Abstract
BACKGROUND:
Cabozantinib, a tyrosine kinase inhibitor of FMS-like tyrosine kinase 3 (FLT3), MET, AXL, vascular endothelial growth factor receptor, and KIT, is approved for use in multiple malignancies. We assessed the safety and tolerability of cabozantinib in AML, given up-regulation of multiple relevant pathways.
METHODS:
Adults were eligible if they were 18 years old or older with relapsed/refractory AML or if they were 70 years old or older with newly diagnosed AML but were ineligible for conventional therapy. Cabozantinib was administered in 28-day cycles, and dose escalation occurred via cohorts. A pharmacodynamic evaluation of serial plasma samples via a plasma inhibitory assay (PIA) was used to assess FLT3-inhibitory activity in FLT3-mutant cell lines.
RESULTS:
Among 18 patients enrolled, 5 were found to harbor FLT3/ITD mutations. Sixteen patients (89%) had relapsed/refractory AML, and most were treated with 2 or more lines of prior treatment. No dose-limiting toxicities (DLTs) were detected at the first dose level (40 mg daily), but 2 patients experienced DLTs at the next level (60 mg daily). The remaining patients were then dosed at 40 mg daily, the maximum tolerated dose (MTD). Additional grade 2 or higher toxicities, possibly/probably related to cabozantinib, included fatigue, nausea, transaminitis, and electrolyte imbalance. No patients had a marrow response according to formal criteria, but 4 had peripheral blast reductions; 2 of these 4 patients transiently cleared circulating blasts. One patient experienced a reduction in marrow blasts, and 1 had stable disease. The FLT3-inhibitory activity of plasma samples, as assessed with the PIA, revealed potent and sustained inhibition in FLT3/ITD and, notably, F691 tyrosine kinase domain (TKD)-mutant cells.
CONCLUSIONS:
Cabozantinib is well tolerated in AML patients at an MTD of 40 mg daily and is a potent inhibitor of FLT3/ITD- and F691 TKD-altered tyrosine kinases.
Keywords: acute myeloid leukemia, FMS-like tyrosine kinase 3 (FLT3), resistance mutations, targeted therapies, tyrosine kinase inhibitors
INTRODUCTION
The successful treatment of acute myeloid leukemia (AML) remains a daunting challenge. Although the majority of adult patients under the age of 60 years achieve a complete remission with conventional cytotoxic chemotherapy, the long-term survival rate remains poor at approximately 30% to 40%.1,2 The prognosis is even worse for patients with higher risk AML, including older patients and those with secondary disease, such as that arising from antecedent myeloid neoplasms.1,3 There is, therefore, an urgent need for novel and effective therapeutic approaches to enhance current paradigms and improve outcomes.
In the last few years, a variety of targeted therapies for AML, including agents inhibiting FMS-like tyrosine kinase 3 (FLT3)4–8 and isocitrate dehydrogenase enzymes,9,10 have emerged with remarkable therapeutic promise. FLT3-activating mutations, consisting of internal tandem duplication (ITD) and tyrosine kinase domain (TKD) variants, affect approximately a third of AML patients and are traditionally associated with proliferative disease, frequent relapses, and poor outcomes.11–13 Most studied FLT3 tyrosine kinase inhibitors potently inhibit autophosphorylation of the FLT3/ITD receptor tyrosine kinase. However, a lesser number effectively inhibit the various FLT3/TKD variants. This is a challenging prospect because these latter mutations often arise as resistance alterations after initial FLT3-targeted therapy.14,15 Nevertheless, a series of inhibitors known to inhibit the FLT3 D835 variant are currently under study.4,16
Cabozantinib is a potent and multitargeted tyrosine kinase inhibitor of FLT3, MET, vascular endothelial growth factor receptor 2 (VEGFR2), and KIT.17,18 It has been extensively studied in multiple solid tumors and is Food and Drug Administration (FDA) approved for use in renal cell and medullary thyroid carcinomas.19–21 However, despite its multiple relevant targets, it has not been previously studied in AML. We assessed the tolerability and safety of cabozantinib in AML and, in this context, investigated its range of activity in FLT3-mutant cell lines to gain an appreciation of its therapeutic promise.
