Abstract
BACKGROUND
Ewing sarcomas (ESs) are highly aggressive malignancy and are predominant in the long bones of extremities of children and young adults with a slight male predilection and rarely presents at extra skeletal locations.
CASE SUMMARY
A 55-year-old woman came to our hospital after finding elevated tumor biomarkers during her physical examination. Her enhanced computed tomography scan showed a jejunal mass. The patient underwent laparoscopic enterectomy. The mass was later diagnosed as ES, evidenced by fluorescence in situ hybridization whereby the GLP ES breakpoint region 1 probe was used, showing that more than 10% of the cells showed a red-green-yellow signal proving the breakpoint rearrangement of the ES breakpoint region 1 gene in chromosome 22.
CONCLUSION
We describe a case of localized ES at the jejunum in China based on the literature.
Keywords: Ewing sarcoma, Small bowel, Fluorescence in situ hybridization, Ewing sarcoma breakpoint region 1 gene, Jejunum, Enterectomy, Case report
Core Tip: Ewing sarcomas (ESs) are a highly aggressive malignancy and are predominant in the long bones of extremities of children and young adults. We hereby present a case of extraosseous ES of the jejunum in a female patient. This case highlights the jejunum as a potential site of ES origin and shows that a surgical approach with adjuvant chemotherapy is beneficial.
INTRODUCTION
Ewing sarcoma (ES) is a small round-cell tumor with simple sarcoma-specific genetic alterations resulting in a TET/FET family member and E26 transformation-specific family member[1]. ESs are rare small round-cell tumors that arise predominantly in children and young adults with a slight male predilection[2-4]. ES most often arises in the mid-shaft or diaphysis of the long bones of the extremities with the spine making up 8% of the primary sites[5]. Extra osseous ES occurs in the soft tissue of the extremities, paravertebral region, and pelvic cavity[6] and has also been discovered in most organs including the pancreas, liver, adrenal gland, esophagus, and uterus[7-13]. Extra skeletal cases are rare, and these patients generally present at an older age and demonstrate a greater overall 5-year survival than skeletal ES tumors[14,15]. Reports of primary liver involvement have been noted, as well as gastrointestinal sites of origin including the stomach, small intestine, and colorectal[16-19]. Nevertheless, ES is extremely rare in the small bowel. Here, we report a case of primary ES in the jejunum with EWS rearrangement.
CASE PRESENTATION
Chief complaints
A 55-year-old otherwise healthy female patient came to our hospital after finding out that she had elevated tumor biomarkers during her annual physical examination.
History of present illness
She had no other complaints. Her sleep and appetite were normal. Her excretion and egestion were all normal.
History of past illness
The patient had a free past medical history.
Personal and family history
The patient grew up in her locality, denies any contact with contaminated water or radiation exposure, and denies smoking and alcohol consumption. She had a gestational history of 1-0-0-1. Her menstruation was 16 (5-6/28-30) 50.
Physical examination
On examination, the patient’s temperature was 37.0 °C, heart rate was 85 beats per min, respiratory rate was 16 breaths per min, and blood pressure was 110/65 mmHg. The Glasgow coma scale was 15/15 without any pathological signs. Her S1 S2 sounds were regular. Her chest was bilaterally clear; no rhonchi or crackles were heard. Abdominal examination revealed a soft and non-tender abdomen. No mass or distension was observed. Bowel sounds were active.
Laboratory examinations
Her carbohydrate antigen 153 (CA-153) level was 38.04 u/mL, CA-199 was 109.5 u/mL, and CA-125 47 was u/mL. The white blood cell count was low at 3.39 × 109/L (normal range 3.50-9.50 × 109/L) and lymphocytes were low at 0.79 × 109/L (normal range 1.10-3.20 × 109/L).
Imaging examinations
Her abdominal computed tomography scan showed a contrast-enhanced mass in the small intestine at the left lower quadrant of the abdomen (Figure 1).
Figure 1.
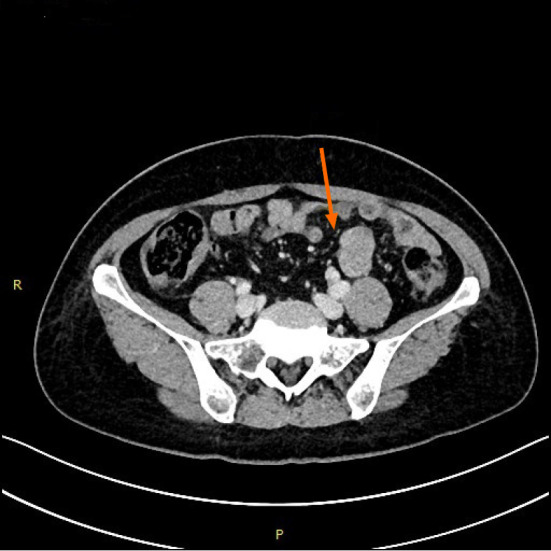
Transverse spiral computed tomography scan of the abdomen, with intravenous contrast enhancement showing dilation of jejunal wall of the left lower quadrant.
