Abstract
Substance use disorders are highly prevalent and continue to be one of the leading causes of disability in the world. Notably, not all people who use addictive drugs develop a substance use disorder. Although substance use disorders are highly heritable, patterns of inheritance cannot be explained purely by Mendelian genetic mechanisms. Vulnerability to developing drug addiction depends on the interplay between genetics and environment. Additionally, evidence from the past decade has pointed to the role of epigenetic inheritance in drug addiction. This emerging field focuses on how environmental perturbations, including exposure to addictive drugs, induce epigenetic modifications that are transmitted to the embryo at fertilization and modify developmental gene expression programs to ultimately impact subsequent generations. This chapter highlights intergenerational and transgenerational phenotypes in offspring following a history of parental drug exposure. Special attention is paid to parental preconception exposure studies of five drugs of abuse (alcohol, cocaine, nicotine, cannabinoids, and opiates) and associated behavioral and physiological outcomes in offspring. The highlighted studies demonstrate that parental exposure to drugs of abuse has enduring effects that persist into subsequent generations. Understanding the contribution of epigenetic inheritance in drug addiction may provide clues for better treatments and therapies for substance use disorders.
1. Introduction
Substance use disorder is a chronic relapsing psychiatric disorder characterized by compulsive use of drugs despite harmful consequences. Addictions are a worldwide human crisis and negatively impact society as well as the families of the abusers (Goldman, Oroszi, & Ducci, 2005). According to the National Survey on Drug Use and Health, 19.3 million Americans aged 18 or older had a substance use disorder in 2018 (Substance Abuse and Mental Health Services Administration, 2019). Drug addiction typically develops in the following stages: initiation, occasional to regular use, dependence, addiction, and relapse (Nielsen, Utrankar, Reyes, Simons, & Kosten, 2012). There are limited effective clinical options available to recover from addiction and there is always a risk for relapse despite years of abstinence. Thus, a better understanding of the processes and biological mechanisms of addiction will help to develop therapies, treatments, and preventive strategies.
Susceptibility to drug addiction is dependent on genetic and environmental factors (Bevilacqua & Goldman, 2009). Evidence from twin and adoption studies has established that addictions have moderate to high heritability that ranges from 39% to 72% (Ducci & Goldman, 2012). Human genome-wide association studies have identified several genes and allelic variants linked with drug addiction (Ducci & Goldman, 2012; Li & Burmeister, 2009), however these genetic variants only account for small proportions of total heritability. This suggests a source of missing inherited information, a phenomenon known as “missing heritability” (Eichler et al., 2010). While there are several possible explanations for missing heritability, a substantial body of evidence supports a role of epigenetics by demonstrating that environmental perturbations induce epigenetic modifications that can be inherited by the next generation. The term epigenetics is defined as the changes in gene expression without alterations in DNA sequence (Bird, 2007). The root of “epigenetics” is derived from the Greek prefix “epi” meaning “above” or “over,” which suggests an additional layer of heritable, regulatory modifiers above the genome. Several human epidemiological studies provide evidence for multigenerational epigenetic inheritance. For instance, 1944–45 Dutch Famine Cohort studies demonstrated that individuals who were exposed prenatally to famine had offspring with increased body weight and body mass index in adulthood (Painter et al., 2008; Veenendaal et al., 2013). The Överkalix study in northern Sweden has reported that longevity of grandchildren was determined by the paternal grandparent’s food supply (Pembrey et al., 2006). Increased vulnerability for psychiatric disorders and post-traumatic stress disorder has been demonstrated in the offspring of female Holocaust survivors (Yehuda, Bell, Bierer, & Schmeidler, 2008). A recent study reported that fathers who were prisoners during the Civil War were more likely to have sons with an increased mortality rate (Costa, Yetter, & DeSomer, 2018). Certainly, these human studies raised the possibility of intergenerational epigenetic inheritance, but they are merely observational, retrospective, difficult to interpret, and lack underlying mechanisms. Therefore, animal studies being conducted under strict environmental conditions, with isogenic backgrounds, have been useful to establish the contribution of epigenetic inheritance to disease risk.
A wide range of animal studies have clearly demonstrated that maternal and paternal environmental insults (such as over/undernutrition, stress, irradiation, traumatic experiences, age of onset, smoking, drug abuse) can negatively impact the quality of gametes, cause epigenetic modifications, and influence the subsequent generations [for excellent reviews, (Day, Savani, Krempley, Nguyen, & Kitlinska, 2016; Lacal & Ventura, 2018; Morgan & Watkins, 2019; van Otterdijk & Michels, 2016)]. Animal studies have provided ample evidence for environmentally induced epigenetic modifications in parental gametes that are passed to offspring, and even grand-offspring, where they can influence gene expression programs. However, the mechanisms of how these epigenetic modifications are sustained between different tissues and through multiple generations remains unclear.
When considering epigenetic inheritance, it is important to clarify the terms intergenerational vs transgenerational inheritance. If males (Fig. 1A) or females (Fig. 1B) are exposed to an environmental stressor prior to mating, all tissues of these F0 animals, including the gametes that will create the F1 generation, are directly exposed. Because the gametes are directly exposed, phenotypes in the F1 generation are considered to be intergenerational. In contrast, the germ cells in the developing F1 animals are not directly exposed to the environmental stressor. Therefore, phenotypes observed in the F2 and beyond generations are considered as transgenerational. When a pregnant female (F0) is exposed to an environmental stressor (Fig. 1C), not only is the female exposed, but all cells of the F1 fetus including the germ cells of the F1 fetus (generally primordial germ cells develop around embryonic day 7.25 (Saitou & Yamaji, 2012)) that will ultimately form the F2 generation are also exposed. The whole process and the pattern of germ cell maturation can be vulnerable to environmental insults (Bale, 2014). Therefore, following exposure of a pregnant female, phenotypes observed in the F1 and F2 offspring constitute intergenerational epigenetic inheritance. Phenotypes observed in the F3 generation are the first generation representing purely transgenerational epigenetic inheritance where that individual or the germ cells that formed that individual had not been directly exposed to the environmental stressor (van Otterdijk & Michels, 2016).
Fig. 1.

Modes of epigenetic inheritance. Phenotypes observed in F1 offspring of males (A) or females (B) prior to mating represent intergenerational effects, while effects observed in F2 and beyond are considered as transgenerational. (C) When a pregnant female is exposed to a drug, her fetus (F1) and its developing germ-line (F2) will be directly be exposed to the drug and therefore phenotypes observed in F1 and F2 offspring represent intergenerational effects. The F3 generation is the first representing true transgenerational epigenetic inheritance following in utero drug exposure. Red stars represent germ cells that were directly exposed to drug. Green stars represent germ cells that developed in the absence of direct drug exposure.
Over the last decade research on epigenetics and drug addiction has advanced rapidly, implicating several mechanisms for mediating how drug exposure modifies the epigenome and thereby alters gene expression within and across generations. This chapter begins with a brief description of epigenetic mechanisms and their role in gene regulation. Next, we review the available literature supporting intergenerational and transgenerational inheritance of behavioral and neurobiological differences associated with a parental history of drug exposure. The focus will be on studies of five prevalent drugs of abuse (alcohol, cocaine, nicotine, cannabinoids, and opioids). While gestational drug exposure has wide ranging effects and several confounds (including direct in utero drug exposure, impact of maternal diet and metabolism, postnatal care, etc.), the current chapter will be limited to studies addressing maternal and paternal drug-exposure prior to mating on the health and behavior of subsequent generations.
2. Overview of epigenetics
The term epigenetics was first introduced in 1942 by Conrad Waddington, as “the study of the mechanisms which explains the causal interactions between genotype and phenotype in the context of development” (Waddington, 1942). Over the following years, various researchers have tried to explain, define, and redefine the term epigenetics resulting into the evolution of the concept (Burggren, 2016). For in depth reviews on the subject, see (Deans & Maggert, 2015; Deichmann, 2016; Nicoglou, 2018). It was not until 1958, another biologist, David Nanney explained epigenetics as “systems controlling the expression of the “specificities library” (that is, the genetic material, DNA or RNA sequence)” (Nanney, 1958). Riggs and Holliday in 1975, independently proposed that DNA methylation influences gene expression and these effects persisted through mitosis (Holliday & Pugh, 1975; Riggs, 1975). However, none of them used the term epigenetics while explaining DNA methylation and they said nothing about the possibility that such DNA methylation patterns could be “heritable” through meiosis too (Nicoglou & Merlin, 2017). In 1994, Holliday provided two definitions of epigenetics, first stated as “the study of the changes in gene expression, which occur in organisms with differentiated cells, and the mitotic inheritance of given patterns of gene expression” and second “nuclear inheritance which is not based on differences in DNA sequence” (Holliday, 1994). In 1996, Russo, Martienssen and Riggs finally streamlined Holliday’s definition of epigenetics as, “the study of mitotically and/or meiotically heritable changes in gene function that cannot be explained by changes in DNA sequence” (Fincham, 1997). There was some debate about whether the definition of epigenetics needs to involve a heritability concept across cell divisions. It has been shown that epigenetic traits can be propagated through meiosis in plants and nematodes (John & Rougeulle, 2018). However, there’s a lot of controversy about whether this is valid for mammals as there is widespread epigenetic reprogramming which includes the near complete erasure of epigenetic marks followed by replacement with a different set of marks (Morgan, Santos, Green, Dean, & Reik, 2005).
Thus, new efforts have been made by some researchers to redefine epigenetics in order to remove the requirement of heritability. In 2007, Bird stated epigenetics as “the structural adaptation of chromosomal regions so as to register, signal or perpetuate altered activity states” (Bird, 2007). Deans and Maggert in 2015 proposed that epigenetics is “the study of mechanisms that cause chromosome-bound, heritable changes to gene expression that are not dependent on changes to DNA sequence” (Deans & Maggert, 2015). Recently, epigenetics is redefined as “the regulatory mechanisms that can perpetuate differential gene expression in the context of same DNA sequence” (Cavalli & Heard, 2019). Overall, the concept of epigenetics has evolved over the years but the definition of epigenetics is still under debate.
Epigenetic mechanisms are involved in several biological processes, including genomic imprinting, X-chromosome silencing, cell differentiation, DNA repair and replication, and the maintenance of genomic stability (Qureshi & Mehler, 2018). The epigenetic mechanisms include histone post-translational modifications, incorporation of histone variants, nucleosome repositioning, DNA methylation and hydroxymethylation, and inheritance of non-coding RNAs (ncRNAs) (John & Rougeulle, 2018). These layers of genomic regulation all work in concert to tightly control gene expression so cellular homeostasis can be maintained (Rivera & Bennett, 2010; Weinhold, 2006). Here, we describe the three major epigenetic mechanisms at play (Fig. 2).
Fig. 2.

Schematics of epigenetic mechanisms of gene regulation. (A) Histone modifications, (B) DNA methylation and (C) non-coding RNA-based pathways constitute three distinct epigenetic mechanisms that are directly impacted by drug exposure and may be involved in the persistent effects of drugs of abuse across generations.
2.1. Histone post-translational modifications
Histones are basic proteins found in eukaryotic cells that are utilized in the nucleus to compact chromosomal DNA (Bannister & Kouzarides, 2011). DNA wraps around eight core histones (dimers of H2A, H2B, H3, and H4) forming structural units referred to as nucleosomes (Bannister & Kouzarides, 2011). Histone epigenetic modifications are covalent, reversible post-translational modifications added onto protein residues (Sadakierska-Chudy & Filip, 2015). In general, histone modifications are catalyzed by particular enzymes that act mostly at histone N-terminal tails, thereby affecting the overall chromatin structure (Alaskhar Alhamwe et al., 2018). Modifications of these tails affect the inter-nucleosomal interactions, thereby impacting overall chromatin structure. Based on differential compaction and functional status, it has been shown that interphase nuclei in eukaryotic cells possess two distinct types of chromatin: euchromatin, a relaxed and transcriptionally active form, and heterochromatin, a more compact and transcriptionally inactive form (Nair, Shoaib, & Sørensen, 2017).
Among numerous types of histone modifications, methylation, acetylation, and phosphorylation are the most studied modifications involved in regulation of transcriptional activity and chromatin reorganization (Swygert & Peterson, 2014). Histone acetylation decreases chromatin compaction, making the DNA more accessible to regulatory proteins and resulting in transcriptional activation (Vaissiere, Sawan, & Herceg, 2008). Histone methylation can cause either gene activation or repression depending on the amino acid residues undergoing modification (Yohn, Bartolomei, & Blendy, 2015). Histone phosphorylation is generally associated with transcriptional activation and mainly known for its contribution to DNA repair in response to cell damage (Alaskhar Alhamwe et al., 2018). Histone ubiquitination varies considerably from the other modifications since it is a very large modification with the ubiquitin moiety comprising of a 76-amino acid polypeptide. It has been demonstrated that ubiquitination of histones along with other histone modifications are inter-connected and act in combination to regulate transcription (Cao & Yan, 2012).
Over the last few years, new histone post-translational modifications such as hydroxylation (addition of 5-hydroxylysine), acylation (addition of acyl groups) and monoaminylation (the covalent linkage of monoamines) to histones have been discovered (reviewed in Chan & Maze, 2020). Most recently, novel modifications like histone serotonylation (covalent linkage of serotonin to histone H3 by transglutaminase 2) (Farrelly et al., 2019) and dopaminylation (addition of dopamine to histone H3) (Lepack et al., 2020) have been identified by a research team led by Dr. Ian Maze. Specifically, histone dopaminylation has been found to be involved in cocaine-induced transcriptional plasticity in the ventral tegmental area (Lepack et al., 2020). However, as this field is emerging and little is known about novel histone modifications, further studies examining the effects across generations would certainly provide inputs in addiction science.
Apart from histone modifications, an alternative means of chromatin remodeling is the incorporation and exchange of histone variants. Histone variants are isoforms of core histones and are found mainly in families of histones H3 and H2A (Henikoff & Smith, 2015). Nucleosomes can be modified by the replacement of canonical histones with histone variants (e.g., H2A.X, H2A.Z, H3.3), that differ in structure and function and result in different post-translational modifications, leading to modification of chromatin architecture and activity of the genomic region such as transcription and DNA repair (Aristizabal et al., 2019; Qureshi & Mehler, 2018). Incorporation of some of the histone variants into nucleosomes may also lead to nucleosome repositioning or remodeling (Henikoff & Smith, 2015; Syed et al., 2009). Nucleosome repositioning at gene promoters is an important aspect of the regulation of gene expression. It is mediated by ATP-dependent chromatin-remodeling enzymes and involves alterations in histone–DNA interactions and plays a role in changing DNA accessibility (Becker & Workman, 2013). The remodeling of nucleosomes may make DNA sequences more accessible to proteins or, conversely, stimulate packing into closely folded structures (Becker & Workman, 2013). Thus, further studies involving structure, function and metabolism of histone variants will provide a basis for understanding the role of chromatin remodeling in various epigenetic and cellular processes (Henikoff & Smith, 2015).
2.2. DNA methylation
DNA methylation is a widespread process by which a methyl group (−CH3) is reversibly incorporated onto DNA cytosines (Jangra et al., 2016; Rivera & Bennett, 2010). This covalent modification on DNA occurs at cytosine guanine dinucleotides (CpG) regions within the genome (Rivera & Bennett, 2010). Methylation of CpG dinucleotides within the gene promoters results in transcriptional repression, whereas unmethylated promoters are transcriptionally active. DNA methylation affects gene expression by preventing the binding of transcription factors to the promoter region and/or by promoting the binding of methyl-CpG-binding proteins, which hamper the access of transcription factors to DNA. These DNA binding proteins can also recruit enzymes that catalyze post-transcriptional modification of histones leading to transcription repression (Mahnke, Miranda, & Homanics, 2017; Rivera & Bennett, 2010). This additional layer of gene expression control is associated with a myriad of key processes such as genomic imprinting, X-chromosome inactivation, repression of transposable elements, and carcinogenesis (Bannister & Kouzarides, 2011; Rivera & Bennett, 2010).
DNA hydroxymethylation (5-hmC), is another type of DNA modification in which the hydrogen atom at the C5-position in cytosine is replaced by a hydroxymethyl group. It is generated through oxidation of 5-methyl cytosine and is considered a possible intermediate step in a DNA demethylation pathway (Richa & Sinha, 2014). It is highly expressed in brain and other tissues of CNS and mostly associated with transcription activation (Shi, Ali, Tang, & Yang, 2017).
2.3. Non-coding RNA regulation
As technologies improve there has been a shift in the understanding of RNA function. While ~75–85% of the genome is transcribed, protein coding RNA (i.e., mRNA) only represents ~2% of the genome (Mayfield, 2017; Rivera & Bennett, 2010). Non-coding RNAs (ncRNAs) are emerging as a diverse class of regulatory molecules that have a wide range of functions (Farris, Arasappan, Hunicke-Smith, Harris, & Mayfield, 2015; Mayfield, 2017). Such ncRNA subtypes include micro RNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA), and are known to regulate gene expression by acting as transcriptional or translational inducers or suppressors (Mayfield, 2017; Yu & Kuo, 2019).
In parallel to DNA epigenetics, there is also an emerging field termed “RNA epitranscriptomics” (Kahl, 2015; Molinie & Giallourakis, 2017). Epitranscriptomics refers to post-transcriptional modifications of RNA that can modulate RNA structure, localization, function, and expression (Dinescu et al., 2019; Kahl, 2015). This provides yet another layer of control by mammalian cells to keep molecular regulation under tight control. One of the most widely studied and characterized RNA epitranscriptomic modifications is the methylation of adenosine at the nitrogen-6 position, yielding the most abundant base modification of RNA, N6-methyladenoside (m6A). The m6A modification is dynamic and can affect various stages of RNA metabolism, such as structure, localization, splicing, translation, and degradation (Li et al., 2019).
