Abstract
Background:
Developing bone is highly adaptable and as such, is susceptible to pathological shape deformation. Thus, it is imperative to quantify if changes in patellofemoral morphology are associated with adolescent-onset patellofemoral pain, as a pathway to improve our understanding of this pain’s etiology.
Purpose:
To quantify and compare patellofemoral morphology in adolescent patients with patellofemoral pain to matched-healthy adolescent controls; and determine if a relationship exists between shape and kinematics (measured during active flexion-extension).
Study Design:
Case-Control
Methods:
Using 3D static MR images acquired during a previous study, we measured patellar, trochlear, and lateral-patellar width; trochlear and patellar depth; Wiberg index; patella-height ratio; lateral trochlear inclination; cartilage and lateral femoral shaft length. Student’s t-test compared shape parameters between adolescents with patellofemoral pain and controls. Pearson’s correlations and step-wise linear regression models explored the relationship between morphology, kinematics (medial-lateral shift/tilt), and pain.
Results:
Relative to controls, adolescents with patellofemoral pain had larger sulci (6.6±0.7 vs. 6.0±1.1; 95% CI: 0.6 mm; p=0.043; d=0.66), lateral-patellar width (23.1±2.4 vs. 21.4±2.6; 95% CI: 1.6 mm; p = 0.033; d=0.70), and patella-trochlear width ratio (1.2±0.1 vs. 1.1±0.1; 95% CI: 0.1; p<0.001; d=1.26). Shape correlated with kinematics in both cohorts and in the entire population. In the patellofemoral pain group, lateral shaft length (r=0.518; p=0.019), Wiberg index (r=0.477; p=0.033), and patella-height ratio (r=−0.582; p=0.007) were correlated with medial shift. A moderate correlation existed between patella-height ratio and lateral patellar tilt (r=0.527; p=0.017). Half of the variation in patellar shift in the patellofemoral pain cohort was explained by the patella-height ratio and Wiberg index (R2=0.487; p=0.003). Linear correlations with pain were not found.
Conclusions:
This study provides direct evidence that patellofemoral morphology is altered and influences maltracking in adolescent patellofemoral pain, highlighting the multifactorial etiology of this pain. Neither morphology nor kinematics (measured during active flexion-extension) correlated with pain. Both increases and decreases in these parameters likely lead to pain, negating a direct linear correlation.
Clinical Relevance:
Morphological changes likely lead to pain both directly through alterations in contact stress and indirectly through altered kinematics during active flexion-extension. These different pathways to pain must be taken into account when designing treatments to best serve a patient’s needs.
Key Terms: patella, shape, kinematics, MRI
Introduction
Patellofemoral (PF) pain reduces the quality of life for thousands of individuals each year45. This is particularly true in the adolescent population where knee pain, specifically PF pain, is one of the most common sources of pain/injury4, 47, 58. PF pain is experienced by 7–29% of all adolescents45, 54. Adolescent pain/injury has an immediate impact on both society and the individual in terms of economic cost31, loss of educational opportunities49, reduced time spent in recreational and competitive sports46, and an inability to maintain a healthy lifestyle44. Additionally, physical symptoms can be just the tip of the iceberg, as many adolescents manifest mental health and self-esteem issues as a result of their injury7. In the short-term, adolescent-onset PF pain can remain unresolved years after symptom development46, which sets it apart from other knee injuries, such as ACL rupture, where pain typically resolves quickly with surgery36. In the long-term, there is mounting evidence that adolescent-onset PF pain is a precursor to osteoarthritis (OA)14, 15, 27. To effectively prevent and treat PF pain, it is imperative that we identify its root cause(s). Consequently, there is a pressing need for more expansive investigation into etiological factors associated with PF pain, particularly in the adolescent population.
The recent literature on adolescents with PF pain has transitioned away from the perspective that this pathology is a simple overuse injury37 and has begun to more fully explore its multifactorial nature50. There is now evidence that alterations in neuromuscular control48, limb alignment57, and patellar tracking8 are associated with adolescent-onset PF pain. This multifaceted character of PF pain and the minimal evidence of a direct relationship between the various etiological factors and reported pain intensity hinder our ability to tailor interventions to each patient’s specific underlying pathology.
The management of adolescent-onset PF pain is further complicated by the fact that the overwhelming majority of PF morphology and kinematic data are based on the adult population. Such data are not directly transferable to adolescents, as maltracking associated with PF pain28 and interventional outcomes39 differ in adolescents. Further, during the rapid musculoskeletal development of adolescence, knee morphology is in transition to its final adult form32. Carlson et al.8 demonstrated a unique spectrum of maltracking patterns in adolescent-onset PF pain and suggested that this spectrum may arise from distinct alterations in bone shape. This is supported by studies in adults with PF pain that demonstrated a correlation between lateral trochlear inclination (LTI) and PF medial-lateral shift11, 29. Similarly, patellar dislocation has clearly been shown to primarily arise from the interplay between trochlear dysplasia, patella alta, and ligament laxity17, 35, 41. Yet, to the best of our knowledge, bone shape in adolescent idiopathic PF pain has yet to be explored.
The purpose of this study is to examine the role that patellar morphology plays in adolescent-onset PF pain and its relationship to PF kinematics and pain intensity. This is a follow up to our previous research55, establishing that a significantly larger patellar volume and smaller femoral trochlear width are associated with PF pain, but these parameters did not correlate to PF maltracking. The primary aim is to measure PF shape variables, including both femoral and patellar parameters, in adolescents with PF pain and compare them to the same parameters measured in adolescents without PF pain or other knee pathologies (matched controls). We hypothesized that patellar and femoral shape would be different in patients with PF pain, relative to controls. A secondary aim is to investigate the relationships between morphology, pain, and kinematics. We hypothesized that alterations in morphology would correlate to both kinematics and patient-reported pain level. Specifically, a lower lateral femoral edge would correlate with lateral maltracking and increased differences in morphology would correlate with increased levels of patient-reported PF pain.
Methods
This Institutional Review Board-approved study was an extension of our previous research55 related to PF size in adolescents with PF pain. For continuity, this study included the same patient and control populations as our previous study55. Demographics and 3D static and dynamic MR data that were acquired during a single visit for each participant during the previous study55 formed the basis for the current analysis. Twenty adolescent females (ages 11–16) with a diagnosis of PF pain, referred from local orthopaedic sports medicine clinics or self-referred, formed the patient cohort (Table 1). To be included, participants in this cohort had to have retro-patellar pain lasting greater than six months prior to enrollment, as well as a confirmed diagnosis of PF pain by an in-house physiatrist. We excluded patients if they had concomitant knee pathology including ligament, meniscus, iliotibial band, cartilage or other lower extremity injury, as well as any prior patellar dislocation, arthritis, traumatic knee injury and/or knee surgery. Twenty age- and body mass index (BMI)-matched female controls (within 6 months and 5 kg/m2, respectively) formed the control cohort. This group of healthy adolescents was recruited from the greater Washington, DC Area utilizing word-of-mouth, flyers, ClinicalTrials.gov, and the resources of the Patient Recruitment Office at the National Institutes of Health (NIH)1. Any individual from either cohort was excluded if they had a contraindication to MR imaging, Beighton score ≥ 556, clinical diagnosis affecting the knee joint (e.g., hypermobility conditions), or a fused femoral growth plate. Before undergoing MR imaging, all participants provided assent (and parental consent), underwent a medical history and physical exam with a focused knee evaluation, and completed a questionnaire regarding their physical activities. The history and physical were used to rule-out any knee pathologies in the control cohort and any concomitant pathologies in the cohort with PF pain. We used a visual analog scale (VAS)43 and anterior knee pain scale (Kujala Score)33 to assess PF pain.
