Abstract
Measles is readily spread to susceptible individuals, but is no longer endemic in the United States. In March 2011, measles was confirmed in a Minnesota child without travel abroad. This was the first identified case-patient of an outbreak. An investigation was initiated to determine the source, prevent transmission, and examine measles-mumps-rubella (MMR) vaccine coverage in the affected community. Investigation and response included case-patient follow-up, post-exposure prophylaxis, voluntary isolation and quarantine, and early MMR vaccine for non-immune shelter residents >6 months and <12 months of age. Vaccine coverage was assessed by using immunization information system records. Outreach to the affected community included education and support from public health, health care, and community and spiritual leaders. Twenty-one measles cases were identified. The median age was 12 months (range, 4 months to 51 years) and 14 (67%) were hospitalized (range of stay, 2–7 days). The source was a 30-month-old US-born child of Somali descent infected while visiting Kenya. Measles spread in several settings, and over 3000 individuals were exposed. Sixteen case-patients were unvaccinated; 9 of the 16 were age-eligible: 7 of the 9 had safety concerns and 6 were of Somali descent. MMR vaccine coverage among Somali children declined significantly from 2004 through 2010 starting at 91.1% in 2004 and reaching 54.0% in 2010 (χ2 for linear trend 553.79; P < .001). This was the largest measles outbreak in Minnesota in 20 years, and aggressive response likely prevented additional transmission. Measles outbreaks can occur if undervaccinated subpopulations exist. Misunderstandings about vaccine safety must be effectively addressed.
Keywords: measles, measles transmission, measles outbreak, measles-mumps-rubella vaccine, vaccine-preventable diseases, vaccine hesitancy, immunization information system, immunization coverage assessment, post-exposure prophylaxis, voluntary isolation and quarantine, undervaccinated subpopulation
Measles, a vaccine-preventable disease, is extremely contagious given that it can be spread via airborne transmission. A susceptible person who has face-to-face contact with an infected person has a 90% likelihood of developing disease.1,2 Measles is still common in many parts of the world, particularly in countries with developing economies. Approximately 20 million cases are estimated to occur globally each year, with 164 000 deaths; most fatalities occur in children under 5 years of age.3
Measles-containing vaccine is very effective; a review of published studies found the median effectiveness of 1 dose of measles-mumps-rubella (MMR) vaccine given ≥12 months of age is 92.5%, and the median effectiveness of 2 doses is 94.1%.4 Widespread use of measles vaccine led to the elimination of endemic measles in the Americas in 2002.5 Measles cases in the United States occur by importation from countries with high measles disease rates or exposure to imported or outbreak-associated cases.
In 2011, 220 measles cases in the United States were reported to the Centers for Disease Control and Prevention (CDC), the highest number since 1996.6 In March 2011, the Minnesota Department of Health (MDH) confirmed measles in a 9-month-old US-born infant who resided at a homeless shelter, with no history of travel abroad. An investigation was initiated to determine the source, prevent transmission, and examine trends in MMR vaccine coverage in the affected community. Although the first identified case had no travel history, the investigation revealed the source case to be an unvaccinated US-born child who was exposed to measles in an endemic region of Africa, and developed disease on return to the United States.7
Minnesota is home to the largest Somali-American community in the United States (estimates range from 20 000 to 60 000), and beginning in 2008, MDH Immunization Program staff were made aware of increasing vaccine hesitancy to MMR vaccine in the Somali-American community because of the misinformation that the vaccine causes autism. Low vaccination rates in the local Somali community, and subsequent exposures among susceptible homeless shelter residents, most of whom were too young for MMR vaccine according to the routine schedule, fueled ongoing transmission of measles.
METHODS
Case Identification
Case-patients were identified by using the 2010 Council of State and Territorial Epidemiologists clinical case definition for measles (fever of ≥101°F [38.3°C], generalized maculopapular rash lasting ≥3 days, and at least 1 of cough, coryza, or conjunctivitis). Confirmed cases were either laboratory confirmed or met the clinical case definition and were epidemiologically linked to a confirmed case. Laboratory confirmation was achieved by serology (positive measles-IgM or a fourfold or more rise in measles-IgG), measles virus isolated in culture, or a positive reverse-transcriptase polymerase chain reaction (PCR). After the first 12 cases were confirmed, refined laboratory testing criteria were used to prioritize testing. Priority was determined by both clinical criteria and known exposure (geographic or to a confirmed case-patient). For example, testing was immediately performed for known exposed individuals who had acute onset of respiratory symptoms and fever, even if rash was not present.
Laboratory Testing
Serology, PCR,8 and/or viral culture (throat, nasal, and urine specimens) were performed at MDH, and equivocal or discrepant results were sent to the CDC for confirmation. Serology from commercial laboratories was confirmed by MDH. Genotyping9 for select specimens was performed at MDH and CDC.