MATERIALS AND METHODS
Clinical Trial Accrual and Treatment
This study was approved by the local institutional review board, was registered at ClinicalTrials.gov (NCT01961765), and was conducted in accordance with the Declaration of Helsinki. Patients were eligible for enrollment if they were 18 years or older with relapsed/refractory AML or if they were 70 years old or older with newly diagnosed disease but were ineligible for or declined conventional therapy. Participants were required to have an Eastern Cooperative Oncology Group performance status of 0 or 1; a cardiac ejection fraction greater than or equal to 50%; and intact organ function, as manifested by aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels less than 3 times the upper limit of normal, a direct bilirubin level less than 1.5 mg/dL, a lipase level less than 2.0 times the upper limit of normal, and a creatinine clearance greater than or equal to 50 mL/min. In addition, because of the range of enzymes inhibited by cabozantinib, which includes VEGFR2, patients with severe hypertension, cavitary lung lesions or significant hemoptysis, active peptic ulcer disease, bowel inflammation, an obstruction or perforation, or recent major surgeries were excluded.
Standard cytogenetic and molecular testing, including testing for FLT3/ITD, FLT3/TKD, nucleophosmin 1 (NPM1), and CCAAT/enhancer-binding protein α (CEBPA), was performed at diagnosis. The study treatment required a white blood cell count less than 30,000/mm3, but concurrent treatment with hydroxyurea was allowed to control the white blood cell count during the first 15 days of the first cycle. The first dose level was 40 mg by mouth daily, a dose previously demonstrated to be relatively well tolerated by patients with solid tumor malignancies. Cabozantinib was administered in 28-day cycles, and dose escalation occurred via 3 cohorts (40, 60, and 80 mg daily). The escalation to each successive dose level was guided by the incidence of dose-limiting toxicities (DLTs) during cycle 1 of the treatment. The definition of a DLT included any grade 3 or higher nonhematologic toxicity (with the exceptions of nausea, vomiting, and diarrhea lasting less than 7 days and oral mucositis, which was required to be grade 4 to be considered a DLT). DLTs also included any grade 2 or higher nonhematologic toxicity that was intolerable or rendered patients unable to take 75% or more of the assigned doses, any grade 4 hematologic toxicity lasting at least 42 days with a nonleukemic marrow cellularity ≤ 5%, and death.
The response was determined by bone marrow biopsy after the first cycle of treatment, and any patient achieving a complete or partial remission or showing stable disease and a clinical benefit could continue in the study until disease progression, intolerable toxicity, or the receipt of 12 cycles.
Correlative Studies
Molm14 cells were obtained from the DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany). The Ba/F3 cell line was transfected with the specific point mutations D835Y and F691L, as described previously.16 All cell lines were cultured with RPMI (Roswell Park Memorial Institute) medium with 10% fetal bovine serum, penicillin/streptomycin, and 2 mM l-glutamine at 37 °C in 5% carbon dioxide. The Ba/F3 ITD/D835Y point mutant was cultured with 1 ng/mL recombinant human interleukin 3.
A pharmacodynamic evaluation of serially obtained patient plasma samples was performed via the use of a plasma inhibitory assay (PIA) to assess FLT3-inhibitory activity in FLT3/ITD cell lines. Plasma samples were collected before dosing, during cycle 1 on days 1, 2, 8, 15, and 28, and during cycle 2 on days 15 and 25. The plasma samples were kept at −80 °C. For each patient, 1 mL of plasma was incubated with Molm14 cells for 1 hour at 37 °C. As described previously,22 immunoprecipitation and immunoblotting were used to analyze the cells for FLT3 phosphorylation. Densitometry was used to compare the relative volumes of the bands for FLT3 phosphorylation with samples from day 1 of cycle 1 as a baseline.
Dose-response studies were performed with Molm14, Ba/F3 ITD/F691L, and Ba/F3 ITD/D835Y cells treated with different doses of cabozantinib or sorafenib in either 1 mL of media or plasma. FLT3 phosphorylation was analyzed via immunoprecipitation and immunoblotting as previously described.