Further diagnostic work-up
The patient underwent minimally invasive exploratory laparotomy. During the exploration, the tumor was located in the distal jejunum. It was well-circumscribed and had a fleshy pink surface similar to that of a gastrointestinal stromal tumor. A segment of the jejunum was resected 5 cm away from the edges of the tumor on both sides and an anastomosis was made using mechanical staple. The patient recovered uneventfully after surgery.
Pathological report
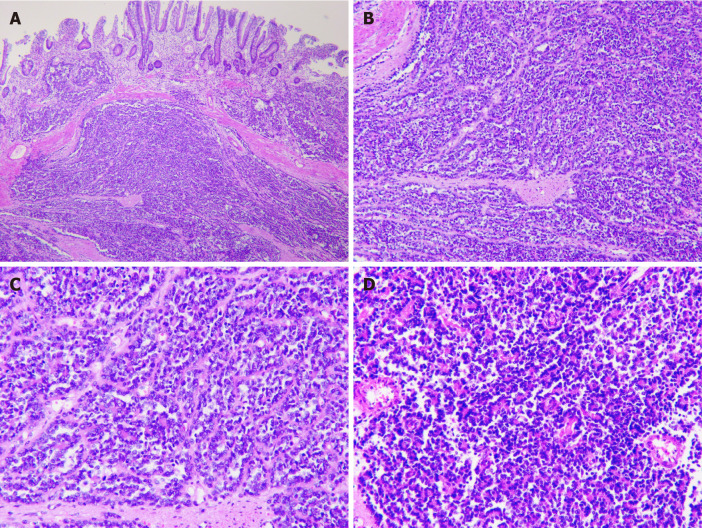
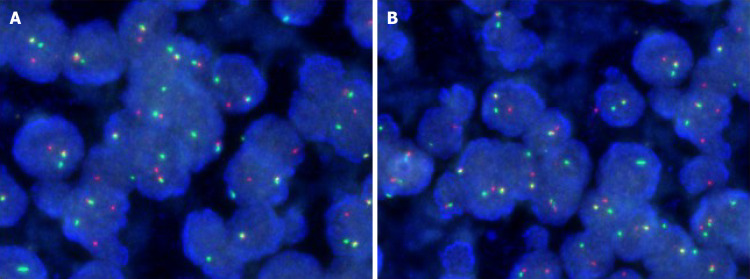
Pathological examination showed that the tumor of 3.5 cm × 3.0 cm × 2.3 cm in size was malignant, as there was invasion of the entire wall of the intestine. The resected sample had negative margins (R0) (Figure 2). Immunohistochemical analysis showed CD117 (-), CD34 (-), DOG-1 (-), Ki67 (35%), CK-pan (partly +), CK-L (+), CD56 (-), Syn (+), Villin (-), CK7 (-), Cg A (-), CD99 (+ +), INI-1 (+), Desmin (-), Inhibin-α (-), ER (-), PR (-), Calretinin (-), WT-1 (-), SF (-), HMB45 (-), S-100 (-), and Melan A (-). Fluorescence in situ hybridization (FISH) for an ES breakpoint region 1 (EWSR1) gene rearrangement (22q11) was performed using GLP EWSR1 probe, showed that more than 10% of the cells had a red-green-yellow signal, demonstrating the breakpoint rearrangement of the EWSR1 gene in chromosome 22 (Figure 3).
Figure 2.
Immunohistochemical analysis. A: Low magnification of the resected sample using formalin-fixed (magnification: × 40); B: Paraffin-embedded sections of tumor stained with hematoxylin and eosin demonstrating sheets of small (magnification: × 100); C: Round-to-spindle, uniform tumor cells with clear cytoplasm (magnification: × 200); D: Higher magnification of C (magnification: × 200).
Figure 3.
Fluorescence in situ hybridization. A: Fluorescence in situ hybridization of the resected tumor showing more than 10% of the cells showed a red-green-yellow signal, proving the breakpoint rearrangement of the Ewing Sarcoma breakpoint region 1 gene; B: More than 10% of the cells from resected sample showing a red-green-yellow signal (magnification: × 200).