3. Drug-induced epigenetic modifications
Studies have shown that epigenetic mechanisms (discussed above) play an important role in the neuroplastic alterations associated with substance abuse. For example, both acute and chronic exposure to cocaine induces histone modifications in the nucleus accumbens (NAc) in rats and is thought to contribute to behavioral neuroadaptation (Kumar et al., 2005; Wang et al., 2010). Acute ethanol exposure in rodents has been associated with altered histone modifications in the amygdala (Pandey, Ugale, Zhang, Tang, & Prakash, 2008) and cerebral cortex (Finegersh & Homanics, 2014a). Chronic ethanol treatment has been shown to cause altered methylation in the N-methyl-d-aspartic acid (NMDA) receptor 2B subunit gene in mouse embryonic cortical neurons (Marutha Ravindran & Ticku, 2005) as well as dysregulation of neurocircuitry and ncRNA transcriptome networks (Farris et al., 2015). In addition to epigenetic changes in brain areas, drug exposure has been reported to cause epigenetic modifications in sperm and ova. For instance, altered histone modifications have been reported in the sperm following voluntary cocaine administration in rats (Vassoler, White, Schmidt, Sadri-Vakili, & Pierce, 2013). Previous studies from our lab (Rompala et al., 2018) and others (Bedi, Chang, Gibbs, Clement, & Golding, 2019) reported that chronic ethanol exposure led to altered small ncRNAs in mouse sperm. For more details regarding drug-induced epigenetic modifications, see: alcohol (Gatta et al., 2019; Jangra et al., 2016; Palmisano & Pandey, 2017; Pandey, Kyzar, & Zhang, 2017; Rompala & Homanics, 2019); cocaine (Ausió, 2016; Engmann et al., 2017; Gajewski et al., 2019; Penrod et al., 2018; Vaillancourt, Ernst, Mash, & Turecki, 2017); cannabinoids (Schrott & Murphy, 2020; Szutorisz & Hurd, 2016, 2018); opiates (Browne, Godino, Salery, & Nestler, 2020; Knothe et al., 2016; Montalvo-Ortiz, Cheng, Kranzler, Zhang, & Gelernter, 2019; Sandoval-Sierra, Salgado García, Brooks, Derefinko, & Mozhui, 2020); nicotine (Jung et al., 2016; Maldonado & Martin, 2016; McCarthy et al., 2018; Pisera-Fuster, Faillace, & Bernabeu, 2020).
It has been suggested that epigenetic marks generated in the germ line as a result of environmental perturbations can shape future generations (Bale, 2015; Skinner, Manikkam, & Guerrero-Bosagna, 2010). Therefore, it is strongly implicated that drugs act as epimutagens and drug-induced epialleles can be transmitted to the next generation, manifesting as behavioral and neurobiological changes across generations. The next section discusses the various physiological and behavioral responses in the offspring as a result of parental drug use.
4. Overview of transgenerational effects of substance abuse
Most paternal preconception studies are focused on chronic substance use, with exposure paradigms lasting upwards of 100 days. This allows for the drug to be present consistently for at least one full cycle of spermatogenesis to exert its full hereditary impact on the maturing sperm. Compared to the mounting research on paternal preconception exposure, maternal and biparental exposure paradigms are not as widely employed. When maternal preconception exposure is exercised however, the paradigm is designed to allow a long exposure followed by a period of abstinence prior to conception. This is done as a means to safeguard maternal behavior toward her offspring from potential alterations due to maternal drug use. Typically, the drug is either presented voluntarily as self-administration or involuntarily through researcher intervention (Table 1). A wide variety of behavioral, drug exposure and molecular changes were investigated. A summary of some key studies that highlight the effects of ethanol, cocaine, nicotine, cannabinoids, and opioids that have been found in offspring following parental exposure, can be found in Tables S1–S5 in Supplementary Material in the online version at https://doi.org/10.1016/bs.irn.2020.08.003, respectively.
Table 1.
Parental administration paradigms.
| Ethanol |
|---|
| Forced administration |
| EtOH intraperitoneal injection |
| Chronic intermittent EtOH exposure via vapor inhalation |
| Intragastric intubation |
| Oral gavage |
| Self-administration |
| EtOH-derived calorie diet |
| Free-access liquid EtOH diet offered as a choice with water |
| Liquid EtOH diet offered intermittently as a choice with water |
| Liquid EtOH drinking offered during the dark cycle |
| Cocaine |
| Forced administration |
| Water-dissolved cocaine |
| Intraperitoneal injection |
| Self-administration |
| Active lever presses infuse cocaine through jugular catheter |
| Nicotine |
| Forced administration |
| Nicotine infusions via osmotic minipump |
| Water-dissolved nicotine |
| Intraperitoneal injection |
| Cigarette smoke inhalation |
| Subcutaneous injection |
| Cannabinoids |
| Forced administration |
| Intraperitoneal injection |
| Oral gavage |
| Subcutaneous injection |
| Opioids |
| Forced administration |
| Subcutaneous injections |
| Water-dissolved morphine |
| Intraperitoneal injections |
5. Ethanol
5.1. Background
Alcohol (ethanol) abuse is the fifth leading risk factor for premature death and disability, as well as the third leading preventable cause of death in the United States (Mokdad, Marks, Stroup, & Gerberding, 2004). Over 14 million adults and over 400,000 adolescents in the U.S. alone suffer from alcohol use disorder (AUD) (Substance Abuse and Mental Health Services Administration, 2017) and ~88,000 people die annually due to alcohol-related causes (Gonzales et al., 2014). While maternal ethanol exposure during pregnancy has been well-characterized and can result in fetal alcohol spectrum disorder (FASD) (Riley, Infante, & Warren, 2011), ethanol exposure prior to conception has received less attention.
AUD has consistently been shown to have high heritability, yet genomewide association studies have only identified a few relevant single nucleotide polymorphisms (Prescott & Kendler, 1999; Rompala & Homanics, 2019; Verhulst, Neale, & Kendler, 2015). So, what is the missing link? Although several explanations have been advanced to explain missing heritability (Crow, 2011; Trerotola, Relli, Simeone, & Alberti, 2015), there is growing evidence that ethanol exposure can induce sperm epimutations which are inherited by the offspring (Chastain & Sarkar, 2017; Finegersh, Rompala, Martin, & Homanics, 2015; Hill, Rompala, Homanics, & Zezza, 2017; Liang et al., 2015; Ouko et al., 2009).
The goal of this section is to summarize the work examining the impact of parental preconception ethanol exposure on offspring development, behavior, and molecular profiles. For more information on the results discussed here, a summary table of the intergenerational effects of ethanol including exposure methods used, effects observed in offspring, and publication information is provided (see Table S1 in Supplementary Material in the online version at https://doi.org/10.1016/bs.irn.2020.08.003).
5.2. Paternal preconception ethanol exposure
5.2.1. Fertility and offspring development
Many studies have reported variations in sperm motility, fertility and fecundity of paternal preconception ethanol (PPE) exposed sires (Abel, 1995, 1993a, 1993b; Bielawski & Abel, 1997; Bielawski, Zaher, Svinarich, & Abel, 2002; Cicero et al., 1994; Emanuele & Emanuele, 2001; Leichter, 1986; Liang et al., 2015; Mankes et al., 1982; Table S1 in Supplementary Material in the online version at https://doi.org/10.1016/bs.irn.2020.08.003). Similarly, PPE exposure has been shown to induce offspring abnormalities throughout development. When pregnancy did occur, increased instances of embryonic death and decreased implantation have been noted from PPE-exposed sires when compared to controls (Mankes et al., 1982), however this was not observed in one study (Randall, Burling, Lochry, & Sutker, 1982). Researchers have also observed that PPE-sired offspring displayed alterations in gestational length (Chang, Wang, Bedi, & Golding, 2019; Meek, Myren, Sturm, & Burau, 2007) and fetal weight (Abel, 1995; Bielawski et al., 2002; Leichter, 1986; Mankes et al., 1982; Randall et al., 1982) when compared to their control counterparts. While there are inconstancies between studies, it is apparent that fetal developmental abnormalities arise due to PPE exposure and may contribute to altered phenotypes displayed by adult offspring.
Similar to the studies mentioned above, it is clear that PPE also impacts newborn development, although inconsistencies across studies are apparent. Litters sired by PPE-exposed fathers largely had no difference in size when compared to control litters (Abel, 1995, 1989, 1993a, 1993b; Abel & Lee, 1988; Bielawski & Abel, 1997; Bielawski et al., 2002; Cake & Lenzer, 1985; Kim et al., 2014; Leichter, 1986), although some reported reductions in number of pups (Cicero et al., 1994; Emanuele & Emanuele, 2001; Liang et al., 2014; Mankes et al., 1982; Meek et al., 2007). Increased number of runts (Abel, 1993b; Bielawski & Abel, 1997; Bielawski et al., 2002) and enhanced newborn mortality (Cicero et al., 1994; Liang et al., 2014; Meek et al., 2007) were also observed. Birthweight and bodyweight of PPE-sired offspring has been reported to be unchanged in numerous studies (Abel, 1995, 1989, 1993b; Abel & Lee, 1988; Abel & Tan, 1988; Bielawski & Abel, 1997; Kim et al., 2014; Liang et al., 2014), but others have reported increased (Finegersh & Homanics, 2014b; Rompala, Finegersh, & Homanics, 2016) or decreased (Ledig et al., 1998; Rompala, Finegersh, Slater, & Homanics, 2017) body weight compared to controls. Changes in tissue weight were observed across multiple organ systems (Abel, 1993b; Abel & Lee, 1988). Of particular interest was brain cortical enlargement (i.e., thickening of cortical layers I–IV and V–VI) in both sexes of PPE-sired offspring relative to controls (Jamerson, Wulser, & Kimler, 2004). As many studies have focused on emotional, cognitive, and reward-related behavior in adult offspring (see below), it is important to note the alterations in brain morphology are present. Behavioral differences between PPE-sired offspring and controls have not been widely explored during adolescence, nevertheless one study reported PPE-sired offspring were hypoactive when compared to controls (Abel, 1989). These results span both voluntary and involuntary ethanol administration with various ethanol doses at differing timepoints and lengths. Additionally, two species and multiple strains have been utilized, allowing for a variety of PPE exposure consequences to be illuminated. Although many discrepancies likely arise because of experimental differences, one can reasonably conclude that PPE exposure can impact offspring development.
5.2.2. Physiological and molecular changes in adult offspring
A plethora of physiological and molecular alterations have been observed in PPE-sired offspring. When compared to controls, PPE-sired offspring displayed changes in multiple enzymes and proteins involved in metabolism and weight gain (Chang et al., 2019; Emanuele et al., 2001; Ledig et al., 1998), alluding to dysregulated metabolic processes. Insulin and glucose tolerance were also found to be different between male and female progeny. Male PPE-sired offspring presented insulin hypersensitivity and upregulated transforming growth factor beta (TGF-β) signaling, whereas only modest decreases in glucose tolerance were observed in females when compared to controls (Chang et al., 2019). Despite identical preconception treatment across sexes, dimorphic phenotypes in response to PPE remain apparent.
In the central nervous system, altered levels of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), as well as their receptors TrkA and p75NTR, were noted in multiple brain regions of PPE-sired offspring relative to controls (Ceccanti et al., 2016). As both of these proteins are important for the growth and maturation of brain circuitry, these results suggest that ancestral ethanol use may impact neuronal survival and differentiation. Bdnf methylation and expression have also been shown to be sexually dimorphic. Even though both male and female PPE-sired offspring expressed decreased methylation of the Bdnf promoter when compared to controls (Finegersh & Homanics, 2014b), only males displayed increased gene expression in the ventral tegmental area (VTA) (Finegersh & Homanics, 2014b; Rompala et al., 2017). That increase was additionally associated with reduced ethanol drinking and lower sensitivity to ethanol-induced behavior in male PPE-sired progeny when compared to controls (Finegersh & Homanics, 2014b; Rompala et al., 2017). This points to a possible Bdnf mechanism underlying behavioral changes.
Dopamine (DA) neurotransmission plays an important role in the development of addiction, therefore investigations into this system were also of high interest. Alterations in dopamine active transporter (DAT) (Ceccanti et al., 2016; Kim et al., 2014), but not DA receptor levels (Ceccanti et al., 2016) may explain some of the differential ethanol behavior in PPE-sired offspring relative to controls.
5.2.3. Drug responses and behavioral changes in adult offspring
It has widely been shown that PPE exposure has persistent effects that impact adult offspring behavior. Locomotor activity was altered in PPE-sired offspring relative to controls (Abel, 1993a, 1994; Abel & Lee, 1988; Abel & Tan, 1988; Jamerson et al., 2004; Kim et al., 2014; Ledig et al., 1998; Meek et al., 2007), with only a few exceptions (Finegersh & Homanics, 2014b; Wozniak, Cicero, Kettinger, & Meyer, 1991). Compared to controls, alterations in both anxiety-like (Ledig et al., 1998; Liang et al., 2014) and depressive-like behavior (Abel & Bilitzke, 1990; Liang et al., 2014) were also noted. Interestingly, the changes in depressive-like behavior were normalized following antidepressant administration (Abel & Bilitzke, 1990), indicating a possible neurochemical shift in PPE-sired offspring. When it comes to cognition, learning, and memory, the results are more variable. Multiple studies have reported that PPE-sired offspring had impaired (Abel, 1994; Abel & Lee, 1988; Hollander, McNivens, Pautassi, & Nizhnikov, 2019; Kim et al., 2014; Liang et al., 2014; Wozniak et al., 1991) or unchanged (Abel & Tan, 1988) performance when compared to controls in learning and memory paradigms. Variations in findings may be due to the experiment being employed, as different aspects of cognition (such as spatial working memory compared to fear memory) may be differentially impacted by PPE application. Finally, compared to controls, heightened aggression and impulsivity (Kim et al., 2014; Meek et al., 2007) was also observed in PPE-sired offspring. Taken together, these results indicate how prevalent the alterations are across a number of behavioral domains. This suggests that multiple neural circuits and neurochemical systems are likely impacted by PPE use. Understanding the underlying biological mechanisms should be an important area of concentration for future researchers.
Overall, alterations in ethanol-drinking behavior were seen predominantly in male PPE-sired progeny compared to controls (Campbell, Flanagan, Marchant, & Lawrence, 2018; Ceccanti et al., 2016; Rompala et al., 2017), while PPE-sired female offspring seem protected from some of the ethanol-drinking behaviors that their male counterparts are vulnerable too (Beeler, Nobile, & Homanics, 2019; Finegersh & Homanics, 2014b; Rompala et al., 2016). Results surrounding the impact of PPE exposure on ethanol drinking were variable and may differ based on the specific drinking assay used and the method and duration of ethanol exposure employed on the sires. PPE chronic intermittent vapor exposure produced a male-specific reduction in ethanol preference and consumption on a home cage two bottle free choice drinking assay compared to control sired offspring (Finegersh & Homanics, 2014a, 2014b; Rompala et al., 2016). PPE every other day two bottle choice drinking produced a male-specific reduction in ethanol consumption on a drinking in the dark assay of binge like consumption but did not alter two bottle choice drinking (Beeler et al., 2019). PPE self-administration produced male offspring (females not tested) that had normal every other day two bottle choice drinking and acquisition of operant self-administration but displayed a reduced context-induced relapse to ethanol seeking (Campbell et al., 2018). In contrast to those studies reporting reduced ethanol consumption, Hollander et al. (2019) reported that PPE via intragastric infusion produced offspring that had increased ethanol intake in 14-day old pups. Ceccanti et al. (2016) reported that PPE via oral forced consumption produced male offspring (females not tested) that were more sensitive than controls to the rewarding effects of low dose ethanol, but developed conditioned place aversion to a high concentration. Thus, there is strong evidence supporting the view that PPE results in changes in ethanol-drinking and reward, with effects often being male-specific. Surprisingly, in most studies, male PPE-sired offspring displayed reductions in ethanol-drinking and reward-related behavior. Therefore, it appears that either female offspring are protected, or male offspring are more sensitive to ancestral ethanol exposure.
5.3. Maternal preconception ethanol exposure
5.3.1. Offspring development
Unfortunately, few studies have investigated maternal preconception ethanol (MPE) exposure. However, it has been observed that MPE offspring display reduced birth weight and body weight throughout development compared to controls (Jabbar et al., 2016).
5.3.2. Physiological and molecular changes in adult offspring
MPE-exposure had negative impacts on stress reactivity and neuronal development. Male MPE offspring had elevated corticotropin-releasing factor (Crf) and reduced Pro-opiomelanocortin (Pomc) expression in the hypothalamus, as well as elevated Crf receptor 1 expression in the hippocampus and amygdala in response to stress compared to controls (Jabbar et al., 2016). MPE-derived males also expressed increased corticostatin, increased corticosterone (CORT) protein levels, and altered methylation of hypothalamic-pituitary-adrenal (HPA) regulatory genes compared to controls, suggesting that offspring present increased vulnerability to stress (Jabbar et al., 2016). Additionally, live imaging and brain analysis revealed increased hypothalamic hypocretin-positive neurogenesis in MPE male offspring compared to controls, which was positively correlated with ethanol intake and MPE-exposure (Collier et al., 2020). These data suggest that MPE exposure can contribute to dysregulation of the stress response and brain development in offspring.
5.3.3. Behavioral changes in adult offspring
Only a few studies of the effects of MPE on offspring behaviors have been reported to date. MPE offspring displayed hyperactivity (Collier et al., 2020), a male-specific increase in anxiety-like behavior (Jabbar et al., 2016), and increased ethanol consumption compared to controls (Collier et al., 2020). Extensive research needs to be conducted to fully characterize the behavioral changes induced by MPE exposure.
5.4. Biparental preconception ethanol exposure
5.4.1. Offspring development
Very few developmental abnormalities have been noted due to biparental ethanol (BPE) exposure. While no changes were observed in most measures, reductions in fetal weight compared to controls were noted (Livy, Maier, & West, 2004). Reduced bodyweight was also observed in post-pubertal BPE offspring compared to controls (Asimes, Kim, Cuarenta, Auger, & Pak, 2018). Behaviorally, juvenile offspring displayed reductions in play behavior (Asimes et al., 2018), indicative of impaired socialization. The majority of these results are consistent with those observed in both PPE and MPE models.
5.4.2. Physiological and molecular changes in adult offspring
Offspring of BPE procreators exhibited phenotypic differences in molecular pathways of both the periphery and central nervous system. Relative to controls, male BPE offspring displayed delayed sexual maturation, as measured by circulating levels of luteinizing hormone and testosterone (Asimes et al., 2018). In the brain, the hypothalamic gene expression seems to be greatly impacted by BPE exposure. DNA methylation patterns differed from controls, and many of these differentially methylated hypothalamic genes showed correlated changes in mRNA gene expression (Asimes et al., 2017). Those genes were found to control a variety of biological processes, including neurogenesis, transcriptional regulation, and reproductive function (Przybycien-Szymanska, Rao, Prins, & Pak, 2014). These data indicate that BPE exposure confers maladaptive epigenetic traits passed down to the next generation via dysregulated gene expression.
5.5. Human studies
While it is a challenge to study and interpret the epigenetic transmission of AUD in humans due to a wide variety of variables that may impact offspring outcomes, strong correlations have been found that support a role of alcohol-induced epigenetic inheritance. Alcoholic fathers expressed altered methylation at a number of genes, including some that are consistent with rodent models of PPE exposure (Hill et al., 2017; Ouko et al., 2009). Children of heavy drinking fathers were inclined to drink more and began drinking at an earlier age than children of non-drinking fathers (Vermeulen-Smit et al., 2012). PPE children also manifest deficits in cognition, attention and visuospatial capacity (Goodwin & Hill, 1975; Tarter, Jacob, & Bremer, 1989) when compared to children from non-alcoholic fathers. It has similarly been observed that heavy paternal alcohol use in the absence of maternal use produces children with characteristics similar to those associated with FASD (Lemoine, Harousseau, Borteyru, & Menuet, 2003). There has been very little research preformed on maternal preconception alcohol consumption in humans, but it has been observed that children of alcoholic mothers who abstained throughout pregnancy still had lower offspring birth weights, again consistent with FASD (Little, 1980; Livy et al., 2004; Ramsay, 2010). Ancestral alcohol exposure in humans has also been associated with methylation and expression of the HRAS oncogene and the TP53 tumor suppressor gene (Hill et al., 2017). These changes suggest that alcohol-induced epigenetic inheritance may contribute to the increased risk of cancer development in individuals with AUD (Hill et al., 2017). Taken together, ancestral exposure to ethanol can have long-lasting effects that impact both epigenetic processes and gene expression that distills into a spectrum of cellular and behavioral abnormalities in descendants.
