) [25]: complete response (CR), defined as disappearance of all target lesions; partial response (PR), defined as at least a 30% decrease in the sum of the diameters of target lesions; progressive disease (PD), defined as at least a 20% increase in the sum of the diameters of target lesions; and stable disease (SD), defined as neither PR nor PD.
Statistical analysis and definitions
Descriptive data with normal distribution are reported as mean ± standard deviation or as percentage; otherwise, they are presented as median (range). We used independent Student’s t-test and Mann-Whitney U test to assess differences between groups for variables that showed normal and abnormal distribution, respectively. Chi-square test was applied to assess differences between two groups for categorical variables. Two-tailed P value of <0.05 was considered statistically significant.
Progression-free survival (PFS) was defined as the time from the date of the first nivolumab administration until radiological disease progression or death, whichever came first. We censored the patients at the date of the last contact or data cutoff for patients who were still alive without radiologically confirmed progression. Overall survival (OS) was calculated from the start of nivolumab treatment until the date of death. Patients who were still alive were censored at the date of the last contact or data cutoff. Survival curves were calculated using the Kaplan-Meier method and compared using log-rank test. Predictive factors of PFS and OS were determined using a Cox regression model. Statistical analyses were performed using the SAS version 9.4 and SPSS software, version 20.0 (SPSS, Inc., Chicago, IL).
Results
Patient data
A total of 90 patients who received nivolumab monotherapy for unresectable HCC were included in the study of AFP response. The baseline characteristics are shown in Table 1. The median AFP level was 466.7 ng/mL, and 14.4% of the patients had low AFP levels (<10 ng/mL) at baseline. Forty (44.4%) patients died during a median observation period of 8.7 (3.1-49.1) months. The median OS and PFS were 16.3 and 4.7 months, respectively.
Table 1.
Baseline characteristics of enrolled patients
| Variables | No. of patients (N=90) | % | |
|---|---|---|---|
| Age (years-old) | 61.4 (26.3-86.0) | 100 | |
| Male gender | 68 | 75.6 | |
| Etiology | |||
| HBV/HCV/NBNC | 59/17/14 | 65.6/18.9/15.6 | |
| Child-Pugh A/B | 76/14 | 84.4/15.6 | |
| ALBI grade I/II/III | 39/48/3 | 43.3/53.3/3.3 | |
| Portal vein thrombosis | |||
| 0/Vp1,2/Vp3,4 | 48/13/29 | 53.3/14.4/32.2 | |
| Extra-hepatic metastasis | 54 | 60.0 | |
| AFP (ng/ml) | 466.7 (2.0-1043407.0) | 100 | |
| <10/10-400/≥ 400 | 13/30/47 | 14.4/30.3/52.2 | |
| BCLC stage B/C | 16/74 | 17.8/82.2 | |
| Lines of systemic therapy | |||
| 1/2/>2 | 37/43/10 | 41.1/47.8/11.1 | |
| Prior locoregional therapy | 71 | 78.9 | |
| Resection/RFA or TACE | 27/44 | 38.0/62.0 | |
| Previous Sorafenib history | 47 | 52.2 | |
| Post PD therapy | 32 | 35.6 | |
| Sorafenib/Chemotherapy | 17 | 53.1 | |
| Regorafenib/Lenvatinib/Carbozantinib | 7 | 21.9 | |
| Atezolizumab plus Bevacizumab | 1 | 3.1 | |
| Others (RT/TACE/Resection) | 7 | 21.9 | |
| Overall IrAE | Any grade | Grade ≥ 3 | |
| Skin rash | 24 | 0 | 26.7/0 |
| Hepatitis | 23 | 7 | 25.6/7.8 |
| Colitis | 1 | 0 | 1.1/0 |
| Pneumonitis | 5 | 1 | 5.6/1.1 |
| Hypothyroidism | 6 | 0 | 6.7/0 |
| Adrenal insufficiency | 2 | 0 | 2.2/0 |
| Mortality | 40 | 44.4 | |
Abbreviations: AFP, alpha-fetoprotein; ALBI, albumin-bilirubin index; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; IrAE, immune related adverse effect; NBNC, non hepatitis B and C virus; PD, progression disease; RFA, radiofrequency ablation; RT, radiotherapy; TACE, transarterial chemoembolization.