Table 1.
Demographics, Pain Assessments, and Clinical Features
| Characteristic | PF Pain (n = 20) | Controls (n = 20) | p value |
|---|---|---|---|
| Age (yrs) | 13.7 ± 1.3 | 13.6 ± 1.3 | 0.728 |
| Age Range (yrs) | 10.3 – 15.7 | 10.2 – 15.9 | - |
| Height (cm) | 160.4 ± 8.0 | 159.2 ± 9.0 | 0.675 |
| Weight (kg) | 49.5 ± 7.1 | 53.2 ± 10.9 | 0.216 |
| BMI (kg/m2) | 19.2 ± 2.3 | 20.9 ± 3.5 | 0.086 |
| Impact Physical Activities (hr/wk) | 7.5 ± 6.3 | 6.4 ± 4.6 | 0.567 |
| Non-impact Physical Activities (hr/wk) | 2.6 ± 3.8 | 3.5 ± 5.6 | 0.594 |
| Kujala Score (out of 100) | 61.5 ± 14.8 | 100 | - |
| Visual Analog Scale (VAS) (out of 10) | |||
| Pain on an average day | 4.4 ± 2.4 | 0 | - |
| Pain at the end of the day | 5.0 ± 3.1 | 0 | - |
| Pain during provocative activities | 7.6 ± 1.8 | 0 | - |
| Positive J-sign | 13 | - | |
| Positive Apprehension Test | 7 | NA | - |
| Lateral Hypermobility (mm) | 6.7 ± 3.1 | - | |
| Q-angle (°) | 13.5 ± 4.0 | - |
Where appropriate, data reported as mean ± 1 SD
All participants were female.
Impact activities require the lower extremities to repeatedly absorb a ground reactive force exceeding body weight (e.g., running, field sports. basketball, volleyball, gymnastics, etc.).
Non-impact activities require the lower extremities to repeatedly absorb a ground reactive force less than body weight (e.g., elliptical training, swimming, biking, etc.).
Kujala score33: measure of anterior knee functional pain; 0 indicates worst function.
VAS score43: 0 indicates no pain and 10 indicates worst pain.
PF = patellofemoral; BMI = body mass index; NA = not applicable.
For static scanning, we positioned participants supine within a 3-Tesla MR scanner (Philips Electronics, Eindhoven, The Netherlands) with their fully extended knee in an 8-channel knee coil. To decrease variability due to limb positioning53, the lower limb was carefully placed in an anatomically neutral position and maintained using a cushioned ankle holder, preventing internal or external rotation of the lower limb. We raised the heel to maintain full knee extension. Once the knee was positioned properly, we captured the following static sagittal MR images: 3D gradient recalled echo (GRE), GRE with fat-saturation (GRE-FS), and proton density weighted (PDW). The output resolution for the GRE and GRE-FS were 0.27×0.27×1.0 mm (512×512 pixels), while the PDW image resolution was 0.27×0.27×1.2 mm (512×512 pixels). Based on the Fourier imaging data, the GRE images were reformatted into axial images (output resolution = 0.27×0.27×1.0 mm). To rule out any undiagnosed knee disorders and to ensure active femoral growth plates, a radiologist, specializing in musculoskeletal imaging, read all MR images.
After static scanning, participants were repositioned for dynamic imaging. We replaced the knee coil with a custom-built coil holder that enabled a pair of large-flex coils to be positioned medial and lateral to the knee and a pair of medium-flex coils to be positioned anteriorly. We placed a triangular cushioned wedge under the knee to support the hip and knee in ~45° of flexion. This configuration allowed participants to actively flex and extend their knee, such that their toes touched the top of the MR scanner’s inner bore at full extension and their heel contacted a small cushion positioned on the MR bed on subsequent flexion. During the motion we acquired a complete set of sagittal plane, cine-phase contrast (CPC) MR images. Post-processing of the CPC velocity images enabled the 3D PF and tibiofemoral joint kinematics to be quantified with an accuracy of <0.3mm5. The CPC MR image set was acquired in a single sagittal scan plane.
Using MIPAV (NIH, Bethesda, MD, USA), we measured 2D femoral and patellar shape parameters in the 3D sagittal and axial GRE images. Reliability was not measured in the current study. However, we followed a similar methodology to our earlier work22 measuring patellofemoral parameters from 3D static MR images, which demonstrated excellent inter-rater reliability (inter-class correlation coefficients: 0.94–0.99). The “lightbox view” tool in MIPAV enabled us to use the sagittal and axial images jointly, when necessary. For the femur, we defined the epicondylar axial (epi_ax) plane (Figure 1B) as the plane containing the greatest femoral width and the superior sulcus image (Figure 1C) as the axial image containing the most superior aspect of the lateral trochlear cartilage. For the patellar measures, we defined the patellar axial (pat_ax) plane as the plane with the greatest patellar width (Figure 1D).
Figure 1. Femoral and Patellar Shape Measures.