Investigations and Interventions
Case-patients and their parents were interviewed regarding symptoms and vaccine history, and to investigate exposures and contacts. The immune status of case-patients was documented by medical records, the Minnesota Immunization Information Connection (MIIC), and school records. Medical records were reviewed for clinical features. To determine exposures, case-patients were asked to describe activities during the 21 days before rash onset. To identify contacts, case-patients were asked to describe activities during the 4 days before through 4 days after rash onset.
Contacts were notified and assessed for evidence of immunity. If case-patient contacts lacked evidence of immunity (ie, <12 months of age, no history of vaccine, disease, or serologic evidence of immunity, and born in 1957 or later) and were eligible, post-exposure prophylaxis (PEP) was recommended (ie, MMR vaccine within 72 hours of exposure for those vaccine-eligible, or immune globulin [IG] within 6 days if immunocompromised, pregnant, or under 1 year of age, as well as healthy susceptible household contacts over 12 months of age who missed the MMR vaccine window). If vaccinated with 1 dose, exposed contacts were advised to receive a second dose of MMR vaccine.
Contacts without evidence of measles immunity were asked to stay home, limit visitors for 21 days after last exposure, and notify public health and medical providers if symptoms developed. In shelter settings where the ability to quarantine exposed individuals was challenging, documenting measles immunity by serology for residents who had unknown immunity was useful to focus recommendations. MDH recommended that an early dose of MMR vaccine be given to Hennepin County shelter residents who were >6 months and <12 months of age and who had not received MMR vaccine. It was recommended that these children restart the 2-dose MMR vaccine series at ≥12 months of age. It also was recommended that children over 13 months of age who resided in Hennepin County and children of Somali descent who had received only 1 dose of MMR at least 28 days preiously receive a second dose.
Immunization Coverage Assessment
MIIC data were queried at the time of the outbreak and again in early 2013 to assess trends in immunizations among Minnesota-born children residing in Hennepin County at the time of analysis according to MIIC records. Birth records linked to MIIC were used to ascertain ethnicity to compare immunization trends between Somali and non-Somali children. Somali children were identified by using the fields “birthplace of mother” and when Somali was specified in race “other.” Starting in 2011, Somali ethnicity of the child was added as a check box.
