Abstract
Numerous prostate cancer (PC) associated genes have been reported in previous genome-wide association studies. Elucidation of prostate cancer pharmacogenomics have enhanced studies into the impact of germline genetic changes on treatment, in addition to evaluating related genomic alterations and biomarkers in prostate tumor tissues. Currently, Abiraterone (Abi) is used as one of the therapeutic options for PC. In this article, germline variants that have been associated with responses to Abi in patients with advanced PC are summarized. These include biomarker genes such as CYP17A1, AR-V7, HSD3B1, SLCO2B1, SULT1E1, and SRD5A2 that are involved in homologous recombination, as well as in gene expression mutations in important signaling pathways, such as WNT and Abi metabolic pathways.
Keywords: Genetic polymorphisms, abiraterone, prostate cancer, androgen receptor, prognostic
Introduction
Abiraterone (Abi), the prodrug Abi acetate (AA), when combined with prednisone and administered orally, is an effective therapeutic option for metastatic castration-resistant prostate cancer (mCRPC) and metastatic castration-sensitive prostate cancer (mCSPC) [1].
In April 2011, the FDA approved Abi, an androgen synthesis inhibitor, in combination with low-dose prednisone for mCRPC patients that had previously received docetaxe containing chemotherapy. This approval was based on the findings of a phase 3, randomized, placebo-controlled trial (COU-AA-301) in male mCRPC patients that were previously treated with docetaxel. This trial reported that median survival time was 15.8 months in the abiraterone group and 11.2 months in the placebo group (HR, 0.74; 95% CI, 0.64-0.86; P < 0.0001). Moreover, the radiologic progression time, PSA decrease and pain relief were also improved [2-4].
The FDA approved the combination of Abi and prednisone after docetaxel on December 10th, 2012. This approval was based on a randomized phase 3 trial of COU-AA--302 in asymptomatic or minimally symptomatic mCRPC patients, with Abi and prednisone vs prednisone alone. After treatment, the primary endpoint of radiological progression free survival in the combination group increased from 8.3 months to 16.5 months (HR, 0.53; P < 0.001). The median follow-up time was 49.2 months (34.7 months vs 30.3 months; HR, 0.81; 95% CI, 0.70-0.93; P = 0.003) [5].
In February 2018, the FDA approved the combination of Abi and prednisone as a therapeutic option for metastatic prostate cancer. This approval was based on two randomized phase 3 clinical trials of abiraterone and low-dose prednisone combined with ADT. Compared to ADT alone, the combination group exhibited improved OS outcomes in newly diagnosed patients with metastatic prostate cancer or high-risk or lymph node positive disease (STAMPEDE and LATITUDE) [6,7].
The adverse events of Abi and prednisone are higher, but generally lighter. These effects are mainly associated with mineralocorticoid excess, hormonal effects and hepatotoxicity. The most common adverse reactions (>5%) were: fatigue (39%); back or joint discomfort (28%-32%); peripheral edema (28%); diarrhea, nausea or constipation (22%); hypokalemia (17%); and hypophosphatemia (24%). The most common adverse drug reactions leading to drug withdrawal were elevated aspartate aminotransferase and/or alanine aminotransferase levels (11%-12%) and heart disease (19%, 6%) [2,3]. Patient reported outcomes improved with abiraterone treatments, with improvements in pain intensity progression, fatigue, decreased function, prostate cancer-related symptoms, and overall health-related QOL [6,7].
Despite the demonstrated benefits of Abi, only a small portion of CRPC male patients responded to the therapy. Compared to standard treatment, median progression-free survival (mPFS) outcome from Abi therapy is minor, at less than 6 months. Moreover, nearly 30% of the patients that used Abi developed primary resistance [8]. Although the mechanisms of resistance to Abi have not been fully established, it has been postulated that they are associated with upregulated systemic and intratumoral androgen biosynthesis [9]. This resistance could also be due to the synthesis of more dihydro-testosterone and testosterone from weak adrenal androgens (i.e., dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS)) by the CRPC cells, or make a new start from cholesterol, react to chronic exposure to an environment of low-testosterone [10,11]. Recently, studies have focused on the effects of germline polymorphisms in androgen biosynthesis, transport, and metabolism-related genes that may influence Abi responses and survival. These polymorphisms include gene mutations of androgen receptors (ARs) and amplification/overexpression, AR splice variants, pathway changes that intersect with AR signals, glucocorticoid receptor overexpression, neuroendocrine differentiation, immune system dysregulation and so on [8]. Studies are evaluating potential biomarkers that can predict therapeutic effects to distinguish among different patients by elucidating on the relationships between candidate gene polymorphisms and clinical outcomes during PC therapy. This could be important in informing individualized Abi treatment.
Many candidate genes involved in metabolism and androgen actions of Abi pathways have been summarized in several reviews. In 2015, Samanta Salvi et al. [12] provided a summary of studies in which the possible roles of genetic variants were clinically investigated based on their predictive significance in gene polymorphisms, prognosis and pathogenesis of prostate tumors. With the elucidation of prostate cancer pharmacogenomics, studies should focus on evaluating the impact of germline changes on therapy, in addition to evaluating related genomic alterations and biomarkers in prostate tumor tissues [13,14]. Eric Johnson et al. [14] summarized the germ-line variants that are associated with therapeutic responses in advanced PC men. With increasing clinical administration of Abi, efforts are aimed at optimizing drug sequencing with a focus on personalizing therapy. Therefore, there is a need to incorporate germline pharmacogenomics into routine clinical use. Currently, reviews on candidate genetic variants of Abi have not been published, however, due to the importance of Abi in PC treatment, such reviews are necessary. This review elucidates on the current status of candidate genes with a clinical impact (Table 1) and provides a reference for the rational clinical use of Abi.
Table 1.
Gene polymorphism-related differences in the outcomes of abiraterone for prostate cancer
| Gene | SNP | CHR | Minor Allele | MAF | Sample Size Studied | Population | Effect on the Response | Ref |
|---|---|---|---|---|---|---|---|---|
| HSD3B1 | rs1047303 | 1 | C | 0.033 | 76 | Caucasian | Not predictive of response to first-line Abi | [31] |
| 1 | C | 0.033 | 102 | Caucasian | The HSD3B1 (1245C) variant allele is associated with A shorter PFS on ADT in patients with mHSPC | [28] | ||
| 1 | C | - | 99 | Japanese | Distinctly better response to Abi | [25] | ||
| 1 | C | - | 30 | NA | Result in a protein variant with increased steady-state levels | [32] | ||
| SLCO2B1 | rs1077858 | 11 | G | 0.353 | 58 | Caucasian | A higher rate of pathologic minimal residual disease on prostatectomy | [39] |
| 11 | G | - | 21 | NA | GG genotype showeded a shorter TTBP and PFS than SNP rs1077858 (AA or GA) allele | [41] | ||
| 11 | G | - | 322 | NA | No significant associations between this SNP and outcomes in mCRPC patients (more line therapy) | [40] | ||
| rs12422149 | 11 | A | 0.118 | 59 | Caucasian | Increased abiraterone levels within prostate tissue (first-line mCRPC) | [39] | |
| 11 | A | - | 323 | NA | Significantly improved median PFS (first-line) | [40] | ||
| rs1789693 | 11 | T | 0.372 | 59 | Caucasian | Increased abiraterone levels within prostate tissue | [39] | |
| 11 | T | - | 323 | NA | No differential association with response (first-line mCRPC) | [40] | ||
| SULT1E1 | rs3775777 | 4 | G | 0.298 | 68 | Caucasian | Significantly associated with increased time to treatment failure | [48] |
| rs4149534 | C | 0.296 | ||||||
| rs10019305 | G | 0.289 | ||||||
| rs3775770 | T | 0.274 | ||||||
| rs4149527 | T | 0.278 | ||||||
| rs3775768 | T | 0.271 | ||||||
| rs3775777 | 4 | G | 0.298 | 68 | Caucasian | Significantly associated with TTF on AA therapy | [48] | |
| rs4149534 | C | 0.296 | ||||||
| rs10019305 | G | 0.289 | ||||||
| rs10019305 | 4 | G | - | 322 | NA | No significant associations between the evaluated SNP and outcomes in mCRPC patients (more line therapy) | [32] | |
| rs4149534 | C | - | ||||||
| rs3775777 | T | - | ||||||
| CYP17A1 | rs2486758 | 10 | C | 0.213 | 87 | NA | Diminished shorter time to biochemical progression and biochemical response | [54] |
| 10 | C | - | 322 | NA | Significant association with TCR (more line therapy) | [32] | ||
| rs10883782 | 10 | G | 0.160 | 109 | French | Associated with rPFS on AA therapy (mCRPC first-line, CRPC-rPFS on AA) | [55] | |
| rs743572 | 10 | G | 0.394 | 64 | Caucasian | No significant associations between these polymorphisms and clinical outcome | [56] | |
| rs10883783 | A | 0.298 | ||||||
| rs17115100 | T | 0.102 | ||||||
| rs284849 | T | 0.177 | ||||||
| CYP17A1 | copy number variations | 10 | - | - | 53 | NA | Associated with the prognosis of mCRPC patients treated with Abi | [53] |
| AKR1C3 | Over expression | 10 | - | - | 117 | Chinese | CRPC-Resistance to abiraterone acetate | [73] |
| SRD5A2 | rs523349 | 2 | C | 0.446 | 86 | Japanese | A worse prognosis in metastatic prostate cancer men for primary ADT | [76] |
| rs2300700 | 2 | A | 0.459 | 322 | Spanish | No significant associations between the SNP and outcomes in mCRPC patients (more line therapy 66) | [32] | |
| SRD5A1 | rs3822430 | 5 | G | 0.379 | 322 | Spanish | Showed a significant association with OS | [32] |
| rs3736316 | 5 | A | 0.399 | 322 | Spanish | Showed a significant association with OS | [32] | |
| GNRH2 | rs6051545 | 20 | T | 0.417 | 80 | Japanese | Higher serum testosterone | [78] |
| AR | [2105T>A (p.L702H)] | X | - | - | 37 | Caucasian | Resistance to abiraterone | [78] |
| [2632A>G (p.T878A)] | X | - | - | 37 | Caucasian | Resistance to Abi | [78] | |
| AR-V7 | X | - | - | 31 | Caucasian | Positive patients had lower PSA response rates than negative | [17] | |
| AR-V9 | X | - | - | 12 | NA | ARV9 expression in metastases related to inital resistance to AA/P | [110] | |
| AR gene amplification | X | - | - | 12 | NA | AA drug resistance (shorter mPSA-PFS and mrPFS) | [110] | |
| SMAD3 | upregulated | 15 | - | - | 18 | NA | Drug-resistant | [108] |
| CCND1 | signaling was enriched | 11 | - | - | 18 | NA | Drug-resistant | [108] |
| DKK4, SFRP2, LRP6 | - | 8, 4, 12 | - | - | 92 | NA | WNT pathway activation resistance in mCRPC | [111] |
| SPOP/CHDI | Mutations/loss | 17 | - | - | 89 | N | A higher response rate to Abi | [118] |
| FOLH1, KLK3, NPY | negative | 11/19/7 | - | - | 50 | NA | Patients with biomarker-negative platelets had the best outcome. FOLH1 and NPY provided Independent predictive information in PFS. KLK2, KLK3, and FOLH1 were associated with short OS | [127] |
| TSPYL1 | rs3828743 | 6 | - | A | 87 | NA | Reduced abiraterone concentrations and increased cell proliferation | [120] |
| UGT1A4 | - | 2 | - | - | 5 | MA | GLucuronide derivatives are detected at variable levels in circulation of treated prostate cancer patients | [121] |
SNP: single-nucleotide polymorphism; CHR: chromosome; MAF: minor allele frequency in Caucasian population. Available online: NCBI (http://www.ncbi.nlm.nih.gov/snp) ; NA: not available.