The overall ORR and disease control rate (DCR) were 23.3% (21/90) and 53.3% (48/90) of the entire cohort, respectively. Patients with objective response had significantly better OS and PFS (median OS: 39.9 vs. 11.5 months, log-rank P=0.001; median PFS: 19.4 vs. 3.6 months, log-rank P<0.001). A total of 72 patients had available first tumor image assessment results within 12 weeks after starting nivolumab monotherapy. Among them, 13 (18.1%) patients had PR and 37 (51.4%) had SD at the initial image assessment.
AFP decrease at week 4 and 12
Forty-seven (52.2%) patients had higher baseline AFP level (≥ 400 ng/ml) before treatment but did not have statistically significant lower ORR than those patients with lower AFP level (<400 ng/ml) (21.3% vs. 25.6%, P=0.630) that baseline AFP level is not associated with response prediction. On the other hand, 34 (37.8%) and 46 (51.1%) patients had an AFP decline of ≥ 10% at week 4 and 12, respectively. Patients with AFP levels decline of ≥ 10% at week 4 and 12 showed significantly higher ORR (week 4: 44.1% vs. 10.7%, P<0.001; week 12: 41.3% vs. 4.5%, P<0.001). Of the 13 patients with baseline AFP <10 ng/ml, a decline of ≥ 10% also showed a trend of higher ORR, although this trend was not statistically significant (week 4: 50.0% vs. 22.2%, P=0.317; week 12: 33.3% vs. 28.6%, P=0.853). There were 18 patients with inconsistent AFP responses between week 4 and 12: three patients initially showed response at week 4, but later became non-respondent at week 12; all these patients died of tumor progression. Another 15 patients had delayed AFP response at week 12: 5 (33.3%) of which had objective image response, and 14 of which (93.3%) were alive until the last follow-up. Comparison of OS between AFP responders and non-responders showed that AFP responders showed no significantly longer OS at week 4 (median OS: 17.1 vs. 14.7 months, log-rank P=0.338) (Figure S1A), but showed significantly longer OS at week 12 (median OS: not reached vs. 7.7 months, log-rank P<0.001, Figure S1B).
The 50-10 rule of AFP response
There were 19 (21.1%), 25 (27.8%), 26 (28.9%), and 20 (22.2%) patients in class I to IV, respectively, according to the AFP response at week 4 and 12 (Figure 1). Class I and II included three patients with CR and 15 patients with PR, whereas class III and IV only had one PR patient. Rapid increases or decreases in AFP of beyond 50% at week 4 were highly associated with AFP response at week 12, and significantly correlated with ORR (class I vs. IV, P<0.001, Figure 1). Patients in class I had the best ORR, followed by class II, III, and IV (47.4%, 36.0%, 7.7%, and 5.0%, P<0.001, Figure 1). Patients in class I and II showed significantly longer median OS than those in class III and VI (OS: not reached and not reached vs. 11.5 and 7.4 months, log-rank P=0.001, Figure 2A). Similarly, patients in class I and II also had longer PFS than those in class III and IV (median: 9.7 and 9.6 vs. 3.1 and 2.6 months, log-rank P<0.001, Figure 2B). When further comparing with class III, patients in class II also had longer OS (median: not-reached vs. 11.5 months, log-rank P=0.022) and PFS (median: 9.6 vs. 3.1 months, log-rank P=0.004). Baseline pretreatment AFP response in class I and II also served as a favorable independent factor of OS (aHR =0.301, P=0.001; Table 2) and PFS (aHR =0.332, P<0.001; Table 3), as determined by Cox regression in multivariate analysis.
Figure 2.