Post-operative course
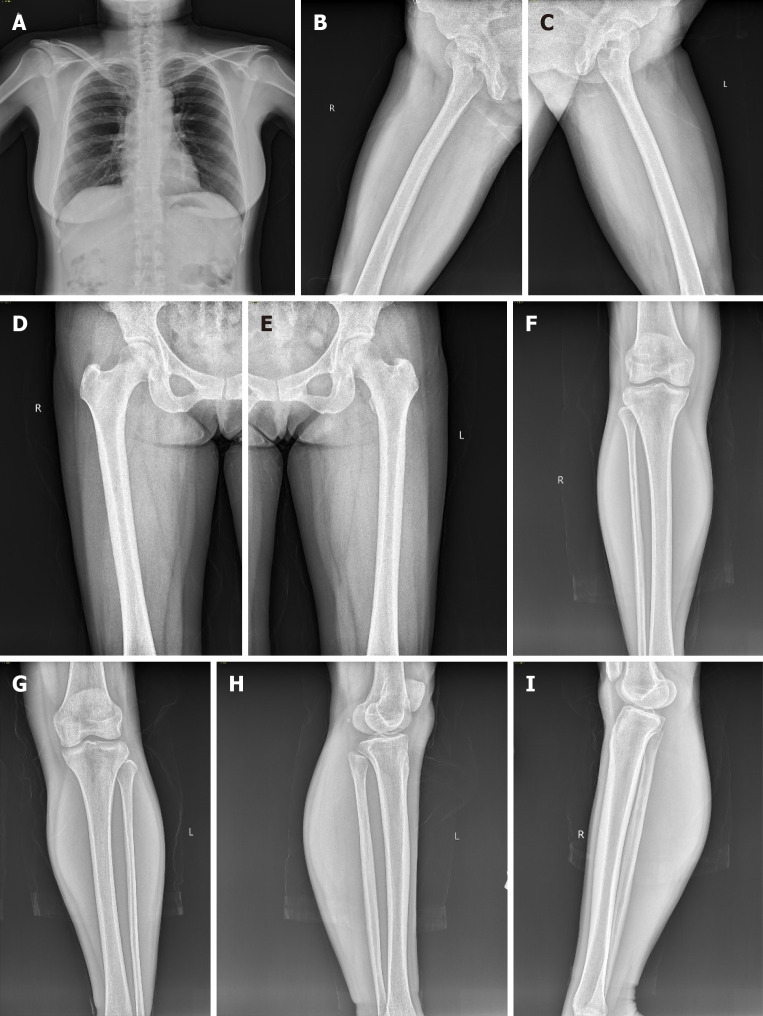
Post-operatively, bone X-rays were done to rule out any primary lesion from her skeletal system (Figure 4). The patient was discharged on post-operative day 8.
Figure 4.
Post-operative bone X-ray which shows no lesion in skeletal system thereby excluding metastasis. A: Anterior-posterior view of the chest; B: Posterior-anterior right upper thigh; C: Posterior-anterior left upper thigh; D: Anterior-posterior right upper thigh; E: Anterior-posterior left upper thigh; F: Anterior-posterior lower leg; G: Anterior-posterior lower leg; H: Medial-lateral lower leg; I: Medial-lateral lower leg.
Further work and follow-up
She was referred to the oncology department for further treatment. The regimen included vincristine, adriamycin, cyclophosphamide, doxorubicin, and addition of ifosfamide and etoposide (VACD-IE), given every 2 wk for 12 cycles. It started 1 mo post-operatively. However, after four cycles, the patient stopped the adjuvant therapy due to a fear of side effects. To date, there has been no sign of relapse and the patient recently showed interest in continuing the adjuvant therapy.
FINAL DIAGNOSIS
Extraosseous ES at the jejunum.
TREATMENT
Minimally invasive exploratory laparotomy. Referred to the oncology department for further treatment. The regimen included VACD-IE, given every 2 wk for 12 cycles. It started 1 mo post-operatively.
OUTCOME AND FOLLOW-UP
After four cycles, the patient stopped the adjuvant therapy due to a fear of side effects. To date, there is no sign of relapse and the patient recently showed interest in continuing the adjuvant therapy.