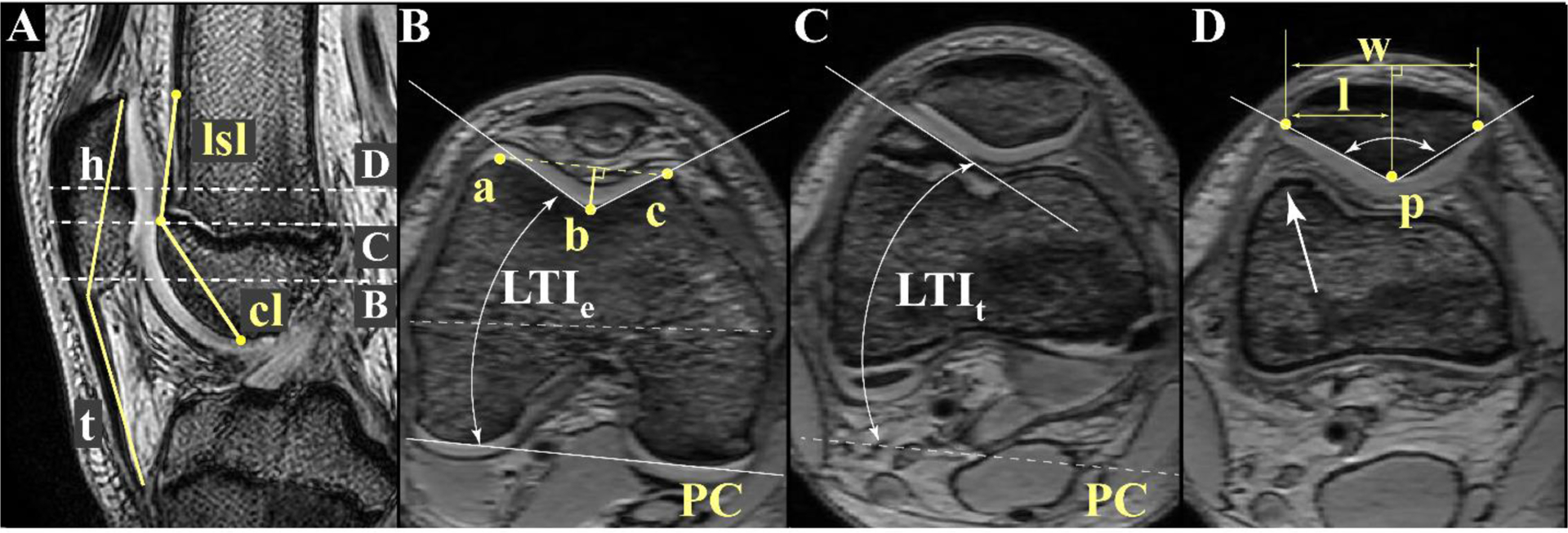
A: Patellofemoral (PF) sagittal reference image. The sagittal plane containing the maximum patellar height (h), measured from the proximal to the distal patellar margins. The three dashed lines depict axial image levels used for quantifying shape. Patella tendon length (t) spans from the tendon’s insertion on the distal patella to its insertion at the tibial tubercle. Patella height ratio = t/h. Lateral shaft length (lsl) captures the length of the shaft above the sulcus containing a raised lateral edge. It is measured as the distance from the superior aspect of the trochlea [one plane superior to image C] to the point on the lateral femoral shaft where the anterior femur becomes flat. The raised lateral edge on the shaft is denoted by the white arrow in image D. Cartilage length (cl) is the distance from the most superior to the most inferior points of the trochlear groove covered by cartilage. B: Epicondylar Image. The axial plane at the widest portion of the femur (epicondylar width, dashed white line). 2D Trochlear width is the distance between the anterior points of the lateral trochlea (a) and the medial trochlea (c), depicted by the dashed yellow line. Point b represents the deepest point in the trochlea at this level. The trochlear length ratio is the ratio of the lateral trochlear length (b-to-a) and medial trochlear length (b-to-c). Sulcus depth is the perpendicular distance from point b to line ac, represented by the solid yellow line. Line PC connects the most posterior points on the medial and lateral posterior condyles. Lateral trochlear inclination (LTIe) is the angle between a line tangent to the lateral trochlear edge and line PC at this level. C: Superior Sulcus. The most superior axial image containing cartilage over the lateral femoral condyle. LTIt is the angle between a line tangent to the lateral trochlear edge (in this image) and line PC (image B). D: Patellar Width. Patellar width (w) is measured from the lateral to medial patellar margins at the greatest patellar width. The lateral patellar width (l) is measured from the lateral patellar margin to the line perpendicular to w that crosses point p (most posterior patellar point). Wiberg index = l/w. Wiberg angle (denoted by double arrow white arc) is the angle defined by two lines: one along the lateral subchondral patellar facet and one along the medial subchondral patellar facet, both originating at point p.
At the epi_ax slice plane, we quantified the trochlear length ratio40, trochlear depth11, trochlear groove width40, and lateral trochlear inclination (LTIe)11 (Figure 1B). In the axial superior (ax_sup) image, lateral trochlear inclination (LTIt)10 was measured (Figure 1C). The 2D trochlear width40 was measured as the distance from the anterior-lateral trochlear condyle to the anterior-medial trochlear condyle. The cartilage length26 was measured as the distance from the most superior to the most inferior point of the sulcus groove covered by cartilage, as visualized in the axial images (Figure 1A). In reviewing static axial images, we noted that certain individuals had a particular large LTIt. This lateral edge continued superiorly onto to shaft as pure bone. We hypothesized the lateral shaft length would influence PF kinematics. Thus, we developed the lateral femoral shaft length, or simply “lateral shaft length,” as a new parameter for this study (Figure 1A). This parameter captured the extent of the shaft in which a raised lateral edge could be defined. Mathematically, the lateral shaft length was the distance from immediately proximal to the superior aspect of the trochlear to the point on the lateral femoral where the anterior aspect of the shaft becomes flat.
The patella measures included 2D patellar width, patellar depth, lateral patellar width, and Wiberg angle24 (Figure 1D). From the trochlear and patellar measures, Wiberg index (lateral patellar width/patellar width), and patellar height ratio (patellar tendon length/patellar height)30 were quantified. Note, we chose to measure 2D patellar and femoral trochlear widths for completeness, although their 3D counterparts were measured previously55.
The paucity of literature focused on adolescent-onset PF pain made an a priori power analysis difficult to perform. Previous work by Kim et al.30 and Mundy et al.38 investigated PF shape parameters (LTI and sulcus depth) in adolescents with knee pain not secondary to PF dislocation. While these study populations cannot be classified as typically developing controls, they were the most appropriate groups available for our power analysis21. To obtain an alpha of 0.05 and a beta of 0.2 for LTI and sulcus depth, 16–20 participants were required for each group to find differences of 4.0° and 1.5mm. No studies on patellar shape or size exist for the adolescents with idiopathic PF pain, so we powered our patellar measures according to a previous adult study on patellar morphology by Fucentese et al.24 Using the same alpha and beta, along with an assumed difference of 9° for Wiberg angle, resulted in 17 individuals for each group being required.
Statistical analyses were performed using SPSS, Version 22.0 (IBM Corp, Armonk, NY). All continuous variables were reported as means, along with their standard deviations (SD) and confidence intervals (CI). For normally distributed demographic variables and shape parameters, a Student’s t-test was used to compare cohorts. Cohen’s d statistic was used to measure effect size12. After ensuring a normal distribution, the relationship between morphology and kinematics was calculated for the PF pain cohort, controls, and for the entire study cohort using Pearson’s correlations. To limit the number of analyses, the correlations focused only on the axial plane kinematics, with medial shift and tilt being positive. A qualitative graphical check was run for all correlations to ensure that outliers did not skew the results. If outliers were found, the correlations were reported both with and without this outlier included. A step-wise regression model using PF shape parameters was constructed. Four participants from the control cohort had missing kinematic data, and as a result, were excluded from correlation calculations and regression modeling. We investigated the relationship between the intensity of pain (VAS during activities that invoke PF pain) and measures of both PF morphology and axial plane kinematics using the Pearson’s correlation, based on data from all subjects with PF pain. Statistical significance was set at p ≤ 0.05.