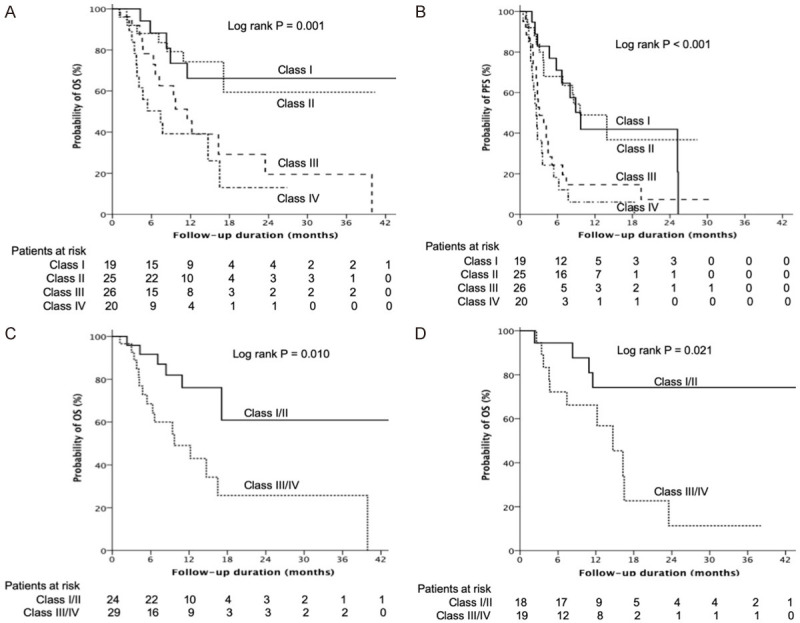
Kaplan-Meier curves. Comparison of (A) overall survival (OS) among the four classes, (B) progression-free survival (PFS) among the four classes, (C) OS between class I/II and III/IV patients who received nivolumab as more than a second-line therapy, and (D) OS between class I/II and III/IV patients with stable disease on the initial image assessment.
Table 2.
Cox’s proportional hazards model for predictors of overall survival
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| AFP 50-10 rule (class I/II vs. class III/IV) | 0.296 | 0.150-0.583 | <0.001 | 0.301 | 0.156-0.614 | 0.001 |
| Age ≥ 60 y/o (vs. <60 y/o) | 1.698 | 0.900-3.205 | 0.102 | |||
| Male (vs. female) | 1.935 | 0.930-4.026 | 0.078 | |||
| Viral infection (vs. others) | 1.958 | 0.765-5.014 | 0.161 | |||
| ALBI grade I (vs. II/III) | 0.573 | 0.298-0.902 | 0.045 | 0.656 | 0.340-1.266 | 0.208 |
| Platelet count ≥ 100K (vs. <100K) | 0.811 | 0.410-1.603 | 0.546 | |||
| Portal vein thrombosis (Vp3/4 vs. Vp1/2) | 1.084 | 0.555-2.120 | 0.813 | |||
| Extrahepatic metastasis (vs. No) | 1.224 | 0.637-2.353 | 0.544 | |||
| AFP ≥ 400 ng/ml (vs. <400 ng/ml) | 1.390 | 0.740-2.611 | 0.306 | |||
| BCLC stage B (vs. C) | 0.979 | 0.404-2.372 | 0.963 | |||
| First line (vs. second line or later) | 1.275 | 0.683-2.378 | 0.445 | |||
| First objective response (vs. No) | 0.505 | 0.195-1.312 | 0.161 | |||
| IrAE (vs. No) | 1.817 | 0.892-3.698 | 0.100 | |||
Abbreviations: AFP, alpha-fetoprotein; ALBI, albumin-bilirubin index; BCLC, Barcelona Clinic Liver Cancer; IrAE, Immunotherapy related adverse events.
Table 3.
Cox’s proportional hazards model for predictors of progression free survival
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| AFP 50-10 rule (class I/II vs. class III/IV) | 0.311 | 0.185-0.524 | <0.001 | 0.332 | 0.194-0.567 | <0.001 |
| Age ≥ 60 y/o (vs. <60 y/o) | 1.255 | 0.762-2.068 | 0.372 | |||
| Male (vs. female) | 1.429 | 0.812-2.514 | 0.216 | |||
| Viral infection (vs. others) | 1.801 | 0.905-3.584 | 0.094 | |||
| ALBI grade I (vs. II/III) | 0.471 | 0.276-0.801 | 0.005 | 0.518 | 0.302-0.888 | 0.017 |
| Platelet count ≥ 100 K (vs. <100 K) | 0.716 | 0.417-1.230 | 0.226 | |||
| Portal vein thrombosis (Vp3/4 vs. Vp1/2) | 1.235 | 0.549-2.777 | 0.610 | |||
| Extrahepatic metastasis (vs. No) | 1.081 | 0.654-1.787 | 0.761 | |||
| AFP ≥ 400 ng/ml (vs. <400 ng/ml) | 1.535 | 0.929-2.535 | 0.094 | |||
| BCLC stage B (vs. C) | 0.972 | 0.504-1.875 | 0.933 | |||
| First-line (vs. second-line) | 0.866 | 0.524-1.430 | 0.574 | |||
| First objective response (vs. No) | 0.438 | 0.204-0.939 | 0.034 | 0.443 | 0.204-0.960 | 0.039 |
| IrAE (vs. No) | 1.548 | 0.942-2.544 | 0.085 | |||
Abbreviations: AFP, alpha-fetoprotein; ALBI, albumin-bilirubin index; BCLC, Barcelona Clinic Liver Cancer; IrAE, Immunotherapy related adverse events.