DISCUSSION
ES harbors multiple balanced translocations, and fusions involving the EWSR1 gene on chromosome 22 exist. The most common translocation is t (11;22), EWSR1-FLI1 fusion (85% of cases), causing overexpression of the FLI-1 protein. The second most common translocation is t (21;22), EWSR1-ERG fusion (5%-10% of cases). Numerous other less common variant translocations exist. Lack of reverse transcription-polymerase chain reaction fusion transcripts for EWSR1-FLI1 and EWSR1-ERG does not exclude the possibility of ES because it does not rule out fusion transcripts that may be present below the limit of detection for the given assay (5%)[20]. It most commonly arises from bone but can develop in extra skeletal sites[21]. ES of the small intestine is extremely rare based on the literature[22-24].
Malignant GIST usually expresses CD117, Dog-1, and CD34, which were all negative in this case. Although both synovial sarcoma and ES/PNET could have genetic rearrangements, the regions of these translocations are quite different. In ES/PNET, Chr22 EWS-FLI or EWS-FEV translocations are commonly reported[25]. However, in synovial sarcoma, SYT-SSX translocation is frequently observed[26]. Clear-cell sarcoma could be ruled out by negative immunohistochemistry for HMB45, S-100, and Melan A. A previous study also indicated the necessity of distinguishing from an intraabdominal desmoplastic small round-cell tumor by histological and immunohistochemical characteristics when ES/PNET occurs in the abdominal cavity[27].
Among the 37 cases found, 3 were derived from the esophagus, 9 from the stomach, 5 were of colorectal origin and 20 arose from the small intestine. Twenty-two cases were in males and fifteen were in females. The age range was 9-years-old to 68-years-old. FISH break-apart EWSR1 was positive in 19 cases, negative in 1 case and was not conducted in 17 cases[9,18,22,23,28-51]. Our patient’s characteristics fell within these demographic data. Demographic research has shown that the frequency of EW is higher in United States Caucasian population than in China[52].
ES predominantly affects children and young adults with a peak incidence between 10 and 20 years of age. About 30% of cases occur in adults over the age of 20 and fewer than 5% occur in adults over the age of 40[53].
to date, the outcome of the 5-year survival rate of metastatic patients is usually poor (< 30%) compared to localized ES (65%-75%), despite the use of chemotherapy[54]. Several studies have indicated that localized extra skeletal ES has a more favorable outcome than skeletal tumors[55,56].
According to National Comprehensive Cancer Network guidelines, postoperative radiation therapy should begin within 60 d of surgery and is given concurrently with consolidation chemotherapy[57]. This explains why our patient was referred to oncology department shortly after surgery for further treatments.
Intergroup Ewing’s Sarcoma Study-I and Intergroup Ewing’s Sarcoma Study-II showed that radiation therapy and chemotherapy with VACD was superior to vincristine, adriamycin, cyclophosphamide (VAC)[58]. The 5-year relapse-free survival rate was 60% and 24 % for VACD and VAC, respectively (P < 0.001). The corresponding overall survival rate was 65% and 28% (P < 0.001). Womer et al[59] reported that VACD-IE given on every 2 wk schedule was found to be more effective and no increase in toxicity.
CONCLUSION
ES is a highly aggressive small round-cell tumor that arises in adults. We have described a patient with ES occurring in the jejunum. This case report helps solidify jejunum as a potential site for ES origin and surgical approach with adjuvant chemotherapy does prove beneficial. However, this is a single case study and conclusion be made only based on our experience.
ACKNOWLEDGEMENTS
We’d like to thank Dr. Ding ZY for the radiological images and Dr. Fang HS for processing the pathological images.
Footnotes
Informed consent statement: Written informed consent was obtained from the participants for publication of this article and any accompanying tables/images. A copy of the written consent is available for review by the Editor of this journal.
Conflict-of-interest statement: The authors declare that they have no competing interests.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: August 4, 2020
First decision: September 17, 2020
Article in press: April 29, 2021
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Altintoprak F S-Editor: Zhang L L-Editor: Filipodia P-Editor: Yuan YY
Contributor Information
Kamleshsingh Shadhu, Department of General Surgery, Gastrointestinal Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China; Pre-registration House Officer, Medical Council of Mauritius, Floreal 0000, Plaine Whilhems, Mauritius.
Dadhija Ramlagun-Mungur, Department of General Surgery, Gastrointestinal Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China; Pre-registration House Officer, Medical Council of Mauritius, Floreal 0000, Plaine Whilhems, Mauritius.
Xiao-Chun Ping, Department of General Surgery, Gastrointestinal Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China. pingxiaochun@jsph.org.cn.
