Abstract
Eosinophils are a myeloid cell subpopulation that mediates type 2 T helper cell immune responses. Unexpectedly, we identified a rapid accumulation of eosinophils in 22 human liver grafts after hepatic transplantation. In contrast, no eosinophils were detectable in healthy liver tissues before transplantation. Studies with two genetic mouse models of eosinophil deficiency and a mouse model of antibody-mediated eosinophil depletion revealed exacerbated liver injury after hepatic ischemia and reperfusion. Adoptive transfer of bone marrow–derived eosinophils normalized liver injury of eosinophil-deficient mice and reduced hepatic ischemia and reperfusion injury in wild-type mice. Mechanistic studies combining genetic and adoptive transfer approaches identified a critical role of suppression of tumorigenicity (ST2)–dependent production of interleukin-13 by eosinophils in the hepatoprotection against ischemia-reperfusion–induced injury. Together, these data provide insight into a mechanism of eosinophil-mediated liver protection that could serve as a therapeutic target to improve outcomes of patients undergoing liver transplantation.
INTRODUCTION
During orthotopic liver transplantation, hepatic ischemia and reperfusion (IR) injury can lead to early allograft dysfunction or primary nonfunction and result in chronic graft rejection or recurrence of viral hepatitis (1, 2). A shortage of donor organs had led to the consideration of using “marginal” allografts from elderly donors, severely steatotic livers, or organs retrieved after circulatory death of the donor (3). However, the utility of “marginal” livers is limited because they are highly susceptible to hepatic IR injury. Given that the treatment modalities to prevent or attenuate hepatic IR injury are extremely limited, studying the underlying pathogenesis to uncover therapeutic targets is an area of intense investigation (4–8).
Eosinophils are bone marrow–derived granulocytes that are involved in host defense against parasitic infections and pathogenesis of allergic diseases (9, 10). However, this classic description has been challenged by recent mechanistic studies using new experimental tools. Evidence now suggests that eosinophils play an important role in modulating T cell responses (11–13) and in promoting tissue repair and resolution of inflammation. In acute peritonitis, eosinophils accumulate in inflamed foci and produce anti-inflammatory mediators and those that promote resolution of inflammation (14). In response to skeletal muscle injury, eosinophils infiltrate the tissue and promote fibroadipogenic progenitor cell proliferation and muscle regeneration (15). Studies of the role of eosinophils in liver injury are limited to just a few reports describing contrasting findings. Eosinophils were associated with a pathological role in concanavalin A– and halothane-induced liver injury in mice (16, 17). However, in liver partial hepatectomy and CCl4-induced injury, eosinophils were shown to play a role in promoting liver regeneration (18).
Here, we report a serendipitous observation that eosinophils rapidly accumulate in the liver after orthotopic liver transplantation in humans. Similarly, hepatic eosinophils appear in the liver of mice exposed to IR injury. Using mice with anti–Siglec-F antibody–induced eosinophil depletion and two strains of mice with genetic deletion of eosinophils, we uncovered a highly protective role of eosinophils during liver IR injury. Our studies provide strong evidence for further exploring eosinophils and interleukin-33 (IL-33) signaling through its receptor, suppression of tumorigenicity (ST2), as approaches to both improve the clinical outcomes of hepatic IR injury and expand the donor pool for liver transplantation.
RESULTS
Eosinophils accumulate in the liver during hepatic IR injury
It is known that hepatic macrophages and neutrophils contribute to tissue inflammation and damage during liver IR injury (19–21). Unexpectedly, we observed another population of innate immune cells, eosinophils, that accumulates in the liver as early as 2 to 3 hours after orthotopic liver transplantation in humans (Fig. 1, A and B). In contrast, very few eosinophils can be detected in healthy liver biopsies. We detected eosinophils by immunohistochemical (IHC) staining using an antibody that recognizes human eosinophil peroxidase (EPX). Because the sizes of the paraffin sections are different, to better quantify and compare the abundance of eosinophils in the liver, we counted the number of cells in six random fields (2448 by 1920 pixels per field) of each section and showed the average number of eosinophils per field (P < 0.0001; Fig. 1C). We next attempted to examine whether there is any correlation between the severity of liver IR injury and the eosinophil frequency. There was no correlation when analyzing all the samples as one group (fig. S1A). Because hepatic steatosis increases susceptibility to IR injury, we next separated the samples into two groups based on the degree of macrosteatosis in the donor tissues. In samples with macrosteatosis equal to or less than 5% as evaluated by an independent liver pathologist, we found that higher numbers of eosinophils in the post-IR liver samples correlated with lower serum alanine aminotransferase (ALT) values on day 1 after liver transplantation (fig. S1B). In those samples with macrosteatosis greater than 5%, no correlation was observed (fig. S1C). In a murine model of hepatic IR injury (Fig. 1D), an increase in eosinophils was detected as early as 1 hour after reperfusion by flow cytometry, and the number continued to rise up to 24 hours (P < 0.01 at 1 hour, P < 0.05 at 4 hours, and P < 0.001 at 8 and 24 hours after reperfusion; Fig. 1, E and F). Eosinophil accumulation in the liver was further confirmed by IHC staining (Fig. 1G).