Statistics
Toxicities of all grades in the study, regardless of attribution, were classified overall and by dose cohort. The efficacy of cabozantinib was assessed for the total number of patients enrolled at the maximum tolerated dose (MTD). Overall survival was defined as the time from the date of registration to death and was censored at the last timepoint the patient was known to be alive. Overall survival was estimated with the Kaplan-Meier method. The median survival was estimated with a 90% confidence interval.
RESULTS
Eighteen patients were enrolled. The median age at registration was 68 years (range, 27-85 years), with the majority of the patients being male (67%) and white (89%). The mutational status was not known for 3 patients. FLT3/ITD mutations were detected in 5 patients (23%), and 1 of these patients had a concurrent FLT3/TKD D835 mutation; NPM1 and CEBPA mutations were detected in no patients (Table 1). Sixteen patients (89%) had relapsed/refractory AML, and 2 (11%) had newly diagnosed disease. The majority of those with relapsed/refractory AML (75%) had been treated with 2 or more lines of prior treatment. Overall, 3 patients received hydroxyurea as a cytoreductive measure during the study.
TABLE 1.
Patient Characteristics (n = 18)
| Characteristic | Value |
|---|---|
| Dose level, No. (%) | |
| 40 mg | 15 (83) |
| 60 mg | 3 (17) |
| Age at registration, median (range), y | 67.8 (27.0-85.1) |
| Sex, No. (%) | |
| Female | 6 (33) |
| Male | 12 (67) |
| Race, No. (%) | |
| White | 16 (89) |
| Asian | 1 (6) |
| >1 race | 1 (6) |
| Ethnicity, No. (%) | |
| Non-Hispanic | 15 (83) |
| Ethnicity not known | 2 (11) |
| Hispanic or Latino | 1 (6) |
| Antecedent myeloid neoplasm, No. (%) | 6 (33) |
| CMML | 1 (6) |
| MDS | 4 (22) |
| MPN | 1 (6) |
| Baseline mutation, No. (%)a | |
| FLT3/ITDb | 5 (33) |
| FLT3/TKDb | 1 (7) |
| NPM1 | 0 (0) |
| CEBPA | 0 (0) |
| Otherc | 5 (33) |
Abbreviations: CMML, chronic myelomonocytic leukemia; FLT3, FMS-like tyrosine kinase 3; ITD, internal tandem duplication; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NPM1, nucleophosmin 1; TKD, tyrosine kinase domain.
Cases 6, 16, and 17 had an unknown mutational status.
One patient demonstrated both ITD and TKD mutations.
Other mutations included NRAS (n = 3), KRAS, SF3B1, NRAS, WT1, and TP53.
Three patients were enrolled at the first dose level (40 mg daily), at which no DLT was detected. One patient at this dose level experienced grade 4 transaminitis, which was deemed unrelated to cabozantinib, but the patient could not be dosed for this reason and was, therefore, replaced. Two of the 3 patients who enrolled at the second dose level (60 mg daily) experienced a DLT: one with grade 3 pancreatitis and the other with grade 3 transaminitis. Three patients were then enrolled at the next lower dose level (40 mg daily); 2 of these patients could not complete the DLT period, one because of disease progression and the other because of an infected aortic ulceration unrelated to the study treatment. These 2 patients were replaced, and no additional DLTs were noted in the remaining patients. The dose of 40 mg daily was, therefore, determined to be the MTD, and 6 additional patients were subsequently treated at this dose in an expansion cohort. Other grade 2 or higher nonhematologic toxicities that were possibly or probably related to cabozantinib, including fatigue, nausea, anorexia, constipation, oral mucositis, hypertension, palmar-plantar erythrodysesthesia, and transaminitis, affected 5 or fewer patients (Table 2), with the most common being nausea (5 of 18 or 28%) and hypertension (5 of 18 or 28%). Grade 2 palmar-plantar erythrodysesthesia affected 2 patients. The most common grade 2 or higher adverse events during the study in each cohort, regardless of attribution, are also provided in Table 3.
TABLE 2.