References
- 1.Kim SK, Park YK. Ewing sarcoma: a chronicle of molecular pathogenesis. Hum Pathol. 2016;55:91–100. doi: 10.1016/j.humpath.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Hense HW, Ahrens S, Paulussen M, Lehnert M, Jürgens H. Factors associated with tumor volume and primary metastases in Ewing tumors: results from the (EI)CESS studies. Ann Oncol. 1999;10:1073–1077. doi: 10.1023/a:1008357018737. [DOI] [PubMed] [Google Scholar]
- 3.Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184–192. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 4.Seker MM, Kos T, Ozdemir N, Seker A, Aksoy S, Uncu D, Zengin N. Treatment and outcomes of Ewing sarcoma in Turkish adults: a single centre experience. Asian Pac J Cancer Prev. 2014;15:327–330. doi: 10.7314/apjcp.2014.15.1.327. [DOI] [PubMed] [Google Scholar]
- 5.Cotterill SJ, Ahrens S, Paulussen M, Jürgens HF, Voûte PA, Gadner H, Craft AW. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 6.Labotka RJ. Principles and Practice of Pediatric Oncology. JAMA . 1994;271:1136–1137. [Google Scholar]
- 7.Murugan P, Rao P, Tamboli P, Czerniak B, Guo CC. Primary Ewing Sarcoma / Primitive Neuroectodermal Tumor of the Kidney: A Clinicopathologic Study of 23 Cases. Pathol Oncol Res. 2018;24:153–159. doi: 10.1007/s12253-017-0228-0. [DOI] [PubMed] [Google Scholar]
- 8.Harimaya K, Oda Y, Matsuda S, Tanaka K, Chuman H, Iwamoto Y. Primitive neuroectodermal tumor and extraskeletal Ewing sarcoma arising primarily around the spinal column: report of four cases and a review of the literature. Spine (Phila Pa 1976) 2003;28:E408–E412. doi: 10.1097/01.BRS.0000085099.47800.DF. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AD, Pambuccian SE, Andrade RS, Dolan MM, Aslan DL. Ewing sarcoma and primitive neuroectodermal tumor of the esophagus: report of a case and review of literature. Int J Surg Pathol. 2010;18:388–393. doi: 10.1177/1066896908316903. [DOI] [PubMed] [Google Scholar]
- 10.Mani S, Dutta D, De BK. Primitive neuroectodermal tumor of the liver: a case report. Jpn J Clin Oncol. 2010;40:258–262. doi: 10.1093/jjco/hyp158. [DOI] [PubMed] [Google Scholar]
- 11.Ren YL, Tang XY, Li T. Ewing sarcoma-primitive neuroectodermal tumor of the uterus: a clinicopathologic, immunohistochemical and ultrastructural study of one case. Arch Gynecol Obstet. 2011;283:1139–1143. doi: 10.1007/s00404-010-1557-3. [DOI] [PubMed] [Google Scholar]
- 12.Bose P, Murugan P, Gillies E, Holter JL. Extraosseous Ewing's sarcoma of the pancreas. Int J Clin Oncol. 2012;17:399–406. doi: 10.1007/s10147-011-0311-6. [DOI] [PubMed] [Google Scholar]
- 13.Abi-Raad R, Manetti GJ, Colberg JW, Hornick JL, Shah JG, Prasad ML. Ewing sarcoma/primitive neuroectodermal tumor arising in the adrenal gland. Pathol Int. 2013;63:283–286. doi: 10.1111/pin.12063. [DOI] [PubMed] [Google Scholar]
- 14.Pradhan A, Grimer RJ, Spooner D, Peake D, Carter SR, Tillman RM, Abudu A, Jeys L. Oncological outcomes of patients with Ewing's sarcoma: is there a difference between skeletal and extra-skeletal Ewing's sarcoma? J Bone Joint Surg Br. 2011;93:531–536. doi: 10.1302/0301-620X.93B4.25510. [DOI] [PubMed] [Google Scholar]
- 15.Applebaum MA, Worch J, Matthay KK, Goldsby R, Neuhaus J, West DC, Dubois SG. Clinical features and outcomes in patients with extraskeletal Ewing sarcoma. Cancer. 2011;117:3027–3032. doi: 10.1002/cncr.25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soulard R, Claude V, Camparo P, Dufau JP, Saint-Blancard P, Gros P. Primitive neuroectodermal tumor of the stomach. Arch Pathol Lab Med. 2005;129:107–110. doi: 10.5858/2005-129-107-PNTOTS. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki Y, Miura Y, Koganemaru S, Suyama K, Inoshita N, Fujii T, Hashimoto M, Tamura T, Takeuchi K, Takano T. Ewing sarcoma of the liver with multilocular cystic mass formation: a case report. BMC Cancer. 