Children who had at least 2 non-influenza vaccinations were included in the analysis to ensure that only active records submitted by a provider were included. Because MIIC includes records automatically uploaded from birth records along with any hepatitis B vaccine given at birth, inclusion of a child who had only 1 vaccination may include children whose records have not been actively updated in MIIC. Excluding records with only 1 vaccination also reduces duplication of records in the denominator of interest.
Only vaccine doses received by age 24 months were counted to assess on-time vaccination. Vaccination rates were compared across birth cohorts from 2004 through 2010 using Epi Info 7.1.1.14 (CDC, Atlanta, GA) χ2 test for trend (Fig 1). A preliminary analysis of MIIC data showed a decline in Somali MMR vaccine coverage rates starting in 2004. In addition, the analysis showed a drop in the same coverage in 2008 coinciding with anecdotal reports of MMR vaccine hesitancy in the local Somali community. Because of this preliminary analysis, 2 birth cohorts were selected (2004–2007 and 2008–2010) and SAS Enterprise Guide 5.1 (SAS Institute, Inc, Cary, NC) was used to generate χ2 test values to compare Somali and non-Somali vaccination coverage within the 2 birth cohorts.
FIGURE 1.
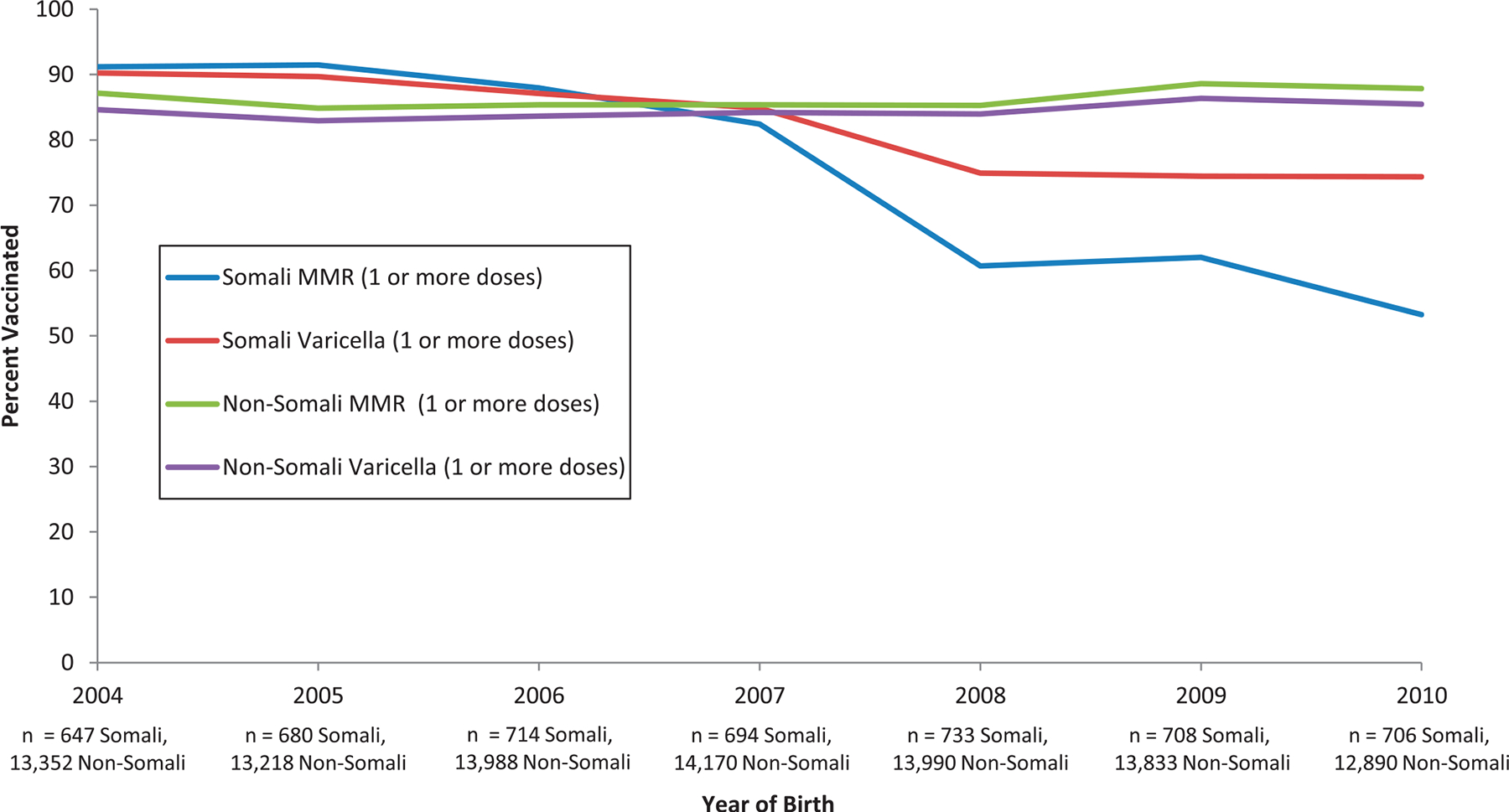
Comparison of 24-month-old children born in Minnesota of Somali descent and non-Somali descent; MMR versus varicella vaccinations, Hennepin County, Minnesota. MMR vaccine coverage among Somali children declined significantly from 2004 through 2010, starting at 91.1% in 2004 and reaching 54.0% in 2010 (P < 0.001). Varicella vaccine also declined significantly from 90.2% to 75.1 % (P < 0.001).
RESULTS
Case Identification
Twenty-one outbreak-associated measles case-patients were identified in Hennepin County from February 15 through April 24, 2011 (Fig2). During the investigation, 211 persons were tested. Two unrelated imported case-patients who had different measles genotypes were also identified.
FIGURE 2.
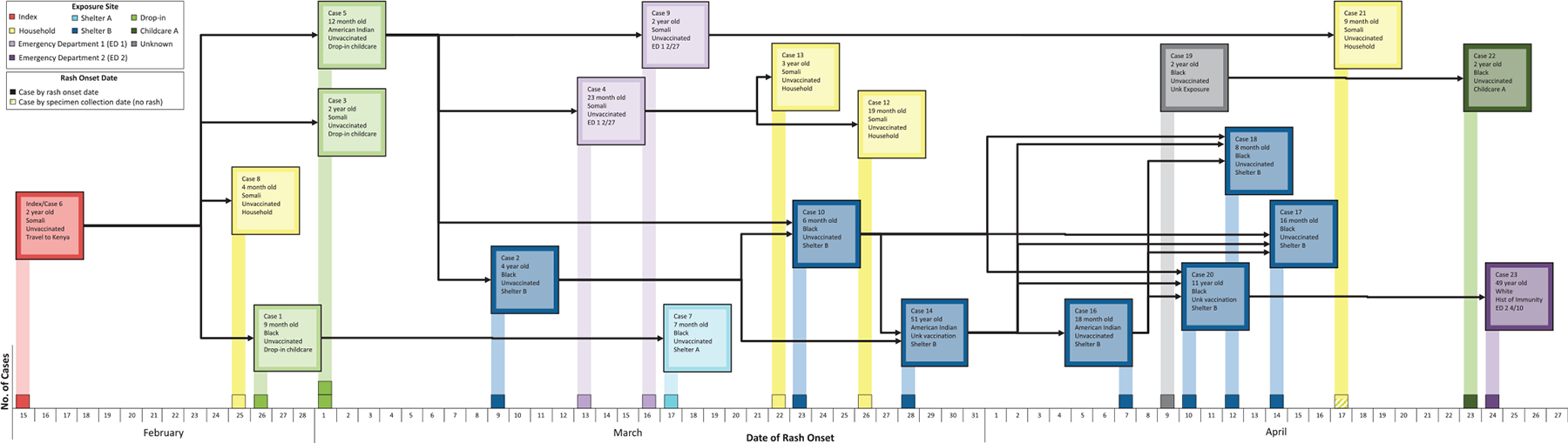
Confirmed measles cases by exposure site and rash onset date, February 15 – April 24, 2011.