Results
The two adolescent cohorts did not differ demographically (Table 1). The two groups did not have any differences in terms of time spent performing physical activities – impact physical activities (p = 0.567) and non-impact physical activities (p = 0.594).
Individuals with PF pain had a deeper sulcus groove compared to the control group (Table 2, 6.6±0.7 vs. 6.0±1.1; 95% CI: 0.6 mm; p=0.043; d=0.66). The 2D patellar width (39.2±2.8 vs. 36.8±3.3; 95% CI: 2.0 mm; p=0.014; d=0.81) and lateral patellar width (23.1±2.4 vs. 21.4±2.6; 95% CI: 1.6 mm; p = 0.033; d=0.70) were significantly larger in the PF pain cohort. Femoral epicondylar width, LTIe, LTIt, trochlear width, and lateral shaft length were not different between the cohorts. Patellar height ratio, cartilage length, patellar-trochlear cartilage overlap, Wiberg angle, and Wiberg index demonstrated no differences between cohorts.
Table 2.
Femoral and Patellar Morphology Parameters
| Parameter | PF Pain | Controls | 95% CI | Cohen’s d | p value |
|---|---|---|---|---|---|
| Femoral Parameters | |||||
| Lateral Shaft Length (mm) | 17.9 ± 3.3 | 17.9 ± 4.0 | 2.3 | 0.00 | 0.999 |
| LTIe (°) | 26.7 ± 2.3 | 26.4 ± 4.2 | 2.2 | 0.08 | 0.808 |
| LTIt (°) | 21.9 ± 4.9 | 24.0 ± 4.9 | 3.2 | −0.42 | 0.190 |
| Sulcus Cartilage (mm) | 27.5 ± 2.6 | 26.2 ± 3.5 | 2.0 | 0.40 | 0.210 |
| Sulcus Depth (mm) | 6.6 ± 0.7 | 6.0 ± 1.1 | 0.6 | 0.66 | 0.043 |
| Trochlear Length Ratio | 1.4 ± 0.2 | 1.3 ± 0.2 | 0.0 | 0.50 | 0.390 |
| Trochlear Width (mm) | 32.0 ± 1.7 | 33.0 ± 1.8 | 1.1 | −0.56 | 0.086 |
| Patellar Parameters | |||||
| Lateral Patellar Width (mm) | 23.1 ± 2.4 | 21.4 ± 2.6 | 1.6 | 0.70 | 0.033 |
| Patellar Cartilage Length (mm) | 26.3 ± 3.5 | 24.6 ± 2.3 | 1.9 | 0.59 | 0.068 |
| Patellar Height Ratio | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.1 | −0.45 | 0.171 |
| Patellar Width (mm) | 39.2 ± 2.8 | 36.8 ± 3.3 | 2.0 | 0.81 | 0.014 |
| Patellar-Femoral Width Ratio | 1.2 ± 0.1 | 1.1 ± 0.1 | 0.1 | 1.26 | <0.001 |
| Patellar-Trochlear Cartilage Overlap (mm) | 4.9 ± 1.6 | 5.0 ± 1.5 | 1.0 | −0.07 | 0.819 |
| Wiberg Angle (°) | 139.3 ± 4.8 | 139.3 ± 7.4 | 4.0 | 0.00 | 0.997 |
| Wiberg Index | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.0 | 0.28 | 0.559 |
Data are reported as mean ± 1 SD. Effect size measured using Cohen’s d statistic12.
LTIe = lateral trochlear inclination, as measured in the axial image containing the epicondylar width; LTIt = lateral trochlear inclination, as measured in the axial image containing the most proximal-lateral aspect of sulcus groove.
Correlations between patellofemoral morphology and PF kinematics existed for the both cohorts and the entire study cohort (Table 3). Lateral shaft length and medial patellar shift were weakly correlated for the entire cohort (r = 0.335; p = 0.038) and moderately correlated in the PF pain cohort (r = 0.518; p = 0.033). Lateral shaft length was also correlated with medial patellar tilt (r = 0.424; p = 0.010) for all participants, but failed to reach significance in the PF pain group. In the PF pain cohort, moderate correlations existed between patellar height ratio and lateral patellar shift (r = 0.582; p = 0.007), as well as between patellar height ratio and lateral patellar tilt (r = 0.527; p = 0.017). Among the entire population, patellar height ratio was correlated with lateral patellar tilt (r = 0.470; p = 0.004), but was not correlated with patellar shift. The VAS score during activities that provoke pain was not correlated with any shape or kinematic parameter. No outliers were found using the graphical evaluation.
Table 3.
Pearson’s Correlation Coefficients (r) for Femoral and Patellar Parameters with Patellofemoral Kinematics
| All Participants | PF Pain | Controls | ||||
|---|---|---|---|---|---|---|
| Shift | Tilt | Shift | Tilt | Shift | Tilt | |
| LTIe | −0.056 | 0.500** | −0.223 | 0.314 | 0.152 | 0.662** |
| Patella-Height Ratio | −0.284 | −0.470** | −0.582** | −0.527* | −0.490 | −0.403 |
| Lateral Shaft Length | 0.335* | 0.424** | 0.518* | 0.273 | 0.231 | 0.582* |
| Sulcus Depth | −0.172 | 0.386* | 0.066 | 0.333 | −0.109 | 0.438 |
| Wiberg Index | 0.134 | −0.303 | 0.477* | −0.097 | −0.274 | −0.512* |
LTIe = lateral trochlear inclination, as measured in the axial image containing the epicondylar width Shift and tilt = medial/lateral shift and tilt. Medial indicates the positive direction of motion.
p≤0.05.
p<0.01.
Based on the regression analysis for all participants, approximately half of the variation in tilt was explained by LTIe, patellar height ratio, and the Wiberg angle (R2 = 0.498; p = <0.001) (Table 4). For lateral shift, approximately 25% of the variation was explained by lateral shaft length and sulcus depth. When focused solely on the PF pain cohort, approximately 50% of the variation in patellar shift was explained using the patellar height ratio and Wiberg index (Table 4, R2 = 0.487; p = 0.003). The prediction of patellar tilt did not improve in this cohort when multiple variables were used.
Table 4.