2015;15:16. doi: 10.1186/s12885-015-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czekalla R, Fuchs M, Stölzle A, Nerlich A, Poremba C, Schaefer KL, Weirich G, Höfler H, Schneller F, Peschel C, Siewert JR, Schepp W. Peripheral primitive neuroectodermal tumor of the stomach in a 14-year-old boy: a case report. Eur J Gastroenterol Hepatol. 2004;16:1391–1400. doi: 10.1097/00042737-200412000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Aboumarzouk OM, Coleman R, Goepel JR, Shorthouse AJ. PNET/Ewing's sarcoma of the rectum: a case report and review of the literature. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.04.2009.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grover M, Bernard CE, Pasricha PJ, Lurken MS, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, McCallum RW, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Ünalp-Arida A, Hamilton FA, Farrugia G NIDDK Gastroparesis Clinical Research Consortium (GpCRC) Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24:531–539, e249. doi: 10.1111/j.1365-2982.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandberg AA, Bridge JA. Updates on cytogenetics and molecular genetics of bone and soft tissue tumors: Ewing sarcoma and peripheral primitive neuroectodermal tumors. Cancer Genet Cytogenet. 2000;123:1–26. doi: 10.1016/s0165-4608(00)00295-8. [DOI] [PubMed] [Google Scholar]
- 22.Shek TW, Chan GC, Khong PL, Chung LP, Cheung AN. Ewing sarcoma of the small intestine. J Pediatr Hematol Oncol. 2001;23:530–532. doi: 10.1097/00043426-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Kim DW, Chang HJ, Jeong JY, Lim SB, Lee JS, Hong EK, Lee GK, Choi HS, Jeong SY, Park JG. Ewing's sarcoma/primitive neuroectodermal tumor (ES/PNET) of the small bowel: a rare cause of intestinal obstruction. Int J Colorectal Dis. 2007;22:1137–1138. doi: 10.1007/s00384-006-0142-5. [DOI] [PubMed] [Google Scholar]
- 24.Li T, Zhang F, Cao Y, Ning S, Bi Y, Xue W, Ren L. Primary Ewing's sarcoma/primitive neuroectodermal tumor of the ileum: case report of a 16-year-old Chinese female and literature review. Diagn Pathol. 2017;12:37. doi: 10.1186/s13000-017-0626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milione M, Gasparini P, Sozzi G, Mazzaferro V, Ferrari A, Casali PG, Perrone F, Tamborini E, Pellegrinelli A, Gherardi G, Arrigoni G, Collini P, Testi A, De Paoli E, Aiello A, Pilotti S, Pelosi G. Ewing sarcoma of the small bowel: a study of seven cases, including one with the uncommonly reported EWSR1-FEV translocation. Histopathology. 2014;64:1014–1026. doi: 10.1111/his.12350. [DOI] [PubMed] [Google Scholar]
- 26.Machado I, Navarro L, Pellin A, Navarro S, Agaimy A, Tardío JC, Karseladze A, Petrov S, Scotlandi K, Picci P, Llombart-Bosch A. Defining Ewing and Ewing-like small round cell tumors (SRCT): The need for molecular techniques in their categorization and differential diagnosis. A study of 200 cases. Ann Diagn Pathol. 2016;22:25–32. doi: 10.1016/j.anndiagpath.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Sutton RJ, Thomas JM. Desmoid tumours of the anterior abdominal wall. Eur J Surg Oncol. 1999;25:398–400. doi: 10.1053/ejso.1999.0664. [DOI] [PubMed] [Google Scholar]
- 28.Adair A, Harris SA, Coppen MJ, Hurley PR. Extraskeletal Ewings sarcoma of the small bowel: case report and literature review. J R Coll Surg Edinb. 2001;46:372–374. [PubMed] [Google Scholar]
- 29.Vignali M, Zacchè MM, Messori P, Natale A, Busacca M. Ewing's sarcoma of the small intestine misdiagnosed as a voluminous pedunculated uterine leiomyoma. Eur J Obstet Gynecol Reprod Biol. 2012;162:234–235. doi: 10.1016/j.ejogrb.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Horie Y, Kato M. Peripheral primitive neuroectodermal tumor of the small bowel mesentery: a case showing perforation at onset. Pathol Int. 2000;50:398–403. doi: 10.1046/j.1440-1827.2000.01045.x. [DOI] [PubMed] [Google Scholar]