For predicting OS, PFS and ORR, respectively, the assessment by the 50-10 rule rather than only AFP ≥ 50% reduction at week 4, ≥ 10% reduction at week 4 or week 12 had best sensitivity (66.0% vs. vs. 28.0% vs. 40.0% vs. 64.0%, 76.9% vs. 30.8% vs. 50.0% vs.76.9%, 90.5% vs. 42.9% vs. 71.4% vs. 85.7%), specificity (70.0% vs. 87.5% vs. 65.0% vs. 67.5%, 67.2% vs. 82.8% vs. 62.5% vs. 59.4%, 72.5% vs. 85.5% vs. 62.3% vs. 60.9%), positive predictive value (PPV) (72.7% vs. 73.4% vs. 58.8% vs. 71.7%, 45.5% vs. 42.1% vs. 29.5% vs.43.5%, 40.9% vs. 47.4% vs. 44.1% vs. 41.3%) and negative predictive value (NPV) (61.4% vs. 49.3% vs. 46.4% vs. 60.9%, 87.0 vs. 74.6% vs. 79.6% vs. 86.4%, and 95.5% vs. 83.1% vs. 89.3% vs. 93.5%) (Table 4).
Table 4.
Sensitivity, specificity, positive predictive value, and negative predictive value of different AFP response assessments for predicting overall survival, progression free survival and objective response rate
| Variables | 50-10 rule at week 4 & 12 | 50% reduction at week 4 | 10% reduction at week 4 | 10% reduction at week 12 |
|---|---|---|---|---|
| OS | ||||
| Sensitivity | 66.0 | 28.0 | 40.0 | 64.0 |
| Specificity | 70.0 | 87.5 | 65.0 | 67.5 |
| PPV | 72.7 | 73.4 | 58.8 | 71.7 |
| NPV | 61.4 | 49.3 | 46.4 | 60.9 |
| PFS | ||||
| Sensitivity | 76.9 | 30.8 | 50.0 | 76.9 |
| Specificity | 67.2 | 82.8 | 62.5 | 59.4 |
| PPV | 45.5 | 42.1 | 29.5 | 43.5 |
| NPV | 87.0 | 74.6 | 79.6 | 86.4 |
| ORR | ||||
| Sensitivity | 90.5 | 42.9 | 71.4 | 85.7 |
| Specificity | 72.5 | 85.5 | 62.3 | 60.9 |
| PPV | 40.9 | 47.4 | 44.1 | 41.3 |
| NPV | 95.5 | 83.1 | 89.3 | 93.5 |
Abbreviations: NPV, negative predictive value; ORR, objective response rate; OS, overall survival; PFS, progression free survival; PPV, positive predictive value.
Subgroup analysis using the 50-10 rule
A total of 53 patients received nivolumab as more than a second-line therapy. ORR was found in 10 of 24 patients (41.7%) in class I and II, and only 2 of 29 patients (6.9%) had an image response in class III and IV. Patients in class I and II had significantly better ORR than those in class III and IV (P=0.003). Patients in class I and II also had better DCR (70.8% vs. 31.0%, P=0.004), longer median PFS (9.6 vs. 2.8 months, log-rank P=0.001), and superior median OS (not reached vs. 9.7 months, log-rank P=0.010, Figure 2C) compared with their counterparts. In patients treated with nivolumab as a first-line therapy, this 50-10 rule also showed similar results of better ORR, DCR, PFS, and OS in class I and II patients than in class III and IV (ORR: 40.0% vs. 5.9%, P=0.016; DCR: 75.0% vs. 41.2%, P=0.037; median PFS: 9.7 vs. 3.6 months, log-rank P=0.001; median OS: not reached vs. 7.4 months, log-rank P=0.004).
In addition, a total of 37 patients showed SD on the initial image evaluation, including 18 (48.6%) patients in class I and II. Six patients had an image response later, all of which were in class I and II and not in class III and IV (33.3% vs. 0%, P=0.008). Patients with initial SD in class I and II (n=18) had significantly longer PFS (13.9 vs. 3.6 months, log-rank P=0.001) and OS (not reached vs. 14.7 months, log-rank P=0.021) (Figure 2D) than those in class III and IV.