The source case-patient was a 30-month-old US-born child of Somali descent, who had returned to Minnesota from Kenya on February 1, 2011. The child developed fever, cough, and vomiting on February 11. On February 14 he attended a drop-in child care center. On February 15, he developed rash, and on February 16 he presented to an emergency department with fever, cough, coryza, vomiting, and rash on the back and trunk. He was diagnosed with bilateral otitis media, bronchiolitis, and dehydration. Measles was not suspected. This child transmitted measles to 3 contacts at a drop-in child care center (including the first identified case-patient) and 1 household contact. The drop-in child care center was available to parents who were accessing services at a county facility. Because of this unique, temporary child care setting, immunization records were not maintained. Subsequent cases occurred in 2 homeless shelters (n = 8), 2 health care facilities (n = 3), 2 households (n = 3), and another conventional child care center (n = 1). One case-patient’s specific exposure was unknown, but he resided in Hennepin County and had a measles strain that was the same genotype as the outbreak strain, and therefore was considered a community exposure (Fig 2). The source case-patient was identified after 5 case-patients already had been confirmed.
Laboratory Testing
Of 21 outbreak cases, 19 were laboratory confirmed and 2 met the clinical case definition and were epidemiologically linked (household contacts) to a confirmed case. Eleven (52%) were confirmed both by serology and PCR, 7 (37%) by PCR only, and 1 (5%) both by PCR and viral culture. The source case was PCR-positive in a nasal wash specimen taken 1 day after rash onset as part of an evaluation that did not include measles. The specimen was subsequently requested by MDH for measles testing. Genotyping was done in 9 cases, including the source case, and was B3. In 3 contacts who received MMR vaccine and who developed a rash within 10 days post-vaccination, PCR testing and genotyping revealed the vaccine strain. Another 6 contacts who received MMR vaccine and who developed a rash greater than 10 days post-vaccination were either measles PCR-negative or the PCR results showed late-cycle threshold values, and there was not enough PCR product to genotype.
Investigations and Interventions
Of 21 outbreak cases-patients, 19 were children (<18 years) and 2 were adults.
The median age of all case-patients was 12 months (range, 4 months to 51 years). Nine of 21 (43%) were black African American, 8 (38%) were black and of Somali descent, 3 (14%) were American Indian, and 1 (5%) was white. This outbreak affected 2 homeless shelters and 8 (38%) case-patients were shelter residents.
Three case-patients had unknown vaccination status, 1 was vaccinated before the recommended age (11months), and 1 was a health care worker who was thought to be immune (IgG-positive documented >10 years previously). Sixteen of 21 (76%) were unvaccinated; 7 of 16 (44%) were too young for routine vaccination. Nine (56%) children were age-eligible for routine vaccination but unvaccinated, 7 because of safety concerns owing to the misinformation that MMR vaccine causes autism; 6 of these children were of Somali descent. Two other children did not refuse but were behind on immunizations.
The most common symptoms were fever of ≥101.1°F, rash, cough, coryza, and conjunctivitis, but other symptoms and complications were common. Fourteen (67%) case-patients were hospitalized; all were being treated for dehydration, and the majority had diarrhea (64%) or vomiting (64%); 6 (43%) had respiratory complications including croup, bronchiolitis, or pneumonia (Table 1). The median length of stay was 4 days (range, 2–7). One case-patient, a 9-month-old unvaccinated infant who was a sibling of another case-patient and measles PCR-positive, had fever of 103°F, cough, and coryza, but rash was never documented (Table 1).
TABLE 1.
Measles Case-Patient Characteristics by Hospitalization Status
| Characteristic | Hospitalized (n = 14) | Non-Hospitalized (n = 7) | Total (n = 21) |
|---|---|---|---|
| Median Age (range) | 1 y (6 m-11 y) | 2 y (4 m-51 y) | 1 y (4 m-51 y) |
| Clinical Presentation | |||
| Median temperature in °C (range) | 39.6 (38.4–40.4) | 38.4 (37.2–39.4) | 39.4 (37.2–40.4) |
| Classic rash progressiona, (%) | 10 (71) | 6 (86) | 16 (76) |
| Any rash, (%) | 14 (100) | 6 (86) | 20 (95) |
| Cough, (%) | 14 (100) | 6 (86) | 20 (95) |
| Coryza, (%) | 14 (100) | 7 (100) | 21 (100) |
| Conjunctivitis, (%) | 14 (100) | 4 (57) | 18 (86) |
| Koplik spots, (%) | 7 (50) | 2 (39) | 9 (43) |
| Complications | |||
| Dehydration, (%) | 14 (100) | 14 (67) | |
| Diarrhea, (%) | 9 (64) | 3 (43) | 12 (57) |
| Vomiting, (%) | 9 (64) | 2 (29) | 11 (52) |
| Otitis, (%) | 6 (43) | 1 (14) | 7 (33) |
| Croup, (%) | 4 (29) | —d | 4 (19) |
| Bronchiolitis, (%) | 2 (14) | — | 2 (10) |
| Pneumonia, (%) | 2 (14) | — | 2 (10) |
| Thrombocytopenia, (%) | 1 (7) | — | 1 (5) |
| Any complications, (%) | 14 (100) | 3 (43) | 17 (81) |
| Underlying conditions, (%) | 3 (21)b | — | 3 (14)b |
| Co-infections, (%) | 1 (7)c | — | 1 (5)c |
From head to trunk and extremities.