Fig. 1. Eosinophils accumulate in the liver during hepatic IR injury.
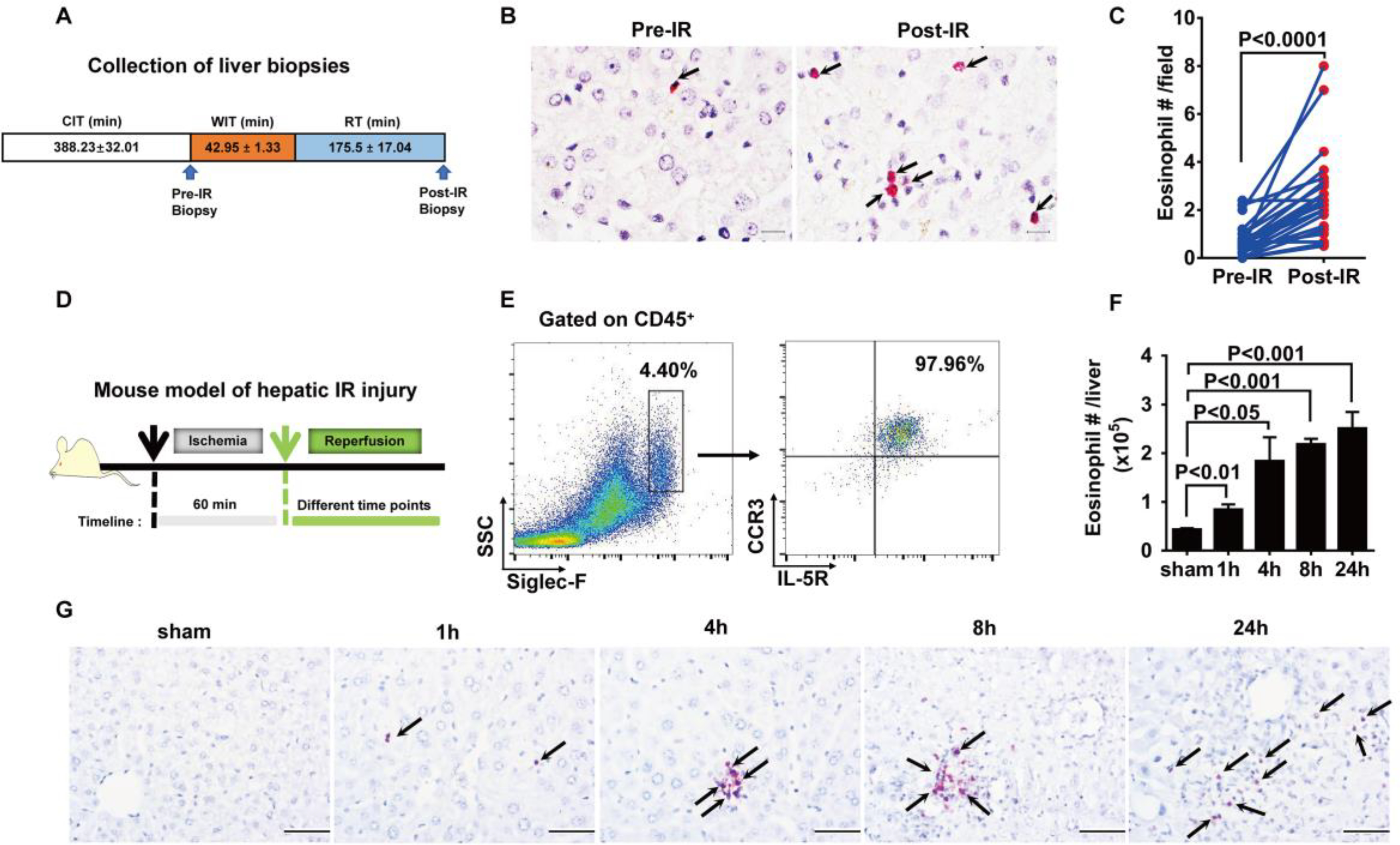
(A) Collection of liver biopsies. (B) Eosinophils in human donor liver biopsies (pre-IR) and biopsies from the same tissues after liver transplantation (post-IR) were detected by IHC staining using anti-human EPX antibody. Scale bars, 25 μm. Red indicates EPX staining, and arrows point to EPX-positive eosinophils. (C) The numbers of eosinophils were quantified (n = 22 patients per group). CIT, cold ischemia time; WIT, warm ischemia time; RT, reperfusion time. (D) Male C57BL/6 mice were subjected to hepatic ischemia for 60 min, and the numbers of eosinophils in the liver were measured at 1, 4, 8, or 24 hours after reperfusion. (E) Representative flow cytometry plots and (F) quantification demonstrate the numbers of eosinophils (SSChiSiglec-F+CCR3+IL-5R+cells) in the liver after IR injury (n = 4 per group). SSC, side scatter. (G) IHC staining of eosinophils using anti-mouse MBP antibody. Arrows point to MBP-positive eosinophils stained red. Scale bars, 200 μm. Images shown are representative of n = 4 mice per time point. A two-tailed paired Student’s t test with Welch’s correction was performed in (C). A one-way ANOVA was performed in (F).
Eosinophils protect against hepatic IR injury
To investigate the functional role of eosinophils in hepatic IR injury, we depleted these cells by an intraperitoneal injection of an anti–Siglec-F antibody as previously reported (22), whereas control mice were treated with an irrelevant immunoglobulin G (IgG) control. As shown in fig. S2, eosinophils were effectively depleted from the bone marrow, blood, and the liver. Eosinophil-depleted mice exhibited much more severe liver IR injury compared with nondepleted mice, as