- 31.Sarangarajan R, Hill DA, Humphrey PA, Hitchcock MG, Dehner LP, Pfeifer JD. Primitive neuroectodermal tumors of the biliary and gastrointestinal tracts: clinicopathologic and molecular diagnostic study of two cases. Pediatr Dev Pathol. 2001;4:185–191. doi: 10.1007/s100240010141. [DOI] [PubMed] [Google Scholar]
- 32.Balasubramanian B, Dinakarababu E, Molyneux AJ. Primary primitive neuroectodermal tumour of the small bowel mesentery: case report. Eur J Surg Oncol. 2002;28:197–198. doi: 10.1053/ejso.2001.1155. [DOI] [PubMed] [Google Scholar]
- 33.Graham DK, Stork LC, Wei Q, Ingram JD, Karrer FM, Mierau GW, Lovell MA. Molecular genetic analysis of a small bowel primitive neuroectodermal tumor. Pediatr Dev Pathol. 2002;5:86–90. doi: 10.1007/s10024-001-0192-1. [DOI] [PubMed] [Google Scholar]
- 34.Maesawa C, Iijima S, Sato N, Yoshinori N, Suzuki M, Tarusawa M, Ishida K, Tamura G, Saito K, Masuda T. Esophageal extraskeletal Ewing's sarcoma. Hum Pathol. 2002;33:130–132. doi: 10.1053/hupa.2002.30219. [DOI] [PubMed] [Google Scholar]
- 35.Tokudome N, Tanaka K, Kai MH, Sueyoshi K, Matsukita S, Setoguchi T. Primitive neuroectodermal tumor of the transverse colonic mesentery defined by the presence of EWS-FLI1 chimeric mRNA in a Japanese woman. J Gastroenterol. 2002;37:543–549. doi: 10.1007/s005350200084. [DOI] [PubMed] [Google Scholar]
- 36.Drut R, Drut M, Müller C, Marrón A. Rectal primitive neuroectodermal tumor. Pediatr Pathol Mol Med. 2003;22:391–398. [PubMed] [Google Scholar]
- 37.Vardy J, Joshua AM, Clarke SJ, Yarrow PM, Lin BP. Small blue cell tumors of the rectum. Case 1. Ewing's sarcoma of the rectum. J Clin Oncol. 2005;23:910–912. doi: 10.1200/JCO.2005.03.096. [DOI] [PubMed] [Google Scholar]
- 38.Kuwabara K, Ishida H, Shirakawa K, Yokoyama M, Nakada H, Hayashi Y, Hashimoto D, Miura I, Itoyama S, Heike Y. Primitive neuroectodermal tumor arising in the colon: report of a case. Surg Today. 2006;36:193–197. doi: 10.1007/s00595-005-3104-6. [DOI] [PubMed] [Google Scholar]
- 39.Sethi B, Smith GT. Primary primitive neuroectodermal tumour arising in the small bowel. Histopathology. 2007;50:665–666. doi: 10.1111/j.1365-2559.2007.02631.x. [DOI] [PubMed] [Google Scholar]
- 40.Colovic RB, Grubor NM, Micev MT, Matic SV, Atkinson HD, Latincic SM. Perigastric extraskeletal Ewing's sarcoma: a case report. World J Gastroenterol. 2009;15:245–247. doi: 10.3748/wjg.15.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rafailidis S, Ballas K, Psarras K, Pavlidis T, Symeonidis N, Marakis G, Sakadamis A. Primary Ewing sarcoma of the stomach--a newly described entity. Eur Surg Res. 2009;42:17–20. doi: 10.1159/000166166. [DOI] [PubMed] [Google Scholar]
- 42.Ankouz A, Elbouhadouti H, Lamrani J, Bouassria A, Louchi A, Taleb KA. [Peripheral primitive neuroectodermal tumor with gastric primary location: about a new case] Pan Afr Med J. 2010;6:15. [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue M, Wakai T, Korita PV, Sakata J, Kurosaki R, Ogose A, Kawashima H, Shirai Y, Ajioka Y, Hatakeyama K. Gastric Ewing sarcoma/primitive neuroectodermal tumor: A case report. Oncol Lett. 2011;2:207–210. doi: 10.3892/ol.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodarte-Shade M, Palomo-Hoil R, Vazquez J, Ancer A, Vilches N, Flores-Gutierrez JP, Sierra M, Garza-Serna U. Primitive Neuroectodermal Tumor (PNET) of the Small Bowel in a Young Adult with Lower Gastrointestinal Bleeding. J Gastrointest Cancer. 2012;43 Suppl 1:S243–S245. doi: 10.1007/s12029-012-9409-y. [DOI] [PubMed] [Google Scholar]
- 45.Aras M, Dede F, Dane F, Aktas B, Turoglu HT. FDG PET/CT appearance of portal vein tumor thrombus in the gastric primitive neuroectodermal tumor: uncommon primary tumor site with rare finding. Clin Nucl Med. 2013;38:47–49. doi: 10.1097/RLU.0b013e3182708530. [DOI] [PubMed] [Google Scholar]