All 3 had asthma or reactive airway disease.
Group A Streptococcus and influenza type B.
None.
Voluntary quarantine was implemented when contacts lacked evidence of immunity. Those living in a private dwelling were able to comply without essential services assistance from public health. Voluntary isolation and quarantine in homeless shelters was complicated by common eating and living areas. One shelter with fewer in-room resources had multiple generations of reported cases.
Most case-patients had multiple health care encounters before measles diagnosis (median, 2 visits; range, 0–5). Over 3009 known exposures occurred. Seventy-six IG doses were administered (43 by a public health agency and 33 by health care institutions) and 3 MMR vaccine doses were given within the 3-day PEP effective time frame (Table 2). One individual who received IG after the recommended time period for effective prophylaxis (day 7 post-exposure) developed measles 31 days after exposure. No persons who received MMR as PEP developed measles. Community vaccination clinics were held, including clinics at shelters and the children’s hospital. A Community Forum was held to engage the Somali community about MMR vaccine safety and measles disease. Somali community leaders, including health care providers, spiritual leaders, and parents participated along with public health leaders. Informational meetings were held and print materials were offered at affected shelters, focusing on measles symptom identification, isolation of sick individuals, access to health care, IG and vaccination administration, and measles antibody testing.
TABLE 2.
Summary of Exposures to Measles Case-Patients
| Casea | Age | Health Care Encounters | Residence Type | Total IG Doses Given as PEP for Exposure to This Case | Total MMR Doses Given as PEP for Exposure to This Case | Health Care Contacts | Community Contacts | Total Contacts | Resultant Cases |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 mos | 4 | Shelter A | 26 | 2 | 8b | 404 | 412 | 1 Shelter A |
| 2 | 4 yrs | 4 | Shelter B | 19 | —h | 56 | 81c | 137 | 2 Shelter B |
| 3 | 2 yrs | 4 | Apartment | — | — | 20 | 6d | 26 | none |
| 4 | 23 mos | 1 | Apartment | 1 | — | 7 | 5d | 12 | 2 household |
| 5 | 12 mos | 1 | Shelter B | — | — | 140 | 404 | 544 | 2 ED 1, and 1 Shelter B |
| 6 | 2 yrs | 2 | Apartment | — | — | 132e | 64d | 196 | 3 temporary drop-in childcaref and 1 household |
| 7 | 7 mos | 1 | Apartment | — | — | 0b | 8d | 8 | none |
| 8 | 4 mos | 1 | Apartment | — | — | 25 | 5d | 30 | none |
| 9 | 19 mos | 4 | Single family | 17 | — | 275 | 3 | 278 | 1 household |
| 10 | 6 mos | 3 | Shelter B | 4 | — | 0 | 45c | 45 | noneg |
| 12 | 19 mos | 1 | Apartment | — | — | 0 | 3d | 3 | none |
| 13 | 3 yrs | 1 | Apartment | — | — | 0 | noned | none | none |
| 14 | 51 yrs | 1 | Shelter B | — | — | 0 | 6c | 6 | 1 Shelter B |
| 16 | 18 mos | 5 | Shelter B | — | — | 0b | 2c | 2 | 3g, Shelter B |
| 17 | 16 mos | 4 | Shelter B | 2 | 1 | 13 | 4c | 17 | none |
| 18 | 8 mos | 3 | Shelter B | — | — | 0 | 44c | 44 | none |
| 19 | 2 yrs | 5 | Single family | — | — | 94 | 113 | 207 | 1 Childcare Center |
| 20 | 11 yrs | 2 | Shelter B | 3 | — | 123 | 179c | 302 | 1 ED 2 |
| 21 | 8 mos | 1 | Single family | — | — | 48 | 2 | 50 | none |
| 22 | 2 yrs | 2 | Single family | — | — | 10 | 116 | 126 | none |
| 23 | 49 yrs | 2 | Single family | 4 | — | 410 | 154 | 564 | none |
Cases 11 and 15 were not epidemiologically linked to the outbreak and are excluded from the table.
Only known susceptible persons.
Does not include repeat exposures of 400 shelter residents.
Apartment residents not included.
Represents best estimate and is likely an underestimate for retrospectively identified case.
Drop-in childcare describes a temporary center used by parents who were accessing county services.
Indicates case(s) where overlap of exposure occurred because the individual lived in a setting with multiple exposures.