6. Cocaine
6.1. Background
Cocaine is an addictive illegal stimulant derived from leaves of the coca plant (Calatayud & Gonzalez, 2003). There are an estimated 15–39 million people worldwide that are classified as problem users (Wang, Kapoor, & Goate, 2012) and roughly 1 out of every 20 Americans 18–25 years old have used cocaine within the past year (Hughes, Williams, Lipari, & Van Horn, 2016). Persistent cocaine use can lead to significant health issues such as drug-induced psychotic symptoms and increased risk for heart, liver, and lung disease (Wang et al., 2012). In addition, repeated cocaine exposure leads to significant changes in gene expression, particularly in the limbic system (Pierce et al., 2018). Cocaine binds to DAT thereby inhibiting DA recycling, leading to a buildup of DA in the synapse, producing the pleasurable effects associated with cocaine (Volkow et al., 2006). As with other drugs of abuse, there is a significant heritable risk for cocaine addiction that cannot be accounted for with traditional Mendelian genetics alone and questions still linger about the mechanisms underlying the heritability of cocaine addiction. A summary table of the intergenerational and transgenerational effects of cocaine including exposure methods used, effects observed in offspring, and publication information is provided (see Table S2 in Supplementary Material in the online version at https://doi.org/10.1016/bs.irn.2020.08.003).
6.2. Paternal preconception cocaine exposure
6.2.1. Fertility and offspring development
Paternal preconception cocaine (PPC) use appears to have only modest impacts on offspring development. PPC sires had no observable change in sperm morphology compared to controls (Abel, Moore, Waselewsky, Zajac, & Russell, 1989), however pregnancy rates were reduced (George et al., 1996). Postnatally, most measures, such as litter size and mortality, did not differ between PPC offspring and controls (Abel et al., 1989), however reduced birthweight of PPC-sired offspring has been reported (George et al., 1996). Additionally, both male and female offspring presented significant fluctuations in bodyweight across their lifespans that differed significantly from controls (Fischer, Rice, Martinez Rivera, Donohoe, & Rajadhyaksha, 2017; Killinger, Robinson, & Stanwood, 2012). Overall, PPC exposure does not appear to cause drastic effects on progeny growth.
6.2.2. Physiological and molecular changes in adult offspring
Voluntary PPC exposure is known to elicit epigenetic remodeling (i.e., alter the processes that control gene expression) in offspring (Wimmer et al., 2017). One area of particular interest is the expression of BDNF, which has implications for neuronal survival and differentiation. PPC-sired offspring expressed higher levels of BDNF than controls (Le et al., 2017; Pierce & Vassoler, 2014), which may be due to increased histone acetylation associated with the Bdnf promoter (Pierce & Vassoler, 2014; Vassoler, White, et al., 2013). This suggests an increase in neuronal cell survival and differentiation in PPC-sired offspring. Researchers have also investigated d-serine and the enzyme responsible for breaking its down, d-amino acid oxidase, as potential mechanisms driving behavioral changes. d-serine is an NMDA receptor agonist and may aid in memory formation. Male PPC-sired offspring showed increased Dao1, the gene encoding d-amino acid oxidase, expression in the hippocampus compared to control counterparts (Wimmer et al., 2017). As d-serine is one of d-amino acid oxidase’s most important targets, this increased expression of Dao1 was coupled with decreased levels of d-serine and impaired long-term potentiation (Wimmer et al., 2017), and may present a biological mechanism through which memory deficits (He, Lidow, & Lidow, 2006; Wimmer et al., 2017) arise in these offspring. More generally, PPC-sired offspring had increased levels of H3K14ac and decreased levels of H3K4me2, H3K18ac, H3K27me2, and H3K20me2 when compared to controls (Wimmer et al., 2019). These histone modifications affect chromatin structure and may lead to altered gene expression. Taken together, these data show that PPC exposure induces epigenetic remodeling in the subsequent generation and these offspring present dysregulation of gene expression and proteins critically important for brain development, learning, and memory.
6.2.3. Drug responses and behavioral changes in adult offspring
PPC-sired offspring behavior was largely unaffected when compared to controls (Table S2 in Supplementary Material in the online version at https://doi.org/10.1016/bs.irn.2020.08.003), however the presenting behavioral changes that did manifest were sexually dimorphic. Male PPC-sired offspring alone exhibited a number of behavioral phenotypes, including hyperactivity, altered anxiety-like behavior, and impaired long-term memory formation when compared to controls (Abel et al., 1989; Fischer et al., 2017; Wimmer et al., 2017). On the other hand, female PPC-sired progeny demonstrated possible modulation of reward-associated behavior such as increased sucrose consumption and reduced cocaine-conditioned place preference relative to controls (Fischer et al., 2017). Only working memory deficits (Abel et al., 1989; He et al., 2006) and increased depressive-like behavior (Killinger et al., 2012; White, Vassoler, Schmidt, Pierce, & Wimmer, 2016) of PPC-sired offspring were consistent between sexes. As with PPE models, there were alterations occurring in numerous behavioral domains in response to PPC exposure.
Cocaine-exposed sires confer altered sensitivity to drugs of abuse in offspring and these changes may persist for multiple generations. As mentioned in the previous section, PPC-sired offspring displayed alterations in reward-associated behavior compared to controls (Fischer et al., 2017). This extended to drugs of abuse as well, as increased responsiveness toward the reward sensitivity to cocaine and amphetamine were observed in PPC-sired offspring relative to controls (Fischer et al., 2017). Additionally, compared to controls male F1 PPC-sired offspring demonstrated decreased cocaine-induced locomotion (Wimmer et al., 2019). Male (but not female) F1 PPC-sired offspring showed reduced cocaine reinforcement (Pierce & Vassoler, 2014; Vassoler, White, et al., 2013) and cocaine self-administration (Le et al., 2017; Vassoler, White, et al., 2013) when compared to controls, suggesting that PPC-sired offspring may be less susceptible to developing cocaine addiction.
Whether a cocaine-related phenotype persists into the F2 PPC generation is dependent on the self- or forced-administration paradigm employed on the F0 sires (Le et al., 2017). The self-administration model employed on the sires impacts the stability of inheriting cocaine-related phenotypes across multiple generations. Grand-offspring of self-administration PPC sires showed increased cocaine consumption and motivation for cocaine reinforcement compared to controls (Le et al., 2017), while forced-administration PPC male grand-offspring surprisingly displayed decreased cocaine preference (Le et al., 2017; Yaw et al., 2019). Taken together, these results suggest that cocaine-induced behavioral alterations can persist across generations in a sexually dimorphic manner, with exacerbated impacts on male progeny. Additionally, it has been demonstrated that the susceptibility or resistance to drug seeking of offspring and grand-offspring was determined in part by sire drug-taking motivation. Thus, depending on sire cocaine use, offspring may be protected or more sensitive to these effects.
6.3. Maternal preconception cocaine exposure
6.3.1. Physiological and molecular changes in adult offspring
Only male offspring were considered under the maternal preconception cocaine (MPC) paradigm, but compared to controls, increased DA receptor D1 transcript levels were noted in the medial prefrontal cortex (mPFC) (Sasaki et al., 2014), showing altered transcriptional regulation.
6.3.2. Drug responses in adult offspring
Very little work has been done investigating MPC on offspring outcomes. To date, it has been reported that male MPC offspring expressed altered psychomotor responses to cocaine (Sasaki et al., 2014), increased reinforcing efficacy of cocaine, and increased cocaine self-administration behavior when compared to controls (Fant et al., 2019).
6.4. Biparental preconception cocaine exposure
Unfortunately, there is no current literature present considering biparental preconception cocaine exposure on offspring. Similar to maternal preconception studies, this exposure paradigm warrants future investigation.
7. Nicotine
7.1. Background
Nicotine remains the most widely abused drug in the world despite sharp declines in many countries (Reitsma et al., 2017). In the US, 15.5% of adults smoked cigarettes in 2016, and over 75% of smokers were daily users (Goldberg & Gould, 2019). Smoking is more common among men than women, and among younger adults aged 18–24 (Kasza et al., 2017). Despite over 4000 chemicals in cigarettes that are toxic, nicotine has repeatedly been identified as having addictive, toxic, and possibly epimutagenic effects (Goldberg & Gould, 2019; Yohn et al., 2015). This presents a pressing problem given the rise of electronic cigarette use in the US (King, Patel, Nguyen, & Dube, 2014).
It has been shown that nicotine causes epigenetic changes in both somatic cells and germline cells within the exposed individual (Goldberg & Gould, 2019; Jenkins et al., 2017; Marczylo, Amoako, Konje, Gant, & Marczylo, 2012). Further, clinical correlation studies have implicated parental and grandparental nicotine use with increased risk of asthma, nicotine abuse, and other severe psychiatric and physiological outcomes in subsequent generations (Bråbäck et al., 2018; Golding et al., 2017; Vandewater, Park, Carey, & Wilkinson, 2013; Wu et al., 2019). Taken together with the substantiation of inter- and transgenerational inheritance from other environmental cues, the epigenetic modifications by nicotine on germline cells and future progeny outcomes merits investigation. A summary of cross-generational effects due to nicotine, including exposure paradigms, assays, and results is provided in Table S3 in Supplementary Material in the online version at https://doi.org/10.1016/bs.irn.2020.08.003.
7.2. Paternal preconception nicotine exposure
7.2.1. Fertility and offspring development
Nicotine has been shown to be epimutagenic and cytotoxic to sperm, thereby reducing sperm concentration and viability (Alkhaled et al., 2018; Beal, Yauk, & Marchetti, 2017; Gu et al., 2016; Jenkins et al., 2017; Marczylo et al., 2012). However, no paternal preconception nicotine (PPN) exposure studies have reported decreased fecundity or salient impacts on pup clinical health.
7.2.2. Molecular and physiological changes
Studies looking at hyperactivity and cognitive deficits of PPN-sired offspring compared to controls (see Section 7.2.3) were shown to be the result of altered DA signaling in the striatum, frontal cortex, and hippocampus (McCarthy et al., 2018; Zhang et al., 2020). Noradrenaline levels and DA receptor subtype mRNA expression in striata were differentially altered in PPN-sired males and females relative to controls (McCarthy et al., 2018). This was possibly due to intergenerational transmission of methylation changes of DA receptor genes in sire sperm, which were found to be correlated with some of the expression changes in offspring (McCarthy et al., 2018). Additional changes in mRNA expression in sperm and PPN progeny brains have also been shown, including increased expression relative to controls of Wnt4 genes such as disheveled protein 2 (Dvl2) or glycogen synthase kinase 3 (Gsk3) genes, which aids in axon differentiation (Dai et al., 2017). These mRNA changes were due to the downregulated expression of miRNA mmu-miR-15b by hypermethylation in F0 sperm and consequently F1 thalamic tissue at similar CpG sites (Dai et al., 2017). The Wnt4-GSK3 signaling pathway is crucial for neurogenesis as well as hippocampal and thalamic functioning. Wnt4 mRNA was shown to be elevated in both F0 sperm and F1 PPN brains compared to controls; PPN resulted in elevated expression of Wnt4 and DVL2 proteins in F1 brain (Dai et al., 2017). These changes were not seen in PPN F1 offspring sperm, which could explain the absence of similar phenotypes in PPN F2 progeny (Dai et al., 2017). Hyperactivity caused by nicotine-induced down-regulation of mmu-miR-15b and subsequent activation of Wnt4 signaling was reversible via viral overexpression of this miRNA in the thalamus (Dai et al., 2017). This led to a decrease in Wnt4 translation and recapitulated a depressive-like phenotype, thereby opposing the nicotine-induced hyperactivity (Dai et al., 2017). Importantly, the examination of a ncRNA shows the pleiotropic side effects of epigenetic modulation by nicotine.
Similarly, PPN-sired offspring were also shown to have decreased expression of DAT in the hippocampus, resulting in increased synaptic DA and D2 receptor activity when compared to controls (Zhang et al., 2020). D2 over-activation led to enhanced GSK3α/β expression via the D2/AKT/GSK3α/β pathway and subsequently hyperactivity, which was attributed to hypermethylation patterns of the Dat gene in both PPN F0 sperm and F1 brain tissue (Zhang et al., 2020) relative to control methylation patterns. This study also showed phenothiazine, a D2 receptor antagonist, administered to male F1 PPN-sired offspring reversed the hyperactive phenotype (Zhang et al., 2020). This receptor blockade modulated the D2-GSK3 pathway by increasing phosphorylation of protein AKT and decreasing molecular GSK3α/β content (Zhang et al., 2020).
Alterations in learning processes in F1 PPN offspring, such as fear conditioning, were attributed to alterations in the hippocampus and cholinergic function (Goldberg et al., 2019). For example, potassium- or nicotine-induced release of acetylcholine in the hippocampus were attenuated in PPN offspring relative to controls. These results may explain the reduction in nicotine self-administration observed (Goldberg et al., 2019). Epigenetic changes and transcriptional activity in hippocampal regions were also affected. Over 1000 genes were differentially regulated across the ventral and dorsal hippocampi, with a number of differentially methylated regions (DMRs) identified (Goldberg et al., 2019). These regions of the hippocampus modulate fear conditioning and pathway analyses of the genes affected implicated alterations in glucocorticoid signaling, neural development, and neural plasticity (Goldberg et al., 2019). Future genome-wide analyses are necessary to fully elucidate the pathways affected and their ultimate outcomes.
Finally, an interesting physiological consequence of PPN was seen in the hepatic metabolic capacity of male PPN-sired F1 mice that otherwise showed no behavioral alterations (Vallaster et al., 2017). Mice exposed to nicotine, or a nicotinic acetylcholine receptor (nAChR) antagonist, sired progeny that showed increased tolerance to lethal doses of nicotine or cocaine (Vallaster et al., 2017). This was shown to be a function of significantly enhanced hepatic clearance capacity by upregulation of 51 genes involved in metabolism, such as cytochrome genes (Vallaster et al., 2017). The upregulation of general metabolism genes in response to either nicotine or its antagonist shows a general adaptation to xenobiotic exposure not specific to nicotine or a given signaling pathway (Vallaster et al., 2017). Almost all of these effects were also sex-specific, showing enhanced vulnerability in male PPN-sired offspring compared to controls.
7.2.3. Drug responses and behavioral changes in adult offspring
Paternal nicotine exposure prior to mating has been demonstrated to result in anxiety-like or hyperactive behavior consistent with attention deficit hyperactivity disorder (Zhang et al., 2020), as well as other cognitive deficits in offspring. One study found, when compared to controls, male PPN-sired offspring had increased locomotor activity that attenuated over time (Hawkey et al., 2019). Interestingly, hyperactivity and increased stereotypy were also observed following PPN exposure (Dai et al., 2017; McCarthy et al., 2018). Changes in locomotor activity represents a significant commonality across various paternal nicotine studies.
In addition to locomotor activity, other behavioral domains can also be affected by PPN exposure (Hawkey et al., 2019). Both male and female F1 and F2 PPN offspring displayed increased cued and contextual fear conditioning as well as male-specific attentional deficits (McCarthy et al., 2018), when compared to controls. Fear conditioning is commonly associated with anxiety- and post-traumatic stress disorder-like phenotypes (Goldberg et al., 2019). F2 male PPN offspring displayed deficits in cognitive flexibility relative to controls, suggesting learning deficits can persist transgenerationally (McCarthy et al., 2018). Taken together, these results suggest that PPN exposure affects a wide range of emotional and cognitive processes in offspring.
Other behavioral or physiological effects have also been reported following PPN exposure, including altered addictive-like behaviors and behavioral responses to nicotine (Goldberg et al., 2019; Yohn, Caruso, & Blendy, 2019). PPN-sired F1 mice showed decreased nicotine self-administration as well as a decreased tendency for relapse-related behavior compared to controls, suggesting a reduction in the reinforcing aspects of nicotine possibly due to aversive memory formation (Goldberg et al., 2019). Transgenerational effects of nicotine manifested as an attenuated locomotor response in PPN-sired F2 males and transient locomotor sensitization in PPN-sired F2 females in response to a nicotine challenge compared to controls (Yohn et al., 2019). These effects on F2 PPN-sired offspring were specific to those offspring derived from F1 males (not females) whose F0 sires were exposed to nicotine (Yohn et al., 2019), showing a paternal lineage of nicotine-related responses. The previous experiment also looked for the additive or modulating effects on offspring phenotype due to exposure to both nicotine and stress across generations. This was accomplished by exposing F0 males to nicotine and F1 males and females to a stress regimen, then assessing behavioral changes in F2 and F3 progeny as a product of both nicotine and stress exposure (Yohn et al., 2019). The effect on nicotine sensitization was different for males and females. For example, F1 stress-exposed females derived from F0 nicotine-exposed sires eventually produced F3 females displaying increased locomotor sensitization to nicotine relative to controls, skipping the F2 generation (Yohn et al., 2019). This effect was not observed in F2 or F3 males from this same lineage. This highlights the importance of sex-specific lineage transmission and supports the idea that nicotine exposure in previous generations can affect responses to nicotine in future generations. Further, since stress alone did not lead to an effect on locomotion, nicotine and stress modulated one another across generations in a sex-dependent manner (Yohn et al., 2019).
7.3. Maternal preconception nicotine exposure
No current maternal preconception nicotine (MPN) exposure studies were identified for the present review. As such, these exposure paradigms warrant future investigation.
7.4. Biparental exposure
7.4.1. Molecular and physiological changes
To study the impact of nicotine exposure in both parents, studies have employed a biparental preconception nicotine (BPN) exposure paradigm to compare with single-parent exposure paradigms. Neurochemical analysis in one study revealed increased activation of Wnt4 and D2/AKT/GSK3 pathways in BPN offspring brains when compared to controls (Zhang et al., 2018). When parental exposure was analyzed separately, PPN-sired offspring showed increased expression of D2 receptor (which was not seen in MPN offspring) leading to modulation of the GSK3 pathway (Zhang et al., 2018). The GSK3 pathway ultimately helps regulate Bdnf expression (Zhang et al., 2018) and appears to be crucial in the epigenetic inheritance of pathological states due to parental nicotine exposure. Future studies are necessary to elucidate the underlying mechanisms governing changes in these pathways, as well as the full implications of altered BDNF on neuroplasticity and behavior due to ancestral nicotine use.