Discussion
In this study, we propose a novel 50-10 rule algorithm based on AFP response at two time points, to include delayed AFP responders. This method can be used to select unresectable HCC patients who will benefit from nivolumab monotherapy with higher ORR. Patients selected by the 50-10 rule also showed superior OS and longer PFS.
There are currently no useful pretreatment biomarkers to predict the image response of unresectable HCC patients undergoing ICI therapy [2,13,14]. Although PD-L1 expression in immune or tumor cells has good association with image response for several cancer types [26-28], the predictive ability of treatment response targeting PD-L1 expression in HCC is restricted to certain HCC variants [29]. AFP response can be associated with ORR in patients who received various systemic therapies including chemotherapy [21], target therapy [22] and recent ICI treatment [23,24]. However, whether we use AFP ≥ 20% decline within 4 weeks conducted by Shao et al [23] or 10-10 rule (baseline AFP ≥ 10 ng/ml and reduction ≥ of 10% within 4 week) conducted by Lee et al [24], these studies enrolled patients only in clinical trials [23] or treated with ICI combined with other therapies [24], which may have led to higher ORR and DCR. Our results supported the association between AFP response and ORR at week 4 according to the 10-10 rule conducted by Lee et al [24]. However, association of AFP response with OS was observed at week 12 only, and not at week 4. The inconsistency between AFP response at week 4 and the ability to predict OS might be due to the delayed response to AFP under monotherapy, and delayed responders could still have an excellent prognosis. Delayed response is rarely reported in previous studies probably because the enhanced anticancer activity of ICI combination therapy resulted in rapid AFP response, which masked the delayed response. Therefore, rapid AFP response at week 4 might not be suitable for predicting the prognosis of patients with HCC only receiving only nivolumab monotherapy. In the current real-world study with HCC patients only receiving nivolumab as monotherapy enrolled, we found that AFP level ≥ 50% rather than 10% reduction at week 4 had better specificity and AFP level ≥ 10% reduction at week 12 rather than week 4 had better sensitivity in predicting OS, PFS and ORR. Therefore, we further conducted a novel 50-10 rule of AFP response evaluated at two time-points (50% AFP level change at week 4 and 10% AFP level change from baseline at week 12, respectively), including 4 classes provides practical guidance for nivolumab monotherapy in unresectable HCC patients especially for those delayed AFP response beyond 4 weeks.
According to the novel 50-10 rule, patients in class I or IV showed a rapid change in AFP level exceeding 50% of the baseline at week 4. The AFP and overall image responses were highly consistent at weeks 4 and 12, which can help predict prognosis earlier at week 4. However, patients with a ± 50% AFP change at week 4 had an uncertain prognosis, as some patients showed delayed AFP response. AFP decline of ≥ 10% at week 12 may be used to predict prognosis; thus, patients with this decline were subdivided into class II and III. AFP responses according to the 50-10 rule was highly consistent with ORR, and could serve as an independent predictor for OS and PFS in multivariate analysis. Therefore, a therapeutic algorithm could be developed using the novel 50-10 rule of AFP response for patients receiving nivolumab monotherapy (Figure 3). Patients in class I and II tended to show objective image response to nivolumab monotherapy, demonstrating the potential for receiving treatment owing to the high ORR and excellent outcomes. On the contrary, for patients in class III and IV, image response should be assessed earlier and treatment policy should be modified owing to the lower possibility of image response. A total of 18 patients in class II had available first tumor image assessment results within 12 weeks after starting nivolumab monotherapy. Although 13 patients showed non-response in initial image evaluation, all the patients kept nivolumab therapy and they still had high ORR (23.1%) and DCR (76.9%) if achieving delayed AFP response at week 12.
Figure 3.