None.
Immunization Coverage Assessment
MMR vaccine coverage among Somali children declined significantly from 2004 through 2010, starting at 91.1% and reaching 54.0% in 2010 (χ2 for linear trend 553.79; P < .001). Varicella vaccine coverage also declined significantly from 90.2% to 75.1% (χ2 for linear trend 137.66; P < .001). Non-Somali coverage rates increased slightly during the same time period from 87.2% to 88.3% (χ2 for linear trend 36.70; P < .001) for MMR and from 84.7% to 85.9% (χ2 for linear trend 34.43; P < .001) for varicella (Fig 1).
Additional analysis comparing Somali with non-Somali coverage for birth cohorts born in 2004–2007 showed that MMR vaccine coverage among Somali children at 88.2% was, in fact, significantly higher than non-Somali children at 85.7% (odds ratio, 1.24; 95% confidence interval, 1.11–1.40), and in 2008 a clear drop occurred in Somali coverage as part of the downward trend of vaccination coverage for this community.
DISCUSSION
This outbreak began with an unvaccinated US-born child who was exposed to measles in an endemic region of Africa and developed disease on return to the United States. Low vaccination rates in the local Somali community, and subsequent exposures among susceptible homeless shelter residents, fueled ongoing transmission of measles. Delay of the source case-patient’s measles diagnosis also may have contributed to transmission before public health interventions. Although post-exposure prophylaxis, vaccination, and voluntary isolation and quarantine were implemented after the first known case, there was ongoing transmission in 1 of the 2 affected shelters. This transmission was attributable to several factors, including exposures that occurred before the first identified case, an exposure of an infant too young for MMR vaccine according to the routine schedule, as well as exposure of an infant who was too young for the early MMR vaccine outbreak recommendation. Other contributing factors were caused by the challenges of quickly assessing and documenting immune status in a large group of individuals living in a temporary, communal setting. These challenges allowed transmission to individuals who initially were assumed to be immune, but who lacked documentation. After ongoing transmission was seen, immune status testing was implemented for those who lacked documentation.
Notably, two-thirds of the cases in this outbreak were hospitalized, and many of these were hospitalized for respiratory complications in addition to dehydration, highlighting that measles is a severe infection even in well-resourced countries.
Minnesota, and in particular Hennepin County, has the largest community of Somali-American persons in the United States; estimates vary from 20 000 to 60 000 persons. The Somali community in Minnesota is a well-established immigrant community formed in the early 1990s. Currently, there are fewer Somali refugees and primary immigrants, but travel to Africa to visit family and friends is common. In 2008, Somali community activists brought to the attention of the local media their perception of high autism rates in Somali children. The MDH Immunization Program also began receiving reports from health care providers regarding MMR vaccine refusal in the same community owing to parental concerns that MMR vaccine causes autism. Reports described local anti-vaccine activists’ impact on the community. In addition, Andrew Wakefield, the former medical researcher known for his now discredited assertion of a link between MMR vaccine and autism,10,11 met with Somali parents of autistic children in Minnesota 3 times since December 2010, and came back to Minnesota during the outbreak on March 23, 2011.12,13 Investigation is underway at the University of Minnesota to evaluate autism prevalence. A coalition was formed to take on the complicated issue of MMR vaccine hesitancy and refusal within the Minnesota Somali community that includes persons from health care, public health, faith-based groups, health plans, and parents.
The Minnesota outbreak, the largest in the United States since 2008, followed the pattern of measles epidemiology in the United States in the post-elimination era; namely, the outbreak began with an imported case that resulted in limited ongoing transmission among unvaccinated contacts, many of whom refused vaccination.14,15 According to the National Immunization Survey (NIS) 2011 results, MMR vaccination rates in the United States for 1 or more doses of MMR vaccine in 19- to 35-month-old children remain high (91.6% [±0.8]) and Minnesota NIS rates are higher than the national average at 96.0% (±3.4).16 However, this outbreak revealed a susceptible subpopulation with lower vaccine coverage. The intersection of this subpopulation with homeless shelter residents who may have continuity of care challenges such as lack of vaccination documentation, and who live together in a communal residence where voluntary isolation and quarantine is challenging, facilitated efficient spread.
Minnesota immunization data revealed a clear drop in MMR vaccination among Hennepin County children of Somali descent born in 2008 through 2010. Varicella vaccine was used as the comparison vaccine because of the parallel schedule, and a less marked decline was found (Fig 1). DTaP (4 doses) and PCV13 (4 doses) coverage rates also declined, although less dramatically, from 2004–2010 (DTaP, 93.9% to 90.45% and PCV13, 93.9% to 80.46) potentially leaving these children at risk for other vaccine-preventable diseases.