7.4.2. Behavioral changes in adult offspring
As previously stated, some BPN exposure studies also include results for single-parent exposures (PPN and MPN) (Zhang et al., 2018). MPN exposure manifested in offspring as a depressive-like response with significantly reduced mobility and sociability compared to control counterparts (Zhang et al., 2018), in contrast to PPN exposures discussed above that tended to result in increased activity and locomotion. Biparental exposure outcomes on offspring showed 70% congruence with maternal-only exposure outcomes, where BPN offspring displayed a largely depressive-like phenotype relative to controls, while 20% showed hyperactivity, congruent with paternal-only exposure. Therefore, maternal lineage showed more pronounced offspring effects than paternal lineage (Zhang et al., 2018). While BPN exposure resulted in modulated GSK3 pathway activity, the increased D2 expression in PPN-sired offspring, but not MPN offspring, may help explain the opposing hyperactive and depressive-like phenotypes in progeny. However, future studies in MPN exposure alone are necessary to fully delineate parental contributions to offspring phenotypes and the molecular mechanisms governing the predominately depressive-like phenotype observed in BPN offspring.
Cognitive deficits were evident following parental nicotine exposure, and complex cognitive functions may be even more vulnerable to biparental exposure. Female F1 BPN offspring exposed to nicotine showed deficits in a serial multiple choice task compared to controls that were also exposed to nicotine, but whose parents were not (Renaud & Fountain, 2016). However, the biological mechanisms of this are unknown. Prospective research should aim to fully characterize the cognitive and behavioral alterations due to biparental nicotine use, especially in regard to addictive-like behaviors, as this remains underexplored.
8. Cannabinoids
8.1. Background
Cannabis is one of the most widely used illicit substances in the world, estimated at over 190 million users worldwide and over 40 million users in the US, in 2016 (Adams et al., 2016). Increasing social and political acquiescence to cannabis use has generated a rise in initiation and diagnosis of cannabis use disorder, most troublingly among younger users aged 12–25, but also in adults (Cerdá et al., 2019). Prevalence disparity by sex is also evident, with males using cannabis at higher rates than females (Adams et al., 2016).
The endocannabinoid (eCB) system consists of endogenous ligands, receptors, and enzymes that mediate cannabinoid (CB) signaling in a variety of neurological and physiological systems (Zou & Kumar, 2018). Exogenous CB ligands include the phytocannabinoids, tetrahydrocannabinol (THC) and cannabidiol (CBD), as well as the synthetic CB WIN55,212–2 (WIN) (Fonseca, Teixeira, & Correia-da-Silva, 2017; Zou & Kumar, 2018). The eCB system functions with other neurotransmitter systems to modulate behaviors like reward-sensitivity, motivation, stress, and emotion (Zou & Kumar, 2018). This has been shown to regulate cross-sensitivity with other substances of abuse (Cecil et al., 2016; Morris, DiNieri, Szutorisz, & Hurd, 2011; Parira, Laverde, & Agudelo, 2017). As such, multiple studies examine the effects of adolescent THC or WIN exposure on offspring behavioral pathologies related to addiction and reward. Given evidence of epigenetic inheritance from other environmental insults as well as amplified incidence and prevalence of cannabinoid use, continued research into the impacts of CB exposure and consequent cross-generational effects is imperative. A summary of cross-generational studies, including assays and results, is provided in Table S4 in Supplementary Material in the online version at https://doi.org/10.1016/bs.irn.2020.08.003.
8.2. Paternal preconception cannabinoid exposure
Although eCBs are expressed in the brain (Kendall & Yudowski, 2016), they are also expressed in testes and sperm (Aquila et al., 2010; Schuel et al., 2002), and exogenous CB exposure shows deleterious impacts on gametes (Aquila et al., 2010; Murphy et al., 2018). One seminal study codified differential DNA methylomes, or the overall genetic methylation pattern, and concordance between rat and human sperm exposed to THC (Murphy et al., 2018). Both rat and human sperm revealed aberrant methylome shifts following THC exposure, mainly toward hypomethylation (Murphy et al., 2018), which related to mRNA expression in offspring tissue (Schrott et al., 2019). This data suggests paternal preconception cannabinoid (PPCB) exposure may cause epimutations within germlines, potentially leading to altered gene expression and behavioral consequences in future generations.
8.2.1. Molecular and physiological changes
Multiple experiments have studied the impact of PPCB exposure on offspring physiology. One study correlated observed behavioral outcomes of PPCB-sired offspring with proteins involved in epigenetic regulation, suggesting the underlying changes in DNA methyltransferases 1 and 3a (DNMT1 and DNMT3a) mRNA expression facilitated an increased anxiety-like response to an applied variable stress regimen (Andaloussi, Taghzouti, & Abboussi, 2019). While all animals exposed to stress had increased circulating CORT and increased expression of DNMT1, PPCB progeny had significantly higher expression of DNMT3a in the prefrontal cortex than controls. This led to increased DNA methylation, which may have altered expression of genes controlling anxiety-like behavior (Andaloussi et al., 2019). However, it is unclear if the resulting anxiogenesis was caused by eCB-HPA axis crosstalk or direct effects on gametes (Andaloussi et al., 2019).
The aforementioned studies scrutinizing epigenetics in rat and human sperm focused on Dlgap2, which encodes discs-large associated protein 2 (Murphy et al., 2018; Schrott et al., 2019). This gene product functions as a neural scaffolding protein to regulate dendritic morphology and synaptic plasticity via DA and glutamatergic signaling (Schrott et al., 2019). Dlgap2 methylation in sperm from cannabis-exposed sires and men correlated with methylation profiles in rat PPCB-sired offspring and human fetal brains, respectively (Schrott et al., 2019). Specifically, the Dlgap2 methylation profile was found to be functionally significant and inversely correlated with mRNA expression levels in human female, but not male, fetal brain tissue (Schrott et al., 2019). Dlgap2 is reported to be an imprinted gene and the significance of methylation-expression in human females, but not males, whose fathers used cannabinoids helps explain possible sex-specific dimorphisms (Schrott et al., 2019). Importantly, this comprehensive study connects epigenomic programming by THC in germline cells to the epigenomic profiles and mRNA expression seen in offspring. However, this relationship is only correlational, and a mechanistic understanding remains unknown. Future studies should identify the mechanisms responsible and establish a causal relation between methylation profiles, mRNA expression, and ultimately behavior. Finally, future studies should explore the relationship between the full F0 and F1 epigenomic profiles from paternal exposure, beyond Dlgap2, and the resulting behavior.
8.2.2. Behavioral changes in adult offspring
Two studies relevant to PPCB exposure explored the impacts on stress response and cognitive function (Andaloussi et al., 2019; Levin et al., 2019). While neither study reported negative effects on pup clinical health or changes in baseline activity, some highly specific neurobehavioral outcomes were observed. One study conducted behavioral assays from adolescence to adulthood in PPCB-sired offspring to help determine stability of behavioral changes (Levin et al., 2019). From a battery of assays, attention deficits and differential habituation to a locomotor behavioral assay were seen in PPCB-sired offspring across the lifespan when compared to controls (Levin et al., 2019). Another study scrutinized habituation to stress by imposing an unpredictable stress regimen on both PPCB-exposed and control adult offspring (Andaloussi et al., 2019). F1 PPCB-exposed male progeny had increased anxiety-like behavior in response to stress, with no deficits in episodic-like memory function, compared to controls (Andaloussi et al., 2019). This highlights the complexity of detecting subtle phenotypic differences that may only surface under particular environmental conditions or developmental stages while sparing other cognitive domains.
8.3. Maternal preconception exposure
8.3.1. Molecular and physiological changes
Compared to PPCB exposure, the consequences of maternal preconception cannabinoid (MPCB) exposure has been studied far less. The mRNA expression levels of the μ-opioid receptor (OPRM1) was increased in the NAc of adult female MPCB offspring following morphine challenge (Vassoler, Johnson, & Byrnes, 2013). Additionally, circulating CORT was augmented by first-time morphine administration (Vassoler, Johnson, et al., 2013). This shows an abnormal transcriptome and stress-reward pathway interaction thought to underlie differences in initial OPRM1 receptor agonism in MPCB offspring.
8.3.2. Behavioral changes in adult offspring
Male MPCB offspring generated enhanced reward-facilitating associations to opioids during both adolescence and adulthood when compared to controls (Byrnes, Johnson, Schenk, & Byrnes, 2012). Adult female MPCB offspring showed enhanced locomotor sensitization to a morphine challenge after withdrawal (Vassoler, Johnson, et al., 2013). Taken together, results from these two studies show an increase in sensitivity to the effects of morphine that are due to MPCB. Effects have also been observed outside the opioidergic system as well, for example, in MPCB offspring response to THC and amphetamine was altered (Pitsilis, Spyridakos, Nomikos, & Panagis, 2017). When paired with intracranial self-stimulation, researchers found reward thresholds associated with THC and amphetamine were shifted toward anhedonia relative to controls in MPCB offspring (Pitsilis et al., 2017). It is possible that CB epigenetic alterations result in modulation of the reward circuitry associated with drug exposure (Pitsilis et al., 2017). However, the underlying neurochemical mechanisms and brain regions involved are unknown.
8.4. Biparental preconception exposure
8.4.1. Molecular and physiological changes
To determine if biparental preconception cannabinoid (BPCB) exposure would result in unique offspring phenotypes, both male and female animals were exposed to THC before being mated. These intergenerational BPCB studies examined sex-specific transcriptomic changes in the striata of adolescent and adult male and female BPCB offspring and found changes in expression of multiple genes compared to control offspring (Szutorisz et al., 2014; Szutorisz, Egervari, Sperry, Carter, & Hurd, 2016). Sex-specific effects included co-expression of Cnr1 and Grin2A (an NMDA receptor subunit) with other synaptic plasticity genes showing a stronger correlation in female than male BPCB offspring (Szutorisz et al., 2016). Further, genes encoding NMDA subunits, AMPA subunits, Cnr1, Dlg4, and others, showed differentially altered expression in striatal locations relative to developmental stage; the shift from adolescence to adulthood marked a concurrent shift from ventral to dorsal striatal dysregulation (Szutorisz et al., 2016). Future studies are necessary to determine if this shift could underlie the transition from casual early exposure to habitual or dependent drug use in CB-exposed offspring.
Delving further into the epigenetics of BPCB exposure, a genome-wide study characterized the DNA methylation profiles in rat NAc of BPCB offspring (Watson et al., 2015). This comprehensive analysis showed 1027 DMRs and 473 differentially methylated genes in BPCB progeny compared to controls, including epimutations in genes that regulate neural plasticity (Watson et al., 2015). Importantly, Dlg4/PSD95 was shown as a crucial epicenter for an associated gene network hub that also includes Dlgap family genes and Grin2A (Szutorisz et al., 2016; Watson et al., 2015). This gene network has also been postulated to help explain opioid sensitization (Szutorisz et al., 2014; Szutorisz & Hurd, 2018) and neuropsychiatric disorders (Schrott et al., 2019). Interestingly, comparisons between F0 sperm epigenetic changes and F1 NAc following BPCB showed 55 overlapping genes epigenetically altered by THC exposure (Murphy et al., 2018; Watson et al., 2015). The fact that multiple studies have shown similar sets of genes as dysregulated following preconception cannabinoid exposure warrants further examination. Since many of these studies focused on DNA methylation, further studies are necessary to codify the full epigenomic profile pursuant to THC action, including those unique to F1 not overlapping with F0 sperm and vice versa. Functional outcomes specific to different DMR epimutations and developmental stages are also needed.
8.4.2. Behavioral changes in adult offspring
Adult BPCB offspring phenotypes indicate that CB exposure in previous generations affects progeny responses to other drugs. For example, male F1 BPCB offspring showed increased motivation to self-administer heroin, as well as altered approach-avoidance behavior and increased stereotypy during acute drug abstinence, relative to controls (Szutorisz et al., 2014). As previously discussed, this is similar to MPCB offspring in regard to sensitivity to opioids. However, in contrast to these reports, another study found adolescent biparental THC exposure resulted in adult F1 males with attenuated motivation to self-administer heroin relative to controls, and no effect on morphine locomotor sensitization in either sex (Hempel et al., 2020). These differences may have been due to the particular CB agent utilized for exposure or strain of rodent tested (Hempel et al., 2020). Future studies should determine the differential effects of THC and WIN relative to strain of animal utilized, as well as the relative contributions of patrilineal transmission on these opioid-responsive phenotypes (Hempel et al., 2020).
9. Opiates
9.1. Background
Opiates are a class of drugs, both legal and illicit, that act by binding to endogenous opioid receptors in the brain and produce analgesic, as well as pleasurable and sedative effects (Pathan & Williams, 2012). It has been widely acknowledged that the United States is currently experiencing an epidemic of opioid addiction and overdose, with an estimated 2 million individuals suffering from opioid use disorder in 2018 (Substance Abuse and Mental Health Services Administration, 2019). In 2018, 69.5% of drug overdoses in the United States, or 46,802 deaths (Hedegaard, Miniño, & Warner, 2020), involved opioids, demonstrating the dramatic impact this epidemic has had on the population. This is not a problem limited to adults, as 108,000 adolescents reported using opioids for recreational purposes in 2018 (Substance Abuse and Mental Health Services Administration, 2019). With the rate of opioid use, particularly among a younger population on the rise, it is important to understand how opiate misuse may impact the germline and affect future generations. A brief summary of the experiments outlined in this chapter is available in Table S5 in Supplementary Material in the online version at https://doi.org/10.1016/bs.irn.2020.08.003. To determine whether time of parental exposure impacts offspring differently, the following studies focused on two distinct developmental time points of the F0: adolescence and adulthood.
9.2. Paternal adolescent opiate exposure
9.2.1. Development
Overall, paternal preconception adolescent opioid (PPAO) exposure has only minor impacts on fertility and developmental outcomes in offspring. Conflicting results are noted regarding litter size, with one study reporting a reduction following opioid exposure (Cicero et al., 1991) and others finding no change in litter size relative to controls (Azadi, Azizi, & Haghparast, 2019; Pachenari, Azizi, Ghasemi, Azadi, & Semnanian, 2018; Vassoler et al., 2020). Beyond this measure, no changes were observed in number of pregnancies, male/female ratio, mortality, or birth weight (Azadi et al., 2019; Cicero et al., 1991; Pachenari et al., 2018; Vassoler et al., 2020).
9.2.2. Molecular and physiological changes
To understand what could be controlling the shift in morphine’s rewarding properties (see below), neuronal activity in multiple brain areas has been assessed. Of particular interest is the VTA, as dopaminergic projections from the VTA activate the NAc and have been implicated in the rewarding effects of drugs of abuse (Wanat, Willuhn, Clark, & Phillips, 2009). Extracellular single unit recordings in male offspring of PPAO sires revealed altered burst firing patterns in these neurons compared to controls (Azadi et al., 2019). Electrophysiological properties of the locus coeruleus (LC), the source of norepinephrine for many brain regions and a site of ubiquitous opioid receptor expression, were also explored (Williams & North, 1984). When performing whole cell patch clamp recordings of LC neurons from PPAO-sired offspring, a number of differences were reported, including a decrease in action potential duration and an increase in after-hyperpolarization amplitude compared to control LC neurons (Pachenari, Azizi, & Semnaniann, 2019). While it has not been directly linked, these physiological differences may underlie the behavioral changes observed in PPAO-sired offspring.
9.2.3. Behavioral changes in adult offspring
Due to the limited number of PPAO studies, the effects on adult offspring behavior remain somewhat poorly defined and deserve additional investigation. Male PPAO-sired offspring did not display changes in locomotor activity in a novel environment (Azadi et al., 2019). While these animals did not differ in their response to the analgesic properties of morphine, they did display reduced responding to its rewarding effects when compared to controls (Azadi et al., 2019). When looking at self-administration behavior, PPAO male and female offspring displayed decreased consumption and motivation to take cocaine, while PPAO female offspring alone increased consumption of morphine when compared to controls (Vassoler et al., 2020). This indicates that these offspring may be protected from developing addiction to some drugs of abuse, while becoming more vulnerable to others. Further exploration of other behaviors, such as anxiety or cognition, will provide a more complete picture of the cross-generational effects.
9.3. Paternal adult opiate exposure
9.3.1. Development
Paternal preconception adult opiate (PPAdO) exposure studies noted smaller litter sizes and an increase in total pup mortality (Cicero, Nock, O’Connor, Adams, & Meyer, 1995; Naquiah et al., 2016). No developmental markers, such as bodyweight, incisor eruption, and eye opening, were affected (Cicero et al., 1995).
9.3.2. Behavioral changes in adult offspring
Male PPAdO-sired offspring and grand-offspring (F2) displayed multiple and persistent behavioral alterations compared to controls. F1 male PPAdO offspring displayed increased anxiety-like behavior as well as aggression toward intruders, when compared to control offspring, behavioral patterns reminiscent of animals undergoing opioid withdrawal (Naquiah et al., 2016). This phenotype persisted into the F2 generation, indicating this was a persistent, transgenerational effect.
Effects of PPAdO on offspring have also been observed on pain and physiological effects of morphine administration. While baseline pain sensitivity did not change, male PPAdO-sired offspring displayed increased sensitivity to the analgesic effects of morphine when compared to controls (Cicero et al., 1995; Vyssotski, 2011). F2 PPAdO-sired males were similar in that altered morphine analgesia was present, but they also displayed higher baseline pain thresholds than controls (Vyssotski, 2011). Female PPAdO-sired offspring of the same litters did not demonstrate any differences relative to controls. Some of these results differed from those of PPAO, specifically those concerning morphine’s analgesic effects, emphasizing that timing of exposure may be critically important in understanding how preconception opioids affect offspring.
9.4. Maternal adolescent opiate exposure
9.4.1. Development and behavioral changes in juvenile offspring
Offspring of maternal preconception adolescent opioid (MPAO) exposure have been extensively studied prior to adulthood, starting as young pups and extending into adolescent behavior. One study found MPAO pups weighed less overall than their control counterparts (Bodi, Vassoler, & Byrnes, 2016), although another found no change in weight between MPAO and control pups (Johnson, Carini, Schenk, Stewart, & Byrnes, 2011). Reductions in ultrasonic vocalizations, generally used as a measure of attachment (Brunelli, Shair, & Hofer, 1994; Hashimoto, Saito, Furudate, & Takahashi, 2001), were also observed (Bodi et al., 2016). As juveniles, offspring displayed alterations in rough and tumble, but not social play when compared to control offspring, suggesting alterations in social behavior (Johnson et al., 2011).