demonstrated by marked increases in ALT (P < 0.0001 and P < 0.05 at 4 and 8 hours after reperfusion, respectively; Fig. 2A), aspartate aminotransferase (AST) (P < 0.05 and P < 0.01 at 4 and 8 hours after reperfusion, respectively; Fig. 2B), and tissue necrosis (P < 0.05; Fig. 2, C and D). Expressions of the proinflammatory cytokines tumor necrosis factor–α (TNF-α) and IL-1β were also increased in the absence of eosinophils (P < 0.001 and P < 0.05; Fig. 2E). As a genetic approach to delete eosinophils, we made use of the PHIL mouse, which expresses a diphtheria toxin A transgene driven by a fragment of the EPX promoter, resulting in eosinophil-specific cytotoxicity and loss of eosinophils (23). With near-absolute loss of eosinophils, the PHIL mice have normal numbers of B cells, T cells, and even mast cells and basophils, which share a direct common precursor with eosinophils (23). Mirroring our studies with antibody-mediated depletion of eosinophils, PHIL mice developed worsened hepatic IR injury compared with wild-type (WT) littermates, evident by increased serum concentrations of ALT (P < 0.005; Fig. 2F), AST (P < 0.05; Fig. 2G), and hepatocyte necrosis (P < 0.0001 and P < 0.0005; Fig. 2, H and I). Moreover, adoptive transfer of WT bone marrow–derived eosinophils (bmEos) to PHIL mice at 24 hours before surgically induced liver ischemia normalized their phenotype to that of the WT mice (Fig. 2, F to I). Furthermore, we transferred hepatic eosinophils isolated from the livers of WT mice 24 hours after hepatic IR injury into PHIL mice and then surgically induced liver ischemia. As shown in fig. S3, adoptive transfer of eosinophils from IR-injured WT livers reduced liver IR injury in eosinophil-deficient PHIL mice.
Fig. 2. Eosinophils protect against hepatic IR injury.