Attributable Grade 2 or Higher Toxicities, Regardless of the Cohort
| Grade 2 | Grade 3 | Grade 4 | ||||
|---|---|---|---|---|---|---|
| Toxicity Description | No. | % | No. | % | No. | % |
| Anemia | 3 | 17 | 1 | 6 | ||
| Anorexia | 2 | 11 | 1 | 6 | ||
| Bilirubinemia | 1 | 6 | ||||
| Constipation | 1 | 6 | ||||
| Fatigue | 1 | 6 | 2 | 11 | ||
| Febrile neutropenia | 3 | 17 | ||||
| Hypertension | 4 | 22 | 1 | 6 | ||
| Oral mucositis | 2 | 11 | ||||
| Nausea | 5 | 28 | ||||
| Neutropenia | 1 | 6 | 2 | 11 | ||
| Palmar-plantar erythrodysesthesia | 2 | 11 | ||||
| Pancreatitis | 1 | 6 | ||||
| Thrombocytopenia | 1 | 6 | 2 | 11 | ||
| Transaminitis | 2 | 11 | 1 | 6 | ||
TABLE 3.
Grade 2 or Higher Toxicities, Regardless of Attribution, by Dose Level
| Dose Level 1 (n = 9) | Dose Level 2 (n = 3) | Expansion (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Toxicity Description | Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 3 |
| Anemia | 2 | 1 | 1 | ||||||
| Anorexia | 2 | 1 | |||||||
| Aortic injury | 1 | ||||||||
| Apnea | 1 | ||||||||
| Back pain | 1 | 1 | |||||||
| Bilirubinemia | 1 | ||||||||
| Catheter-related infection | 1 | ||||||||
| Colitis | 1 | ||||||||
| Constipation | 1 | ||||||||
| Dehydration | 1 | ||||||||
| Delirium | 1 | ||||||||
| Disseminated intravascular coagulation | 1 | 1 | |||||||
| Dyspnea | 1 | ||||||||
| Prolonged QT interval | 1 | ||||||||
| Epistaxis | 1 | ||||||||
| Fatigue | 1 | 1 | 1 | ||||||
| Febrile neutropenia | 1 | 2 | |||||||
| Generalized muscle weakness | 1 | ||||||||
| Hyperglycemia | 1 | ||||||||
| Hypertension | 3 | 1 | 1 | ||||||
| Hypokalemia | 1 | ||||||||
| Hyponatremia | 1 | ||||||||
| Hypophosphatemia | 1 | 1 | |||||||
| Lower gastrointestinal hemorrhage | 1 | ||||||||
| Lung infection | 2 | 1 | 1 | ||||||
| Nausea | 3 | 2 | |||||||
| Neutropenia | 1 | 1 | 1 | ||||||
| Oral mucositis/bleeding | 2 | 2 | |||||||
| Palmar-plantar erythrodysesthesia | 1 | 1 | |||||||
| Pancreatitis | 1 | ||||||||
| Rectal pain | 1 | ||||||||
| Transaminitis | 1 | 1 | 1 | 1 | |||||
| Thrombocytopenia | 1 | 1 | 1 | ||||||
Sixteen patients could be assessed for a response during the study. No patients had a marrow response according to formal criteria. Four patients had a reduction in peripheral blasts during treatment, and 2 of these patients transiently cleared their circulating blasts (1 with an FLT3/ITD mutation). The reductions in peripheral blasts for these patients are represented in Figure 1. Three of the 4 patients, including the FLT3-mutant patient (patient 4 in Fig. 1), experienced reductions in blasts by day 14, and the remaining patient had a reduction in blasts by day 28. Two of the 4 patients were noted to have recurrent increases in peripheral blasts by day 49, and the other 2 were taken off the protocol-based therapy by their treating physician, despite a persistent reduction in peripheral blasts, because of persistent disease in the marrow. One of these 4 patients also experienced a reduction in marrow blasts (patient 1). Patients 1 and 4 also had slight increases in hemoglobin and platelets during the time of, and concurrent with, the reduction in their peripheral blasts. An additional patient experienced stable marrow disease. None of these patients had received concurrent hydroxyurea. Sixteen patients (89%) died either during the study or during the follow-up period. The median survival for all enrolled patients was 3.88 months (90% confidence interval, 1.12-4.73 months), and the median survival for those enrolled at the MTD was also 3.88 months (90% confidence interval, 0.92-5.33 months).
Figure 1.

Cabozantinib treatment led to a reduction in circulating blasts in 4 patients in the study, as represented here. Patient 4 harbored harbored a FLT3/ITD mutation.