9.4.2. Molecular and physiological changes
In addition to the behavioral changes described above, molecular and physiological alterations have been studied in the offspring of dams exposed to opioids during adolescence. One area of interest is the release of hormones, such as CORT and insulin, and glucose. When compared to controls, male MPAO offspring displayed increases in the plasma levels of all three when maintained on a high-fat diet, indicating metabolic dysfunction (Toorie et al., 2019). CORT levels have also been found to be responsive to drugs of abuse, with control animals demonstrating a dose-responsive increase in plasma CORT (Vassoler, Johnson-Collins, Carini, & Byrnes, 2014). However, male MPAO offspring had an overall reduction in CORT release following acute morphine administration (Vassoler et al., 2014; Vassoler, Toorie, & Byrnes, 2018). This is in contrast to what was observed following cocaine administration, where female MPAO offspring demonstrated a significant increase in plasma CORT levels not observed in controls (Vassoler, Toorie, & Byrnes, 2019). Taken together, this suggests an overall dysregulation of release and control levels of multiple peripheral regulatory factors in MPAO offspring.
As stated previously, exogenous opiates such as morphine or heroin exert their effects by binding to endogenous opioid receptors in the brain (Pathan & Williams, 2012). Studies have investigated whether expression of these receptors, as well as DA receptors implicated in reward and drug addiction (Wise & Rompre, 1989), changed in response to MPAO. While no changes were observed in DA receptor 2 and κ-opioid receptor in the NAc (Byrnes, Johnson, Carini, & Byrnes, 2013), expression of the gene encoding the μ-opioid receptor Oprm1 in F1 MPAO offspring is not as clear. Changes appear to be brain region and sex-specific, with one study reporting no differences between control and MPAO offspring in the VTA (Vassoler et al., 2014), while another noted reductions in expression in the paraventricular nucleus of F1 female MPAO offspring (Vassoler et al., 2018). F2 male MPAO progeny, displayed lower Oprm1 expression compared to controls (Vassoler et al., 2018). Protein expression of the μ-opioid receptor was also significantly impacted by MPAO. In NAc, only female MPAO offspring displayed an increase, while both sexes demonstrated reduced μ-opioid protein in VTA (Vassoler, Wright, & Byrnes, 2016). These changes may underly some of the altered responses to morphine observed.
Researchers have also identified a number of genes and proteins that appear to be responsive to maternal adolescent preconception opiate exposure. POMC neurons are directly regulated by μ-opioid receptor activity (Shiraishi, Yanagita, Fujita, & Bungo, 2008), presenting themselves as a prime subject for investigation. At baseline, POMC mRNA and protein expression did not differ between MPAO and control offspring (Vassoler et al., 2014, 2019). In response to morphine, which would directly activate these neurons, male MPAO offspring showed a significant elevation in POMC expression relative to controls (Vassoler et al., 2014). A similar trend was seen following cocaine administration, with a male-specific increase in POMC protein levels observed in MPAO offspring compared to controls (Vassoler et al., 2019). F1 and F2 MPAO offspring also deviated from controls and displayed dysregulation of genes related to synaptic plasticity and neural remodeling (Vassoler et al., 2017). This altered gene expression suggests a possible mechanism underlying the aberrant behavior observed in MPAO offspring, although the downstream consequences of these changes still need to be investigated.
9.4.3. Drug responses and behavioral changes in adult offspring
As adults, numerous studies have reported sexually dimorphic changes in basal locomotor and anxiety-like behaviors. Female MPAO offspring show decreased locomotion (Byrnes, 2005; Byrnes, Babb, Scanlan, & Byrnes, 2011) and increased anxiety-like behaviors when compared to controls (Byrnes, 2005). Interestingly, male offspring did not demonstrate these behavioral alterations, in contrast to the increase in anxiety-like behavior observed following PPAdO (Naquiah et al., 2016). This indicates that the protective effect previously observed in female offspring may be limited to paternal exposure paradigms.
To determine if MPAO offspring display altered response to drug administration, multiple studies have investigated the effects of acute or repeated morphine exposure in offspring on analgesia and locomotor behavior. As previously described, morphine is a powerful analgesic used to treat severe pain, but also imparts a sedative effect on patients taking the drug (Pathan & Williams, 2012). Adult male, but not female, MPAO offspring were more sensitive to the sedative effects of acute morphine and developed tolerance to the analgesic and sedative effects quicker than controls (Byrnes, 2005; Byrnes et al., 2011). Female MPAO offspring displayed increased sensitivity to the rewarding effects of low doses of morphine in the conditioned place paradigm relative to controls (Vassoler et al., 2016). This contrasts with results of PPAO and suggests that female MPAO offspring may be more vulnerable to developing an opioid addiction.
In addition, both male and female MPAO offspring displayed altered self-administration behavior. When given the opportunity to self-administer morphine, both male and female F1 MPAO offspring showed a reduction in consumption as well as motivation to work for the drug compared to controls (Vassoler et al., 2017). During extinction trials, male MPAO offspring demonstrated reduced time to reach extinction criteria, while female MPAO offspring responded less during the reinstatement phase (Vassoler et al., 2017). When testing self-administration behavior in the F2 MPAO offspring, females consumed less morphine, while male MPAO offspring showed decreased responding during reinstatement relative to controls (Vassoler et al., 2017). Taken together, it would appear that MPAO offspring are less likely to become addicted to opioids, although this conclusion runs counter to conclusions drawn by other studies of maternal adolescent opioid exposure.
Studies looking at the self-administration of cocaine by MPAO offspring showed an opposite effect than results from morphine self-administration. Both male and female MPAO offspring had increased motivation to take cocaine compared to controls (Vassoler et al., 2019). Additionally, female MPAO offspring took longer to reach criteria during extinction trials, while male MPAO offspring demonstrated greater responding during the reinstatement trial compared to controls (Vassoler et al., 2019). This suggests that MPAO animals may be more sensitive to the rewarding effects of cocaine and more susceptible to the development of addiction to this drug. The differential results seen with morphine and cocaine self-administration may be indicative of molecular changes in neural circuitry associated with these two drugs of abuse, leaving MPAO offspring more or less susceptible to addiction depending on the drug. It will be interesting to see how offspring of adolescent exposed dams respond to other drugs of abuse like alcohol, nicotine, and cannabinoids.
9.5. Maternal adult opiate exposure
9.5.1. Molecular and physiological changes
Very few studies have focused on the effects of maternal preconception adult opiate (MPAdO) exposure on subsequent generations. F1 and F2 male MPAdO offspring displayed increased mRNA expression of Mecp2 and Hdac2 in the hippocampus relative to controls (Moulaei et al., 2018), both of which code for proteins that modify chromatin and inhibit transcription (Chahrour et al., 2008; Choudhary et al., 2009; Ng et al., 1999). Expression of these genes has been found to be impacted by alcohol and cocaine (Agudelo et al., 2011; Cassel et al., 2006), so it seems that these genetic changes may be long lasting and heritable following MPAdO exposure as well.
9.5.2. Behavioral changes in adult offspring
In addition to the aforementioned physiological differences, learning and memory was assessed in MPAdO offspring. Male offspring displayed memory impairments in the Morris water maze test compared to controls and these deficits persisted into the F2 generation (Moulaei et al., 2018). Neither F1 nor F2 MPAdO females displayed cognitive dysfunction.
9.6. Biparental opiate exposure
9.6.1. Molecular and physiological changes
Delving into the molecular and physiological changes that occur in offspring of biparental preconception opioid (BPO) exposure, male BPO offspring displayed increases in mRNA expression of all three opioid receptor subtypes in the NAc relative to controls (Ashabi et al., 2018). This was accompanied by increased protein levels of the μ-opioid receptor, as well as phosphorylated CREB and phosphorylated ERK1/2 (Ashabi et al., 2018), both of which are involved in downstream intracellular signaling following activation of opioid receptors (Al-Hasani & Bruchas, 2011). Levels of TNF-α, a proinflammatory cytokine, and S100B, a protein involved in the production of proinflammatory cytokines (Bianchi, Kastrisianaki, Giambanco, & Donato, 2011; Donato et al., 2009), were also assessed. Compared to their control counterparts, hippocampal TNF-α was significantly increased, while S100B was significantly decreased, in both male and female BPO offspring (Amri, Sadegh, Moulaei, & Palizvan, 2018) indicating neuroinflammatory dysregulation. BPO offspring, compared to controls, also displayed reduced mRNA and protein expression of insulin growth factor 2 (IGF-2) (Li et al., 2014), a protein involved in synapse formation and dendritic spine maturation (Schmeisser et al., 2012). Supporting its role in dendritic spine morphology, dendritic spines in BPO offspring were shorter and had decreased branching compared to controls (Li et al., 2014). Functionally, these changes may mediate the reduction in neuronal firing observed in the PFC and NAc of BPO male offspring (Ashabi et al., 2018). Epigenetically, reductions in acetylated histone 3 were observed in the PFC and hippocampus of BPO-exposed offspring across their lifespan relative to controls (Sadat-Shirazi et al., 2020). As mentioned in Section 2, histone acetylation relaxes the chromatin structure and may lead to increased transcription (Gates et al., 2017). These data suggest alterations to numerous molecular and neurochemical pathways, including intracellular signaling and neuronal maturation, may be unique to BPO exposure.
9.6.2. Behavioral changes in juvenile offspring
BPO offspring displayed a sexually dimorphic array of behavioral alterations. Compared to controls, male adolescent BPO offspring exhibited increased anxiety-like behavior, a phenotype that persisted into adulthood, while female offspring showed increased depressive-like behavior (Pooriamehr, Sabahi, & Miladi-Gorji, 2017). Interestingly, when given the option to voluntarily consume morphine, all BPO offspring consumed more morphine than controls (Pooriamehr et al., 2017), a finding that contrasts the MPO findings previously discussed (Vassoler et al., 2017).
9.6.3. Behavioral changes in adult offspring
Although some of the behavioral results of BPO offspring were similar to those previously described by single-parent opioid use, some inconsistencies do exist. Male BPO offspring displayed increased anxiety-like behavior (Li et al., 2014; Sabzevari et al., 2019) and increased sensitivity to the analgesic effects of morphine than controls (Ashabi et al., 2018), both of which were previously reported with other exposure paradigms (Byrnes, 2005; Cicero et al., 1995; Naquiah et al., 2016; Pooriamehr et al., 2017; Vyssotski, 2011). Similar to findings previously discussed (Vyssotski, 2011), male BPO offspring had a higher basal pain threshold (Ashabi et al., 2018) compared to controls. Results regarding learning and memory deficits of BPO offspring vary, with deficits being observed in novel object recognition (Sadat-Shirazi et al., 2020), but not the Morris water maze (Li et al., 2014). These discrepancies emphasize the importance of understanding how BPO may lead to different phenotypes than those recorded following single-parent exposure.
Environmental enrichment (EE) has been posed as a promising intervention for both F0’s as well as their offspring following BPO. EE generally consists of allowing the animals to have access to a large cage with numerous toys for an extended period of time each day. This has been employed at two different time periods (one for each generation) with promising results in the F1 offspring. EE was provided during withdrawal from opiates in the F0 generation prior to mating, or to the F1 offspring during adolescence. In both scenarios, BPO offspring with either form of EE were behaviorally indistinguishable from controls (Li et al., 2014; Pooriamehr et al., 2017). This indicates that the intervention prevented the increase in anxiety-like behavior observed in BPO offspring. While more research is needed on the topic, this evidence supports EE as a promising strategy for preventing behavioral and physiological dysregulation in the offspring of parents exposed to opioids prior to mating.
10. Conclusions and future directions
The extensive body of literature reviewed here firmly establishes that preconception exposure to drugs of abuse, including alcohol, cocaine, nicotine, cannabinoids and opiates, have a wide range of impacts on future generations. These effects range from behavioral to molecular and physiological changes, with many displaying sex-specific alterations. Even with a plethora of studies having been completed in this field, much remains to be explored.
While many of the results discussed in this chapter are inconsistent or even contradictory, a few similarities do arise. Preconception exposure, particularly in the case of ethanol, cocaine, and opiates, in a laboratory setting decreases the consumption of the respective drugs in the offspring (Beeler et al., 2019; Le et al., 2017; Rompala et al., 2016, 2017; Vassoler, White, et al., 2013). This indicates that offspring of sires or dams exposed to drugs may be less likely to develop an addiction to that particular drug in the future, which may make sense from an evolutionary perspective. However, offspring of morphine exposed dams showed a higher propensity to take cocaine (Vassoler et al., 2019), demonstrating that this protective effect may not generalize to all drugs of abuse. Another area that revealed consistencies across studies involves HPA axis regulation. Multiple studies showed that preconception exposure to drugs of abuse increased CORT levels, as well as expression of Crf in the brain (Jabbar et al., 2016; Toorie et al., 2019; Vassoler et al., 2014, 2018; Vassoler, Johnson, et al., 2013), pointing to a disruption in the stress response in the offspring. Expression of receptors and transporters related to DA release and action were altered as well (Azadi et al., 2019; Ceccanti et al., 2016; Kim et al., 2014; McCarthy et al., 2018; Sasaki et al., 2014; Zhang et al., 2018, 2020), indicating changes in dopaminergic signaling and reward circuitry in the brain. As these results were the most consistent across exposure paradigms and drugs, they may be interesting areas to focus on in future research.
As alluded to above, many inconsistencies have been observed in studies across different drugs of abuse, and even following exposure to the same drug. There are a large number of experimental variables which may account for some of these inconsistencies, including the wide variety of exposure paradigms that were employed, the dose of drug, age during and length of exposure, and different species and strains of rodents. Additionally, the drug exposure in dams and sires was a mixture of forced or voluntary consumption. All of the aforementioned factors may lead to contradictory or inconsistent results.
To move the field forward, researchers in the drug abuse field should begin to focus on the epigenetic mechanisms that mediate the preconception drug exposure effects that persist across generations (Fig. 3). The prevailing theory in the epigenetic inheritance field is that environmental perturbations, such as exposure to drugs, leads to changes in the ncRNA content of sperm. The altered abundance of specific ncRNAs in sperm impact development of the embryo following fertilization and ultimately, behavioral and physiological outcomes in the offspring. Studies looking at this causal link have been performed in the areas of metabolic disease. Researchers identified ncRNAs that were dysregulated in the sperm of male animals with metabolic diseases and injected those ncRNAs into fertilized embryos sired by healthy parents. The offspring born from the injected embryos displayed behavioral and physiological changes comparable to those observed in offspring born from sires with metabolic disease (Chen et al., 2016; Grandjean et al., 2015). Similar findings have also been shown following paternal preconception stress (Gapp & Bohacek, 2018; Gapp et al., 2014; Rodgers, Morgan, Leu, & Bale, 2015) and environmental enrichment (Benito et al., 2018) indicating this mechanism of inheritance may be common across multiple preconception environmental exposures. Performing similar studies in the drug abuse field would confirm whether this mechanism is also at play following preconception drug exposure. While a few studies have demonstrated that alcohol changes the ncRNA content of sperm (Bedi et al., 2019; Rompala et al., 2018), this has not been demonstrated for other drugs of abuse and no studies in the drug abuse field have established causality of any sperm ncRNA changes. Additionally, epididymal extracellular vesicles, or epididymosomes, appear to be a critical component of this epigenetic inheritance mechanism as well. As epididymosomes provide the majority of RNA that is found in mature sperm, and studies have shown that environmental perturbations including drug exposure can alter the content of these vesicles (Reilly et al., 2016; Rompala et al., 2018; Sharma et al., 2016, 2018), further investigation is needed to establish the contribution of epididymosomes to the intergenerational effects of drugs exposure. This mechanism of ncRNA transfer from epididymosome to sperm has recently been demonstrated to be sufficient to impart the intergenerational effects of paternal preconception stress (Chan & Maze, 2020). Additionally, it has been shown that incubation of sperm with epididymosomes from CIE mice is sufficient to impact behavior in the next generation (Rompala, Ferguson, & Homanics, 2020), lending further support to the theory that ncRNA transfer from extracellular vesicles could be controlling these intergenerational effects following paternal preconception drug exposure.
Fig. 3.
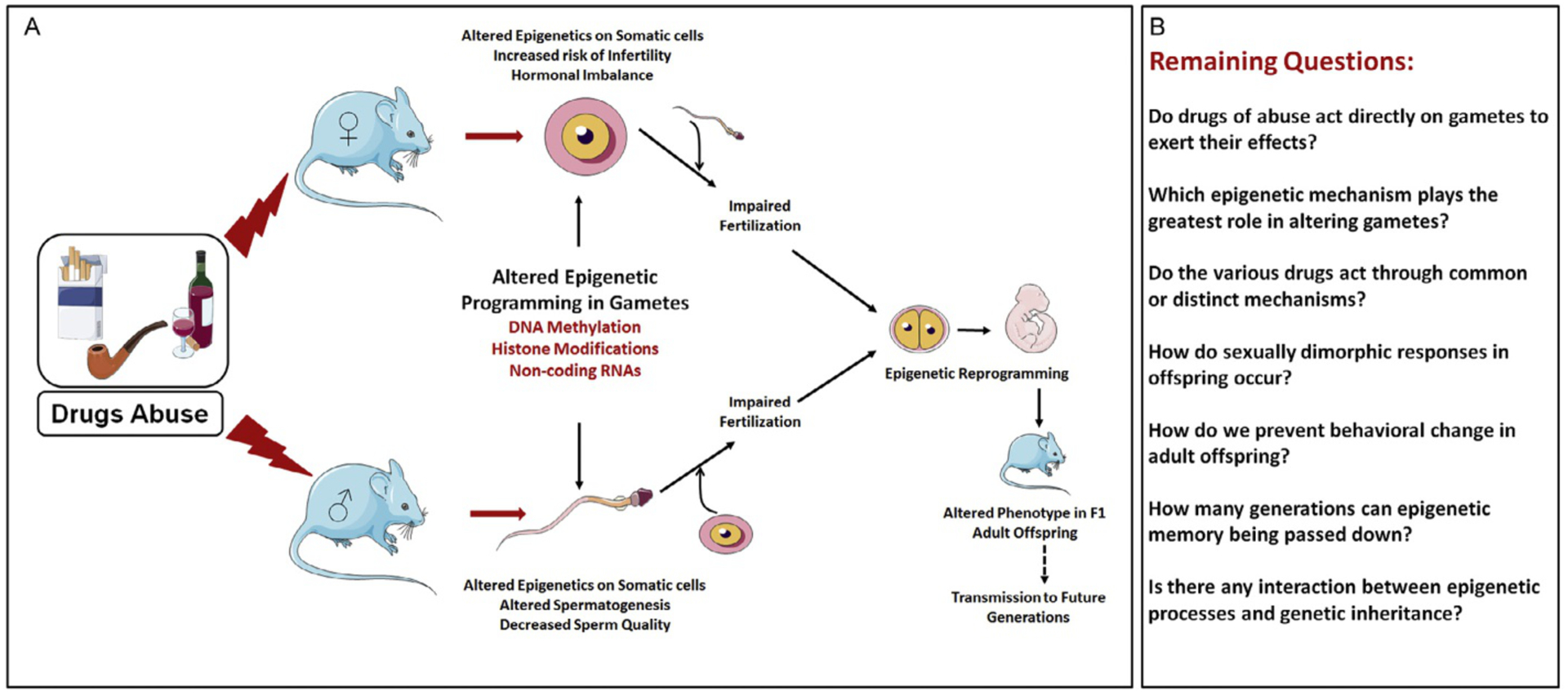
Mechanisms for germ-line mediated epigenetic inheritance caused by drugs abuse. (A) Environmental perturbations like exposure to drugs may lead to altered epigenetic programming in somatic cells and gametes, which further impact development of the embryo following fertilization and ultimately influence behavioral and physiological outcomes in the offspring and future generations. (B) Remaining questions that need to be answered to more fully understand epigenetic-based inheritance which could provide insights for better treatments and therapies for substance use disorders.