Regression Analysis For All Participants and PF Pain Cohort
| Cohort | Kinematic Variable | Parameter | R2 | p value |
|---|---|---|---|---|
| Lateral Shaft Length | 0.086 | 0.045 | ||
| Shift | Sulcus Depth | 0.190 | 0.012 | |
| All Participants | LTIe | 0.250 | 0.002 | |
| Tilt | Patellar Height Ratio | 0.393 | <0.001 | |
| Wiberg Angle | 0.498 | <0.001 | ||
| Patellar Height Ratio | 0.339 | 0.007 | ||
| PF Pain | Shift | Wiberg Index | 0.487 | 0.003 |
| Tilt | Patellar Height Ratio | 0.277 | 0.017 |
Results from a step-wise linear regression are provided.
Discussion
Our study enhances the clinical understanding of PF pain by providing direct evidence that patellofemoral morphology is altered and is correlated with maltracking in adolescents with idiopathic PF pain. The totality of our work in this population8, 55 reaches beyond a single etiological factor, exposing significant associations between PF pain and shape deformation, maltracking, and size. Although PF shape is altered in these adolescents with PF pain and is correlated with their PF kinematics, neither shape nor kinematics correlate to the patients’ pain levels. Thus, pain is not dependent on a linear, unidirectional change in a single variable. Instead, an intricate interplay between morphology and kinematics likely creates an envelope of pathology, with both increases and decreases in specific parameters combining with other alterations (e.g., alterations in soft tissue forces) to cumulate in PF pain. Such a multifaceted etiology makes the diagnosis of a single pathological factor for PF pain incredibly challenging in most patients.
Although numerous authors have emphasized the importance of understanding PF morphology as it relates to maltracking and PF pain3, 16, 20, data comparing morphology in patients with idiopathic PF pain to healthy controls remains sparse. The current work in idiopathic adolescent-onset PF pain agrees with previous adult studies in finding minimal shape changes associated with PF pain19, 26, 34. The significant differences in sulcus depth and lateral patellar width may appear small, but are large in comparison to the overall cartilage thickness. The difference between cohorts for these variables are approximately 19% and 53% of the average patellar cartilage thickness in adult females (3.25mm13). In opposition to adult studies, neither LTIe nor LTIt correlate with PF kinematics and trochlear depth is increased in adolescent-onset PF pain. The lack of trochlea dysplasia in isolated adolescent-onset PF pain clearly disagrees with studies focused on PF pain secondary to dislocation2, 6, 42. Together, these comparisons support a distinction previously asserted by Grelsamer25, who postulated that individuals with patellar symptoms can be divided into two groups: “those who feel the patella slip [dislocate], and those who simply have pain.” As such, clinical interventions must be designed specifically for each cohort and future studies must maintain a distinction between these cohorts.
In general, changes in patellar shape are not associated with PF pain, similar to adults34. One exception is that the lateral patellar width is significantly longer in the adolescents with PF pain. This is in opposition to Eijkenboom et al.18, who utilized a general linear model to detect differences in axial and sagittal plane patellar shape modes across adults with PF pain, OA, and controls. They reported a narrower patella with a proportionally shorter lateral edge in adults with PF pain, relative to controls. Variations in imaging modality and analysis methodology are unlikely causes for these differences. The most likely explanation is that patients with PF pain secondary to dislocation were not expressly excluded in their study cohort. Previous studies24, 42 have clearly demonstrated that patients with a history of patellar dislocation have a smaller patellar width. It is also possible that the variance is due to morphological differences between adolescents and adults, but further research is needed in this area.
At first glance, it is easy to negate the importance of morphological and kinematic changes associated with PF pain due to a lack of correlation between these variables and reported PF pain intensity. However, this would be shortsighted. Similar to previous studies26, 59, 25–50% of the variability in PF axial-plane kinematics can be explained using a combination of shape parameters, highlighting a complex interplay between shape and kinematics. For example, lateral shaft length and Wiberg index both demonstrated no significant differences between cohorts and neither correlates with pain. However, both correlate with medial PF shift. Thus, an increase and/or decrease in these parameters may foster PF pain by fostering either medial or lateral maltracking. To put this in context of our current population, the two patients who demonstrated a slightly medial shift pattern at dynamic full extension, had the third and fourth longest shaft lengths of any participant (21.2 and 21.6 mm) along with a higher than average LTI and trochlear depth. Here, the pain is most likely due to impingement resulting in excessive contact forces (Figure 2 A–C). On the other side of the spectrum, seven patients with PF pain, classified as extreme maltrackers (lateral maltracking >2 SD from control average)8, all tended to have shorter lateral shaft lengths, smaller lateral patellar widths, and no consistent pattern in the change for LTI (Figure 2 D–F). Thus, the lack, but not complete loss, of boney constraint, along with a likely soft tissue force imbalance promoted extreme lateral shift. Pain for this group most likely arose from two sources: the shear forces induced by the large patellar movements and the cartilage-to-bone contact arising from the constraint forces of the lateral femoral shaft. In addition, some individuals with extreme maltracking may also exhibit and incongruent patellar-to-trochlear fit in deeper flexion. This combination of multiple subtle changes leading to pain was defined by Selfe and colleagues50 under their mosaic hypothesis. This hypothesis states that patients with PF pain often had multiple subtle variations in their clinical exam (i.e. muscle tightness, hip abductor weakness, pronation, etc.), and the summation of these mild differences lead to pain.
Figure 2. Examples of Patellofemoral (PF) Contact for Two Adolescents with PF Pain.