In addition, FLT3-inhibitory activity, as determined with the ex vivo PIA of serially collected patient plasma samples, consistently revealed potent and sustained inhibition of FLT3 autophosphorylation in FLT3/ITD-mutant cell lines by day 8 of cycle 1 (Fig. 2). Additional studies revealed that cabozantinib had very high potency under both medium and plasma conditions (with half-maximal inhibitory concentration [IC50] values of 0.6 and 34 nM, respectively) against FLT3/ITD (Fig. 3). This degree of potency is akin to that demonstrated with the potent and selective FLT3/ITD inhibitor quizartinib23 (with IC50 values of 1 and 18 nM, respectively). Cabozantinib did not reveal any activity against the FLT3 D835 TKD point mutant cells in media (Fig. 4) but, intriguingly, was potently active against the F691L-mutant BAF3 cell line under both medium and plasma conditions with an IC50 value of 2.86nM in media, which was significantly lower than that of other studied FLT3 inhibitors16 (Fig. 5A). Cabozantinib also effectively suppressed FLT3/F691L in serial plasma samples from patients in the study, as assessed with the PIA, with potent inhibition by day 8 (Fig. 5B).
Figure 2.
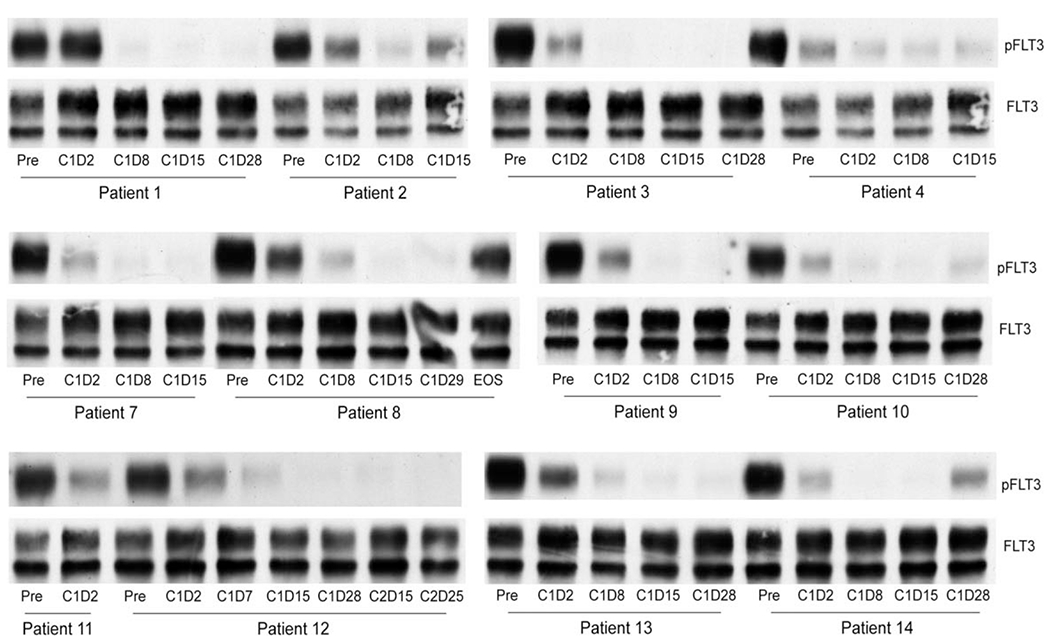
Cabozantinib suppressed FLT3-internal tandem duplication autophosphorylation in all samples assessed with the plasma inhibitor assay. FLT3 indicates FMS-like tyrosine kinase 3; pFLT3, phosphorylated FMS-like tyrosine kinase 3.
Figure 3.

CZT demonstrated high potency against FLT3/internal tandem duplication under medium and plasma conditions. CZT indicates cabozantinib; FLT3, FMS-like tyrosine kinase 3; IC50, half-maximal inhibitory concentration; pFLT3, phosphorylated FMS-like tyrosine kinase 3.
Figure 4.

Like sorafenib, cabozantinib does not have potent activity against FLT3/D835Y variant receptor tyrosine kinase. FLT3 indicates FMS-like tyrosine kinase 3; pFLT3, phosphorylated FMS-like tyrosine kinase 3.