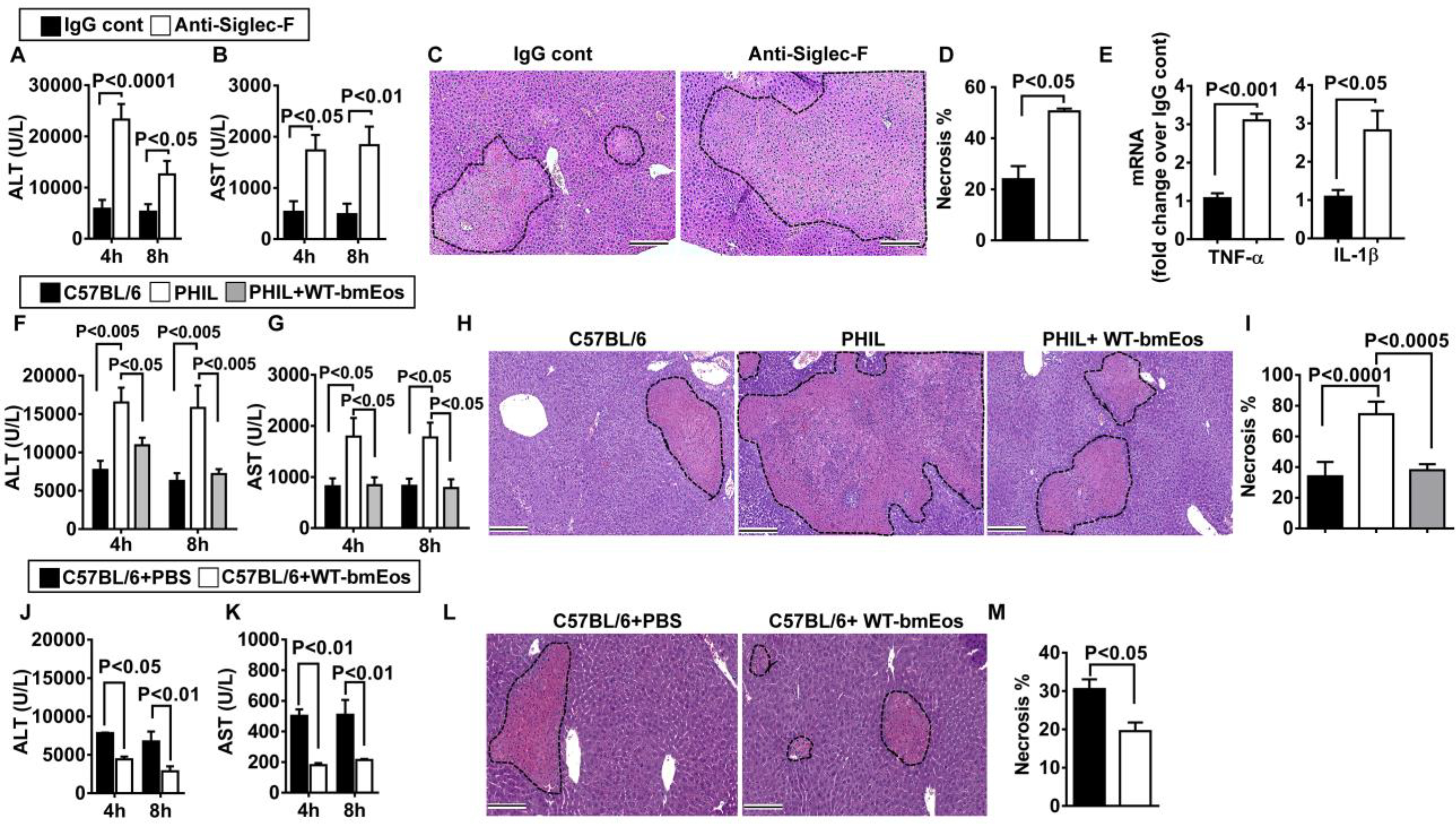
(A to E) Eosinophils were depleted by anti–Siglec-F antibody in male C57BL/6 mice, and control mice were treated with IgG (n = 5 in IgG control and n = 3 in anti–Siglec-F treatment group), and serum concentrations of ALT (A) and AST (B) were measured at 4 and 8 hours after reperfusion. (C) Liver necrosis (scale bars, 200 μm) was evaluated and quantified (D) at 24 hours after reperfusion. (E) mRNA expression of cytokines in liver nonparenchymal cells was measured at 4 hours after reperfusion (n = 6 mice per group). (F to I) WT littermates, eosinophil-deficient PHIL mice, and PHIL mice receiving WT-bmEos (2 × 107) were subjected to hepatic IR injury (n = 4 mice per group). Serum concentrations of ALT (F) and AST (G) were measured at 4 and 8 hours after reperfusion. (H) Liver necrosis (scale bars, 200 μm) was examined and quantified (I) at 24 hours after reperfusion. (J to M) WT C57BL/6 mice (n = 4 mice per group) received WT-bmEos or PBS as a control. Serum concentrations of ALT (J) and AST (K) were measured at 4 and 8 hours after reperfusion. (L) Liver necrosis (scale bars, 200 μm) was examined and quantified (M) 24 hours after reperfusion. A two-tailed unpaired Student’s t test with Welch’s correction was performed in (D), (E), and (M). A one-way ANOVA was performed in (I). A two-way ANOVA was performed in (A), (B), (F), (G), (J), and (K).