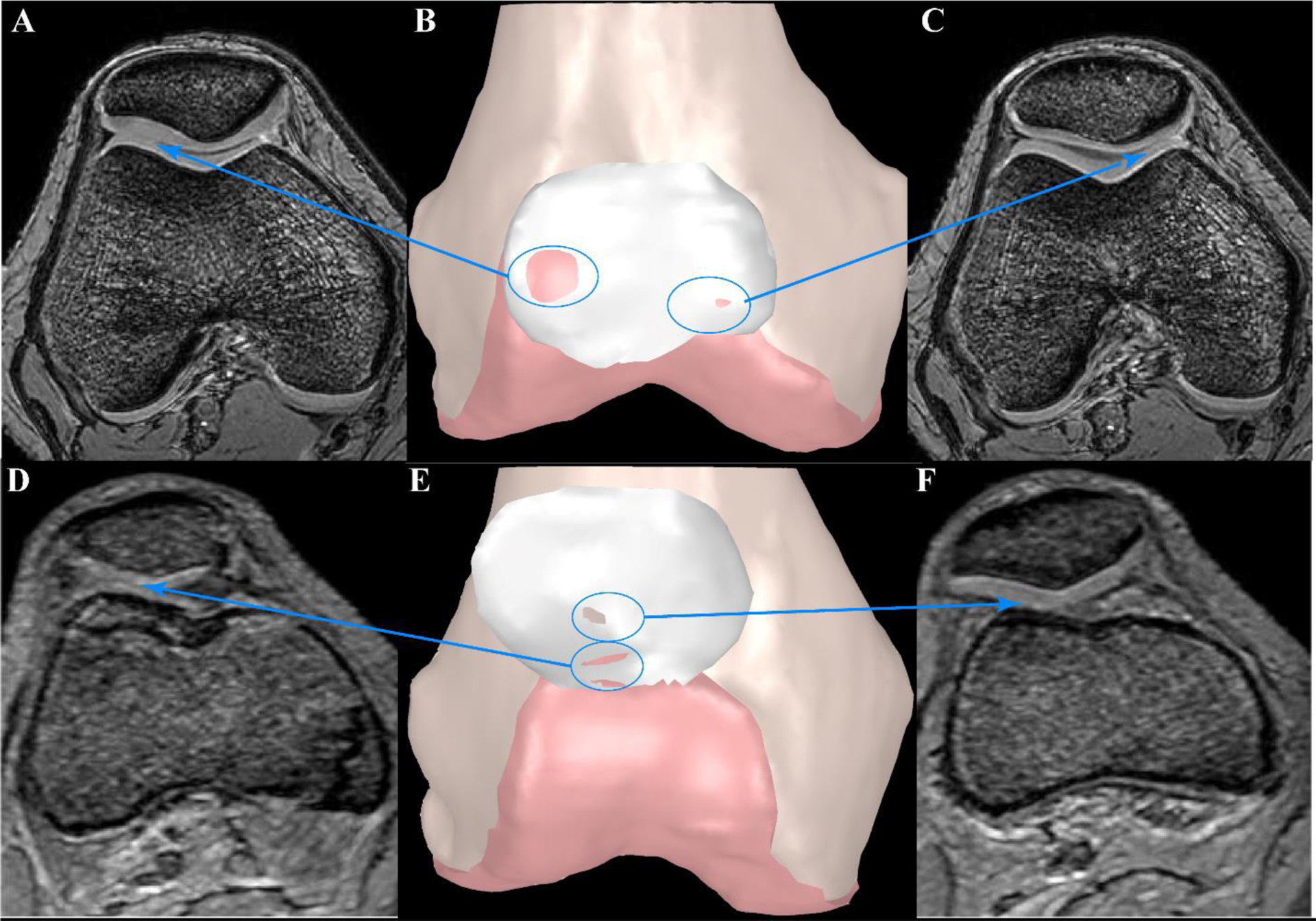
Static GRE images were used to create 3D bone models (B, E). Femoral and patellar cartilage surfaces were outlined in MIPAV and imported into Geomagic Wrap to create 3D surface models. Contact between surfaces is shown with the underlying surface color appearing on the patellar cartilage. A, C, D, and F represent the axial MR images at the level where contact between surfaces can be visualized. Patient 1 (A-C) demonstrates minimal lateral maltracking (−0.7mm at full extension) with average values (relative to PF pain cohort) for Wiberg index, lateral shaft length, and trochlear-patellar ratio. PF contact is not evenly distributed across the cartilage surfaces. Instead, the contact is concentrated primarily at the anterior-lateral trochlear, with a gap in contact in the central groove. The resulting stress concentration, particular when the quadriceps become active is a likely source of pain. Patient 2 (D-F) exhibits extreme lateral maltracking (−9.5 mm at full extension), patella alta, a short lateral shaft length, and the smallest lateral patellar width. There is cartilage-cartilage contact (D, E) and cartilage-bone contact (E, F). The patella’s path from centered in the groove at deep flexion to extreme lateral maltracking at full extension would foster excessive cartilage sheer forces. Additionally, the femoral shaft provides a force that resists further lateral shift. This combination of sheer force and bone-to-cartilage contact are likely sources of pain.
The correlation between medial shift/tilt and lateral femoral shaft length raises a serious concern that direct cartilage-to-bone contact is a likely source of adolescent-onset PF pain. To determine if the lateral shaft truly influences the PF kinematics, a post-hoc review of the dynamic images for both cohorts was conducted. Patellar cartilage contact with the lateral femoral shaft could be visually detected in 55% of the individuals with PF pain and 38% of the controls during terminal knee extension. The exact reason why this would cause pain in one group and not the other is beyond the scope of the current work, but differences in soft tissue forces are a likely source. This cartilage-to-bone contact has important clinical implications, particularly for those individuals with extreme maltracking, where the cartilage-to-bone contact involves a large shear component. In such individuals, if conservative treatments fail, surgical correction to re-center the patella within the groove may be necessary to ensure both joint health and proper maturation of the final joint morphology23.
For all patients suffering from PF pain, conservative treatment remains the logical first step. If this fails, a deep exploration of the patient’s pain etiology is warranted when planning more aggressive intervention. For example, in terms of maltracking, stabilization surgery may benefit the more extreme maltracking group who have issues with soft tissue imbalance51, while mild force redirection may benefit the less extreme lateral maltrackers9. For those patients whose pain appears to arise from altered contact due to shape deformation, there are no accepted surgical interventions available. Future cadaver and modelling studies investigating surgical intervention (e.g., patellar reshaping or resurfacing) are necessary to explore options for pain relief in these patients.
The primary limitation in this study is the isolated focus on adolescent females with idiopathic PF pain, which prevents the generalization of our results to males or other PF pain populations (e.g., dislocators, adults, etc.). However, this restriction limits confounding factors. The second limitation is sample size. While we adequately powered our study for the PF shape deformation, our correlations may be limited by the size of the study population. This study is designed to investigate the influence of bone shape on kinematics, yet the opposite – kinematics influencing bone shape – may also be true52. The possibility of this latter hypothesis is important to recognize, but is not the focus of this study, as a longitudinal study design is required to address this question. Unfortunately, as pain is a subjective experience, there is no objective measure or tool to gauge it. Thus, we must hypothesize the source of pain utilizing statistical differences, correlations, regressions, and visualization. Further, this study focused on specific aspects of the potential biological causes of PF pain. As there are clearly other biological factors (e.g., muscle strength, neurological control, overall limb alignment, etc.) and other non-biological factors (psychological, social, etc.) that may foster or result from PF pain, future work is needed to paint a more detailed picture of the etiology of adolescent-onset PF pain. Lastly, lateral shaft length is a PF parameter that has not been previously investigated. Consequently, there are no comparative studies. However, the sound methodology used in this study to measure lateral shaft length should be confirmed in future studies.
In conclusion, our findings provide direct evidence that PF shape is altered in adolescents with PF pain and support the hypothesis that the pathway to pain is not based on a unidirectional change in a single parameter. Instead, an envelope of pathology exists where, most often, subtle changes in multiple variables combine and likely foster pain. Reduced shaft length with a shorter lateral patella appears to enable excessive shear contact forces, with bone-to-cartilage contact occurring in terminal extension; whereas an increase in both these parameters appears to lead to impingement of the patella, resulting in increased cartilage stress. Of particular concern is the evidence that more than half of our adolescents with PF pain demonstrate cartilage-to-bone contact in terminal extension. Such an interaction not only potentially leads to pain, but could foster joint degeneration14, 15, 18. These different pathways leading to pain, call on the medical community to search for optimal interventions that lead to symptom resolution. Overall, the patient-specific etiology of PF pain should be emphasized when designing treatments to best serve their needs39.