Figure 5.

(A) In contrast to other studied FLT3 inhibitors, CZT has significantly more potent activity against the Ba/F3 internal tandem duplication/F691L-mutant cell line under both medium and plasma conditions. (B) Cabozantinib potently suppressed FLT3/F691L in samples assessed with the plasma inhibitory assay. CZT indicates cabozantinib; FLT3, FMS-like tyrosine kinase 3; IC50, half-maximal inhibitory concentration; pFLT3, phosphorylated FMS-like tyrosine kinase 3.
DISCUSSION
We herein report the results of a phase 1 trial of the multikinase inhibitor cabozantinib in patients with AML. Cabozantinib is a potent inhibitor of multiple relevant tyrosine kinase targets in human malignancies and, after extensive clinical study, is now approved for use by the FDA for the treatment of advanced renal cell and medullary thyroid carcinomas. In our population of predominantly relapsed/refractory AML patients, we found that cabozantinib was relatively well tolerated with an MTD of 40 mg daily. Two DLTs—grade 3 pancreatitis and grade 3 transaminitis—were seen at a dose of 60 mg daily. Although marrow responses were not identified by formal criteria, 4 patients, including 1 patient with a FLT3/ITD mutation, had reductions in leukemic blasts in the peripheral blood or marrow.
It is noteworthy that the approved recommended doses for the use of cabozantinib in renal cell and medullary thyroid carcinomas, 60 and 140 mg daily, respectively, are higher than the determined MTD in this study of AML. However, in trials of solid tumor malignancies, dose reductions due to toxicity have been common at higher doses of cabozantinib. In the randomized phase 3 clinical trial of cabozantinib for medullary thyroid carcinoma, despite dosing being initiated at 140 mg daily, 65% required dosing holds, and 79% required dose reductions. In that study, up to 2 dose reductions were allowed down to a minimum of 60 mg daily.24 Similarly, in randomized phase 2 and 3 studies of cabozantinib for renal cell carcinoma, with patients starting at a dose of 60 mg daily, dose reductions occurred in 58% and 60% of patients, respectively, because of adverse events.21,25 In this light, it is perhaps not surprising that the maximum tolerated dose for patients with relapsed or refractory AML, who would be expected to have a more profound acute comorbidity burden than those with solid tumor malignancies, would be lower. Nevertheless, we found that the MTD dose of 40 mg daily was well tolerated in our patient population, with 15 patients being administered this dose with no DLT. Whether a higher dose of cabozantinib would have led to a higher response rate is unclear. It is also possible that a different dosing schedule (eg, 3 weeks of treatment followed by 1 week off vs the continuous-dosing approach studied on this trial) would allow a higher dose of cabozantinib to be administered. This can perhaps be tested in future advanced-phase safety and efficacy trials of cabozantinib.
Importantly, we were able to further characterize the range of activity of cabozantinib as a FLT3 inhibitor, which is particularly relevant to the treatment of AML. Approximately a third of patients harbor FLT3-activating mutations, the majority of which are ITD alterations, with the rest being point mutations affecting the TKD of the enzyme.14,15,26,27 Both mutation types, FLT3/ITD and FLT3/TKD, lead to constitutive activation of the enzyme, but only the former is clearly associated with a poorer prognosis in AML.26,27 Over the last decade, multiple inhibitors of FLT3-activating mutations have emerged and have been studied in clinical trials with varying degrees of selectivity for the FLT3 target.4–6,8 Midostaurin, a less specific FLT3 inhibitor, was recently shown to improve survival when it was combined with conventional chemotherapy for newly diagnosed FLT3-mutant patients, and it is now approved for use in this setting.8 Potent and selective FLT3 inhibitors such as quizartinib, crenolanib, and gilteritinib all lead to impressive responses as monotherapy in patients with FLT3 mutations.4–6
However, there is a differential pattern of activity for FLT3 inhibitors against the various TKD point mutations, and this finding is increasingly relevant in the emerging era of FLT3-targeted therapies. TKD point mutations can exist at diagnosis but can also emerge as a mechanism of resistance after initial FLT3-inhibitor therapy for ITD-mutant disease. Patients treated with potent FLT3 inhibitors such as sorafenib and quizartinib for FLT3/ITD AML have been demonstrated to develop secondary TKD point mutations that are associated with disease progression and therapeutic resistance.28,29 The majority of mutations are D835 alterations, but other resistant TKD variants, including the F691 alteration, are increasingly being detected. Several FLT3 inhibitors, including crenolanib, midostaurin, and gilteritinib, have activity against D835 FLT3/TKD AML,16,30 whereas other compounds, such as quizartinib and sorafenib, have minimal activity against this point mutation. In addition, with very limited exceptions,31 most FLT3 inhibitors currently under study do not effectively inhibit the F691 TKD alteration,16,32 which is also increasingly being seen after initial FLT3-targeted therapy. As a result, patients with FLT3/TKD AML upon presentation or disease progression may respond only to certain FLT3 inhibitors,4,16 most of which are currently unavailable for conventional use.