The ability of other modes of epigenetic inheritance, such as DNA methylation and histone modifications, to be responsible for intergenerational effects of drug exposure on offspring has been understudied. Studies have indicated changes in DNA methylation patterns in the sperm and offspring of chronically drinking fathers (Jabbar et al., 2016; Knezovich & Ramsay, 2012; Ouko et al., 2009). It should be noted, though, that studies have failed to directly link these methylation changes to phenotypic outcomes (Chang et al., 2019), indicating that DNA methylation may not be a major contributor to intergenerational inheritance in the case of drugs of abuse. Paternal preconception cocaine has been found to enrich multiple types of histone post-translational modifications (Vassoler, White, et al., 2013; Wimmer et al., 2017), suggesting an important role for histone modifications in cocaine exposure, although that has yet to be causally linked to phenotypic changes in adult offspring. Evidence supporting DNA methylation and histone modifications as drivers of intergenerational inheritance is not as strong as the evidence supporting ncRNA mechanisms, and more research needs to be conducted.
While explanations for maternal epigenetic inheritance are much less developed, changes in DNA methylation, histone modifications, and ncRNA content of oocytes are all plausible mechanisms. It remains to be seen whether drugs of abuse act directly on the oocytes themselves, or if an intermediate mechanism is at play in this phenomenon. Studies of preconception maternal stress, specifically in Holocaust survivors, have found alterations in the DNA methylation patterns of HPA axis genes in offspring (Yehuda et al., 2016, 2014), indicating that the stress response and glucocorticoids may be implicated in intergenerational changes. For more in depth analyses of the possible mechanisms of intergenerational inheritance, see (Lempradl, 2020; Perez & Lehner, 2019; Skvortsova, Iovino, & Bogdanović, 2018). Nevertheless, seeing as this field of research is still in its infancy, a number of avenues exist that have the potential to profoundly expand addiction research.
In addition to exploring the mechanisms described above, there are other areas of research that require further study. The primary focus of much of this research has been on paternal preconception exposure, with the exception of opiates. Continuing to explore the effects of maternal, as well as biparental, exposure is incredibly important. With the widespread use of drugs such as alcohol and cannabis among the adolescent population, understanding how adolescent vs adult use may differentially impact offspring should also be explored. Finally, only a few groups have attempted to develop intervention strategies to prevent the changes we have discussed here. While a deep understanding of the underlying mechanisms of inter- and transgenerational inheritance of preconception drug exposure may be necessary to develop targeted interventions, promising results from environmental enrichment studies demonstrate that useful intervention strategies may already exist.
Overall, preconception drug exposure has been definitively shown to impact subsequent generations, with changes in drug-taking behavior of the offspring, the stress response, and dopamine signaling being some of the most consistent findings. Despite this wealth of knowledge, the mechanisms by which these alterations occur are poorly understood. The prevalence of drug use in our society, in adolescent as well as adult populations, underscores the importance of continuing to research this phenomenon, as well as develop intervention strategies to counteract these effects.
Supplementary Material
Acknowledgment
This work was supported by grants from the NIH (AA10422, AA020889, T32 GM08424, and T32 NS007433-22).
References
- Abel EL (1989). Paternal alcohol consumption: Effects of age of testing and duration of paternal drinking in mice. Teratology, 40, 8. [DOI] [PubMed] [Google Scholar]
- Abel EL (1993a). Paternal alcohol exposure and hyperactivity in rat offspring: Effects of amphetamine. Neurotoxicology, 15, 5. [DOI] [PubMed] [Google Scholar]
- Abel EL (1993b). Rat offspring sired by males treated with alcohol. Alcohol, 10(3), 237–242. [DOI] [PubMed] [Google Scholar]
- Abel EL (1994). Effects of physostigmine on male offspring sired by alcohol-treated fathers. Alcoholism: Clinical and Experimental Research, 18(3), 648–652. [DOI] [PubMed] [Google Scholar]
- Abel E (1995). A surprising effect of paternal alcohol treatment on rat fetuses. Alcohol, 12(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Abel E, & Bilitzke P (1990). Paternal alcohol exposure: Paradoxical effect in mice and rats. Psychopharmacology, 100(2), 159–164. [DOI] [PubMed] [Google Scholar]
- Abel EL, & Lee JA (1988). Paternal alcohol exposure affects offspring behavior but not body or organ weights in mice. Alcoholism: Clinical and Experimental Research, 12(3), 349–355. [DOI] [PubMed] [Google Scholar]
- Abel EL, Moore C, Waselewsky D, Zajac C, & Russell L (1989). Effects of cocaine hydrochloride on reproductive function and sexual behavior of male rats and on the behavior of their offspring. Journal of Andrology, 10(1), 17–27. [DOI] [PubMed] [Google Scholar]
- Abel EL, & Tan S (1988). Effects of paternal alcohol consumption on pregnancy outcome in rats. Neurotoxicology and Teratology, 10(3), 187–192. [DOI] [PubMed] [Google Scholar]
- Adams K, Adams T, Aldworth J, Asman K, Barnett S, & Bishop E (2016). Results from the 2016 national survey on drug use and health: Detailed tables. Center for Behavioral Health Statistics and Quality (CBHSQ), Substance Abuse and Mental Health Services Administration (SAMHSA), US Department of Health and Human Services (HHS), RTI International. [Google Scholar]
- Agudelo M, Gandhi N, Saiyed Z, Pichili V, Thangavel S, Khatavkar P, et al. (2011). Effects of alcohol on histone deacetylase 2 (HDAC2) and the neuroprotective role of trichostatin a (TSA). Alcoholism: Clinical and Experimental Research, 35(8), 1550–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaskhar Alhamwe B, Khalaila R, Wolf J, von Bulow V, Harb H, Alhamdan F, et al. (2018). Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy, Asthma and Clinical Immunology, 14, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, & Bruchas MR (2011). Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology: The Journal of the American Society of Anesthesiologists, 115(6), 1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhaled Y, Laqqan M, Tierling S, Lo Porto C, Amor H, & Hammadeh M (2018). Impact of cigarette-smoking on sperm DNA methylation and its effect on sperm parameters. Andrologia, 50(4), e12950. [DOI] [PubMed] [Google Scholar]
- Amri J, Sadegh M, Moulaei N, & Palizvan MR (2018). Transgenerational modification of hippocampus TNF-α and S100B levels in the offspring of rats chronically exposed to morphine during adolescence. The American Journal of Drug and Alcohol Abuse, 44(1), 95–102. [DOI] [PubMed] [Google Scholar]
- Andaloussi ZIL, Taghzouti K, & Abboussi O (2019). Behavioural and epigenetic effects of paternal exposure to cannabinoids during adolescence on offspring vulnerability to stress. International Journal of Developmental Neuroscience, 72, 48–54. [DOI] [PubMed] [Google Scholar]
- Aquila S, Guido C, Santoro A, Perrotta I, Laezza C, Bifulco M, et al. (2010). Human sperm anatomy: Ultrastructural localization of the cannabinoid1 receptor and a potential role of anandamide in sperm survival and acrosome reaction. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology, 293(2), 298–309. [DOI] [PubMed] [Google Scholar]
- Aristizabal MJ, Anreiter I, Halldorsdottir T, Odgers CL, McDade TW, Goldenberg A, et al. (2019). Biological embedding of experience: A primer on epigenetics. Proceedings of the National Academy of Sciences, 201820838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashabi G, Sadat-Shirazi M-S, Akbarabadi A, Vousooghi N, Kheiri Z, Toolee H, et al. (2018). Is the nociception mechanism altered in offspring of morphine-abstinent rats? The Journal of Pain, 19(5), 529–541. [DOI] [PubMed] [Google Scholar]
- Asimes A, Kim CK, Cuarenta A, Auger AP, & Pak TR (2018). Binge drinking and intergenerational implications: Parental preconception alcohol impacts offspring development in rats. Journal of the Endocrine Society, 2(7), 672–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimes A, Torcaso A, Pinceti E, Kim CK, Zeleznik-Le NJ, & Pak TR (2017). Adolescent binge-pattern alcohol exposure alters genome-wide DNA methylation patterns in the hypothalamus of alcohol-naïve male offspring. Alcohol, 60, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausió J (2016). MeCP2 and the enigmatic organization of brain chromatin. Implications for depression and cocaine addiction. Clinical Epigenetics, 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadi M, Azizi H, & Haghparast A (2019). Paternal exposure to morphine during adolescence induces reward-resistant phenotype to morphine in male offspring. Brain Research Bulletin, 147, 124–132. [DOI] [PubMed] [Google Scholar]
- Bale TL (2014). Lifetime stress experience: Transgenerational epigenetics and germ cell programming. Dialogues in Clinical Neuroscience, 16(3), 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL (2015). Epigenetic and transgenerational reprogramming of brain development. Nature Reviews. Neuroscience, 16(6), 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, & Kouzarides T (2011). Regulation of chromatin by histone modifications. Cell Research, 21(3), 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MA, Yauk CL, & Marchetti F (2017). From sperm to offspring: Assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutation Research, Reviews in Mutation Research, 773, 26–50. [DOI] [PubMed] [Google Scholar]
- Becker PB, & Workman JL (2013). Nucleosome remodeling and epigenetics. Cold Spring Harbor Perspectives in Biology, 5(9), a017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi Y, Chang RC, Gibbs R, Clement TM, & Golding MC (2019). Alterations in sperm-inherited noncoding RNAs associate with late-term fetal growth restriction induced by preconception paternal alcohol use. Reproductive Toxicology, 87, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler E, Nobile ZL, & Homanics GE (2019). Paternal preconception every-other-day ethanol drinking alters behavior and ethanol consumption in offspring. Brain Sciences, 9(3), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito E, Kerimoglu C, Ramachandran B, Pena-Centeno T, Jain G, Stilling RM, et al. (2018). RNA-dependent intergenerational inheritance of enhanced synaptic plasticity after environmental enrichment. Cell Reports, 23(2), 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, & Goldman D (2009). Genes and addictions. Clinical Pharmacology and Therapeutics, 85(4), 359–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R, Kastrisianaki E, Giambanco I, & Donato R (2011). S100B protein stimulates microglia migration via rage-dependent up-regulation of chemokine expression and release. Journal of Biological Chemistry, 286(9), 7214–7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski DM, & Abel EL (1997). Acute treatment of paternal alcohol exposure produces malformations in offspring. Alcohol, 14(4), 397–401. [DOI] [PubMed] [Google Scholar]
- Bielawski DM, Zaher FM, Svinarich DM, & Abel EL (2002). Paternal alcohol exposure affects sperm cytosine methyltransferase messenger RNA levels. Alcoholism: Clinical and Experimental Research, 26(3), 347–351. [PubMed] [Google Scholar]
- Bird A (2007). Perceptions of epigenetics. Nature, 447(7143), 396–398. [DOI] [PubMed] [Google Scholar]
- Bodi CM, Vassoler FM, & Byrnes EM (2016). Adolescent experience affects postnatal ultrasonic vocalizations and gene expression in future offspring. Developmental Psychobiology, 58(6), 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bråbäck L, Lodge CJ, Lowe AJ, Dharmage SC, Olsson D, & Forsberg B (2018). Childhood asthma and smoking exposures before conception—A three-generational cohort study. Pediatric Allergy and Immunology, 29(4), 361–368. [DOI] [PubMed] [Google Scholar]
- Browne CJ, Godino A, Salery M, & Nestler EJ (2020). Epigenetic mechanisms of opioid addiction. Biological Psychiatry, 87(1), 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli SA, Shair HN, & Hofer MA (1994). Hypothermic vocalizations of rat pups (Rattus norvegicus) and direct maternal search behavior. Journal of Comparative Psychology, 108(3), 298. [DOI] [PubMed] [Google Scholar]
- Burggren W (2016). Epigenetic inheritance and its role in evolutionary biology: Re-evaluation and new perspectives. Biology (Basel), 5(2), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM (2005). Transgenerational consequences of adolescent morphine exposure in female rats: Effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology, 182(4), 537–544. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Babb JA, Scanlan VF, & Byrnes EM (2011). Adolescent opioid exposure in female rats: Transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behavioural Brain Research, 218(1), 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Carini LM, & Byrnes EM (2013). Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology, 227(2), 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Schenk ME, & Byrnes EM (2012). Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. Journal of Psychopharmacology, 26(10), 1348–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cake H, & Lenzer I (1985). On effects of paternal ethanol treatment on fetal outcome. Psychological Reports, 57, 7. [DOI] [PubMed] [Google Scholar]
- Calatayud J, & Gonzalez A (2003). History of the development and evolution of local anesthesia since the coca leaf. Anesthesiology: The Journal of the American Society of Anesthesiologists, 98(6), 1503–1508. [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Flanagan JP, Marchant NJ, & Lawrence AJ (2018). Reduced alcohol-seeking in male offspring of sires exposed to alcohol self-administration followed by punishment-imposed abstinence. Pharmacology Research & Perspectives, 6(2), e00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, & Yan Q (2012). Histone ubiquitination and deubiquitination in transcription, DNA damage response, and cancer. Frontiers in Oncology, 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, Dietrich J-B, et al. (2006). Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Molecular Pharmacology, 70(2), 487–492. [DOI] [PubMed] [Google Scholar]
- Cavalli G, & Heard E (2019). Advances in epigenetics link genetics to the environment and disease. Nature, 571(7766), 489–499. [DOI] [PubMed] [Google Scholar]
- Ceccanti M, Coccurello R, Carito V, Ciafrè S, Ferraguti G, Giacovazzo G, et al. (2016). Paternal alcohol exposure in mice alters brain NGF and BDNF and increases ethanol-elicited preference in male offspring. Addiction Biology, 21(4), 776–787. [DOI] [PubMed] [Google Scholar]
- Cecil C, Walton E, Smith R, Viding E, McCrory E, Relton C, et al. (2016). DNA methylation and substance-use risk: A prospective, genome-wide study spanning gestation to adolescence. Translational Psychiatry, 6(12), e976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá M, Mauro C, Hamilton A, Levy NS, Santaella-Tenorio J, Hasin D, et al. (2019). Association between recreational marijuana legalization in the united states and changes in marijuana use and cannabis use disorder from 2008 to 2016. JAMA Psychiatry, 77(2), 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. (2008). MeCP2, a key contributor to neurological disease, activates and represses transcription. Science, 320(5880), 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JC, & Maze I (2020). Nothing is yet set in (hi)stone: Novel post-translational modifications regulating chromatin function. Trends in Biochemical Sciences. 10.1016/j.tibs.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RC, Wang H, Bedi Y, & Golding MC (2019). Preconception paternal alcohol exposure exerts sex-specific effects on offspring growth and long-term metabolic programming. Epigenetics & Chromatin, 12(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain LG, & Sarkar DK (2017). Alcohol effects on the epigenome in the germline: Role in the inheritance of alcohol-related pathology. Alcohol, 60, 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. (2016). Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science, 351(6271), 397–400. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. (2009). Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science, 325(5942), 834–840. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Adams ML, Giordano A, Miller BT, O’Connor L, & Nock B (1991). Influence of morphine exposure during adolescence on the sexual maturation of male rats and the development of their offspring. Journal of Pharmacology and Experimental Therapeutics, 256(3), 1086–1093. [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O’Connor L, Adams M, & Meyer ER (1995). Adverse effects of paternal opiate exposure on offspring development and sensitivity to morphine-induced analgesia. Journal of Pharmacology and Experimental Therapeutics, 273(1), 386–392. [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O’Connor L, Adams ML, Sewing BN, & Meyer ER (1994). Acute alcohol exposure markedly influences male fertility and fetal outcome in the male rat. Life Sciences, 55(12), 901–910. [DOI] [PubMed] [Google Scholar]
- Collier AD, Min SS, Campbell SD, Roberts MY, Camidge K, & Leibowitz SF (2020). Maternal ethanol consumption before paternal fertilization: Stimulation of hypocretin neurogenesis and ethanol intake in zebrafish offspring. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 96, 109728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DL, Yetter N, & DeSomer H (2018). Intergenerational transmission of paternal trauma among us civil war ex-pows. Proceedings of the National Academy of Sciences of the United States of America, 115(44), 11215–11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T (2011). The missing genes: What happened to the heritability of psychiatric disorders? Molecular Psychiatry, 16(4), 362. [DOI] [PubMed] [Google Scholar]
- Dai J, Wang Z, Xu W, Zhang M, Zhu Z, Zhao X, et al. (2017). Paternal nicotine exposure defines different behavior in subsequent generation via hyper-methylation of mmu-mir-15b. Scientific Reports, 7(1), 7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J, Savani S, Krempley BD, Nguyen M, & Kitlinska JB (2016). Influence of paternal preconception exposures on their offspring: Through epigenetics to phenotype. American Journal of Stem Cells, 5(1), 11–18. [PMC free article] [PubMed] [Google Scholar]
- Deans C, & Maggert KA (2015). Whatdoyoumean,“epigenetic”? Genetics,199(4),887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann U (2016). Epigenetics: The origins and evolution of a fashionable topic. Developmental Biology, 416(1), 249–254. [DOI] [PubMed] [Google Scholar]
- Dinescu S, Ignat S, Lazar AD, Constantin C, Neagu M, & Costache M (2019). Epitranscriptomic signatures in lncRNAs and their possible roles in cancer. Genes, 10(1), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, et al. (2009). S100b’s double life: Intracellular regulator and extracellular signal. Biochimica et Biophysica Acta, Molecular Cell Research, 1793(6), 1008–1022. [DOI] [PubMed] [Google Scholar]
- Ducci F, & Goldman D (2012). The genetic basis of addictive disorders. Psychiatric Clinics of North America, 35(2), 495–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, et al. (2010). Missing heritability and strategies for finding the underlying causes of complex disease. Nature Reviews. Genetics, 11(6), 446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele MA, & Emanuele N (2001). Alcohol and the male reproductive system. Alcohol Research and Health, 25(4), 282–287. [PMC free article] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Steiner J, Colantoni A, Van Thiel DH, & Emanuele MA (2001). Peripubertal paternal EtOH exposure. Endocrine, 14(2), 213–219. [DOI] [PubMed] [Google Scholar]