To further corroborate these results, we used another strain of eosinophil-deficient mice (ΔdblGata1). The ΔdblGata1 mice carry a deletion of a high-affinity Gata1-binding site in the promoter of Gata-1, which is critical for eosinophil development (24). Similar to PHIL mice, ΔdblGata-1 mice also developed exacerbated liver IR injury compared to WT Balb/c mice (fig. S4). To investigate whether eosinophils confer further protection in WT mice, we adoptively transferred WT-bmEos to WT mice 24 hours before ischemia surgery. The recipient mice showed reductions in ALT (P < 0.05 and P < 0.01 at 4 and 8 hours after reperfusion, respectively; Fig. 2J), AST (P < 0.01; Fig. 2K) concentrations, and liver necrosis (P < 0.05; Fig. 2K) relative to control mice that did not receive bmEos. Together, these data revealed a role for eosinophils in mediating liver protection during hepatic IR injury.
ST2 plays a critical role in the hepatoprotective effect of eosinophils
To characterize the molecular mechanisms accounting for the protective function of eosinophils, we compared gene expression profiles between liver nonparenchymal cells from eosinophil-depleted and nondepleted mice at 4 hours after reperfusion. As expected, nonparenchymal cells from eosinophil-depleted mice showed decreased expression of eosinophil-related genes, such as EPX, IL-5, and IL-13 (fig. S5A). We found a reduction of ST2 expression in eosinophil-depleted nonparenchymal cells, and this was validated by quantitative polymerase chain reaction (qPCR; P < 0.05; fig. S5B). ST2, also named as IL-1 receptor like-1 (IL-1rl1), is the receptor for IL-33. ST2 is known to be highly expressed on type 2 T helper T cells (TH2), mast cells, and eosinophils (25, 26). Flow cytometric analysis of ST2 protein expression demonstrated that almost all eosinophils (Siglec-F+) expressed ST2 (Fig. 3A). Eosinophils accounted for most of the ST2+ cells in the liver during IR injury. In contrast, there were reduced proportions of ST2+ cells in other cell types of the liver, including neutrophils, endothelial cells, macrophages, T and B lymphocytes, natural killer cells, and natural killer T cells (fig. S5C). Group 2 innate lymphoid cells (ILC2s) are known to express ST2. However, the number of ILC2 cells detectable by flow cytometry (Lin-Sca+CD127+KLRG1+) was extremely low in the liver and it did not increase after liver IR injury. These data led to our hypothesis that ST2 plays an important role in the protective function of eosinophils during hepatic IR injury. Compared with WT mice, ST2−/− mice had much fewer eosinophils in the liver (Fig. 3B) and developed increased IR-related tissue damage. ST2−/− mice also had higher serum concentrations of ALT (P < 0.05) and AST (P < 0.05) and more severe liver necrosis (P < 0.001; Fig. 3, C to F). To determine whether the exacerbated IR injury in ST2−/− mice was related to eosinophils, we transferred WT-bmEos to ST2−/− mice at 24 hours before hepatic IR. As shown in fig. S6, more total eosinophils accumulated in the liver after adoptive transfer of naïve bmEos as compared to mice not receiving bmEos. Further, we adoptively transferred naïve bmEos or bmEos after stimulation with the ST2 ligand, IL-33, 24 hours before hepatic IR. We found that hepatic IR injury in the recipient ST2−/− mice was attenuated by adoptive transfer of either naïve or IL-33–stimulated bmEos derived from WT, but not from ST2−/− mice (P < 0.0001; Fig. 3, G to J), suggesting that ST2 function is eosinophil intrinsic.
Fig. 3. ST2 plays a critical role in the hepatoprotective effect of eosinophils.