Our data suggest that cabozantinib is highly active against FLT3/ITD cell lines but also remarkably active, as demonstrated by the PIA and on Ba/F3 mutant cell lines, against the F691-altered enzyme. The IC50 against that variant for cabozantinib in media, 2.86 nM, is significantly lower than that seen with other studied FLT3/TKD inhibitors such as crenolanib and gilteritinib. In contrast, cabozantinib did not show efficacy in its inhibition of D835-mutant cell lines. Among the 5 FLT3-mutant patients in our study, 1 demonstrated a peripheral clearance of leukemic blasts after 4 weeks. However, it is important to note that our population was heavily pretreated; 1 FLT3/ITD patient was inevaluable and was replaced early in the study; and another patient harbored both FLT3/ITD and FLT3/TKD/D835 mutations, the latter of which appears to be resistant to inhibition by cabozantinib.
The potent inhibition of the TKD F691 FLT3 variant is an important and potentially clinically impactful finding. Cabozantinib is now approved by the FDA for use in multiple solid tumor malignancies. FLT3-mutant AML patients who progress beyond initial lines of therapy because of the emergence of resistant F691 point mutations have limited conventional and trial-based options. For such individuals, cabozantinib could be further explored as a therapeutic approach for treating FLT3-mutant disease because of its potency in inhibiting both ITD and this specific TKD alteration. Although the current clinical trial, being a small dose-escalation study, was limited by its size and scope, future and larger efficacy trials of cabozantinib can further explore this potential therapeutic promise by specifically studying patients with FLT3-mutant AML.
In summary, the tyrosine kinase inhibitor cabozantinib is well tolerated at a dose of 40 mg daily in patients with AML, with the most common related toxicities being nausea and transaminitis. Unlike other potent FLT3 inhibitors currently under study, cabozantinib also effectively inhibits the FLT3/TKD/F691 resistance mutation and may, therefore, play a role in future therapeutic paradigms.
Acknowledgments
FUNDING SUPPORT
Donna S. Neuberg reports a Dana-Farber/Harvard Cancer Center Support Grant (5P30 CA006516) during the conduct of the study.
CONFLICT OF INTEREST DISCLOSURES
Amir T. Fathi has participated in advisory boards for Agios and Pfizer; has provided consulting for Seattle Genetics, Amgen, Medimmune, and Celgene; and has received grant support to conduct clinical trials from Takeda, Celgene, Exelixis, and Seattle Genetics. Traci M. Blonquist has received a salary partly supported by a grant from the pharmaceutical industry paid to the Dana-Farber Cancer Institute pediatric acute lymphoblastic leukemia consortium. Malgorzata McMasters reports that her spouse is employed by Merck & Co, Inc, and Vertex Pharmaceuticals. Gabriela Hobbs has received grant support to conduct clinical trials from Bayer, Kura, and Stemline. Andrew M. Brunner reports clinical trial support from Celgene, Takeda, and Novartis. Mark J. Levis has provided consulting for Novartis, Daiichi, Astellas, Arog, and Agios and reports grants from Novartis and Astellas.