Action mechanisms of Abi
Abi, an androgen receptor drug, blocks androgen synthesis in various pathways, including in the testis, adrenal glands, peripheral tissues, and adrenal tumor cells. It selectively inhibits CYP450 17α-hydroxy/17,20-lyase (CYP17A1), an enzyme involved in androgen biosynthesis. Abi exhibits the same 3β-hydroxyl and δ 5-steroid structure as DHEA and other 3β-hydroxysteroid dehydrogenase (3β-HSD) catalytic substrates. Therefore, it can be metabolized by 3β-HSD while still retaining its properties as a CYP17A1 inhibitor, and gains the ability required for effective androgen biosynthesis to act as an AR antagonist and inhibitors of others enzyme. In the past few years, Abi associated metabolites such as 5α-Abi and D4-Abi (D4A), which exhibit significant pharmacological activities, have been shown to be formed by steroidogenic enzymes [15]. 3β-HSD converts Abi to D4A first, which antagonizes the AR while Abi blocks CYP17A1 and steroid-5α-reductase (Figure 1) [16]. Then, D4A is irreversibly converted to 3-keto-5β-Abi or 3-keto-5α-Abi. Both metabolites are then converted to their 3β-OH and 3α-OH derivatives. In total, six downstream D4A metabolites are formed (Figure 2). 5α-Abi is directly metabolized to D4A, with both acting as androgen receptor agonists. However, the 5β-Abi metabolite is not active [15].
Figure 1.
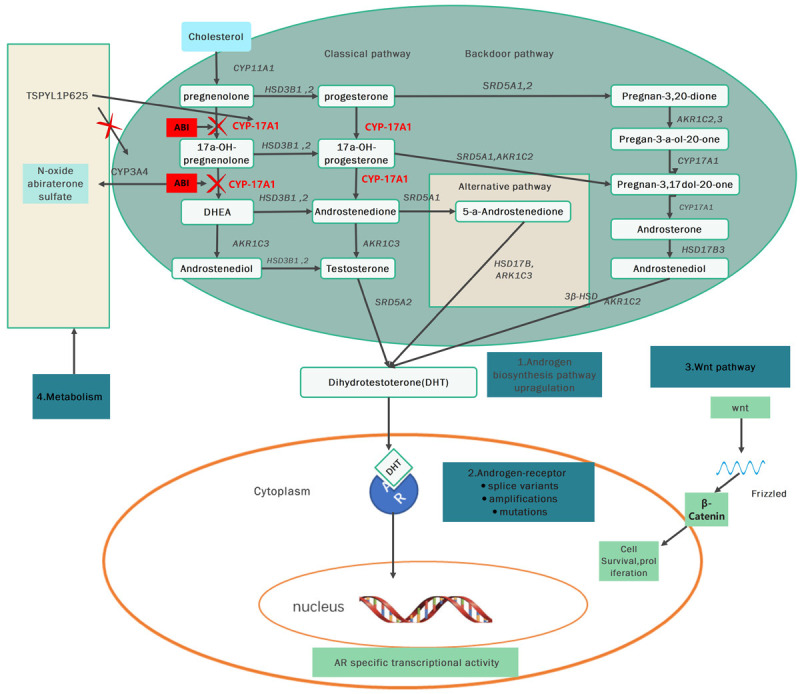
Schematic of the mechanisms of signaling pathways of Abi. Declined synthesis of the androgens in prostate cancer adrenal glands, and related tissue because of inhibiting the enzymes CYP17, 20 lyase and 17α hydroxylase irreversibly by ABI. ABI: abiraterone; CYP17A1: CYP450 17α-hydroxy/17,20-lyase; AR: androgen receptor; TSPYL: testis-specific-encoding-like; DHT: Dihydrotestosterone; AKR1C3: Aldo-Keto Reductase Family 1 Member C3.
Figure 2.
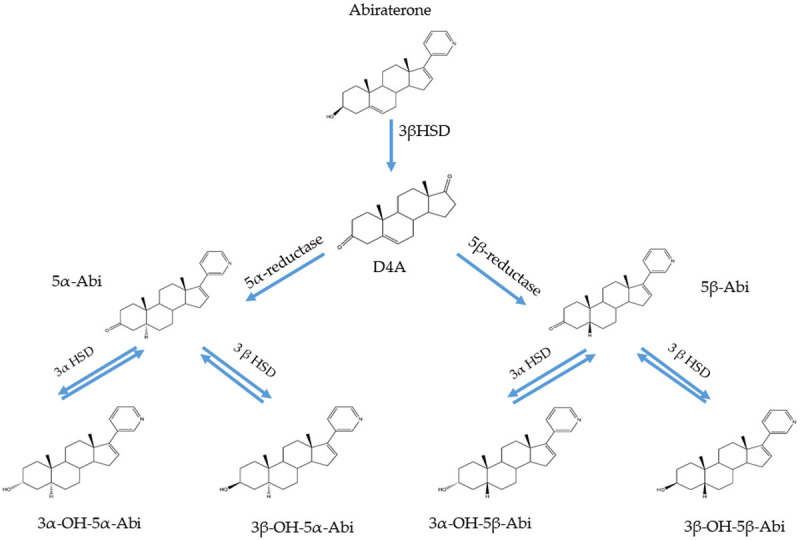
Genesis of 5α- and 5β-reduced Abi metabolites in patients treated with Abi. The structurally similar conversion from abiraterone to D4A results in the reduction of D4A5α- and 5β at C5, with a total of six additional abiraterone metabolites. D4A: Δ4-abiraterone; 3βHSD: 3β-hydroxysteroid dehydrogenase; 3αHSD: 3α-hydroxysteroid dehydrogenase.
Genetic testing
A series of genomic and other molecular analyses have been performed on tumor samples to inform therapeutic decisions by identifying known predictive markers for improving the diagnosis and treatment of PC. To elucidate on the molecular pathological mechanisms in tumor tissues, invasive procedures are often required, which are not always feasible, and continuous monitoring of tumor genotypes is not possible. Currently, cfDNA and CTCs are used to evaluate genetic and epigenetic changes using the NGS of complete exome DNA to establish the transcribed coding and non-coding RNA profiles [17-19]. Matti Annala et al. [19] reported the relative influence of frequent circulating tumor DNA modifications on patient responses to the most extensively used therapeutic options (such as enzalutamide and Abi) for advanced prostate cancer. They used serum samples obtained from a phase II trial, set up genomic drivers of resistance to first line AR treatment in mCRPC and evaluated the possible minimally invasive biomarkers. Studies have reported the potential prognostic values of CTC and AR markers [20-23]. Therefore, development of non-invasive liquid biopsy markers to elucidate on tissue-based information is still a priority, and CTC-based AR-V7 expression is the first such marker that can accurately predict ARSI responses in mCRPC individuals [17].
Polymorphisms associated with abi responses
HSD3B1
The enzyme 3β-HSD1, which catalyzes adrenal androgen precursors into dihydrotestosterone (DHT) is encoded by the HSD3B1 gene. However, due to an amino acid change (p.367T>N) or a missense SNP (rs1047303, NM_000862.3:c.1100C>A) in exon 4 of the HSD3B1 gene, the 3β-HSD1 protein is resistant to ubiquitination and degradation. This results in the accumulation of enzymes, increased intracellular conversion of DHT precursors to DHT and associated progression to CRPC [24]. DHT synthesis is enhanced by variant HSD3B1 (1245C) alleles which predict metastatic disease resistance to ADT and biochemical recurrence after prostate cancer resection. Patients with HSD3B1 (1245C) allele mutations have significantly worse prognostic outcomes after ADT than those without [25], indicating that the HSD3B1 variant status is correlated with shortened ADT response time.
Neeraj Agarwal et al. [26] reported that 10% of males with homozygous HSD3B1 (1245C) mutant alleles have suboptimal responses to ADT alone. These patients may benefit more from a prior therapy of docetaxel or from participating in prior deeper androgen blockade trials using novel androgen signaling inhibitors. This could have occurred because of the ability of Abi metabolites to act as androgen signaling agonists (3-keto-5α-Abi) and antagonists (D4A) at the same time [27]. Formerly, inheritance of the HSD3B1 variant had been associated with extended responses of non-steroidal drugs to CYP17A1 inhibition, further implying increased tumor dependence on external androgens in males with HSD3B1 mutation [28].
A study [29] involving 76 mCRPC men treated with AA tested the hypothesis that the HSD3B1 (1245C) variant forecasts clinical responses to treatment and can inform on individualized therapy for patients with advanced PC. It was found that the HSD3B1 (1245C) variant did not predict the response of patients using Abi as initial treatment. This outcome could be because Abi metabolites can act as agonists (3-keto-5α-Abi) and at the same time, as antagonists (D4A) during androgen signaling. HSD3B1 (1245C) synthesis predicts faster clinical resistance and sensitivity to extraadrenal androgen synthesis inhibition [30]. Therefore, HSD3B1 (1245C) looks like to limit a subfraction of patients who benefit from blocking androgens of extragonadal.
Masaki Shiota et al. [25] evaluated the relationships between HSD3B1 genotypes, clinical consequences and clinic-pathological parameters such as PFS, tFFS, OS and PSA responses in 203 Japanese men. A total of 104 men were allocated to the primary ADT cohort, while 99 men were allocated to the Abi group, with most patients in each cohort having metastatic disease. They reported that the prognosis of HSD3B1 mutation carriers was worse in the ADT group, involving 104 mHSPC patients. However, the 99 mCRPC patients with the HSD3B1 variant showed better clinical responses to Abi therapy. Therefore, the HSD3B1 genotype is a potential biomarker for ADT and Abi. It is recommended to apply Abi and ADT in advanced mHSPC patients.
In contrast, a study [31] involving mCRPC patients receiving Abi as first-, second- or third-line therapeutic options showed that there were no significant associations between HSD3B1 (rs1047303) and clinical outcomes. Therefore, they determined whether the inheritance of HSD3B1 (1245C) is associated with increased 3-keto-5α-Abi synthesis [32]. They found that individuals who inherited 0, 1, 2 copies of HSD3B1 (1245C) have a gradual increase in normalized 3-keto-5α-Abi. These patients were more likely to benefit by inhibiting CYP17A1, however, Abi benefits were partially offset by elevated 3-keto-5α-Abi levels.
Even Although the HSD3B1 (1245C) allele increases the rate by which adrenal androgen precursors are converted to DHT, AR antagonists compete with intratumoral androgens and may weaken the effect of the mutant allele. Therefore, the high exposure rate to early ADT and the frequent use of AR antagonists during ADT rescue treatments may change the genotypic effect on composite TTP and OS.
Recently, large individual differences in the metabolic ratios of Δ4A/Abi (CV = 140%) when 3β-HSD1 transforms Abi into Δ4A have been reported [33]. An increase in the Abi to Δ4A ratio may predict the heterozygous or homozygous variant of the patient (1245C), and is also associate with individual differences. In addition, inheritance of HSD3B1 (1245C) variants is associated with elevated AR agonist 5-α-Abi levels. These results suggest that plasma exposure to Abi affects pharmacodynamic activities in mCRPC patients treated with Abi than with Δ4A. Furthermore, Δ4A level or ratio may be a substitute of endogenous 3β-HSD1 activity, which partly depends on HSD3B1 genotype inherit [34].