NIS data found that among the vaccine hesitant, parents were more likely to vaccinate if they reported that health care providers influenced their decision-making.17 In contrast, providers serving Somali children reported during the outbreak that parents were not responsive to their recommendations even when additional information was provided. According to national exemption data, Minnesota refusal rates are near the national median. The 2011–2012 school year exemption data show that the percentage of kindergarten students whose parents claimed exemptions to all required vaccines (polio, DTaP, MMR, HepB, and varicella) was 1.6%. The range of state estimates is <0.1% to 7.0% (median, 1.5%).18 Investigating successful messaging as well as engaging community leaders and health care providers are important strategies to address vaccine hesitancy.
A recent study to investigate different approaches to vaccine hesitancy highlights the complexity of messaging, particularly in those who have the most negative attitudes about vaccines.19 This study emphasized careful pretesting of messages, because strategies such as portraying disease risk in text and visuals can backfire. This finding is consistent with communication science literature showing that fear campaigns must be accompanied by messaging that helps individuals feel able to avert the threat.20 This is particularly relevant to the perceived threat of autism in the Somali community. Somali parent interviews conducted by MDH immunization staff have revealed that parents are more fearful of autism than measles (unpublished data). Meszaros et al describe how the cognitive process in such situations can lead to errors of omission over errors of commission. Parents would rather not vaccinate and risk a rare disease than choose vaccination, an action they believe could cause autism.21 If averting the threat of measles means that they take on the threat of autism, measles disease risk messaging will not be effective.
Community leaders including imams are important community figures who may be helpful in advising public health staff.22 Additionally, the local Somali community has a strong oral tradition used to inform and advise one another. Brunson describes the strong influence of parental social networks. In particular nonconformers to vaccination have a greater social network to support that behavior.23 Learning more about how Somali parents talk about MMR vaccination with each other will help change the conversation. The MDH immunization program is committed to using culturally appropriate approaches. Two Somali staff recently were hired to work directly with the Somali community, and a peer-to-peer education class was developed. The class aims to first address the fear driving the decision process, and focuses on child growth and development and autism before broaching the topic of immunizations.
Assessment of vaccine coverage is limited in several ways. First, although >89.7% of pediatric health care providers in Hennepin County routinely use MIIC, it is not yet universally adopted. Also, MIIC stores only current addresses and does not commonly receive address changes from providers indicating that a child has moved out of the county or state. This limitation may lead to an artificially inflated denominator that may depress MIIC vaccination rates. Because the assessment was a point estimate (2013), movement in and out of Hennepin County may have affected rates in either direction. Also, foreign-born children were not included in the analysis, because Minnesota birth records were used to match MIIC records for ethnicity data. Although not included in the analysis, the number of Somali refugees born 2004–2010 arriving in Minnesota and age-eligible for vaccine was negligible (MDH refugee health database, eSHARE unpublished data).
Because there is not a birth record field for race or ethnicity of the child, birth record fields “birthplace of mother” and mother’s race from written-in race information were used to place children in Somali and non-Somali groups. Although the “birthplace of mother” field is well populated (99.6%), there may have been Somali mothers identified as “African American” only, which would place their children in the non-Somali group.
CONCLUSIONS
This outbreak began with an unvaccinated child whose diagnosis initially was missed, and spread was facilitated through an undervaccinated subpopulation and through contacts in homeless shelters where prevention efforts were challenging because of many factors, including the communal setting. Although measles is no longer endemic in the United States, outbreaks can readily occur if widespread immunization is not maintained. Health care providers, together with public health and community leaders, must address vaccine hesitancy to ensure high immunization rates in all communities, including subpopulations. Public health and health care intervention fora highly contagious disease such as measles is time-consuming and expensive, but likely prevents additional transmission. The potential for sustained transmission increases when undervaccinated and difficult to isolate groups intersect. As this outbreak highlights, a parental decision to vaccinate or not impacts not just their child but others in the community in which they live.
ACKNOWLEDGMENTS
We thank the following for their contributions: Emily Banerjee, MPH, Vicki Buttery, MS, Rachel Ostadkar, BS, Stacy Holzbauer, DVM, MPH, Sarah Solarz, MPH, Sudha Setty, MPH, Heather Pint, RN, PHN, Jill Marette, RN, PHN, Beth Parilla, MPH, CarolHajicek, BA, Elly Pretzel, Kathy Como-Sabetti, MPH, Franci Livingston, JD, MPH, Sarah Chute, MPP, Alisa Johnson, MA, Stephanie Able, RN, BS, Steven Swanson, MD, Mary Ellen Bennett, RN, MPH, JaneHarper, BSN, MS, Margaret Roddy, MPH, and Kristen Ehresmann, RN, MPH.
FUNDING:
This investigation was supported by the Centers for Disease Control and Prevention Public Health Emergency Preparedness Grant (U90TP516981) and the Immunization Grant Program (5H23IP522551–09).
FINANCIAL DISCLOSURE:
The authors have indicated they have no financial relationships relevant to this article to disclose.