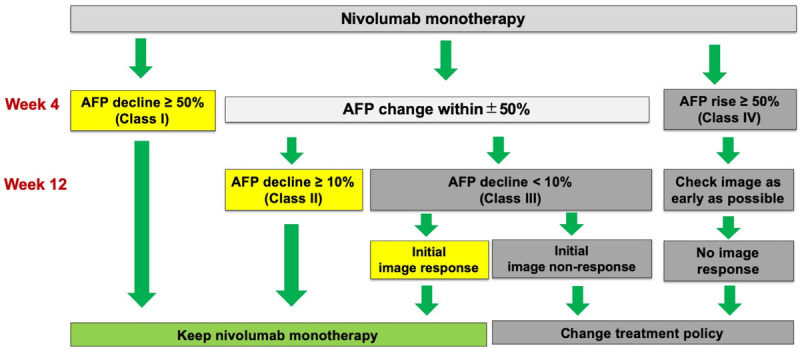
Algorithm of treatment decision based on alfa-fetoprotein response according to the 50-10 rule.
Currently, there are many options of systemic treatment for unresectable HCC [2,16,28,30]. Nivolumab has been approved as a second-line treatment for HCC according to recently published guidelines, and may serve as an alternative to a first-line therapy in real-world practice. However, the ORR of nivolumab monotherapy is only 15-20% [13,17]. Combination therapies with ICI plus vascular endothelial growth factor antagonists such as atezolizumab plus bevacizumab [31], ICI plus tyrosine kinase inhibitors such as pembrolizumab plus lenvatinib [32], or combination therapy with two ICIs such as nivolumab plus ipilimumab [33] can provide >30% ORR. Nevertheless, combination therapies require higher medical costs and probably pose higher risks of drug toxicity [28]. Therefore, nivolumab is preferred over combination therapies, as it has similar ORR and overall survival when compared to combination therapies, but lower medical cost and possibly lower risk of high-grade adverse effects. The ORR of patients who received nivolumab monotherapy in our study was consistent with that in clinical trials. Thus, we propose that the 50-10 rule of AFP response can be used to select potential nivolumab responders among class I and II patients.
The 50-10 rule of AFP response has good discriminative ability for patients receiving nivolumab monotherapy either as a first-line or more than a second-line therapy. According to the 50-10 rule of AFP response, class I and II patients are those with an ORR above 40% both in the first- and more than a second-line therapy. Furthermore, class I and II patients, who showed SD at the initial image examination after the first 12 weeks of nivolumab monotherapy, are recommended to maintain nivolumab monotherapy according to the 50-10 rule of AFP response, as the ORR may be higher than 30% and the cumulative 2-year OS rate may exceed 70%.
However, this study has several limitations. First, this was a retrospective study, in which several patients received the first image assessment beyond the first 12 weeks, and only 72 patients had the first image evaluation within the first 12 weeks. Second, most of our patients (65.6%) had chronic hepatitis B virus infection as the underlying hepatic disease. Our results should be interpreted with caution when investigating other populations. Third, only 14.4% of patients with baseline AFP levels below 10 ng/mL were enrolled in our study. Generally, up to 30% of HCC patients have low AFP level at the time of diagnosis, even those with advanced HCC [34] and the level usually remain low during treatment. The small number of enrolled patients with low baseline AFP levels may indicate that pretreatment AFP level has a limited role in predicting the prognosis of patients who received nivolumab therapy for unresectable HCC. Although patients with a baseline AFP level lower than 10 ng/mL with a 10% reduction still have a similar trend in ORR, caution should be exercised when applying the 50-10 rule in these patients owing to the small sample size and possible amplification of laboratory errors.
In conclusion, the novel 50-10 rule of AFP is a useful tool for predicting the prognosis of patients who received nivolumab monotherapy and those with AFP delayed response. A rapid decline in AFP level of more than 50% from baseline at week 4 is a predictor of good prognosis. Patients with AFP change at week 4 within ± 50% from baseline should be checked for AFP level at week 12 to help predict prognosis. Besides, the 50-10 rule of AFP response could serve as a practical guidance to determine patients who will benefit from nivolumab monotherapy as first- or more than a second-line treatment for unresectable HCC or those who should shift early to combined therapy if feasible. It could also guide the treatment of patients who had SD at the initial image assessment within the first 12 weeks of nivolumab monotherapy. This recommendation, however, still needs to be validated in a larger prospective cohort.
Acknowledgements
The authors thank all the members of the Cancer Center, Chang Gung Memorial Hospital, for their invaluable help including Ching-Ting Wang and Hsiu-Ying Chai. The Linkou Chang-Gung Memorial Hospital Institutional Review Board approved this study (IRB number: 202000764B0). This study was supported by grants from Chang Gung Medical Research Fund (CMRPG3J1341, CORPG3G0871, CORPG3H0641, CORPG3H0651, CORPG3H0661, CORPG3H0671), National Science Council, Taiwan (NMRPG3H0471).