There are divergent opinions on whether HSD3B1 gene polymorphisms can be used as biomarkers for AA to treat prostate cancer. Some studies [25,26] have confirmed that therapeutic outcomes are predictive when AA is used as a first-line therapeutic option for CSPC. However, for men exposed to ADT from non-steroidal androgen drugs, HSD3B1 cannot predict clinical outcomes, possibly because HSD3B1 may have been affected by previous ADT treatments [27]. Therefore, further studies are needed to evaluate the impact of this gene in patients with different stages of prostate cancer.
SLCO2B1
Solute carrier organic anion transporter family member 2B1 (SLCO2B1) is involved in the transport of hormones such as testosterone and dehydroepiandrosterone sulfate (DHEAS) as well as drugs such as AA [35]. Therefore, germline variations in SLCO2B1 can alter therapeutic responses to therapies targeting the androgen axis. This outcome has been observed in multiple studies [36-38] in which SLCO2B1 SNPs have been associated with resistance to ADT. The organic anion-transporting polypeptides (OATPs) transport a variety of compounds, including adrenal androgens, which are encoded by SLCO genes.
Elahe A Mostaghel et al. [39] reported that SLCO2B1, rs12422149 and rs1789693 SNPs are associated with elevated Abi levels within the prostate tissue and a higher rate of pathologic minimal residual disease on prostatectomy.
A study [40] evaluated the predictive value of SLCO2B1 germline variants (rs1789693 and rs12422149) on PFS in mCRPC men administered with first-line Abi. Men heterozygous for the rs12422149 variant allele had a significantly improved median PFS compared to those homozygous for wild-type rs12422149 allele. There were no differential associations in responses to treatment with Abi for patients with the rs1789693 genotype. Germline variant alleles of SLCO2B1 (rs12422149) are frequent and are potential predictors for improved responses to first-line Abi in mCRPC male patients.
Silvana Giacinti et al. [41] hypothesized that germline variants of the androgen transporter gene (SLCO2B1) may influence responses to Abi in mCRPC male patients by altering the stock ability of adrenal precursors to prostate cancer cells. Three single nucleotide polymorphisms (SNPs), intronic SNPs (rs1789693 and rs1077858) and an exonic SNP (rs12422149), were genotyped in 21 mCRPC male patients who had been treated with Abi. Patients carrying the SLCO2B1 rs1077858 risk genotype (GG) showed a shorter PFS and TTBP than patients with the primary AA or GA allele. Therefore, SLCO2B1 genetic variants may be pharmacogenomic determinants of resistance to Abi in mCRPC. This phenomenon has been elucidated [42]. It was reported that there are clear differences in SLCO expression between Gleason score 4 and 3 tumors, ADT-treated and untreated tissues as well as between PCa and NP samples. Although the study involved a small sample, these results showed that steroid ADT uptake and response may be influenced by baseline and changes in ADT-induced PCa OATP expression. as well as uptake and response of drugs transport by OATP-mediated such as Abi and docetaxel, which are now commonly used in combination with ADT in mCSPC patients.
Costantine Albany et al. [43] reported that SLCO2B1, KIF3C CYP19A, and ESR1 polymorphisms are significantly associated with PFS during Abi therapy (P≤0.025; q-value < 0.69). This result showed the importance of gene polymorphisms in individualized treatment with Abi. There is a need to determine whether correlations of more than one polymorphism with longer TTP and PFS is a predictor for better responses to treatment.
SULT1E1
Estrogen sulfotransferase (SULT1E1) belongs to the cytosolic sulfotransferase superfamily, which are Phase II drug-metabolizing enzymes. They mediate sulfate conjugation that is important in xenobiotic detoxification and regulate multiple signaling molecules [44]. In the human reproductive tissue, SULT1E1 catalyzes the sulfation of estrogenic compounds [45]. Estrogen has a key role in PC pathogenesis and outcomes [46]. AA inhibition of DHEA sulfonation has been confirmed in enzymatic cultures containing human liver or intestine tissue cytosol or recombinant human SULT2A1, SULT2B1b or SULT1E1 enzymes [47].
A study [48] evaluated the correlation between time to treatment failure with Abi and 832 SNPs in 61 candidate androgen pathway genes from 68 mCRPC patients. After correcting for multiple testing and controlling for other clinical variables, 6 SNPs (rs3775777, rs4149534, rs10019305, rs3775770, rs4149527, and rs3775768) in one gene, SULT1E1, were significantly correlated with increased time of treatment failure. Another study [49] arrived at a similar conclusion, where estrogen sulfotransferase genes, rs3775777, rs4149534 in SULT1E1 were significantly correlated with TTF in Abi treatment and may act as prognostic markers for efficacy upon treatment with Abi in Caucasian mCRPC male patients. These SNPs are potential predictive markers for Abi and should be validated in a larger cohort.
CYP17A1
The human CYP17A1 gene is localized on chromosome 10q24.3, spans 6.6 kb and contains eight exons and seven introns. In adrenals and gonads, an identical 2.1 kb mRNA is transcribed from this gene [50]. In the adrenals, the expression levels of CYP17A1 are regulated by the adrenocorticotropic hormone (ACTH), and by gonadotropic hormone in the testes and ovaries. Due to its activity on 17-hydroxylase and 17,20-lyase, which play vital roles in hormonal production pathways, the CYP17A1 gene is very important in the production of androgens and glucocorticoids [51].
As a CYP17A1 inhibitor, alterations in CYP17A1 have been implicated in resistance to Abi [52]. A study [53] showed that CYP17A1 copy number variations affects the prognosis of mCRPC patients treated with Abi.
Moritz Binder et al. [54] evaluated the associations between four CYP17A1 tag SNPs and responses to Abi in 87 male mCRPC patients. Four SNPs (rs743572, rs4919685, rs2486758, and rs17115100) provided a 100% coverage of CYP17A1 common genetic variants (minor allele frequency 0.05). A single SNP (rs2486758) was confirmed to be associated with diminished shorter time to biochemical progression and biochemical responses.
Nine SNPs of 6 candidate genes have been previously analyzed [32]. They include CYP17A1 (rs2486758), SRD5A1 (rs3822430 and rs3736316), SRD5A2 (rs2300700), SCLO2B1 (rs1077858), SULT1E1 (rs10019305, rs3775777 and rs4149rs104) and HSD3B1 (rs1047303). A single SNP of CYP17A1 (rs2486758) was significantly correlated with TCR (time to castration resistance). There were no significant associations between most of the evaluated SNPs and outcomes in Abi treated mCRPC male patients. Unlike other studies, patients involved in this study received Abi as the first-, second- or third-line therapeutic option.
The ABIGENE study [55], a multicentric prospective non-randomized pharmacogenetic study, evaluated mCRPC patients treated with AA+prednisone as first-line therapy. Based on the PCWG2 criteria, they found that the association between 13 SNPs in genes (CYP17A1, SLCO2B1 and SLCO2B3) are associated with Abi pharmacology and radiographic progression-free survival (rPFS). During Abi treatment, SNP CYP17A1 (rs10883782) was associated with rPFS. Statistical analyses did not reveal significant associations between rs10883783, rs743572, rs284849 and rs17115100 polymorphisms in CYP17A1 and prognosis. However, patients with the TT genotype of rs10883783 exhibited longer PFS than patients with the AA or TA genotype by 3-months [56].
In summary, single SNP (rs2486758) in CYP17A1 was significantly correlated with poor clinical outcomes and resistance to Abi treatment. More studies will transform these conclusions into decision-making indicators for clinical treatment.
AKR1C3
Aldo-Keto Reductase Family 1 Member C3 (AKR1C3) is a protein-coding gene. The diseases associated with AKR1C3 include prostate disease and endometrial cancer. Annotations in Gene Ontology (GO) related to this gene include the activity of oxidoreductase and aldo-keto reductase (NADP). AKR1C3 plays a key role in all DHT pathways, including catalysis of conversions from Δ4-androstene-3,17-dione (Δ4-AD) to T, 5-Adione to DHT, and DHEA to 5-Adiol [57].
Overall gene expression analyses revealed that the steroid biosynthetic pathway is activated in prostate cancer cells resistant to Abi [58]. One of the key steroid-like gene enzymes, AKR1C3, has been found to be significantly elevated in Abi-resistant cells. In addition, AKR1C3 is highly expressed in metastatic and recurrent prostate cancer. Moreover, compared to parental cells, androgen precursors, such as cholesterol, dehydroepiandrosterone and progesterone, as well as androgens, are highly upregulated in Abi-resistant prostate cancer cells. The overexpression of AKR1C3 confers resistance to Abi. AKR1C3 expression has been observed in prostate cancer cell samples grown in androgen-depleted media [59], in xenografts from castrate mice [60-62], in tumor samples of patients with soft-tissue metastases [10] and in nine clinical studies [10,18,59,63-67]. These results suggest that AKR1C3 activation is a critical resistance mechanism associated with Abi resistance.
AKR1C3 is also associated with the backdoor pathway, where it converts androsterone to 3α-diol [67-69]. AKR1C3 expression is up-regulated by ADT and is inhibited by androgens, and its overexpression is part of the mechanisms associated with Abi resistance [61]. The TMPRSS2-ERG fusion protein binds the AKR1C3 promoter to enhance the expression of AKR1C3. The pre-pass mechanism has been proposed, in which, when the tumor starts to synthesize more T and DHT, TMPRSS2-ERG expression is elevated, which replaces the AR in the AKR1C3 promoter, thereby enhancing intratumoral androgen biosynthesis [66]. Tamae D. et al. [70] reported that one of the mechanisms involved in Abi resistance is that DHEA-SO4 residues and AKR1C3 overexpression after CYP17 inhibition form a storm for AA drug resistance.
Some studies [71,72] have reported that AKR1C3 detection of prostate re-Bx in mCRPC tissue is associated with early Abi resistance. That is, AKR1C3 significantly shortens mPSA PFS and mrPFS. The expression of AKR1C3 is not correlated with PSA response and OS. These findings inform clinical decisions on the best personalized treatment for mCRPC patients, and help clinicians predict Abi effectiveness, therefore, it is recommended to routinely describe it in the pathology report.
Studies [73,74] have also evaluated the effects of AKR1C3 on the therapeutic effect of corticosteroid conversion in predicting mCRPC patients receiving Abi treatment. One study showed that AKR1C3 expression by mCRPC in prostate re Bx tissue is associated with the shortening of PSA-PFS caused by the conversion of glucocorticoid from prednisone to dexamethasone. These conclusions have a certain reference value for mCRPC patients, especially for the individualized choice of corticosteroid conversion therapy.
In summary, activation of AKR1C3 enhances androgen secretion, which is a key mechanism for Abi resistance. Therefore, targeting AKR1C3 activation is a potential treatment strategy for patients with metastatic prostate cancer who are resistant to Abi and corticosteroid conversion therapies.