- 46.Insabato L, Guadagno E, Natella V, Somma A, Bihl M, Pizzolorusso A, Mainenti PP, Apice G, Tornillo L. An unusual association of malignant gastrointestinal neuroectodermal tumor (clear cell sarcoma-like) and Ewing sarcoma. Pathol Res Pract. 2015;211:688–692. doi: 10.1016/j.prp.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Kim SB, Lee SH, Gu MJ. Esophageal subepithelial lesion diagnosed as malignant gastrointestinal neuroectodermal tumor. World J Gastroenterol. 2015;21:5739–5743. doi: 10.3748/wjg.v21.i18.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boland JM, Folpe AL. Oncocytic variant of malignant gastrointestinal neuroectodermal tumor: a potential diagnostic pitfall. Hum Pathol. 2016;57:13–16. doi: 10.1016/j.humpath.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 49.Khuri S, Gilshtein H, Sayidaa S, Bishara B, Kluger Y. Primary Ewing Sarcoma/Primitive Neuroectodermal Tumor of the Stomach. Case Rep Oncol. 2016;9:666–671. doi: 10.1159/000449126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maxwell AW, Wood S, Dupuy DE. Primary extraskeletal Ewing sarcoma of the stomach: a rare disease in an uncommon location. Clin Imaging. 2016;40:843–845. doi: 10.1016/j.clinimag.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Song MJ, An S, Lee SS, Kim BS, Kim J. Primitive Neuroectodermal Tumor of the Stomach: A Case Report. Int J Surg Pathol. 2016;24:543–547. doi: 10.1177/1066896916639371. [DOI] [PubMed] [Google Scholar]
- 52.Li FP, Tu JT, Liu FS, Shiang EL. Rarity of Ewing's sarcoma in China. Lancet. 1980;1:1255. doi: 10.1016/s0140-6736(80)91719-5. [DOI] [PubMed] [Google Scholar]
- 53.Bacci G, Balladelli A, Forni C, Ferrari S, Longhi A, Bacchini P, Alberghini M, Fabbri N, Benassi M, Briccoli A, Picci P. Adjuvant and neoadjuvant chemotherapy for Ewing sarcoma family tumors in patients aged between 40 and 60: report of 35 cases and comparison of results with 586 younger patients treated with the same protocols in the same years. Cancer. 2007;109:780–786. doi: 10.1002/cncr.22456. [DOI] [PubMed] [Google Scholar]
- 54.Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, Delattre O, Paulussen M, Picci P, Sundby Hall K, van den Berg H, Ladenstein R, Michon J, Hjorth L, Judson I, Luksch R, Bernstein ML, Marec-Bérard P, Brennan B, Craft AW, Womer RB, Juergens H, Oberlin O. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J Clin Oncol. 2015;33:3036–3046. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 55.Cash T, McIlvaine E, Krailo MD, Lessnick SL, Lawlor ER, Laack N, Sorger J, Marina N, Grier HE, Granowetter L, Womer RB, DuBois SG. Comparison of clinical features and outcomes in patients with extraskeletal vs skeletal localized Ewing sarcoma: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2016;63:1771–1779. doi: 10.1002/pbc.26096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galyfos G, Karantzikos GA, Kavouras N, Sianou A, Palogos K, Filis K. Extraosseous Ewing Sarcoma: Diagnosis, Prognosis and Optimal Management. Indian J Surg. 2016;78:49–53. doi: 10.1007/s12262-015-1399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denbo JW, Shannon Orr W, Wu Y, Wu J, Billups CA, Navid F, Rao BN, Davidoff AM, Krasin MJ. Timing of surgery and the role of adjuvant radiotherapy in ewing sarcoma of the chest wall: a single-institution experience. Ann Surg Oncol. 2012;19:3809–3815. doi: 10.1245/s10434-012-2449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nesbit ME Jr, Gehan EA, Burgert EO Jr, Vietti TJ, Cangir A, Tefft M, Evans R, Thomas P, Askin FB, Kissane JM. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol. 1990;8:1664–1674. doi: 10.1200/JCO.1990.8.10.1664. [DOI] [PubMed] [Google Scholar]
- 59.Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP, Weiss AR. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]