What is known about the subject:
Patellofemoral pain is assumed multifactorial in nature with variables like trochlear shape, patella alta, and muscle imbalance contributing to the problem. Little research on patellar shape and its role in the exact etiology exists.
What this study adds to existing knowledge:
This study adds to the literature by focusing on an understudied, but considerably affected population: adolescents with idiopathic patellofemoral pain. We went beyond focusing on a single etiological factor, and demonstrated significant relationships among shape deformation, maltracking, and bone size in patients with patellofemoral pain. The relationship between these variables in adolescents with patellofemoral pain support the concept that their pain is the result of an envelope of pathology, with both increases and decreases in specific parameters combining with other alterations to cause this pathology.
Acknowledgements:
The Division of Intramural Research of the National Institute of Health (NIH) Clinical Center, Bethesda, MD, USA supported this research. CNF acknowledges funding support through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions from the Doris Duke Charitable Foundation, Genentech, American Association for Dental Research, the Colgate-Palmolive Company, Elsevier, alumni of student research programs, and other individual supporters via contributions to the Foundation for the National Institutes of Health. For a complete list, please visit the Foundation website at: https://fnih.org/what-we-do/current-education-and-training-programs/mrsp. We thank the NIH Clinical Center Radiology Department for their technical support this work.
Resources
- 1.Patient Recruitment at the NIH Clinical Center. Available at: https://clinicalcenter.nih.gov/recruit/index.html. Accessed June 12, 2019.
- 2.Aglietti P, Cerulli G. Chondromalacia and recurrent subluxation of the patella: a study of malalignment, with some indications for radiography. Ital J Orthop Traumatol. 1979;5(2):187–201. [PubMed] [Google Scholar]
- 3.Agnihotri G, Kalyan GS. Demystifying the complex patellar form via ratios and indices. Mymensingh Med J. 2014;23(4):787–791. [PubMed] [Google Scholar]
- 4.Barber Foss KD, Myer GD, Hewett TE. Epidemiology of basketball, soccer, and volleyball injuries in middle-school female athletes. Phys Sportsmed. 2014;42(2):146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behnam AJ, Herzka DA, Sheehan FT. Assessing the accuracy and precision of musculoskeletal motion tracking using cine-PC MRI on a 3.0T platform. J Biomech. 2011;44(1):193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biedert R, Sigg A, Gal I, Gerber H. 3D representation of the surface topography of normal and dysplastic trochlea using MRI. Knee. 2011;18(5):340–346. [DOI] [PubMed] [Google Scholar]
- 7.Boykin RE, McFeely ED, Shearer D, et al. Correlation between the Child Health Questionnaire and the International Knee Documentation Committee score in pediatric and adolescent patients with an anterior cruciate ligament tear. J Pediatr Orthop. 2013;33(2):216–220. [DOI] [PubMed] [Google Scholar]
- 8.Carlson VR, Boden BP, Sheehan FT. Patellofemoral Kinematics and Tibial Tuberosity-Trochlear Groove Distances in Female Adolescents With Patellofemoral Pain. Am J Sports Med. 2017;45(5):1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson VR, Sheehan FT, Shen A, Yao L, Jackson JN, Boden BP. The Relationship of Static Tibial Tubercle-Trochlear Groove Measurement and Dynamic Patellar Tracking. Am J Sports Med. 2017;45(8):1856–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrillon Y, Abidi H, Dejour D, Fantino O, Moyen B, Tran-Minh VA. Patellar instability: assessment on MR images by measuring the lateral trochlear inclination-initial experience. Radiology. 2000;216(2):582–585. [DOI] [PubMed] [Google Scholar]
- 11.Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging-based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374–384. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J Statistical power analysis for the behavioral sciences. Hillsdale, N.J: L. Erlbaum Associates; 1988. [Google Scholar]
- 13.Coleman JL, Widmyer MR, Leddy HA, et al. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech. 2013;46(3):541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins NJ, Oei EHG, de Kanter JL, Vicenzino B, Crossley KM. Prevalence of radiographic and MRI features of patellofemoral osteoarthritis in young and middle-aged adults with persistent patellofemoral pain. Arthritis Care Res (Hoboken). 2018. [DOI] [PubMed] [Google Scholar]
- 15.Conchie H, Clark D, Metcalfe A, Eldridge J, Whitehouse M. Adolescent knee pain and patellar dislocations are associated with patellofemoral osteoarthritis in adulthood: A case control study. Knee. 2016;23(4):708–711. [DOI] [PubMed] [Google Scholar]
- 16.Coupal TM, Munk PL, Ouellette HA, Al-Shikarchy H, Mallinson PI, Choudur H. Popping the cap: the constellation of MRI findings in patellofemoral syndrome. Br J Radiol. 2018;91(1089):20170770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19–26. [DOI] [PubMed] [Google Scholar]
- 18.Eijkenboom JFA, Waarsing JH, Oei EHG, Bierma-Zeinstra SMA, van Middelkoop M. Is patellofemoral pain a precursor to osteoarthritis?: Patellofemoral osteoarthritis and patellofemoral pain patients share aberrant patellar shape compared with healthy controls. Bone Joint Res. 2018;7(9):541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erkocak OF, Altan E, Altintas M, Turkmen F, Aydin BK, Bayar A. Lower extremity rotational deformities and patellofemoral alignment parameters in patients with anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):3011–3020. [DOI] [PubMed] [Google Scholar]
- 20.Esfandiarpour F, Lebrun CM, Dhillon S, Boulanger P. In-vivo patellar tracking in individuals with patellofemoral pain and healthy individuals. J Orthop Res. 2018. [DOI] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 2007;39(1):175–191. [DOI] [PubMed] [Google Scholar]
- 22.Freedman BR, Sheehan FT. Predicting three-dimensional patellofemoral kinematics from static imaging-based alignment measures. J Orthop Res. 2013;31(3):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu K, Duan G, Liu C, Niu J, Wang F. Changes in femoral trochlear morphology following surgical correction of recurrent patellar dislocation associated with trochlear dysplasia in children. Bone Joint J. 2018;100(6):811–821. [DOI] [PubMed] [Google Scholar]
- 24.Fucentese SF, von Roll A, Koch PP, Epari DR, Fuchs B, Schottle PB. The patella morphology in trochlear dysplasia--a comparative MRI study. Knee. 2006;13(2):145–150. [DOI] [PubMed] [Google Scholar]
- 25.Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-a(11):1639–1650. [PubMed] [Google Scholar]