SRD5A
Genetic variations in genes associated with androgen production pathways such as GNRH2 (rs6051545) and SRD5A2 (rs523349) are related to serum testosterone levels and prognosis during ADT [75-77]. In addition, SRD5A2 gene polymorphism is correlated with the prognosis of metastatic PC after primary ADT. SRD5A2 encodes 5α-reductase 2, which can convert testosterone to 10 times stronger DHT [75]. M Shiota et al. determined whether serum testosterone concentration or body mass index (BMI) in patients with metastatic PC and primary ADT is correlated with prognosis. In addition, the association between serum testosterone levels and SRD5A2 polymorphism was examined during ADT. The CC SRD5A2 (RS523349) allele encodes a less active 5-α-reductase, which is associated with decreased serum testosterone levels in the course of ADT. These findings suggest that significant inhibition of SERUM testosterone by ADT is correlated with SRD5A2 polymorphism [75].
It has been reported [76] that a greater active 5α-reductase variant encoded by the GG allele of SRD5A2 (rs5233499) is associated with poor clinical outcomes in patients with metastatic PC treated with essential ADT. Moreover, patients with the CC allele, which encodes the less active 5α-reductase, have lower plasma testosterone concentrations and better clinical outcomes upon ADT therapy. It is advised that distinction of blood testosterone levels during ADT treatment might also mediate the prognostic impact of SRD5A2 polymorphism. It has not been established whether plasma testosterone concentrations in the course of ADT therapy is an independent prognostic for SRD5A2.
GNRH2
Gonadotrophin-releasing hormone (GnRH) is a decapeptide that is synthesized by the hypothalamus. Two subtypes of GnRH, GnRH1 and GNRH2, are expressed in the trophoblast and syncytial trophoblast of human placenta, respectively. The GNRH2 gene is located on chromosome 20p13 and has 70% homology with the GnRH1 gene, and consists of 4 exons [77].
A Japanese study [78] measured serum testosterone levels of 80 mCRPC patients on ADT treatment. Compared to the CC allele, the CT/TT and CT alleles in the GNRH2 gene (rs6051545) were associated with elevated plasma testosterone concentrations. During ADT treatment, CT alleles were associated with a high progression risk after adjusting for age and plasma testosterone. Therefore, it is concluded that the rs6051545 (GNRH2) gene mutation may lead to insufficient serum testosterone suppression during ADT, leading to the missing effect of androgen deprivation therapy in mCRPC male patients.
Androgen receptor (AR) gene mutations and amplifications
Abnormal AR gene mutations are rare. However, in rapid autopsy diagnosed metastatic tumors before hormonal therapy, up to 60% of patients were found to have these mutations [79]. Two AR point mutations, 2632A>G and 2105T>A, are associated with Abi resistance and are activated by progesterone or prednisone former, respectively [80-83]. Findings from related biomarker studies revealed that the AR gene status in plasma DNA is correlated with poor prognosis of CRPC patients on Abi.
Androgen-receptor splice variant (ARV)
The ARV level is associated with PC process, and there is a significant elevation in ARV expression during ADT [81-87]. This may be attributed to the activation of AR in the ligand resulting in the absence of an AR clipping variant of the ligand binding domain (LBD) as a transcription factor to maintain continuous activity in a ligand non-dependent manner [88]. ARV-7 and ARV-567 are the most commonly expressed variants that are associated with PC progression during ADT [89]. Abi therapy is correlated with an increased truncated variant expression, and ARV expression can mediate resistance to treatments targeting FL AR and mCRPC cell line in CRPC xenografts [90].
AR-V7
AR-V7, a special AR-V, develops from contiguous splicing of AR exons 1, 2, and 3 and cryptic exon 3 [91]. Due to selective splicing of 30 terminal cryptic exons, AR-Vs lacks the full-length AR COOH terminal LBD [92]. These 30 terminal cryptic exons encode short carboxy-terminal extensions. The expression levels of exon CE3 as the 30-terminal exon of AR-V7 has been used for RT-PCR, in RNA sequencing (RNA-seq) and in in situ hybridization (ISH) to detect the mRNA expression levels of AR-V7 in all kinds of biological samples from CRPC patients [93-102]. Positivity of AR-V7 expression in CTCs is correlated with resistance to Abi, but not to taxane therapy [17,94]. AR-V7 is associated with CRPC pathogenesis, and its prognostic value in CRPC should be further elucidated. In CRPC patients treated with androgen receptor signaling (ARS) inhibitors, AR-V7 positive is associated with poor PSA response and PFS prognosis. However, it does not have an effect on the OS of chemotherapy patients. Even though AR-V7 detection based on circulating tumor cells (CTC) has been shown to predict patient’s responses to second-generation androgen receptor therapy, AR-V7 is rarely expressed in mCRPC patients, suggesting that other factors mediate resistance.
A study [103] reported that PSA response rates of AR-V7-positive patients to androgen receptor signal suppression therapy was significantly lower than that of AR-V7-negative patients. The OR of PSA response in AR-V7-positive patients has been found to be 0.07 (95% CI, 0.02-0.35; P = 0.0010) in patients treated with Abi. In global case series or in male patients allocated into three groups based on basic PSA levels, when CTC negative to CTC positive/AR-V7 negative to CTC positive/AR-V7 positive, all approved treatment results deteriorated. Pierangela Sepe et al. [104] reported that when individualized biomarker-driven therapy is extended to all patients, priority should be given to combining the predictive effect of CTC status with AR-V7 detection.
A study [17] involving Abi-treated CRPC patients reported that AR-V7 was positive in CTCs in 31 patients, and that these patients had low PSA response rates than patients without AR-V7 expression. The median clinical or radiographic PFS in patients without AR-V7 expression was longer than in the AR-V7-positive group in men with Abi. In addition, 9-15% of mCRPC patients were positive for AR-V7 expression at initial treatment, and AR-V7 exhibited an increasing trend during Abi treatment, supporting the hypothesis that AR-V7 is associated with both intrinsic and acquired resistance of patients to Abi [105,106].
These studies imply that AR-V7 is a potential predictive biomarker in precision therapy. Inhibiting the transcriptional activity of AR-V7 and reducing the recruitment of AR-V7 to PSA promoters can be a vital therapeutic strategy, and may also be an advantageous way for overcoming Abi resistance [107].
Sumanta Kumar Pal et al. [108] reported contrasting findings to those of previous CRPC studies. They did not report significant differences in AR-V7 levels between drug resistance and drug sensitive patients and neither did they report that high AR-V7 baseline levels imply a weak response to Abi. All four patients with elevated AR-V7 baseline levels initially responded to Abi, even though they progressed to resistance within one year of initial Abi.
Even though evidence suggests that AR-V7 expression levels cannot be used as predictive markers for targeted AR therapy, studies [109,110] have reported that this is not always exact and individuals with positive AR-V7 expression may still benefit from Abi. In addition, this suggests that other mechanisms other than AR splicing variant expression may lead to resistance to these drugs.
AR-V9
Abi acetate can be used as a target for transcriptional reactivation of AR in some patients, and in most cases, the transcriptional activity of AR persists [110]. These two AR variants, AR-V9 and AR-V7, have a common 30-terminal recessive exon, which predisposes AR-V9 to experimental manipulations that were previously thought to be AR-V7 specific. Since AR-V9 promotes the growth of prostate cancer cells that do not rely on ligands, elevated mRNA expression levels of AR-V9 in CRPC metastases is a predictor of initial resistance to Abi. Therefore, AR-V9 may be an important part of CRPC resistance. A study [110] assessing mCRPC individuals begin with pre-chemotherapy Abi+P accepted biopsies of metastatic site before and after 12 weeks-therapy. Compound progression included PSA, RECIST, bone scan and symptoms (by PCWG2), which were evaluated at 12 weeks (primary endpoint). The associations between resistance at 12 weeks of Abi therapy and these parameters, including mRNA expression of pre-AA/P ARFL (full-length AR), AR-V3, AR-V7, AR-V9, AR-V23, ARV45, four cell cycle division genes, PSA/testosterone levels at initial diagnosis, chromogranin-A (CHGA) along with Gleason score (GS), tumor volume and time from start of hormonal therapy to mCRPC stage were evaluated by logistic regression models. It was found that elevated AR-V9 mRNA expression levels in metastases is correlated with early resistance to AA+prednisone. This finding should be validated in similar studies.
Androgen receptor amplifications
Even though findings from AR amplification testing were not superior to standard prognostic biomarkers, the LBD truncated AR gene is rearranged in patients with primary drug resistance. These studies confirmed the driver genes for resistance to first-line AR treatment in patients of mCRPC, and identified the potential biomarkers for minimally invasive testing [19].
Signaling pathway
TGFβ/SMAD3 and CCND1
From the RNA-seq findings of CTCs, classic mutations correlated with CRPC and new mutations were identified, including in AR ligand binding domains that help escape AR targeted drugs. Pathway evaluations of differentially regulated genes [111] revealed that cyclin D1 (CCND1) and transforming growth factor β (TGFβ) signaling pathways are substantially upregulated in drug resistance. These findings indicate that Abi-sensitive and Abi-resistant states represented by RNA-seq of CTCs are potential resistant mechanisms. Moreover, CCND1 signaling and TGFβ/SMAD family member 3 (SMAD3) play key roles in driving oncogenic conversion after AR-targeted therapy.
Wnt pathway
The WNT pathway is associated with mCRPC drug resistance [112], and can induce tumor transformation from epithelial to mesenchymal states. Mesenchymal transformed cancer cells stimulate the invasion of adjacent epithelial cancer cells by secreting WNT5B [113].
Manish Kohli et al. [111] reported that activation of the Wnt/catenin pathway is associated with primary AA/P resistance. They also found that Wnt/β-catenin pathway associated genes often have mutations, and the negative regulators of the Wnt pathway (SFRP2, LRP6 and DKK4) are often deleted in non-responders. Gene expression analysis showed that expression levels of cell cycle regulation genes in non-responders increased significantly, and at the same time, the expression levels of the Wnt/catenin pathway inhibitors decreased significantly. This discovery provides the possibility for establishing predictive biomarkers to regulate target pathways to overcome molecular resistance to Abi.
Some studies have reported that the expression of Wnt transcripts, such as WNT5A and WNT7B, and genes related to classical Wnt signaling, including LEF1 and FZD4 in resistant samples are significantly elevated [96,108,112]. However, they did not observe elevated expression levels of non-canonical Wnt pathways such as target “cell division control protein 42 homolog” (CDC42), RAC, or RHOA. Moreover, these non-classical pathways have not yet been shown to be enriched in drug-resistant samples. Therefore, the activation levels and clinical relevance of Wnt pathway in drug-resistant prostate cancer should be further elucidated.
Genome-wide analysis of patients with initial Abi resistance revealed that there is a frequency of variation in the WNT pathway. Therefore, the value of WNT pathway in Abi resistance should be further evaluated in studies.
SPOP
Tumor genome-wide and exome sequencing studies have shown that SPOP is the most commonly mutated gene in primary prostate cancer. It is a substrate of cullin-3 (CUL3) ring box 1 (RBX1) E3 ubiquitin ligase (CRL) complex object recognition subunit [114]. In prostate cancer, SPOP mutations are associated with a structurally labeled substrate binding region known as the region of methyldopa and tumor necrosis factor receptor-related factor (TRAF) homology (MATH) [115-117]. These findings suggest that the pathophysiology originating from SPOP mutations may be mediated by impaired substrate ubiquitination.