ABBREVIATIONS
- CDC
Centers for Disease Control and Prevention
- IG
immune globulin
- MDH
Minnesota Department of Health
- MIIC
Minnesota Immunization Information Connection
- MMR
measles-mumps-rubella
- NIS
National Immunization Survey
- PCR
positive reverse-transcriptase polymerase chain reaction
- PEP
post-exposure prophylaxis
Footnotes
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Ehresmann KR, Hedberg CW, Grimm MB, Norton CA, MacDonald KL, Osterholm MT. An outbreak of measles at an international sporting event with airborne transmission in a domed stadium. J Infect Dis. 1995;171(3):679–683 [DOI] [PubMed] [Google Scholar]
- 2.Siegel JD, Rhinehart E, Jackson M, Chiarello L, and the Healthcare Infection Control Practices Advisory Committee. 2007. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Available at: www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf. Accessed June 20, 2012
- 3.World Health Organization Measles Fact Sheet. Available at: www.who.int/mediacentre/factsheets/fs286/en/. Accessed June 30, 2012
- 4.Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature. 1985;318(6044):323–329 [DOI] [PubMed] [Google Scholar]
- 5.Uzicanin A, Zimmerman L. Field effectiveness of live attenuated measles-containing vaccines: a review of published literature. J Infect Dis. 2011;204(suppl 1):S133–S148 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Measles—United States, 2011. MMWR Morb Mortality Wkly Rep. 2012;61(15):253–257 [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Notes from the field: measles outbreak—Hennepin County, Minnesota, February–March 2011. MMWR Morb Mortality Wkly Rep. 2011;60(13):421. [PubMed] [Google Scholar]
- 8.Hummel KB, Lowe L, Bellini WJ, Rota PA. Development of quantitative gene-specific real-time RT-PCR assays for the detection of measles virus in clinical specimens. J Virol Methods. 2006;132(1–2):166–173 [DOI] [PubMed] [Google Scholar]
- 9.Tipples GA, Gray M, Garbutt M, Rota PA; Canadian Measles Surveillance Program. Genotyping of measles virus in Canada: 1979–2002. J Infect Dis. 2004;189(suppl 1): S171–S176 [DOI] [PubMed] [Google Scholar]
- 10.DeStefano F. Vaccines and autism: evidence does not support a causal association. Clin Pharmacol Ther. 2007;82(6):756–759 [DOI] [PubMed] [Google Scholar]
- 11.Retraction—Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 2010;375(9713):445. [DOI] [PubMed] [Google Scholar]
- 12.Lerner M. Anti-vaccine doctor meets with Somalis. Star Tribune. March 24, 2011. Available at: www.startribune.com/lifestyle/health/118547569.html. Accessed July 10, 2013
- 13.Perry S. Fear and frustration dominated Somali community forum on measles, vaccines, and autism. Minnpost. March 28, 2011. Available at: www.minnpost.com/second-opinion/2011/03/fear-and-frustration-dominated-somali-community-forum-measles-vaccines-and-au. Accessed July 10, 2013
- 14.Sugerman DE, Barskey AE, Delea MG, et al. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics. 2010;125(4):747–755 [DOI] [PubMed] [Google Scholar]
- 15.Chen SY, Anderson S, Kutty PK, et al. Health care-associated measles outbreak in the United States after an importation: challenges and economic impact. J Infect Dis. 2011;203(11):1517–1525 [DOI] [PubMed] [Google Scholar]
- 16.Estimated Vaccination Coverage with Individual Vaccines and Selected Vaccination Series Among Children 19–35 Months of Age by State and Local Area US, National Immunization Survey, Q1/2011-Q4/2011. Available at: www.cdc.gov/vaccines/stats-surv/nis/data/tables_2011.htm#overall. Accessed May 30, 2013
- 17.Smith PJ, Kennedy AM, Wooten K, Gust DA, Pickering LK. Association between health care providers’ influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics. 2006;118(5). Available at: www.pediatrics.org/cgi/content/full/118/5/e1287 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Vaccination Coverage Among Children in Kindergarten—United States, 2011–12 School Year. MMWR Morb Mortality Wkly Rep. 2012;61(33):647–652. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm6133a2.htm?s_cid=mm6133a2_w. Accessed June 10, 2013 [PubMed] [Google Scholar]
- 19.Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4). Available at: www.pediatrics.org/cgi/content/full/133/4/e835 [DOI] [PubMed] [Google Scholar]
- 20.Witte K, Allen M. A meta-analysis of fear appeals: implications for effective public health campaigns. Health Educ Behav. 2000; 27(5):591–615 [DOI] [PubMed] [Google Scholar]
- 21.Meszaros JR, Asch DA, Baron J, et al. Cognitive processes and the decisions of some parents to forego pertussis vaccination for their children. J Clin Epidemiol. 1996;49(6): 697–703.21 [DOI] [PubMed] [Google Scholar]
- 22.Padela AI, Killawi A, Heisler M, Demonner S, Fetters MD. The role of imams in American Muslim health: perspectives of Muslim community leaders in Southeast Michigan. J Relig Health. 2011;50(2):359–373 [DOI] [PubMed] [Google Scholar]
- 23.Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1397 [DOI] [PubMed] [Google Scholar]