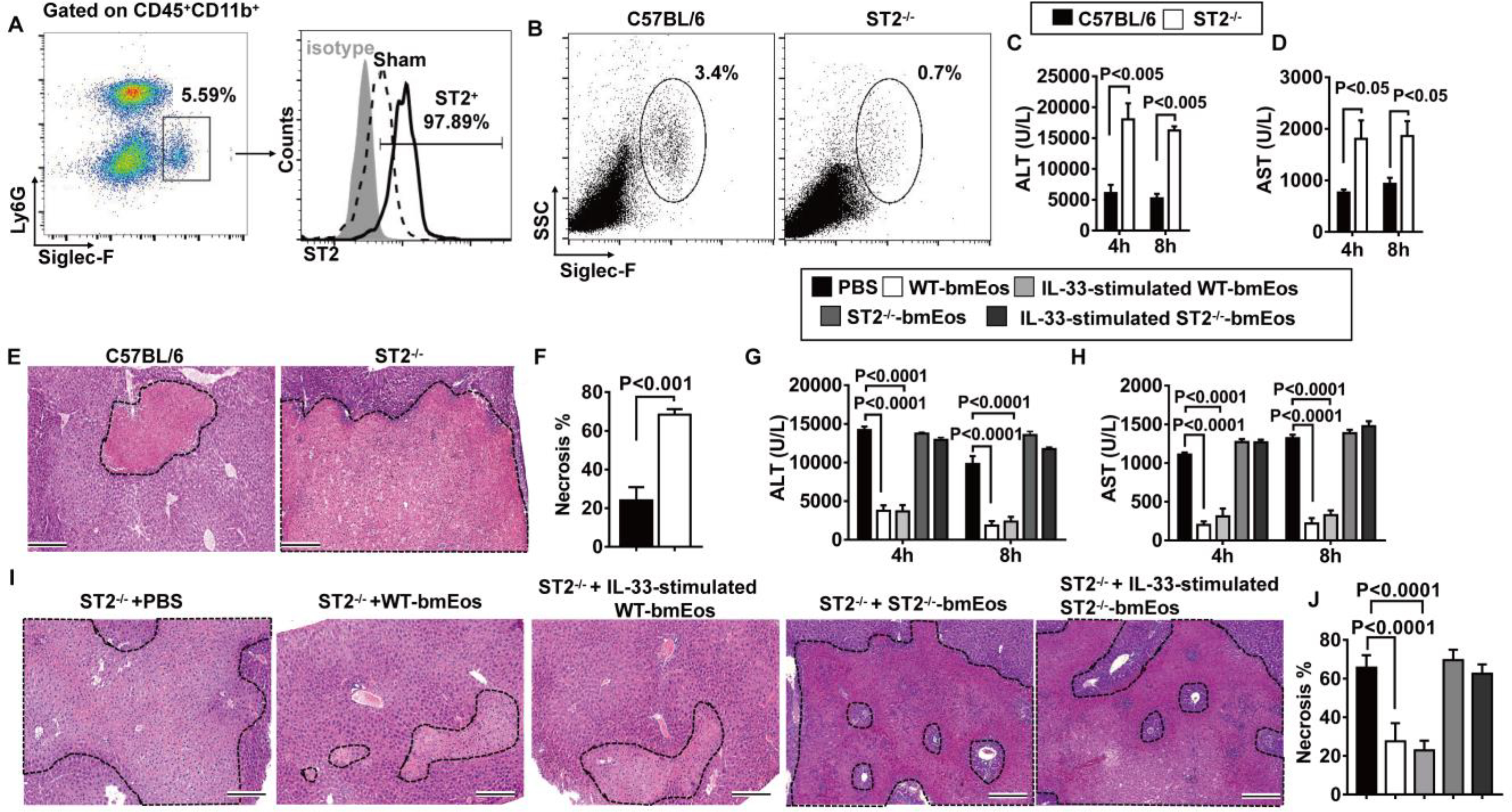
(A) Liver nonparenchymal cells were isolated at 24 hours after reperfusion. ST2 expressions on eosinophils were measured by flow cytometry. Data shown represent samples from four mice. (B to F) WT and ST2−/− mice were subjected to hepatic IR surgery (n = 4 mice per group). Hepatic eosinophil accumulation, shown as proportions among CD45+ nonparenchymal cells, was measured at 4 hours after reperfusion (B). Serum concentrations of ALT (C) and AST (D) were measured at 4 and 8 hours after reperfusion. Liver necrosis (scale bars, 200 μm) was examined at 24 hours after reperfusion (E) and quantified (F). (G to J) ST2−/− mice were adoptively transferred with WT-bmEos, IL-33–stimulated WT-bmEos, ST2−/−-bmEos, IL-33–stimulated ST2−/−-bmEos, or PBS as control at 24 hours before hepatic IR surgery (n = 3 in the ST2−/− + PBS, n = 4 in all remaining groups). Serum concentrations of ALT (G) and AST (H) were measured at 4 and 8 hours after reperfusion. Liver necrosis (scale bars, 200 μm) was examined at 24 hours after reperfusion (I) and quantified (J). A two-tailed unpaired Student’s t test with Welch’s correction was performed in (F). A two-way ANOVA was performed in (C), (D), (G), (H), and (J).