SPOP mutations and CHD1 deletions frequently occur in prostate cancer, with lower frequencies reported in CRPC. A study [118] evaluated the key molecular characteristics of mCRPC for CHD1 deficiency/SPOP mutations, and showed that they are associated with a high probability of benefit from Abi therapy. CHD1 deficiency is significantly correlated with SPOP mutations, while ERG re-scheduling is negatively associated with SPOP mutations and CHD1 deficiency, suggesting that these genomic bits have selective roles in prostate cancer progression.
Studies have determined that SPOP mutations appear in the early stages of prostate tumors and are associated with the loss of CHD1, thus defining a subclass of the disease. It has been reported that SPOP mutations are associated with increased androgen receptor (AR) signaling pathways. Therefore, it has been hypothesized that SPOP-mutated prostate cancer is highly sensitive to AR blockade during Abi treatment. It has been reported [119] that this subclass of prostate cancer is very sensitive to Abi’s AR signal blockade, and that most tumors with SPOP mutations/CHD1 deletion respond to it.
A study [119] reported that most of the PTEN in the wild-type background of CHD1 and SPOP in mCRPC of localized prostate cancer was missing, and two patients with both CHD1 and PTEN missing at the same time were reported, indicating that the combination of these two proteins’ basic relationships are not universal. Moreover, this study reported several SPOP mutations including R121P, G148E, E50K, S105F, Q120R, and A187T that were not reported in previous systematic prostate cancer studies.
Other metabolic pathways
TSPYL
The testis-specific y-encoding-like protein (TSPYL) gene family includes TSPYL1 to TSPYL6. Among them, TSPYL1, 2 and 4 regulate the expression of many CYP genes, such as CYP3A4 and CYP17A1, which encode enzymes that catalyze Abi metabolism and key enzymes for androgen biosynthesis, respectively. In addition, a common SNP of TSPYL1, rs3828743 (G/a) (Pro62Ser), suppresses the ability of TSPYL1 to inhibit the expression of CYP3A4, resulting in a decrease in Abi concentration and an increase in cell proliferation. SNP genotype A is significantly associated with adverse reactions. A prospective clinical trial of 87 mCRPC men administered with Abi acetate/prednisone revealed a significant correlation between poor clinical responses and short PFS. Therefore, as a new CYP gene transcription regulator, the TSPYLs gene affects responses to drug therapy because genetic changes significantly regulate CYP450 gene transcription [120].
UGT1A4
A study [121] showed that Abi and its metabolites undergo glucuronidation in the liver, and different levels of glucuronic acid derivatives are detected in the blood of PC men. UDP-glucuronosyltransferase (UGT)1A4 is a key enzyme. Mutations of this enzyme were shown to affect this metabolic pathway in vitro, suggesting that it may affect the metabolism and function of Abi in patients. These drug compounds inhibit the effects of drugs and steroidal glucuronic acid, and may affect the UGT-related agent metabolism system and the pre-receptor control of androgen metabolism in patients.
CYP450
Abi can be extensively metabolized through a variety of pathways, but mainly through the transformation of SULT2A1 to Abi sulfate (M45), and to N-oxide Abi sulfate (M31) by SULT2A1 and CYP3A4 [122,123]. M31 and M45 are the major metabolites that are not active, and they account for 40% of Abi in blood serum [123]. In vitro studies indicated that Abi is a strong inhibitor of CYP1A2 and CYP2D6 as well as a moderate inhibitor of CYP2C9, CYP2C19, and CYP3A4 [124]. In vitro, Abi sulfate and N-oxide Abi sulfate inhibited OATP1B1, the hepatic uptake transporter [125], which indicates potential interactions between drugs and OATP substrates. However, currently, there is no evidence of drug interactions due to transporter induction or inhibition [125].
FASN overexpression
Abnormal regulation of lipid metabolism caused by overexpression of fatty acid synthase (FASN) is an important sign of prostate cancer progression. FASN is a key enzyme for restarting fatty acid synthesis. FASN and AR-FL have been detected in 87% of mCRPC metastases, with AR-V7 being found in 39% of bone metastases, and they were always co-expressed with FASN. FASN/AR-V7 double-positive metastases have been reported in 77% of Abi treatment cases. These findings provide compelling reasons for the use FASN inhibitors in mCRPC, including in those who overexpress AR-V7 [126].
KLK3, FOLH1, and NPY
Results [127] from the Abi-treated cohort revealed that detectable biomarkers (FOLH1, KLK3 and NPY) are associated with short PFS. Patients with negative platelet biomarkers have the best clinical outcomes. FOLH1 and NPY as biomarkers have been shown to have independent predictive values in the multivariate analysis of PFS. Three biomarkers (KLK2, KLK3 and FOLH1) are associated with a short OS. Introducing the three biomarkers of KLK3, FOLH1 and NPY in one panel at the same time can predict long-term and short-term responders with a sensitivity of 87% and a specificity of 82%.
Conclusions
Approval of Abi for mCRPC has greatly enriched the treatment strategy for PC. However, many germline variants have been shown to affect clinical responses in men with advanced PC to systemic treatment. Associations between germline variants such as HSD3B1, SLCO2B1, SULT1E1, CYP17A1, SRD5A2, AR-V7 and clinical responses to ADT in CSPC have been extensively validated in independent cohorts, but gene mutations in the WNT signaling pathway, SPOP, KLK3, FOLH1, NPY and metabolic pathways are worthy of attention.
Since genetic polymorphisms have been shown to exhibit contradictory effects on the clinical outcomes of Abi in the treatment of PC, larger-scale studies should be performed to evaluate genetic polymorphisms of Abi as biomarkers in clinical practice. The correlation between SNPs and treatment outcomes can be used as prognostic and predictive biomarkers for patient stratification and to distinguish between individualized therapy and follow-up plans. Therefore, studies should aim at establishing a corresponding model to determine the influence of the clinical outcomes of genes that can be applied for clinical, individualized treatment.
Acknowledgements
This research was funded by Beijing Hope Run Special Fund of Cancer Foundation of China, grant number NO. LC2018A25; CAMS Innovation Fund for Medical Sciences (CIFMS) (NO. 2016-I2M-1-001).
Disclosure of conflict of interest
None.
References
- 1.Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Ippolito JE, Kane CJ, Kuettel MR, Lang JM, McKenney J, Netto G, Penson DF, Plimack ER, Pow-Sang JM, Pugh TJ, Richey S, Roach M, Rosenfeld S, Schaeffer E, Shabsigh A, Small EJ, Spratt DE, Srinivas S, Tward J, Shead DA, Freedman-Cass DA. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 2.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F, Mainwaring P, Harland S, Goodman OB Jr, Sternberg CN, Li JH, Kheoh T, Haqq CM, de Bono JS COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 3.Logothetis CJ, Basch E, Molina A, Fizazi K, North SA, Chi KN, Jones RJ, Goodman OB, Mainwaring PN, Sternberg CN, Efstathiou E, Gagnon DD, Rothman M, Hao Y, Liu CS, Kheoh TS, Haqq CM, Scher HI, de Bono JS. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13:1210–1217. doi: 10.1016/S1470-2045(12)70473-4. [DOI] [PubMed] [Google Scholar]
- 4.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ, Carles J, Flaig TW, Taplin ME, Higano CS, de Souza P, de Bono JS, Griffin TW, De Porre P, Yu MK, Park YC, Li J, Kheoh T, Naini V, Molina A, Rathkopf DE COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 6.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, De Porre P, Kheoh T, Park YC, Todd MB, Chi KN LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 7.Chi KN, Protheroe A, Rodriguez-Antolin A, Facchini G, Suttman H, Matsubara N, Ye Z, Keam B, Damiao R, Li T, McQuarrie K, Jia B, De Porre P, Martin J, Todd MB, Fizazi K. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol. 2018;19:194–206. doi: 10.1016/S1470-2045(17)30911-7. [DOI] [PubMed] [Google Scholar]
- 8.Buttigliero C, Tucci M, Bertaglia V, Vignani F, Bironzo P, Di Maio M, Scagliotti GV. Understanding and overcoming the mechanisms of primary and acquired resistance to abiraterone and enzalutamide in castration resistant prostate cancer. Cancer Treat Rev. 2015;41:884–892. doi: 10.1016/j.ctrv.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Antonarakis ES. Current understanding of resistance to abiraterone and enzalutamide in advanced prostate cancer. Clin Adv Hematol Oncol. 2016;14:316–319. [PubMed] [Google Scholar]
- 10.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvi S, Conteduca V, Gurioli G, Calistri D, Casadio V, De Giorgi U. Impact of candidate genetic polymorphisms in prostate cancer: an overview. Mol Diagn Ther. 2016;20:1–12. doi: 10.1007/s40291-015-0169-9. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto N, Shiota M, Tomisaki I, Minato A. Gene polymorphism-related individual and interracial differences in the outcomes of androgen deprivation therapy for prostate cancer. Clin Genitourin Cancer. 2017;15:337–342. doi: 10.1016/j.clgc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Johnson E, Nussenzveig R, Agarwal N, Swami U. Germline variants and response to systemic therapy in advanced prostate cancer. Pharmacogenomics. 2020;21:75–81. doi: 10.2217/pgs-2019-0125. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Alyamani M, Li J, Rogacki K, Abazeed M, Upadhyay SK, Balk SP, Taplin ME, Auchus RJ, Sharifi N. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533:547–551. doi: 10.1038/nature17954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Bishop AC, Alyamani M, Garcia JA, Dreicer R, Bunch D, Liu J, Upadhyay SK, Auchus RJ, Sharifi N. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523:347–351. doi: 10.1038/nature14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsiades N, Sung CC, Schultz N, Danila DC, He B, Eedunuri VK, Fleisher M, Sander C, Sawyers CL, Scher HI. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 2012;72:6142–6152. doi: 10.1158/0008-5472.CAN-12-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annala M, Vandekerkhove G, Khalaf D, Taavitsainen S, Beja K, Warner EW, Sunderland K, Kollmannsberger C, Eigl BJ, Finch D, Oja CD, Vergidis J, Zulfiqar M, Azad AA, Nykter M, Gleave ME, Wyatt AW, Chi KN. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018;8:444–457. doi: 10.1158/2159-8290.CD-17-0937. [DOI] [PubMed] [Google Scholar]