- Engmann O, Labonté B, Mitchell A, Bashtrykov P, Calipari ES, Rosenbluh C, et al. (2017). Cocaine-induced chromatin modifications associate with increased expression and three-dimensional looping of auts2. Biological Psychiatry, 82(11), 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant B, Wimmer ME, Swinford-Jackson SE, Maurer J, Van Nest D, & Pierce RC (2019). Preconception maternal cocaine self-administration increases the reinforcing efficacy of cocaine in male offspring. Psychopharmacology, 236(12), 3429–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly LA, Thompson RE, Zhao S, Lepack AE, Lyu Y, Bhanu NV, et al. (2019). Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature, 567(7749), 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SP, Arasappan D, Hunicke-Smith S, Harris RA, & Mayfield RD (2015). Transcriptome organization for chronic alcohol abuse in human brain. Molecular Psychiatry, 20(11), 1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham JRS (1997). Epigenetic mechanisms of gene regulation. Edited by Russo VEA, Martienssen RA and Riggs AD. Cold Spring Harbor Laboratory Press, 1996. 693+xii pages. Price $125. Isbn 0 87969 490 4. Genetical Research, 69(2), 159–162. [Google Scholar]
- Finegersh A, & Homanics GE (2014a). Acute ethanol alters multiple histone modifications at model gene promoters in the cerebral cortex. Alcoholism: Clinical and Experimental Research, 38(7), 1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh A, & Homanics GE (2014b). Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS One, 9(6), e99078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh A, Rompala GR, Martin DI, & Homanics GE (2015). Drinking beyond a lifetime: New and emerging insights into paternal alcohol exposure on subsequent generations. Alcohol, 49(5), 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DK, Rice RC, Martinez Rivera A, Donohoe M, & Rajadhyaksha AM (2017). Altered reward sensitivity in female offspring of cocaine-exposed fathers. Behavioural Brain Research, 332, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca B, Teixeira N, & Correia-da-Silva G (2017). Cannabinoids as modulators of cell death: Clinical applications and future directions. In Nilius B, Gudermann Th., Jahn R, Lill R, Petersen OH, & de Tombe P (Eds.), Vol. 173. Reviews of physiology, biochemistry & pharmacology (pp. 63–88). Springer. [DOI] [PubMed] [Google Scholar]
- Gajewski PA, Eagle AL, Williams ES, Manning CE, Lynch H, McCornack C, et al. (2019). Epigenetic regulation of hippocampal fosb expression controls behavioral responses to cocaine. Journal of Neuroscience, 39(42), 8305–8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, & Bohacek J (2018). Epigenetic germline inheritance in mammals: Looking to the past to understand the future. Genes, Brain, and Behavior, 17(3), e12407. [DOI] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, et al. (2014). Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nature Neuroscience, 17(5), 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates LA, Shi J, Rohira AD, Feng Q, Zhu B, Bedford MT, et al. (2017). Acetylation on histone H3 lysine 9 mediates a switch from transcription initiation to elongation. Journal of Biological Chemistry, 292(35), 14456–14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta E, Grayson DR, Auta J, Saudagar V, Dong E, Chen Y, et al. (2019). Genome-wide methylation in alcohol use disorder subjects: Implications for an epigenetic regulation of the cortico-limbic glucocorticoid receptors (nr3c1). Molecular Psychiatry. 10.1038/s41380-019-0449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George VK, Li H, Teloken C, Grignon DJ, Lawrence WD, & Dhabuwala C (1996). Effects of long-term cocaine exposure on spermatogenesis and fertility in peripubertal male rats. The Journal of Urology, 155(1), 327–331. [PubMed] [Google Scholar]
- Goldberg LR, & Gould TJ (2019). Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. European Journal of Neuroscience, 50(3), 2453–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LR, Zeid D, Kutlu MG, Cole RD, Lallai V, Sebastian A, et al. (2019). Paternal nicotine enhances fear memory, reduces nicotine administration, and alters hippocampal genetic and neural function in offspring. Addiction Biology. 10.1111/adb.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, et al. (2017). Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Scientific Reports, 7, 46179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, & Ducci F (2005). The genetics of addictions: Uncovering the genes. Nature Reviews. Genetics, 6(7), 521–532. [DOI] [PubMed] [Google Scholar]
- Gonzales K, Roeber J, Kanny D, Tran A, Saiki C, Johnson H, et al. (2014). Alcohol-attributable deaths and years of potential life lost—11 states, 2006–2010. MMWR. Morbidity and Mortality Weekly Report, 63(10), 213–216. [PMC free article] [PubMed] [Google Scholar]
- Goodwin DW, & Hill SY (1975). Chronic effects of alcohol and other psychoactive drugs on intellect, learning and memory. In Rankin JG (Ed.), Alcohol, drugs and brain damage (pp. 55–69). Toronto: Addiction Research Foundation. [Google Scholar]
- Grandjean V, Fourré S, De Abreu DAF, Derieppe M-A, Remy J-J, & Rassoulzadegan M (2015). RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Scientific Reports, 5, 18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Xu W, Nie D, Zhang D, Dai J, Zhao X, et al. (2016). Nicotine induces Nme2-mediated apoptosis in mouse testes. Biochemical and Biophysical Research Communications, 472(4), 573–579. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Saito TR, Furudate S-I, & Takahashi KW (2001). Prolactin levels and maternal behavior induced by ultrasonic vocalizations of the rat pup. Experimental Animals, 50(4), 307–312. [DOI] [PubMed] [Google Scholar]
- Hawkey AB, White H, Pippen E, Greengrove E, Rezvani AH, Murphy SK, et al. (2019). Paternal nicotine exposure in rats produces long-lasting neurobehavioral effects in the offspring. Neurotoxicology and Teratology, 74, 106808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Lidow IA, & Lidow MS (2006). Consequences of paternal cocaine exposure in mice. Neurotoxicology and Teratology, 28(2), 198–209. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Miniño AM, & Warner M (2020). Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, no 356. Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- Hempel BJ, Crissman ME, Imanalieva A, Melkumyan M, Weiss TD, Winston CA, et al. (2020). Cross-generational THC exposure alters heroin reinforcement in adult male offspring. Drug and Alcohol Dependence, 212, 107985. [DOI] [PubMed] [Google Scholar]
- Henikoff S, & Smith MM (2015). Histone variants and epigenetics. Cold Spring Harbor Perspectives in Biology, 7(1), a019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Rompala G, Homanics GE, & Zezza N (2017). Cross-generational effects of alcohol dependence in humans on HRAS and TP53 methylation in offspring. Epigenomics, 9(9), 1189–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander J, McNivens M, Pautassi RM, & Nizhnikov ME (2019). Offspring of male rats exposed to binge alcohol exhibit heightened ethanol intake at infancy and alterations in t-maze performance. Alcohol, 76, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R (1994). Epigenetics: An overview. Developmental Genetics, 15(6), 453–457. [DOI] [PubMed] [Google Scholar]
- Holliday R, & Pugh JE (1975). DNA modification mechanisms and gene activity during development. Science, 187(4173), 226–232. [PubMed] [Google Scholar]
- Hughes A, Williams MR, Lipari RN, & Van Horn S (2016). State estimates of past year cocaine use among young adults: 2014 and 2015. In The CBHSQ report Substance Abuse and Mental Health Services Administration (US). [PubMed] [Google Scholar]
- Jabbar S, Chastain LG, Gangisetty O, Cabrera MA, Sochacki K, & Sarkar DK (2016). Preconception alcohol increases offspring vulnerability to stress. Neuropsychopharmacology, 41(11), 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamerson PA, Wulser MJ, & Kimler BF (2004). Neurobehavioral effects in rat pups whose sires were exposed to alcohol. Brain Research. Developmental Brain Research, 149(2), 103–111. [DOI] [PubMed] [Google Scholar]
- Jangra A, Sriram CS, Pandey S, Choubey P, Rajput P, Saroha B, et al. (2016). Epigenetic modifications, alcoholic brain and potential drug targets. Annals of Neurosciences, 23(4), 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T, James E, Alonso D, Hoidal J, Murphy P, Hotaling J, et al. (2017). Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology, 5(6), 1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John RM, & Rougeulle C (2018). Developmental epigenetics: Phenotype and the flexible epigenome. Frontiers in Cell and Development Biology, 6, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, & Byrnes EM (2011). Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Frontiers in Psychiatry, 2, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Hsieh LS, Lee AM, Zhou Z, Coman D, Heath CJ, et al. (2016). An epigenetic mechanism mediates developmental nicotine effects on neuronal structure and behavior. Nature Neuroscience, 19(7), 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl G (2015). The dictionary of genomics, transcriptomics and proteomics, 4 volume set. John Wiley & Sons. [Google Scholar]
- Kasza KA, Ambrose BK, Conway KP, Borek N, Taylor K, Goniewicz ML, et al. (2017). Tobacco-product use by adults and youths in the united states in 2013 and 2014. New England Journal of Medicine, 376(4), 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DA, & Yudowski GA (2016). Cannabinoid receptors in the central nervous system: Their signaling and roles in disease. Frontiers in Cellular Neuroscience, 10, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killinger CE, Robinson S, & Stanwood GD (2012). Subtle biobehavioral effects produced by paternal cocaine exposure. Synapse, 66(10), 902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Choi CS, Park JH, Joo SH, Kim SY, Ko HM, et al. (2014). Chronic exposure to ethanol of male mice before mating produces attention deficit hyperactivity disorder-like phenotype along with epigenetic dysregulation of dopamine transporter expression in mouse offspring. Journal of Neuroscience Research, 92(5), 658–670. [DOI] [PubMed] [Google Scholar]
- King BA, Patel R, Nguyen KH, & Dube SR (2014). Trends in awareness and use of electronic cigarettes among us adults, 2010–2013. Nicotine & Tobacco Research, 17(2), 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezovich JG, & Ramsay M (2012). The effect of preconception paternal alcohol exposure on epigenetic remodeling of the h19 and rasgrf1 imprinting control regions in mouse offspring. Frontiers in Genetics, 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe C, Oertel BG, Ultsch A, Kettner M, Schmidt PH, Wunder C, et al. (2016). Pharmacoepigenetics of the role of DNA methylation in μ-opioid receptor expression in different human brain regions. Epigenomics, 8(12), 1583–1599. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. (2005). Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron, 48(2), 303–314. [DOI] [PubMed] [Google Scholar]
- Lacal I, & Ventura R (2018). Epigenetic inheritance: Concepts, mechanisms and perspectives. Frontiers in Molecular Neuroscience, 11, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q, Yan B, Yu X, Li Y, Song H, Zhu H, et al. (2017). Drug-seeking motivation level in male rats determines offspring susceptibility or resistance to cocaine-seeking behaviour. Nature Communications, 8, 15527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledig M, Misslin R, Vogel E, Holownia A, Copin J, & Tholey G (1998). Paternal alcohol exposure: Developmental and behavioral effects on the offspring of rats. Neuropharmacology, 37(1), 57–66. [DOI] [PubMed] [Google Scholar]
- Leichter J (1986). Effect of paternal alcohol ingestion on fetal growth in rats. Growth, 50(2), 228–233. [PubMed] [Google Scholar]
- Lemoine P, Harousseau H, Borteyru J, & Menuet J (2003). Children of alcoholic parents—Observed anomalies: Discussion of 127 cases. Therapeutic Drug Monitoring, 25(2), 132–136. [DOI] [PubMed] [Google Scholar]
- Lempradl A (2020). Germ cell-mediated mechanisms of epigenetic inheritance. In Paper presented at the seminars in cell & developmental biology. [DOI] [PubMed] [Google Scholar]
- Lepack AE, Werner CT, Stewart AF, Fulton SL, Zhong P, Farrelly LA, et al. (2020). Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science, 368(6487), 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Hawkey AB, Hall BJ, Cauley M, Slade S, Yazdani E, et al. (2019). Paternal THC exposure in rats causes long-lasting neurobehavioral effects in the offspring. Neurotoxicology and Teratology, 74, 106806. [DOI] [PubMed] [Google Scholar]
- Li MD, & Burmeister M (2009). New insights into the genetics of addiction. Nature Reviews. Genetics, 10(4), 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-Q, Luo Y-W, Bi F-F, Cui T-T, Song L, Cao W-Y, et al. (2014). Development of anxiety-like behavior via hippocampal IGF-2 signaling in the offspring of parental morphine exposure: Effect of enriched environment. Neuropsychopharmacology, 39(12), 2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang X, Qi Z, Sang Y, Liu Y, Xu B, et al. (2019). The role of mRNA m(6)a methylation in the nervous system. Cell & Bioscience, 9, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Diao L, Jiang N, Zhang J, Wang H-J, Zhou W-H, et al. (2015). Chronic exposure to ethanol in male mice may be associated with hearing loss in offspring. Asian Journal of Andrology, 17(6), 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Diao L, Liu J, Jiang N, Zhang J, Wang H, et al. (2014). Paternal ethanol exposure and behavioral abnormities in offspring: Associated alterations in imprinted gene methylation. Neuropharmacology, 81, 126–133. [DOI] [PubMed] [Google Scholar]
- Little RE (1980). Maternal alcohol and tobacco use and nausea and vomiting during pregnancy: Relation to infant birthweight. Acta Obstetricia et Gynecologica Scandinavica, 59(6), 495–497. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Maier SE, & West JR (2004). Long-term alcohol exposure prior to conception results in lower fetal body weights. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 71(3), 135–141. [DOI] [PubMed] [Google Scholar]
- Mahnke AH, Miranda RC, & Homanics GE (2017). Epigenetic mediators and consequences of excessive alcohol consumption. Alcohol (Fayetteville, NY), 60, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, & Martin M (2016). Epigenetics, behavior and early nicotine. Nature Neuroscience, 19(7), 863–864. [DOI] [PubMed] [Google Scholar]
- Mankes R, LeFevre R, Benitz KF, Rosenblum I, Bates H, Walker A, et al. (1982). Paternal effects of ethanol in the long-evans rat. Journal of Toxicology and Environmental Health, Part A: Current Issues, 10(6), 871–878. [DOI] [PubMed] [Google Scholar]
- Marczylo EL, Amoako AA, Konje JC, Gant TW, & Marczylo TH (2012). Smoking induces differential mirna expression in human spermatozoa: A potential transgenerational epigenetic concern? Epigenetics, 7(5), 432–439. [DOI] [PubMed] [Google Scholar]
- Marutha Ravindran CR, & Ticku MK (2005). Role of CpG islands in the up-regulation of NMDA receptor NR2B gene expression following chronic ethanol treatment of cultured cortical neurons of mice. Neurochemistry International, 46(4), 313–327. [DOI] [PubMed] [Google Scholar]
- Mayfield RD (2017). Emerging roles for ncRNAs in alcohol use disorders. Alcohol, 60, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Morgan TJ Jr., Lowe SE, Williamson MJ, Spencer TJ, Biederman J, et al. (2018). Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS Biology, 16(10), e2006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek LR, Myren K, Sturm J, & Burau D (2007). Acute paternal alcohol use affects offspring development and adult behavior. Physiology & Behavior, 91(1), 154–160. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2004). Actual causes of death in the united states, 2000. JAMA, 291(10), 1238–1245. [DOI] [PubMed] [Google Scholar]
- Molinie B, & Giallourakis CC (2017). Genome-wide location analyses of n6-methyladenosine modifications (m6A-seq). In Lusser A (Ed.), RNA methylation (pp. 45–53). Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo-Ortiz JL, Cheng Z, Kranzler HR, Zhang H, & Gelernter J (2019). Genomewide study of epigenetic biomarkers of opioid dependence in European–American women. Scientific Reports, 9(1), 4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan HD, Santos F, Green K, Dean W, & Reik W (2005). Epigenetic reprogramming in mammals. Human Molecular Genetics, 14(suppl_1), R47–R58. [DOI] [PubMed] [Google Scholar]
- Morgan HL, & Watkins AJ (2019). Transgenerational impact of environmental change. Advances in Experimental Medicine and Biology, 1200, 71–89. [DOI] [PubMed] [Google Scholar]
- Morris CV, DiNieri JA, Szutorisz H, & Hurd YL (2011). Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. European Journal of Neuroscience, 34(10), 1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulaei N, Mondanizadeh M, Salmani ME, Palizvan MR, Khansarinejad B, & Sadegh M (2018). Transgenerational consequences of prepregnancy chronic morphine use on spatial learning and hippocampal Mecp2 and Hdac2 expression. Neuroreport, 29(9), 739–744. [DOI] [PubMed] [Google Scholar]
- Murphy SK, Itchon-Ramos N, Visco Z, Huang Z, Grenier C, Schrott R, et al. (2018). Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics, 13(12), 1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair N, Shoaib M, & Sørensen CS (2017). Chromatin dynamics in genome stability: Roles in suppressing endogenous DNA damage and facilitating DNA repair. International Journal of Molecular Sciences, 18(7), 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney DL (1958). Epigenetic control systems. Proceedings of the National Academy of Sciences of the United States of America, 44(7), 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naquiah MZF, James RJ, Suratman S, Lee LS, Hafidz MIM, Salleh MZ, et al. (2016). Transgenerational effects of paternal heroin addiction on anxiety and aggression behavior in male offspring. Behavioral and Brain Functions, 12(1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H-H, Zhang Y, Hendrich B, Johnson CA, Turner BM, Erdjument-Bromage H, et al. (1999). MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nature Genetics, 23(1), 58. [DOI] [PubMed] [Google Scholar]
- Nicoglou A (2018). Waddington’s epigenetics or the pictorial meetings of development and genetics. History and Philosophy of Life Sciences, 40(4), 61. [DOI] [PubMed] [Google Scholar]
- Nicoglou A, & Merlin F (2017). Epigenetics: A way to bridge the gap between biological fields. Studies in History and Philosophy of Biological and Biomedical Sciences, 66, 73–82. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Utrankar A, Reyes JA, Simons DD, & Kosten TR (2012). Epigenetics of drug abuse: Predisposition or response. Pharmacogenomics, 13(10), 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouko LA, Shantikumar K, Knezovich J, Haycock P, Schnugh DJ, & Ramsay M (2009). Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and ig-dmr in male gametes—Implications for fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research, 33(9), 1615–1627. [DOI] [PubMed] [Google Scholar]
- Pachenari N, Azizi H, Ghasemi E, Azadi M, & Semnanian S (2018). Exposure to opiates in male adolescent rats alters pain perception in the male offspring. Behavioural Pharmacology, 29(2), 255–260. [DOI] [PubMed] [Google Scholar]
- Pachenari N, Azizi H, & Semnaniann S (2019). Adolescent morphine exposure in male rats alters the electrophysiological properties of locus coeruleus neurons of the male offspring. Neuroscience, 410, 108–117. [DOI] [PubMed] [Google Scholar]
- Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, & Roseboom TJ (2008). Transgenerational effects of prenatal exposure to the dutch famine on neonatal adiposity and health in later life. BJOG: An International Journal of Obstetrics and Gynaecology, 115(10), 1243–1249. [DOI] [PubMed] [Google Scholar]
- Palmisano M, & Pandey SC (2017). Epigenetic mechanisms of alcoholism and stress-related disorders. Alcohol, 60, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Kyzar EJ, & Zhang H (2017). Epigenetic basis of the dark side of alcohol addiction. Neuropharmacology, 122, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, & Prakash A (2008). Brain chromatin remodeling: A novel mechanism of alcoholism. Journal of Neuroscience, 28(14), 3729–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parira T, Laverde A, & Agudelo M (2017). Epigenetic interactions between alcohol and cannabinergic effects: Focus on histone modification and DNA methylation. Journal of Alcoholism and Drug Dependence, 5(2), 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan H, & Williams J (2012). Basic opioid pharmacology: An update. British Journal of Pain, 6(1), 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, et al. (2006). Sex-specific, male-line transgenerational responses in humans. European Journal of Human Genetics, 14(2), 159–166. [DOI] [PubMed] [Google Scholar]