IL-33 signaling through ST2 in eosinophils protects against hepatic IR injury
The ligand of ST2, IL-33, is known as an alarmin and is released during tissue damage. To investigate the involvement of IL-33, we first measured the serum concentration of IL-33 after hepatic IR injury. As shown in Fig. 4A, IL-33 is elevated after hepatic IR injury in WT and ST2−/− mice (P < 0.01 at 4 hours after reperfusion in WT mice, P < 0.01 at 4 hours, P < 0.001 at 8 hours, and P < 0.0001 at 24 hours after reperfusion in ST2−/− mice). Moreover, similar to ST2−/− mice, IL-33−/− mice had impaired eosinophil accumulation in the liver (Fig. 4B) and developed exacerbated hepatic IR injury, evident from increased values of ALT (P < 0.05; Fig. 4C), AST (P < 0.01; Fig. 4D), and worsened histology (P < 0.0001; Fig. 4, E and F), suggesting a protective role for IL-33. To address whether IL-33 protects the liver through ST2 signaling in eosinophils, we adoptively transferred naïve WT-bmEos or IL-33–stimulated WT-bmEos to IL-33−/− mice. Control mice were injected with phosphate-buffered saline (PBS). The data showed that only IL-33–stimulated WT-bmEos, but not naïve WT-bmEos, could reduce liver injury in IL-33−/− mice (P < 0.0005 for ALT concentrations, P < 0.0001 and P < 0.0005 for AST concentrations, and P < 0.01 for the percentage of necrosis; Fig. 4, G to J). These data, together with those from the adoptive transfer of bmEos to ST2−/− mice, suggest that the IL-33–ST2 axis is critically involved in the hepatoprotective function of eosinophils.
Fig. 4. IL-33 signaling through ST2 in eosinophils protects against hepatic IR injury.
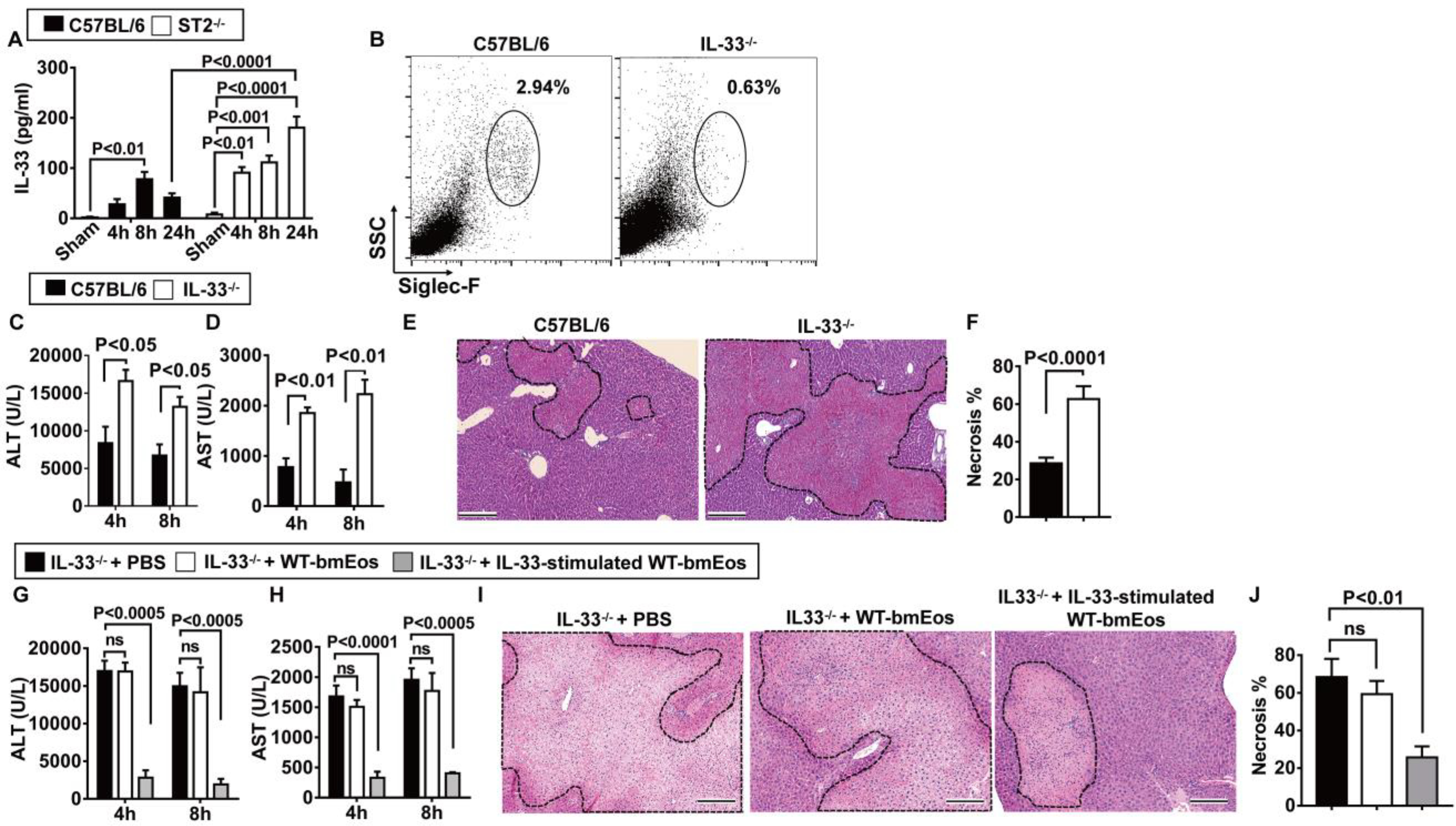
(A) Serum concentrations of IL-33 were measured in C57BL/6 and ST2−/− mice at various times after reperfusion (n = 4 in sham group, n = 6 in all remaining groups). (B to F) WT and IL-33−/− mice were subjected to hepatic IR surgery. Hepatic eosinophil accumulation, shown as proportions among CD45+ nonparenchymal cells, was measured at 4 hours after reperfusion (n = 8 in WT and n = 6 in IL-33−/− group) (B). Serum concentrations of ALT (C) and AST (D) were measured at 4 and 8 hours after reperfusion. Liver necrosis (scale bars, 200 μm) was examined at 