- 20.Chung JS, Wang Y, Henderson J, Singhal U, Qiao Y, Zaslavsky AB, Hovelson DH, Spratt DE, Reichert Z, Palapattu GS, Taichman RS, Tomlins SA, Morgan TM. Circulating tumor cell-based molecular classifier for predicting resistance to abiraterone and enzalutamide in metastatic castration-resistant prostate cancer. Neoplasia. 2019;21:802–809. doi: 10.1016/j.neo.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM, Loda M, Witte ON. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 1998;95:1735–40. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, Raitano A, Witte ON, Said JW, Loda M, Reiter RE. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19:1288–96. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- 23.Hara N, Kasahara T, Kawasaki T, Bilim V, Obara K, Takahashi K, Tomita Y. Reverse transcription-polymerase chain reaction detection of prostate-specific antigen, prostate-specific membrane antigen,and prostate stem cell antigen in one milliliter of peripheral blood: value for the staging of prostate cancer. Clin Cancer Res. 2002;8:1794–1799. [PubMed] [Google Scholar]
- 24.Agarwal N, Hahn AW, Gill DM, Farnham JM, Poole AI, Cannon-Albright L. Independent validation of effect of hsd3b1 genotype on response to androgen-deprivation therapy in prostate cancer. JAMA Oncol. 2017;3:856–857. doi: 10.1001/jamaoncol.2017.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiota M, Narita S, Akamatsu S, Fujimoto N, Sumiyoshi T, Fujiwara M, Uchiumi T, Habuchi T, Ogawa O, Eto M. Association of missense polymorphism in HSD3B1 with outcomes among men with prostate cancer treated with androgen-deprivation therapy or abiraterone. JAMA Netw Open. 2019;2:e190115. doi: 10.1001/jamanetworkopen.2019.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen WS, Feng EL, Aggarwal R, Foye A, Beer TM, Alumkal JJ, Gleave M, Chi KN, Reiter RE, Rettig MB, Evans CP, Small EJ, Sharifi N, Zhao SG. Germline polymorphisms associated with impaired survival outcomes and somatic tumor alterations in advanced prostate cancer. Prostate Cancer Prostatic Dis. 2020;23:316–323. doi: 10.1038/s41391-019-0188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn AW, Gill DM, Nussenzveig RH, Poole A, Farnham J, Cannon-Albright L, Agarwal N. Germline variant in HSD3B1 (1245A>C) and response to abiraterone acetate plus prednisone in men with new-onset metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2018;16:288–292. doi: 10.1016/j.clgc.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Almassi N, Reichard C, Li J, Russell C, Perry J, Ryan CJ, Friedlander T, Sharifi N. HSD3B1 and response to a nonsteroidal CYP17A1 inhibitor in castration-resistant prostate cancer. JAMA Oncol. 2018;4:554–557. doi: 10.1001/jamaoncol.2017.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hettel D, Sharifi N. HSD3B1 status as a biomarker of androgen deprivation resistance and implications for prostate cancer. Nat Rev Urol. 2018;15:191–196. doi: 10.1038/nrurol.2017.201. [DOI] [PubMed] [Google Scholar]
- 30.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, Vessella R, Nelson PS, Kapur P, Guo X, Mirzaei H, Auchus RJ, Sharifi N. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aragon IM, Cendón Y, Lorente D, Mejorada RL, Laorden NR, Colmenero AM, Puente J, Villatoro R, Estevez SV, Piulats JM, Lainez N, Fernandez-Parra E, del Alba AG, Jorge AH, Olmos B, Rivera L, Pacheco MI, Hidalgo DO, Castro E. Implications of single nucleotide polymorphisms (SNPs) in androgen related-genes in outcome of metastatic castration-resistant prostate cancer (mCRPC) patients treated with abiraterone (Abi) and enzalutamide (Enza) Ann Oncol. 2019:30. [Google Scholar]
- 32.Emamekhoo H, Alyamani M, Park S, Taylor J, Almassi N, Upadhyay S, Tyler AJ, Berk M, Hwang TH, Grivas P, Rini BI, Garcia JA, Auchus RJ, Sharifi N. HSD3B1 genotype and abiraterone (Abi) metabolites in patients (pts) with prostate cancer (PCa) J. Clin. Oncol. 2018;36:325. [Google Scholar]
- 33.Blanchet B, Carton E, Alyamani M, Golmard L, Huillard O, Thomas-Scheomann A, Vidal M, Goldwasser F, Sharifi N, Alexandre J. A PK/PD study of Delta-4 abiraterone metabolite in metastatic castration-resistant prostate cancer patients. Pharmacol Res. 2018;136:56–61. doi: 10.1016/j.phrs.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 34.van Nuland M, Groenland SL, Bergman AM, Steeghs N, Rosing H, Venekamp N, Huitema ADR, Beijnen JH. Exposure-response analyses of abiraterone and its metabolites in real-world patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2020;23:244–251. doi: 10.1038/s41391-019-0179-5. [DOI] [PubMed] [Google Scholar]
- 35.Hagenbuch B, Stieger B. The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med. 2013;34:396–412. doi: 10.1016/j.mam.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M, Xie W, Mostaghel E, Nakabayashi M, Werner L, Sun T, Pomerantz M, Freedman M, Ross R, Regan M, Sharifi N, Figg WD, Balk S, Brown M, Taplin ME, Oh WK, Lee GS, Kantoff PW. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2011;29:2565–2573. doi: 10.1200/JCO.2010.31.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright JL, Kwon EM, Ostrander EA, Montgomery RB, Lin DW, Vessella R, Stanford JL, Mostaghel EA. Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev. 2011;20:619–627. doi: 10.1158/1055-9965.EPI-10-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Harshman LC, Xie W, Nakabayashi M, Qu F, Pomerantz MM, Lee GS, Kantoff PW. Association of SLCO2B1 genotypes with time to progression and overall survival in patients receiving androgen-deprivation therapy for prostate cancer. J. Clin. Oncol. 2016;34:352–359. doi: 10.1200/JCO.2015.62.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mostaghel EA, Cho E, Zhang A, Alyamani M, Kaipainen A, Green S, Marck BT, Sharifi N, Wright JL, Gulati R, True LD, Loda M, Matsumoto AM, Tamae D, Penning TN, Balk SP, Kantoff PW, Nelson PS, Taplin ME, Montgomery RB. Association of tissue abiraterone levels and SLCO genotype with intraprostatic steroids and pathologic response in men with high-risk localized prostate cancer. Clin Cancer Res. 2017;23:4592–4601. doi: 10.1158/1078-0432.CCR-16-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahn AW, Gill DM, Poole A, Nussenzveig RH, Wilson S, Farnham JM, Stephenson RA, Cannon-Albright LA, Maughan BL, Agarwal N. Germline variant in SLCO2B1 and response to abiraterone acetate plus prednisone (AA) in new-onset metastatic castration-resistant prostate cancer (mCRPC) Mol Cancer Ther. 2019;18:726–729. doi: 10.1158/1535-7163.MCT-18-0739. [DOI] [PubMed] [Google Scholar]
- 41.Giacinti S, Bassanelli M, Roberto M, Strigari L, Nunzio CD, Aschelter AM, Marchetti P. Polymorphisms of the androgen transporting gene SLCO2B1 and response to abiraterone acetate in mCRPC patients. J. Clin. Oncol. 2017;35:174. [Google Scholar]
- 42.Alsinnawi M, Zhang A, Bianchi-Frias D, Burns J, Cho E, Zhang X, Sowalsky A, Ye H, Slee AE, True L, Porter C, Taplin ME, Balk S, Nelson PS, Montgomery RB, Mostaghel EA. Association of prostate cancer SLCO gene expression with Gleason grade and alterations following androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2019;22:560–568. doi: 10.1038/s41391-019-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albany C, Daignault-Newton S, Skaar TC, Ipe J, Siddiqui J, Twardowski P, Stein MN, Kunju LP, Chinnaiyan AM, Montgomery RB, Antonarakis ES, Shevrin DH, Whang YE, Caram MV, Smith DC, Feng FYC, Stadler WM, Hussain M. Genetic polymorphisms to predict progression-free survival in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving abiraterone therapy: results from the NCI 9012 trial. J. Clin. Oncol. 2017;35:145. [Google Scholar]
- 44.Chen Y, Zhou TY, Zhang SF, Chen GP. Biochanin a induction of sulfotransferases in rats. J Biochem Mol Toxicol. 2010;24:102–114. doi: 10.1002/jbt.20318. [DOI] [PubMed] [Google Scholar]
- 45.Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Suzuki T, Fukuda T, Ito A, Endo M, Moriya T, Arai Y, Sasano H. Steroid sulfatase and estrogen sulfotransferase in human prostate cancer. Prostate. 2006;66:1005–1012. doi: 10.1002/pros.20426. [DOI] [PubMed] [Google Scholar]
- 47.Yip CKY, Bansal S, Wong SY, Lau AJ. Identification of galeterone and abiraterone as inhibitors of dehydroepiandrosterone sulfonation catalyzed by human hepatic cytosol, SULT2A1, SULT2B1b, and SULT1E1. Drug Metab Dispos. 2018;46:470–482. doi: 10.1124/dmd.117.078980. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal N, Alex AB, Farnham JM, Patel S, Gill D, Buckley TH, Stephenson RA, Cannon-Albright L. Inherited variants in SULT1E1 and response to abiraterone acetate by men with metastatic castration refractory prostate cancer. J Urol. 2016;196:1112–1116. doi: 10.1016/j.juro.2016.04.079. [DOI] [PubMed] [Google Scholar]
- 49.Buckley TH, Alex A, Farnham JM, Gill D, Patel SB, Teerlink C, Albright FS, Stephenson RA, Cannon-Albright LA, Agarwal N. Association of single nucleotide polymorphisms (SNPs) in SULT1E1 with response to treatment with abiraterone acetate (AA) in men with metastatic castration refractory prostate cancer (mCRPC) J. Clin. Oncol. 2016;34:222. [Google Scholar]
- 50.Auchus RJ. Steroid 17-hydroxylase and 17,20-lyase deficiencies, genetic and pharmacologic. J Steroid Biochem Mol Biol. 2017;165:71–78. doi: 10.1016/j.jsbmb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porubek D. CYP17A1: a biochemistry, chemistry, and clinical review. Curr Top Med Chem. 2013;13:1364–1384. doi: 10.2174/1568026611313120002. [DOI] [PubMed] [Google Scholar]
- 52.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salvi S, Casadio V, Conteduca V, Burgio SL, Menna C, Bianchi E, Rossi L, Carretta E, Masini C, Amadori D, Calistri D, Attard G, De Giorgi U. Circulating cell-free AR and CYP17A1 copy number variations may associate with outcome of metastatic castration-resistant prostate cancer patients treated with abiraterone. Br J Cancer. 2015;112:1717–1724. doi: 10.1038/bjc.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Binder M, Zhang BY, Hillman DW, Kohli R, Kohli T, Lee A, Kohli M. Common genetic variation in CYP17A1 and response to abiraterone acetate in patients with metastatic castration-resistant prostate cancer. Int J Mol Sci. 2016;17:1097. doi: 10.3390/ijms17071097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borchiellini D, Mahammedi H, Viotti J, Gravis G, Roubaud G, Beuzeboc P, Largillier R, Linassier C, Khalil A, Kaphan R, Joly F, Eymard JC, Falkowski S, Bompas E, Zanetta S, Schiappa R, Maurin M, Etienne-Grimaldi MC, Milano GA, Ferrero JM. Association of single nucleotide polymorphisms (SNPs) in CYP17A1 and SLCO2B1 genes and clinical outcome in metastatic castration-resistant prostate cancer patients treated with abiraterone acetate: results of the ABIGENE prospective study. J. Clin. Oncol. 2018;36:358. [Google Scholar]