- 26.Harbaugh CM, Wilson NA, Sheehan FT. Correlating femoral shape with patellar kinematics in patients with patellofemoral pain. J Orthop Res. 2010;28(7):865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter DJ, Zhang YQ, Niu JB, et al. Patella malalignment, pain and patellofemoral progression: the Health ABC Study. Osteoarthritis Cartilage. 2007;15(10):1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson JN, Sheehan FT, Boden BP, Shen A, Carlson V, Alter KE. Adolescents and Adults with Patellofemoral Pain Have Different Pathological Knee Kinematics. Pm r. 2016;8(9s):S155. [DOI] [PubMed] [Google Scholar]
- 29.Keser S, Savranlar A, Bayar A, Ege A, Turhan E. Is there a relationship between anterior knee pain and femoral trochlear dysplasia? Assessment of lateral trochlear inclination by magnetic resonance imaging. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):911–915. [DOI] [PubMed] [Google Scholar]
- 30.Kim HK, Shiraj S, Anton C, Horn PS. The patellofemoral joint: do age and gender affect skeletal maturation of the osseous morphology in children? Pediatr Radiol. 2014;44(2):141–148. [DOI] [PubMed] [Google Scholar]
- 31.Knowles SB, Marshall SW, Miller T, et al. Cost of injuries from a prospective cohort study of North Carolina high school athletes. Inj Prev. 2007;13(6):416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo K, Teshima T, Hirose N, Tsunoda N. Growth changes in morphological and mechanical properties of human patellar tendon in vivo. J Appl Biomech. 2014;30(3):415–422. [DOI] [PubMed] [Google Scholar]
- 33.Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159–163. [DOI] [PubMed] [Google Scholar]
- 34.Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003(414):172–182. [DOI] [PubMed] [Google Scholar]
- 35.Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575–581. [DOI] [PubMed] [Google Scholar]
- 36.Lutz C, Baverel L, Colombet P, et al. Pain after out-patient vs. in-patient ACL reconstruction: French prospective study of 1076 patients. Orthop Traumatol Surg Res. 2016;102(8s):S265–s270. [DOI] [PubMed] [Google Scholar]
- 37.Milgrom C, Finestone A, Eldad A, Shlamkovitch N. Patellofemoral pain caused by overactivity. A prospective study of risk factors in infantry recruits. J Bone Joint Surg Am. 1991;73(7):1041–1043. [PubMed] [Google Scholar]
- 38.Mundy A, Ravindra A, Yang J, Adler BH, Klingele KE. Standardization of patellofemoral morphology in the pediatric knee. Pediatr Radiol. 2016;46(2):255–262. [DOI] [PubMed] [Google Scholar]
- 39.O’Neill DB, Micheli LJ, Warner JP. Patellofemoral stress. A prospective analysis of exercise treatment in adolescents and adults. Am J Sports Med. 1992;20(2):151–156. [DOI] [PubMed] [Google Scholar]
- 40.Pfirrmann CW, Zanetti M, Romero J, Hodler J. Femoral trochlear dysplasia: MR findings. Radiology. 2000;216(3):858–864. [DOI] [PubMed] [Google Scholar]
- 41.Post WR, Fithian DC. Patellofemoral Instability: A Consensus Statement From the AOSSM/PFF Patellofemoral Instability Workshop. Orthop J Sports Med. 2018;6(1):2325967117750352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prakash J, Seon JK, Woo SH, Jin C, Song EK. Comparison of Radiological Parameters between Normal and Patellar Dislocation Groups in Korean Population: A Rotational Profile CT-Based Study. Knee Surg Relat Res. 2016;28(4):302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. [DOI] [PubMed] [Google Scholar]
- 44.Rathleff CR, Olesen JL, Roos EM, Rasmussen S, Rathleff MS. Half of 12–15-year-olds with knee pain still have pain after one year. Dan Med J. 2013;60(11):A4725. [PubMed] [Google Scholar]
- 45.Rathleff MS. Patellofemoral pain during adolescence: much more prevalent than appreciated. Br J Sports Med. 2016;50(14):831–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathleff MS, Rathleff CR, Olesen JL, Rasmussen S, Roos EM. Is Knee Pain During Adolescence a Self-limiting Condition? Prognosis of Patellofemoral Pain and Other Types of Knee Pain. Am J Sports Med. 2016;44(5):1165–1171. [DOI] [PubMed] [Google Scholar]
- 47.Rathleff MS, Roos EM, Olesen JL, Rasmussen S. High prevalence of daily and multi-site pain--a cross-sectional population-based study among 3000 Danish adolescents. BMC Pediatr. 2013;13:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathleff MS, Samani A, Olesen JL, et al. Neuromuscular activity and knee kinematics in adolescents with patellofemoral pain. Med Sci Sports Exerc. 2013;45(9):1730–1739. [DOI] [PubMed] [Google Scholar]
- 49.Roth-Isigkeit A, Thyen U, Stoven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115(2):e152–162. [DOI] [PubMed] [Google Scholar]
- 50.Selfe J, Janssen J, Callaghan M, et al. Are there three main subgroups within the patellofemoral pain population? A detailed characterisation study of 127 patients to help develop targeted intervention (TIPPs). Br J Sports Med. 2016;50(14):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheehan FT, Borotikar BS, Behnam AJ, Alter KE. Alterations in in vivo knee joint kinematics following a femoral nerve branch block of the vastus medialis: Implications for patellofemoral pain syndrome. Clin Biomech (Bristol, Avon). 2012;27(6):525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheehan FT, Brainerd EL, Troy KL, Shefelbine SJ, Ronsky JL. Advancing quantitative techniques to improve understanding of the skeletal structure-function relationship. J Neuroeng Rehabil. 2018;15(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shibanuma N, Sheehan FT, Stanhope SJ. Limb positioning is critical for defining patellofemoral alignment and femoral shape. Clin Orthop Relat Res. 2005(434):198–206. [DOI] [PubMed] [Google Scholar]
- 54.Smith BE, Selfe J, Thacker D, et al. Incidence and prevalence of patellofemoral pain: A systematic review and meta-analysis. PLoS One. 2018;13(1):e0190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith RM, Boden BP, Sheehan FT. Increased Patellar Volume/Width and Decreased Femoral Trochlear Width Are Associated With Adolescent Patellofemoral Pain. Clin Orthop Relat Res. 2018;476(12):2334–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119–123, 123.e111–114. [DOI] [PubMed] [Google Scholar]
- 57.Steinberg N, Tenenbaum S, Hershkovitz I, Zeev A, Siev-Ner I. Lower extremity and spine characteristics in young dancers with and without patellofemoral pain. Res Sports Med. 2017;25(2):166–180. [DOI] [PubMed] [Google Scholar]
- 58.Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006–2010/2011. Med Sci Sports Exerc. 2013;45(3):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teng HL, Chen YJ, Powers CM. Predictors of patellar alignment during weight bearing: an examination of patellar height and trochlear geometry. Knee. 2014;21(1):142–146. [DOI] [PubMed] [Google Scholar]