Disclosure of conflict of interest
None.
Abbreviations
- AFP
alpha-fetoprotein
- ALBI
albumin-bilirubin index
- BCLC
Barcelona Clinic Liver Cancer
- CR
complete response
- ER
early responder
- ECOG
Eastern Cooperative Oncology Group
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ICI
immune checkpoint inhibitor
- IrAE
immune-related adverse effect
- NBNC
non-hepatitis B and C virus
- PD
progressive disease
- PR
partial response
- RR
rapid responder
- RT
radiotherapy
- SD
stable disease
Supporting Information
References
- 1.Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:871–873. doi: 10.1093/annonc/mdy510. [DOI] [PubMed] [Google Scholar]
- 4.Lin SM, Lu SN, Chen PT, Jeng LB, Chen SC, Hu CT, Yang SS, Le Berre MA, Liu X, Mitchell DY, Prins K, Grevel J, Pena CA, Meinhardt G. HATT: a phase IV, single-arm, open-label study of sorafenib in Taiwanese patients with advanced hepatocellular carcinoma. Hepatol Int. 2017;11:199–208. doi: 10.1007/s12072-016-9774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao YY, Wang SY, Lin SM Diagnosis Group; Systemic Therapy Group. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2021;120:1051–1060. doi: 10.1016/j.jfma.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 9.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klumpen HJ. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 13.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 16.Vogel A, Cervantes A, Chau I, Daniele B, Llovet J, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E, Committee EG. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–iv255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 17.Yau T, Hsu C, Kim TY, Choo SP, Kang YK, Hou MM, Numata K, Yeo W, Chopra A, Ikeda M, Kuromatsu R, Moriguchi M, Chao Y, Zhao H, Anderson J, Cruz CD, Kudo M. Nivolumab in advanced hepatocellular carcinoma: sorafenib-experienced Asian cohort analysis. J Hepatol. 2019;71:543–552. doi: 10.1016/j.jhep.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, Lam KC, Chan AT, Mok TS, Yeo W. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J. Clin. Oncol. 2009;27:446–452. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 19.Chen LT, Liu TW, Chao Y, Shiah HS, Chang JY, Juang SH, Chen SC, Chuang TR, Chin YH, Whang-Peng J. alpha-fetoprotein response predicts survival benefits of thalidomide in advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2005;22:217–226. doi: 10.1111/j.1365-2036.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- 20.Vora SR, Zheng H, Stadler ZK, Fuchs CS, Zhu AX. Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist. 2009;14:717–725. doi: 10.1634/theoncologist.2009-0038. [DOI] [PubMed] [Google Scholar]
- 21.Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH, Cheng AL. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116:4590–4596. doi: 10.1002/cncr.25257. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Kim BK, Kim SU, Park JY, Kim do Y, Ahn SH, Han KH. Early alpha-fetoprotein response predicts survival in patients with advanced hepatocellular carcinoma treated with sorafenib. J Hepatocell Carcinoma. 2015;2:39–47. doi: 10.2147/JHC.S79353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao YY, Liu TH, Hsu C, Lu LC, Shen YC, Lin ZZ, Cheng AL, Hsu CH. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39:2184–2189. doi: 10.1111/liv.14210. [DOI] [PubMed] [Google Scholar]
- 24.Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC, Chen SC, Hou MC, Huang YH. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (Basel) 2020;12:182. doi: 10.3390/cancers12010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72:307–319. doi: 10.1016/j.jhep.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, Lafdil F, Pawlotsky JM. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64:2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 30.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 31.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 32.Llovet J, Shepard KV, Finn RS, Ikeda M, Sung M, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Mody K, Kubota T, Saito K, Siegel AB, Dubrovsky L, Zhu AX. A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): updated results. Ann Oncol. 2019;30:v286–v287. [Google Scholar]
- 33.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, He AR, El-Rayes BF, Acosta-Rivera M, Neely J, Shen Y, Baccan C, Cruz CMD, Hsu C. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J. Clin. Oncol. 2019;37(Suppl):4012. [Google Scholar]
- 34.Colombo M. Screening for cancer in viral hepatitis. Clin Liver Dis. 2001;5:109–122. doi: 10.1016/s1089-3261(05)70156-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