- 56.Salvi S, Casadio V, Burgio SL, Conteduca V, Rossi L, Menna C, Carretta E, Costantini M, Zoli W, De Giorgi U. CYP17A1 polymorphisms and clinical outcome of castration-resistant prostate cancer patients treated with abiraterone. Int J Biol Markers. 2016;31:e264–269. doi: 10.5301/jbm.5000197. [DOI] [PubMed] [Google Scholar]
- 57.Yepuru M, Wu Z, Kulkarni A, Yin F, Barrett CM, Kim J, Steiner MS, Miller DD, Dalton JT, Narayanan R. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin Cancer Res. 2013;19:5613–5625. doi: 10.1158/1078-0432.CCR-13-1151. [DOI] [PubMed] [Google Scholar]
- 58.Liu C, Lou W, Yang J, Pan CX, Dall’Era M, Evans C, Gao A. MP57-19 targeting akr1c3 activation by indomethacin overcomes resistance to enzalutamide and abiraterone. J Urol. 2017;197:e771. [Google Scholar]
- 59.Hofland J, van Weerden WM, Dits NF, Steenbergen J, van Leenders GJ, Jenster G, Schroder FH, de Jong FH. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70:1256–1264. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- 60.Tian Y, Zhao L, Zhang H, Liu X, Zhao L, Zhao X, Li Y, Li J. AKR1C3 overexpression may serve as a promising biomarker for prostate cancer progression. Diagn Pathol. 2014;9:42. doi: 10.1186/1746-1596-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu C, Armstrong CM, Lou W, Lombard A, Evans CP, Gao AC. Inhibition of AKR1C3 activation overcomes resistance to abiraterone in advanced prostate cancer. Mol Cancer Ther. 2017;16:35–44. doi: 10.1158/1535-7163.MCT-16-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fankhauser M, Tan Y, Macintyre G, Haviv I, Hong MK, Nguyen A, Pedersen JS, Costello AJ, Hovens CM, Corcoran NM. Canonical androstenedione reduction is the predominant source of signaling androgens in hormone-refractory prostate cancer. Clin Cancer Res. 2014;20:5547–5557. doi: 10.1158/1078-0432.CCR-13-3483. [DOI] [PubMed] [Google Scholar]
- 63.Hamid AR, Pfeiffer MJ, Verhaegh GW, Schaafsma E, Brandt A, Sweep FC, Sedelaar JP, Schalken JA. Aldo-keto reductase family 1 member C3 (AKR1C3) is a biomarker and therapeutic target for castration-resistant prostate cancer. Mol Med. 2013;18:1449–1455. doi: 10.2119/molmed.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jernberg E, Thysell E, Bovinder Ylitalo E, Rudolfsson S, Crnalic S, Widmark A, Bergh A, Wikstrom P. Characterization of prostate cancer bone metastases according to expression levels of steroidogenic enzymes and androgen receptor splice variants. PLoS One. 2013;8:e77407. doi: 10.1371/journal.pone.0077407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pfeiffer MJ, Smit FP, Sedelaar JP, Schalken JA. Steroidogenic enzymes and stem cell markers are upregulated during androgen deprivation in prostate cancer. Mol Med. 2011;17:657–664. doi: 10.2119/molmed.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Powell K, Semaan L, Conley-LaComb MK, Asangani I, Wu YM, Ginsburg KB, Williams J, Squire JA, Maddipati KR, Cher ML, Chinni SR. ERG/AKR1C3/AR constitutes a feed-forward loop for AR signaling in prostate cancer cells. Clin Cancer Res. 2015;21:2569–2579. doi: 10.1158/1078-0432.CCR-14-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byrns MC, Mindnich R, Duan L, Penning TM. Overexpression of aldo-keto reductase 1C3 (AKR1C3) in LNCaP cells diverts androgen metabolism towards testosterone resulting in resistance to the 5alpha-reductase inhibitor finasteride. J Steroid Biochem Mol Biol. 2012;130:7–15. doi: 10.1016/j.jsbmb.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3β-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3/17-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 69.Hardy DO, Ge RS, Catterall JF, Hou YT, Penning TM, Hardy MP. Identification of the oxidative 3β-hydroxysteroid dehydrogenase activity of rat leydig cells as type ii retinol dehydrogenase. Endocrinology. 2000;141:1608–1617. doi: 10.1210/endo.141.5.7445. [DOI] [PubMed] [Google Scholar]
- 70.Tamae D, Mostaghel E, Montgomery B, Nelson PS, Balk SP, Kantoff PW, Taplin ME, Penning TM. The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer. Chem Biol Interact. 2015;234:332–338. doi: 10.1016/j.cbi.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao J, Zhang M, Liu J, Liu Z, Shen P, Nie L, Guo W, Cai D, Liu J, Armstrong CM, Sun G, Chen J, Zhu S, Dai J, Zhang H, Zhao P, Zhang X, Yin X, Zhu X, Ni Y, Chen N, Zeng H. AKR1C3 expression in primary lesion rebiopsy at the time of metastatic castration-resistant prostate cancer is strongly associated with poor efficacy of abiraterone as a first-line therapy. Prostate. 2019;79:1553–1562. doi: 10.1002/pros.23875. [DOI] [PubMed] [Google Scholar]
- 72.Zhao J. The value of AKR1C3 in predicting the therapeutic efficacy of abiraterone for patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2019;37:313. [Google Scholar]
- 73.Ni Y, Zhao J, Chen J, Sun G, Zhu S, Zhang X, Dai J, Wang Z, Zhang H, Zhu X, Chen N, Shen P, Zeng H. The effect of AKR1C3 on the switch from prednisone to dexamethasone in metastatic castration-resistant prostate cancer patients receiving abiraterone. J. Clin. Oncol. 2020;38:133. [Google Scholar]
- 74.Romero-Laorden N, Lozano R, Jayaram A, Lopez-Campos F, Saez MI, Montesa A, Gutierrez-Pecharoman A, Villatoro R, Herrera B, Correa R, Rosero A, Pacheco MI, Garces T, Cendon Y, Nombela MP, Van de Poll F, Grau G, Rivera L, Lopez PP, Cruz JJ, Lorente D, Attard G, Castro E, Olmos D. Phase II pilot study of the prednisone to dexamethasone switch in metastatic castration-resistant prostate cancer (mCRPC) patients with limited progression on abiraterone plus prednisone (SWITCH study) Br J Cancer. 2018;119:1052–1059. doi: 10.1038/s41416-018-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shiota M, Fujimoto N, Yokomizo A, Takeuchi A, Kashiwagi E, Dejima T, Kiyoshima K, Inokuchi J, Tatsugami K, Eto M. The prognostic impact of serum testosterone during androgen-deprivation therapy in patients with metastatic prostate cancer and the SRD5A2 polymorphism. Prostate Cancer Prostatic Dis. 2016;19:191–196. doi: 10.1038/pcan.2016.2. [DOI] [PubMed] [Google Scholar]
- 76.Shiota M, Fujimoto N, Yokomizo A, Takeuchi A, Itsumi M, Inokuchi J, Tatsugami K, Uchiumi T, Naito S. SRD5A gene polymorphism in Japanese men predicts prognosis of metastatic prostate cancer with androgen-deprivation therapy. Eur J Cancer. 2015;51:1962–1969. doi: 10.1016/j.ejca.2015.06.122. [DOI] [PubMed] [Google Scholar]
- 77.Fernald RD, White RB. Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front Neuroendocrinol. 1999;20:224–240. doi: 10.1006/frne.1999.0181. [DOI] [PubMed] [Google Scholar]
- 78.Shiota M, Fujimoto N, Takeuchi A, Kashiwagi E, Dejima T, Inokuchi J, Tatsugami K, Yokomizo A, Kajioka S, Uchiumi T, Eto M. The association of polymorphisms in the gene encoding gonadotropin-releasing hormone with serum testosterone level during androgen deprivation therapy and prognosis of metastatic prostate cancer. J Urol. 2018;199:734–740. doi: 10.1016/j.juro.2017.09.076. [DOI] [PubMed] [Google Scholar]
- 79.Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, Etzioni R, Bolouri H, Montgomery B, White T, Lucas JM, Brown LG, Dumpit RF, DeSarkar N, Higano C, Yu EY, Coleman R, Schultz N, Fang M, Lange PH, Shendure J, Vessella RL, Nelson PS. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22:369–378. doi: 10.1038/nm.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakazawa M, Antonarakis ES, Luo J. Androgen receptor splice variants in the era of enzalutamide and abiraterone. Horm Cancer. 2014;5:265–273. doi: 10.1007/s12672-014-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, Bergh A, Wikstrom P. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Z, Chen S, Sowalsky AG, Voznesensky OS, Mostaghel EA, Nelson PS, Cai C, Balk SP. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res. 2014;20:1590–1600. doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, Vakar-Lopez F, Vessella RL, Plymate SR. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS One. 2011;6:e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamamoto Y, Loriot Y, Beraldi E, Zhang F, Wyatt AW, Al Nakouzi N, Mo F, Zhou T, Kim Y, Monia BP, MacLeod AR, Fazli L, Wang Y, Collins CC, Zoubeidi A, Gleave M. Generation 2.5 antisense oligonucleotides targeting the androgen receptor and its splice variants suppress enzalutamide-resistant prostate cancer cell growth. Clin Cancer Res. 2015;21:1675–1687. doi: 10.1158/1078-0432.CCR-14-1108. [DOI] [PubMed] [Google Scholar]
- 87.Dehm SM, Tindall DJ. Alternatively spliced androgen receptor variants. Endocr Relat Cancer. 2011;18:R183–196. doi: 10.1530/ERC-11-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carreira S, Romanel A, Goodall J, Grist E, Ferraldeschi R, Miranda S, Prandi D, Lorente D, Frenel JS, Pezaro C, Omlin A, Rodrigues DN, Flohr P, Tunariu N, S de Bono J, Demichelis F, Attard G. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med. 2014;6:254ra125. doi: 10.1126/scitranslmed.3009448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romanel A, Gasi Tandefelt D, Conteduca V, Jayaram A, Casiraghi N, Wetterskog D, Salvi S, Amadori D, Zafeiriou Z, Rescigno P, Bianchini D, Gurioli G, Casadio V, Carreira S, Goodall J, Wingate A, Ferraldeschi R, Tunariu N, Flohr P, De Giorgi U, de Bono JS, Demichelis F, Attard G. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re310. doi: 10.1126/scitranslmed.aac9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen EJ, Sowalsky AG, Gao S, Cai C, Voznesensky O, Schaefer R, Loda M, True LD, Ye H, Troncoso P, Lis RL, Kantoff PW, Montgomery RB, Nelson PS, Bubley GJ, Balk SP, Taplin ME. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin Cancer Res. 2015;21:1273–1280. doi: 10.1158/1078-0432.CCR-14-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 92.Henzler C, Li Y, Yang R, McBride T, Ho Y, Sprenger C, Liu G, Coleman I, Lakely B, Li R, Ma S, Landman SR, Kumar V, Hwang TH, Raj GV, Higano CS, Morrissey C, Nelson PS, Plymate SR, Dehm SM. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat Commun. 2016;7:13668. doi: 10.1038/ncomms13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao H, Coram MA, Nolley R, Reese SW, Young SR, Peehl DM. Transcript levels of androgen receptor variant AR-V1 or AR-V7 do not predict recurrence in patients with prostate cancer at indeterminate risk for progression. J Urol. 2012;188:2158–2164. doi: 10.1016/j.juro.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 94.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bernemann C, Schnoeller TJ, Luedeke M, Steinestel K, Boegemann M, Schrader AJ, Steinestel J. Expression of AR-V7 in circulating tumour cells does not preclude response to next generation androgen deprivation therapy in patients with castration resistant prostate cancer. Eur Urol. 2017;71:1–3. doi: 10.1016/j.eururo.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 96.Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, Arora KS, Desai N, Dahl DM, Sequist LV, Smith MR, Kapur R, Wu CL, Shioda T, Ramaswamy S, Ting DT, Toner M, Maheswaran S, Haber DA. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351–6. doi: 10.1126/science.aab0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakazawa M, Lu C, Chen Y, Paller CJ, Carducci MA, Eisenberger MA, Luo J, Antonarakis ES. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol. 2015;26:1859–1865. doi: 10.1093/annonc/mdv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJ, Mathijssen RH, Hamberg P, Meulenbeld HJ, De Laere B, Dirix LY, van Soest RJ, Lolkema MP, Martens JW, van Weerden WM, Jenster GW, Foekens JA, de Wit R, Sleijfer S. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015;68:939–945. doi: 10.1016/j.eururo.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 99.Saylor PJ, Lee RJ, Arora KS, Deshpande V, Hu R, Olivier K, Meneely E, Rivera MN, Ting DT, Wu CL, Miyamoto DT. Branched chain RNA in situ hybridization for androgen receptor splice variant AR-V7 as a prognostic biomarker for metastatic castration-sensitive prostate cancer. Clin Cancer Res. 2017;23:363–369. doi: 10.1158/1078-0432.CCR-16-0237. [DOI] [PubMed] [Google Scholar]