- Penrod RD, Carreira MB, Taniguchi M, Kumar J, Maddox SA, & Cowan CW (2018). Novel role and regulation of hdac4 in cocaine-related behaviors. Addiction Biology, 23(2), 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MF, & Lehner B (2019). Intergenerational and transgenerational epigenetic inheritance in animals. Nature Cell Biology, 21(2), 143–151. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Fant B, Swinford-Jackson SE, Heller EA, Berrettini WH, & Wimmer ME (2018). Environmental, genetic and epigenetic contributions to cocaine addiction. Neuropsychopharmacology, 43(7), 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, & Vassoler FM (2014). Reduced cocaine reinforcement in the male offspring of cocaine-experienced sires. Neuropsychopharmacology, 39(1), 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisera-Fuster A, Faillace MP, & Bernabeu R (2020). Pre-exposure to nicotine with nocturnal abstinence induces epigenetic changes that potentiate nicotine preference. Molecular Neurobiology, 57(4), 1828–1846. [DOI] [PubMed] [Google Scholar]
- Pitsilis G, Spyridakos D, Nomikos GG, & Panagis G (2017). Adolescent female cannabinoid exposure diminishes the reward-facilitating effects of δ9-tetrahydrocannabinol and d-amphetamine in the adult male offspring. Frontiers in Pharmacology, 8, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooriamehr A, Sabahi P, & Miladi-Gorji H (2017). Effects of environmental enrichment during abstinence in morphine dependent parents on anxiety, depressive-like behaviors and voluntary morphine consumption in rat offspring. Neuroscience Letters, 656, 37–42. [DOI] [PubMed] [Google Scholar]
- Prescott CA, & Kendler KS (1999). Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry, 156(1), 34–40. [DOI] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Rao YS, Prins SA, & Pak TR (2014). Parental binge alcohol abuse alters F1 generation hypothalamic gene expression in the absence of direct fetal alcohol exposure. PLoS One, 9(2), e89320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, & Mehler MF (2018). Epigenetic mechanisms underlying nervous system diseases. Handbook of Clinical Neurology, 147, 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay M (2010). Genetic and epigenetic insights into fetal alcohol spectrum disorders. Genome Medicine, 2(4), 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Burling TA, Lochry EA, & Sutker PB (1982). The effect of paternal alcohol consumption on fetal development in mice. Drug and Alcohol Dependence, 9(1), 89–95. [DOI] [PubMed] [Google Scholar]
- Reilly JN, McLaughlin EA, Stanger SJ, Anderson AL, Hutcheon K, Church K, et al. (2016). Characterisation of mouse epididymosomes reveals a complex profile of micrornas and a potential mechanism for modification of the sperm epigenome. Scientific Reports, 6(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma MB, Fullman N, Ng M, Salama JS, Abajobir A, Abate KH, et al. (2017). Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the global burden of disease study 2015. The Lancet, 389(10082), 1885–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud SM, & Fountain SB (2016). Transgenerational effects of adolescent nicotine exposure in rats: Evidence for cognitive deficits in adult female offspring. Neurotoxicology and Teratology, 56, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richa R, & Sinha RP (2014). Hydroxymethylation of DNA: An epigenetic marker. EXCLI Journal, 13, 592–610. [PMC free article] [PubMed] [Google Scholar]
- Riggs AD (1975). X inactivation, differentiation, and DNA methylation. Cytogenetics and Cell Genetics, 14(1), 9–25. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, & Warren KR (2011). Fetal alcohol spectrum disorders: An overview. Neuropsychology Review, 21(2), 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera RM, & Bennett LB (2010). Epigenetics in humans: An overview. Current Opinion in Endocrinology, Diabetes and Obesity, 17(6), 493–499. [DOI] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, & Bale TL (2015). Transgenerational epigenetic programming via sperm microrna recapitulates effects of paternal stress. Proceedings of the National Academy of Sciences of the United States of America, 112(44), 13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Ferguson C, & Homanics GE (2020). Coincubation of sperm with epididymal extracellular vesicle preparations from chronic intermittent ethanol-treated mice is sufficient to impart anxiety-like and ethanol-induced behaviors to adult progeny. Alcohol, 87, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Finegersh A, & Homanics GE (2016). Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol, 53, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Finegersh A, Slater M, & Homanics GE (2017). Paternal preconception alcohol exposure imparts intergenerational alcohol-related behaviors to male offspring on a pure C57BL/6J background. Alcohol, 60, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, & Homanics GE (2019). Intergenerational effects of alcohol: A review of paternal preconception ethanol exposure studies and epigenetic mechanisms in the male germline. Alcoholism, Clinical and Experimental Research, 43(6), 1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Mounier A, Wolfe CM, Lin Q, Lefterov I, & Homanics GE (2018). Heavy chronic intermittent ethanol exposure alters small noncoding RNAs in mouse sperm and epididymosomes. Frontiers in Genetics, 9, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabzevari S, Rohbani K, Sadat-Shirazi M-S, Babhadi-Ashar N, Shakeri A, Ashabi G, et al. (2019). Morphine exposure before conception affects anxiety-like behavior and crf level (in the CSF and plasma) in the adult male offspring. Brain Research Bulletin, 144, 122–131. [DOI] [PubMed] [Google Scholar]
- Sadakierska-Chudy A, & Filip M (2015). A comprehensive view of the epigenetic landscape. Part II: Histone post-translational modification, nucleosome level, and chromatin regulation by ncRNAs. Neurotoxicity Research, 27(2), 172–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadat-Shirazi M-S, Asgari P, Mahboubi S, Zadeh-Tehrani SN, Ashabi G, Rohbani K, et al. (2020). Effect of morphine exposure on novel object memory of the offspring: The role of histone H3 and δfosb. Brain Research Bulletin, 156, 141–149. [DOI] [PubMed] [Google Scholar]
- Saitou M, & Yamaji M (2012). Primordial germ cells in mice. Cold Spring Harbor Perspectives in Biology, 4(11), a008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Sierra JV, Salgado García FI, Brooks JH, Derefinko KJ, & Mozhui K (2020). Effect of short-term prescription opioids on DNA methylation of the oprm1 promoter. Clinical Epigenetics, 12(1), 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Constantinof A, Pan P, Kupferschmidt DA, McGowan PO, & Erb S (2014). Cocaine exposure prior to pregnancy alters the psychomotor response to cocaine and transcriptional regulation of the dopamine D1 receptor in adult male offspring. Behavioural Brain Research, 265, 163–170. [DOI] [PubMed] [Google Scholar]
- Schmeisser MJ, Baumann B, Johannsen S, Vindedal GF, Jensen V, Hvalby ØC, et al. (2012). Iκb kinase/nuclear factor κb-dependent insulin-like growth factor 2 (Igf2) expression regulates synapse formation and spine maturation via Igf2 receptor signaling. Journal of Neuroscience, 32(16), 5688–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrott R, Acharya K, Itchon-Ramos N, Hawkey AB, Pippen E, Mitchell JT, et al. (2019). Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics, 15(1–2), 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrott R, & Murphy SK (2020). Cannabis use and the sperm epigenome: A budding concern? Environmental Epigenetics, 6(1), dvaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuel H, Burkman LJ, Lippes J, Crickard K, Forester E, Piomelli D, et al. (2002). N-acylethanolamines in human reproductive fluids. Chemistry and Physics of Lipids, 121(1–2), 211–227. [DOI] [PubMed] [Google Scholar]
- Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, et al. (2016). Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science, 351(6271), 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, et al. (2018). Small RNAs are trafficked from the epididymis to developing mammalian sperm. Developmental Cell, 46(4), 481–494. e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi DQ, Ali I, Tang J, & Yang WC (2017). New insights into 5hmc DNA modification: Generation, distribution and function. Frontiers in Genetics, 8, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi J. i., Yanagita K, Fujita M, & Bungo T (2008). M-opioid receptor agonist diminishes pomc gene expression and anorexia by central insulin in neonatal chicks. Neuroscience Letters, 439(3), 227–229. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, & Guerrero-Bosagna C (2010). Epigenetic transgenerational actions of environmental factors in disease etiology. Trends in Endocrinology and Metabolism, 21(4), 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvortsova K, Iovino N, & Bogdanović O (2018). Functions and mechanisms of epigenetic inheritance in animals. Nature Reviews Molecular Cell Biology, 19(12), 774–790. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2017). Key substance use and mental health indicators in the united states: Results from the 2016 national survey on drug use and health. Center for Behavioral Health Statistics and Quality (CBHSQ). HHS Publication No. SMA 17–5044, NSDUH Series H-52. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2019). Key substance use and mental health indicators in the united states: Results from the 2018 national survey on drug use and health. Center for Behavioral Health Statistics and Quality (CBHSQ). HHS Publication No. PEP19–5068, NSDUH Series H-54. [Google Scholar]
- Swygert SG, & Peterson CL (2014). Chromatin dynamics: Interplay between remodeling enzymes and histone modifications. Biochimica et Biophysica Acta (BBA)—Bioenergetics, 1839(8), 728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SH, Boulard M, Shukla MS, Gautier T, Travers A, Bednar J, et al. (2009). The incorporation of the novel histone variant h2al2 confers unusual structural and functional properties of the nucleosome. Nucleic Acids Research, 37(14), 4684–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, et al. (2014). Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology, 39(6), 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, Egervari G, Sperry J, Carter JM, & Hurd YL (2016). Cross-generational THC exposure alters the developmental sensitivity of ventral and dorsal striatal gene expression in male and female offspring. Neurotoxicology and Teratology, 58, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, & Hurd YL (2016). Epigenetic effects of cannabis exposure. Biological Psychiatry, 79(7), 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, & Hurd YL (2018). High times for cannabis: Epigenetic imprint and its legacy on brain and behavior. Neuroscience & Biobehavioral Reviews, 85, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Jacob T, & Bremer DA (1989). Cognitive status of sons of alcoholic men. Alcoholism: Clinical and Experimental Research, 13(2), 232–235. [DOI] [PubMed] [Google Scholar]
- Toorie AM, Vassoler FM, Qu F, Schonhoff CM, Bradburn S, Murgatroyd CA, et al. (2019). A history of opioid exposure in females increases the risk of metabolic disorders in their future male offspring. Addiction Biology. 10.1111/adb.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trerotola M, Relli V, Simeone P, & Alberti S (2015). Epigenetic inheritance and the missing heritability. Human Genomics, 9(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt K, Ernst C, Mash D, & Turecki G (2017). DNA methylation dynamics and cocaine in the brain: Progress and prospects. Genes (Basel), 8(5), 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaissiere T, Sawan C, & Herceg Z (2008). Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutation Research, 659(1–2), 40–48. [DOI] [PubMed] [Google Scholar]
- Vallaster MP, Kukreja S, Bing XY, Ngolab J, Zhao-Shea R, Gardner PD, et al. (2017). Paternal nicotine exposure alters hepatic xenobiotic metabolism in offspring. eLife, 6, e24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Otterdijk SD, & Michels KB (2016). Transgenerational epigenetic inheritance in mammals: How good is the evidence? FASEB Journal, 30(7), 2457–2465. [DOI] [PubMed] [Google Scholar]
- Vandewater EA, Park SE, Carey FR, & Wilkinson AV (2013). Intergenerational transfer of smoking across three generations and forty-five years. Nicotine & Tobacco Research, 16(1), 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Johnson NL, & Byrnes EM (2013). Female adolescent exposure to cannabinoids causes transgenerational effects on morphine sensitization in female offspring in the absence of in utero exposure. Journal of Psychopharmacology, 27(11), 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Johnson-Collins NL, Carini LM, & Byrnes EM (2014). Next generation effects of female adolescent morphine exposure: Sex-specific alterations in response to acute morphine emerge before puberty. Behavioural Pharmacology, 25(2), 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Oliver DJ, Wyse C, Blau A, Shtutman M, Turner JR, et al. (2017). Transgenerational attenuation of opioid self-administration as a consequence of adolescent morphine exposure. Neuropharmacology, 113, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Toorie AM, & Byrnes EM (2018). Transgenerational blunting of morphine-induced corticosterone secretion is associated with dysregulated gene expression in male offspring. Brain Research, 1679, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Toorie AM, & Byrnes EM (2019). Increased cocaine reward in offspring of females exposed to morphine during adolescence. Psychopharmacology, 236(4), 1261–1272. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Toorie AM, Teceno DN, Walia P, Moore DJ, Patton TD, et al. (2020). Paternal morphine exposure induces bidirectional effects on cocaine versus opioid self-administration. Neuropharmacology, 162, 107852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, & Pierce RC (2013). Epigenetic inheritance of a cocaine-resistance phenotype. Nature Neuroscience, 16(1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Wright SJ, & Byrnes EM (2016). Exposure to opiates in female adolescents alters mu opiate receptor expression and increases the rewarding effects of morphine in future offspring. Neuropharmacology, 103, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenendaal MV, Painter RC, de Rooij SR, Bossuyt PM, van der Post JA, Gluckman PD, et al. (2013). Transgenerational effects of prenatal exposure to the 1944–45 dutch famine. BJOG: An International Journal of Obstetrics and Gynaecology, 120(5), 548–553. [DOI] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, & Kendler KS (2015). The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychological Medicine, 45(5), 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen-Smit E, Koning IM, Verdurmen JE, Van der Vorst H, Engels RC, & Vollebergh WA (2012). The influence of paternal and maternal drinking patterns within two-partner families on the initiation and development of adolescent drinking. Addictive Behaviors, 37(11), 1248–1256. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, et al. (2006). Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. The Journal of Neuroscience, 26(24), 6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyssotski DL (2011). Transgenerational epigenetic compensation. Evolocus, 1, 1–6. [Google Scholar]
- Waddington CH (1942). Canalization of development and the inheritance of acquired characters. Nature, 150(3811), 563–565. [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, & Phillips PE (2009). Phasic dopamine release in appetitive behaviors and drug addiction. Current Drug Abuse Reviews, 2(2), 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-C, Kapoor M, & Goate AM (2012). The genetics of substance dependence. Annual Review of Genomics and Human Genetics, 13, 241–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J, et al. (2010). Chronic cocaine-induced H3 acetylation and transcriptional activation of camkiialpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology, 35(4), 913–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CT, Szutorisz H, Garg P, Martin Q, Landry JA, Sharp AJ, et al. (2015). Genome-wide DNA methylation profiling reveals epigenetic changes in the rat nucleus accumbens associated with cross-generational effects of adolescent THC exposure. Neuropsychopharmacology, 40(13), 2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold B (2006). Epigenetics: The science of change. Environmental Helath Persepectives, 114(3), A160–A167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SL, Vassoler FM, Schmidt HD, Pierce RC, & Wimmer ME (2016). Enhanced anxiety in the male offspring of sires that self-administered cocaine. Addiction Biology, 21(4), 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, & North R (1984). Opiate-receptor interactions on single locus coeruleus neurones. Molecular Pharmacology, 26(3), 489–497. [PubMed] [Google Scholar]
- Wimmer M, Briand L, Fant B, Guercio L, Arreola A, Schmidt H, et al. (2017). Paternal cocaine taking elicits epigenetic remodeling and memory deficits in male progeny. Molecular Psychiatry, 22(11), 1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer ME, Vassoler FM, White SL, Schmidt HD, Sidoli S, Han Y, et al. (2019). Impaired cocaine-induced behavioral plasticity in the male offspring of cocaine-experienced sires. European Journal of Neuroscience, 49(9), 1115–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, & Rompre P-P (1989). Brain dopamine and reward. Annual Review of Psychology, 40(1), 191–225. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Cicero TJ, Kettinger L III, & Meyer ER (1991). Paternal alcohol consumption in the rat impairs spatial learning performance in male offspring. Psychopharmacology, 105(2), 289–302. [DOI] [PubMed] [Google Scholar]
- Wu C-C, Hsu T-Y, Chang J-C, Ou C-Y, Kuo H-C, Liu C-A, et al. (2019). Paternal tobacco smoke correlated to offspring asthma and prenatal epigenetic programming. Frontiers in Genetics, 10, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaw AM, Prosser RA, Jones PC, Garcia BJ, Jacobson DA, & Glass JD (2019). Epigenetic effects of paternal cocaine on reward stimulus behavior and accumbens gene expression in mice. Behavioural Brain Research, 367, 68–81. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bell A, Bierer LM, & Schmeidler J (2008). Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of holocaust survivors. Journal of Psychiatric Research, 42(13), 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, et al. (2016). Holocaust exposure induced intergenerational effects on fkbp5 methylation. Biological Psychiatry, 80(5), 372–380. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Lehrner A, Desarnaud F, Bader HN, Makotkine I, et al. (2014). Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in holocaust survivor offspring. American Journal of Psychiatry, 171(8), 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn NL, Bartolomei MS, & Blendy JA (2015). Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Progress in Biophysics and Molecular Biology, 118(1–2), 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn NL, Caruso MJ, & Blendy JA (2019). Effects of nicotine and stress exposure across generations in C57BL/6 mice. Stress, 22(1), 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C-Y, & Kuo H-C (2019). The emerging roles and functions of circular RNAs and their generation. Journal of Biomedical Science, 26(1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Xu W, He G, Zhang D, Zhao X, Dai J, et al. (2018). Maternal nicotine exposure has severe cross-generational effects on offspring behavior. Behavioural Brain Research, 348, 263–266. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhang D, Dai J, Cao Y, Xu W, He G, et al. (2020). Paternal nicotine exposure induces hyperactivity in next-generation via down-regulating the expression of DAT. Toxicology, 431, 152367. [DOI] [PubMed] [Google Scholar]
- Zou S, & Kumar U (2018). Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. International Journal of Molecular Sciences, 19(3), 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