REFERENCES
- 1.Karp JE, Smith MA. The molecular pathogenesis of treatment-induced (secondary) leukemias: foundations for treatment and prevention. Semin Oncol. 1997;24:103–113. [PubMed] [Google Scholar]
- 2.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. [DOI] [PubMed] [Google Scholar]
- 3.Bao T, Smith BD, Karp JE. New agents in the treatment of acute myeloid leukemia: a snapshot of signal transduction modulation. Clin Adv Hematol Oncol. 2005;3:287–296. [PubMed] [Google Scholar]
- 4.Altman JK, Perl AE, Cortes JE, et al. Antileukemic activity and tolerability of ASP2215 80 mg and greater in FLT3 mutation-positive subjects with relapsed or refractory acute myeloid leukemia: results from a phase 1/2, open-label, dose-escalation/dose-response study [abstract 321]. Blood. 2015;126:321. [Google Scholar]
- 5.Levis MJ, Perl AE, Dombret H, et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation [abstract 673]. Blood. 2012;120:673. [Google Scholar]
- 6.Randhawa JK, Kantarjian HM, Borthakur G, et al. Results of a phase II study of crenolanib in relapsed/refractory acute myeloid leukemia patients (pts) with activating FLT3 mutations [abstract 389]. Blood. 2014;124:389. [Google Scholar]
- 7.Rollig C, Serve H, Huttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16:1691–1699. [DOI] [PubMed] [Google Scholar]
- 8.Stone RM, Mandrekar S, Sanford BL, et al. The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose C consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18-60 with FLT3 mutations (muts): an international prospective randomized (rand) P-controlled double-blind trial (CALGB 10603/RATIFY [Alliance]) [abstract 6]. Blood. 2015;126:6.26138538 [Google Scholar]
- 9.DiNardo CD, de Botton S, Stein EM, et al. Determination of IDH1 mutational burden and clearance via next-generation sequencing in patients with IDH1 mutation–positive hematologic malignancies receiving AG-120, a first-in-class inhibitor of mutant IDH1 [abstract 1070]. Blood 2016;128:1070. [Google Scholar]
- 10.Stein E, DiNardo C, Altman JK, et al. Safety and efficacy of AG-221, a potent inhibitor of mutant IDH2 that promotes differentiation of myeloid cells in patients with advanced hematologic malignancies: results of a phase 1/2 trial [abstract 323]. Blood. 2015;126:323. [Google Scholar]
- 11.Fathi AT, Chabner BA. FLT3 inhibition as therapy in acute myeloid leukemia: a record of trials and tribulations. Oncologist. 2011;16:1162–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. [DOI] [PubMed] [Google Scholar]
- 13.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983–988. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. [DOI] [PubMed] [Google Scholar]
- 16.Galanis A, Ma H, Rajkhowa T, et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood. 2014;123:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grullich C Cabozantinib: a MET, RET, and VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res. 2014;201:207–214. [DOI] [PubMed] [Google Scholar]
- 18.Tannir NM, Schwab G, Grunwald V. Cabozantinib: an active novel multikinase inhibitor in renal cell carcinoma. Curr Oncol Rep. 2017;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–927. [DOI] [PubMed] [Google Scholar]
- 22.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108:3477–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levis M Quizartinib for the treatment of FLT3/ITD acute myeloid leukemia. Future Oncol. 2014;10:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. [DOI] [PubMed] [Google Scholar]
- 27.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. [DOI] [PubMed] [Google Scholar]
- 28.Moore AS, Faisal A, Gonzalez de Castro D, et al. Selective FLT3 inhibition of FLT3-ITD + acute myeloid leukaemia resulting in secondary D835Y mutation: a model for emerging clinical resistance patterns. Leukemia. 2012;26:1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry EV, Clark JJ, Cools J, Roesel J, Gilliland DG. Uniform sensitivity of FLT3 activation loop mutants to the tyrosine kinase inhibitor midostaurin. Blood. 2007;110:4476–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zirm E, Spies-Weisshart B, Heidel F, et al. Ponatinib may overcome resistance of FLT3-ITD harbouring additional point mutations, notably the previously refractory F691I mutation. Br J Haematol. 2012;157:483–492. [DOI] [PubMed] [Google Scholar]
- 32.Williams AB, Nguyen B, Li L, et al. Mutations of FLT3/ITD confer resistance to multiple tyrosine kinase inhibitors. Leukemia. 2013;27:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