- 100.Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, Miccoli M, Galli L, Falcone A, Jenster GW, van Schaik RH, Danesi R. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. Eur Urol. 2017;71:680–687. doi: 10.1016/j.eururo.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 101.Qu F, Xie W, Nakabayashi M, Zhang H, Jeong SH, Wang X, Komura K, Sweeney CJ, Sartor O, Lee GM, Kantoff PW. Association of AR-V7 and prostate specific antigen RNA levels in blood with efficacies of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clin Cancer Res. 2017;23:726–734. doi: 10.1158/1078-0432.CCR-16-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Todenhofer T, Azad A, Stewart C, Gao J, Eigl BJ, Gleave ME, Joshua AM, Black PC, Chi KN. AR-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. J Urol. 2017;197:135–142. doi: 10.1016/j.juro.2016.06.094. [DOI] [PubMed] [Google Scholar]
- 103.Li H, Wang Z, Tang K, Zhou H, Liu H, Yan L, Guan W, Chen K, Xu H, Ye Z. Prognostic value of androgen receptor splice variant 7 in the treatment of castration-resistant prostate cancer with next generation androgen receptor signal inhibition: a systematic review and meta-analysis. Eur Urol Focus. 2018;4:529–539. doi: 10.1016/j.euf.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 104.Sepe P, Verzoni E, Miodini P, Claps M, Ratta R, Martinetti A, Mennitto R, Sottotetti E, Procopio G, Cappelletti V, Daidone MG. Could circulating tumor cells and arv7 detection improve clinical decisions in metastatic castration-resistant prostate cancer? The istituto nazionale dei tumori (INT) experience. Cancers (Basel) 2019;11:980. doi: 10.3390/cancers11070980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daniel M, Dehm SM. Lessons from tissue compartment-specific analysis of androgen receptor alterations in prostate cancer. J Steroid Biochem Mol Biol. 2017;166:28–37. doi: 10.1016/j.jsbmb.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chan SC, Selth LA, Li Y, Nyquist MD, Miao L, Bradner JE, Raj GV, Tilley WD, Dehm SM. Targeting chromatin binding regulation of constitutively active AR variants to overcome prostate cancer resistance to endocrine-based therapies. Nucleic Acids Res. 2015;43:5880–5897. doi: 10.1093/nar/gkv262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, Gao AC. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin Cancer Res. 2014;20:3198–3210. doi: 10.1158/1078-0432.CCR-13-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pal SK, Patel J, He M, Foulk B, Kraft K, Smirnov DA, Twardowski P, Kortylewski M, Bhargava V, Jones JO. Identification of mechanisms of resistance to treatment with abiraterone acetate or enzalutamide in patients with castration-resistant prostate cancer (CRPC) Cancer. 2018;124:1216–1224. doi: 10.1002/cncr.31161. [DOI] [PubMed] [Google Scholar]
- 109.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–711. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kohli M, Ho Y, Hillman DW, Van Etten JL, Henzler C, Yang R, Sperger JM, Li Y, Tseng E, Hon T, Clark T, Tan W, Carlson RE, Wang L, Sicotte H, Thai H, Jimenez R, Huang H, Vedell PT, Eckloff BW, Quevedo JF, Pitot HC, Costello BA, Jen J, Wieben ED, Silverstein KAT, Lang JM, Wang L, Dehm SM. Androgen receptor variant AR-V9 is coexpressed with AR-V7 in prostate cancer metastases and predicts abiraterone resistance. Clin Cancer Res. 2017;23:4704–4715. doi: 10.1158/1078-0432.CCR-17-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kohli M, Wang L, Dehm S, Hillman DW, Sicotte H, Gormley M, Bhargava V, Ricci DS, Li W, Tan W, Costello BA, Pitot HC, Dronca RS, Ho TH, Bryce AH, Zhenqing Y, Vedell PT, Barman P, Carlson R, Wang L. Association of Wnt pathway activation with prechemotherapy abiraterone acetate resistance in metastatic castration-resistant prostate cancer (mCRPC) by genome-wide analysis of metastases. J. Clin. Oncol. 2017;35:175. [Google Scholar]
- 112.Kato S, Hayakawa Y, Sakurai H, Saiki I, Yokoyama S. Mesenchymal-transitioned cancer cells instigate the invasion of epithelial cancer cells through secretion of WNT3 and WNT5B. Cancer Sci. 2014;105:281–289. doi: 10.1111/cas.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hjorth-Jensen K, Maya-Mendoza A, Dalgaard N, Sigurethsson JO, Bartek J, Iglesias-Gato D, Olsen JV, Flores-Morales A. SPOP promotes transcriptional expression of DNA repair and replication factors to prevent replication stress and genomic instability. Nucleic Acids Res. 2018;46:9484–9495. doi: 10.1093/nar/gky719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi Q, Zhu Y, Ma J, Chang K, Ding D, Bai Y, Gao K, Zhang P, Mo R, Feng K, Zhao X, Zhang L, Sun H, Jiao D, Chen Y, Sun Y, Zhao SM, Huang H, Li Y, Ren S, Wang C. Prostate cancer-associated SPOP mutations enhance cancer cell survival and docetaxel resistance by upregulating Caprin1-dependent stress granule assembly. Mol Cancer. 2019;18:170. doi: 10.1186/s12943-019-1096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N, Nickerson E, Chae SS, Boysen G, Auclair D, Onofrio RC, Park K, Kitabayashi N, MacDonald TY, Sheikh K, Vuong T, Guiducci C, Cibulskis K, Sivachenko A, Carter SL, Saksena G, Voet D, Hussain WM, Ramos AH, Winckler W, Redman MC, Ardlie K, Tewari AK, Mosquera JM, Rupp N, Wild PJ, Moch H, Morrissey C, Nelson PS, Kantoff PW, Gabriel SB, Golub TR, Meyerson M, Lander ES, Getz G, Rubin MA, Garraway LA. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Theurillat JP, Udeshi ND, Errington WJ, Svinkina T, Baca SC, Pop M, Wild PJ, Blattner M, Groner AC, Rubin MA, Moch H, Prive GG, Carr SA, Garraway LA. Prostate cancer. Ubiquitylome analysis identifies dysregulation of effector substrates in SPOP-mutant prostate cancer. Science. 2014;346:85–89. doi: 10.1126/science.1250255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, Zimmermann M, Bond R, Shou J, Li C, Blattner M, Lonard DM, Demichelis F, Coarfa C, Rubin MA, Zhou P, O’Malley BW, Mitsiades N. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc Natl Acad Sci U S A. 2013;110:6997–7002. doi: 10.1073/pnas.1304502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.An J, Ren S, Murphy SJ, Dalangood S, Chang C, Pang X, Cui Y, Wang L, Pan Y, Zhang X, Zhu Y, Wang C, Halling GC, Cheng L, Sukov WR, Karnes RJ, Vasmatzis G, Zhang Q, Zhang J, Cheville JC, Yan J, Sun Y, Huang H. Truncated ERG oncoproteins from TMPRSS2-ERG fusions are resistant to SPOP-mediated proteasome degradation. Mol Cell. 2015;59:904–916. doi: 10.1016/j.molcel.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 119.Boysen G, Rodrigues DN, Rescigno P, Seed G, Dolling D, Riisnaes R, Crespo M, Zafeiriou Z, Sumanasuriya S, Bianchini D, Hunt J, Moloney D, Perez-Lopez R, Tunariu N, Miranda S, Figueiredo I, Ferreira A, Christova R, Gil V, Aziz S, Bertan C, de Oliveira FM, Atkin M, Clarke M, Goodall J, Sharp A, MacDonald T, Rubin MA, Yuan W, Barbieri CE, Carreira S, Mateo J, de Bono JS. SPOP-mutated/CHD1-deleted lethal prostate cancer and abiraterone sensitivity. Clin Cancer Res. 2018;24:5585–5593. doi: 10.1158/1078-0432.CCR-18-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qin S, Liu D, Kohli M, Wang L, Vedell PT, Hillman DW, Niu N, Yu J, Weinshilboum RM, Wang L. TSPYL family regulates CYP17A1 and CYP3A4 expression: potential mechanism contributing to abiraterone response in metastatic castration-resistant prostate cancer. Clin Pharmacol Ther. 2018;104:201–210. doi: 10.1002/cpt.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vaillancourt J, Turcotte V, Caron P, Villeneuve L, Lacombe L, Pouliot F, Levesque E, Guillemette C. Glucuronidation of abiraterone and its pharmacologically active metabolites by UGT1A4, influence of polymorphic variants and their potential as inhibitors of steroid glucuronidation. Drug Metab Dispos. 2020;48:75–84. doi: 10.1124/dmd.119.088229. [DOI] [PubMed] [Google Scholar]
- 122.FDA. Clinical pharmacology and biopharmaceutics review: zytiga (abiraterone acetate) Silver Spring: US FDA; 2010. pp. 1–86. [Google Scholar]
- 123.Acharya M, Gonzalez M, Mannens G, De Vries R, Lopez C, Griffin T, Tran N. A phase I, open-label, single-dose, mass balance study of 14C-labeled abiraterone acetate in healthy male subjects. Xenobiotica. 2013;43:379–389. doi: 10.3109/00498254.2012.721022. [DOI] [PubMed] [Google Scholar]
- 124.European Medicines Agency. Zytiga-H-C-2321-PSUV-19. European public assessment report: scientific conclusions and grounds recommending the variation to the terms of the marketing authorisation. London: European Medicines Agency; 2014. pp. 1–11. [Google Scholar]
- 125.European Medicines Agency. European Public Assessment Report (EPAR): zytiga (abiraterone acetate) London: European Medicines Agency; 2016. [Google Scholar]
- 126.Zadra G, Ribeiro CF, Chetta P, Ho Y, Cacciatore S, Gao X, Syamala S, Bango C, Photopoulos C, Huang Y, Tyekucheva S, Bastos DC, Tchaicha J, Lawney B, Uo T, D’Anello L, Csibi A, Kalekar R, Larimer B, Ellis L, Butler LM, Morrissey C, McGovern K, Palombella VJ, Kutok JL, Mahmood U, Bosari S, Adams J, Peluso S, Dehm SM, Plymate SR, Loda M. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2019;116:631–640. doi: 10.1073/pnas.1808834116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tjon-Kon-Fat LA, Lundholm M, Schröder M, Wurdinger T, Thellenberg-Karlsson C, Widmark A, Wikström P, Nilsson RJA. Platelets harbor prostate cancer biomarkers and the ability to predict therapeutic response to abiraterone in castration resistant patients. Prostate. 2018;78:48–53. doi: 10.1002/pros.23443. [DOI] [PubMed] [Google Scholar]