24 hours after reperfusion (E) and quantified (F) (n = 4 in WT and n = 3 in IL-33−/− group). (G to J) IL-33−/− mice were adoptively transferred with WT-bmEos, IL-33–stimulated WT-bmEos, or PBS as control at 24 hours before hepatic IR surgery (n = 3 in IL-33−/− + PBS, n = 4 in all remaining groups). Serum concentrations of ALT (G) and AST (H) were measured at 4 and 8 hours after reperfusion. Liver necrosis (scale bars, 200 μm) was examined at 24 hours after reperfusion (I) and quantified (J). A two-tailed unpaired Student’s t test with Welch’s correction was performed in (F). A one-way ANOVA was performed in (J). A two-way ANOVA was performed in (A), (C), (D), (G), and (H).
Eosinophils suppress neutrophils through IL-13 production
Our microarray analyses revealed that expression of myeloperoxidase (MPO) was higher in liver nonparenchymal cells from eosinophil-depleted mice as compared to nondepleted mice (fig. S5A), which was confirmed by qPCR analysis (P < 0.05; fig. S7A). Furthermore, IHC staining for MPO+ cells (P < 0.001; Fig. 5, A and B) and flow cytometric analysis of Ly6G+ cells (P < 0.01; Fig. 5, C and D) revealed that neutrophils were much more abundant in the liver of anti–Siglec-F–treated compared with control IgG–treated mice after IR injury. Similarly, the numbers of MPO+ cells were higher in eosinophil-deficient PHIL and ΔdblGata1 compared with their WT counterparts after IR injury (P < 0.0001; fig. S7B). Neutrophils are known to play a critical role in causing liver inflammation and hepatocyte death during IR injury (19, 20). The pathological effect of neutrophils in vivo is principally mediated by their production of oxidants including hydrogen peroxide and hypochlorous acid (HOCl) (27). Using a monoclonal antibody that recognizes HOCl protein adducts, we detected an increase of HOCl-modified proteins in hepatocytes of anti–Siglec-F–treated than control IgG-treated mice (fig. S7C), as well as in PHIL and ΔdblGata1 mice compared with their WT counterparts (fig. S7, D and E). These data together suggest that the protective role of eosinophils is related to the suppression of neutrophils during hepatic IR injury.
Fig. 5. Eosinophils suppress neutrophils through IL-13 production.
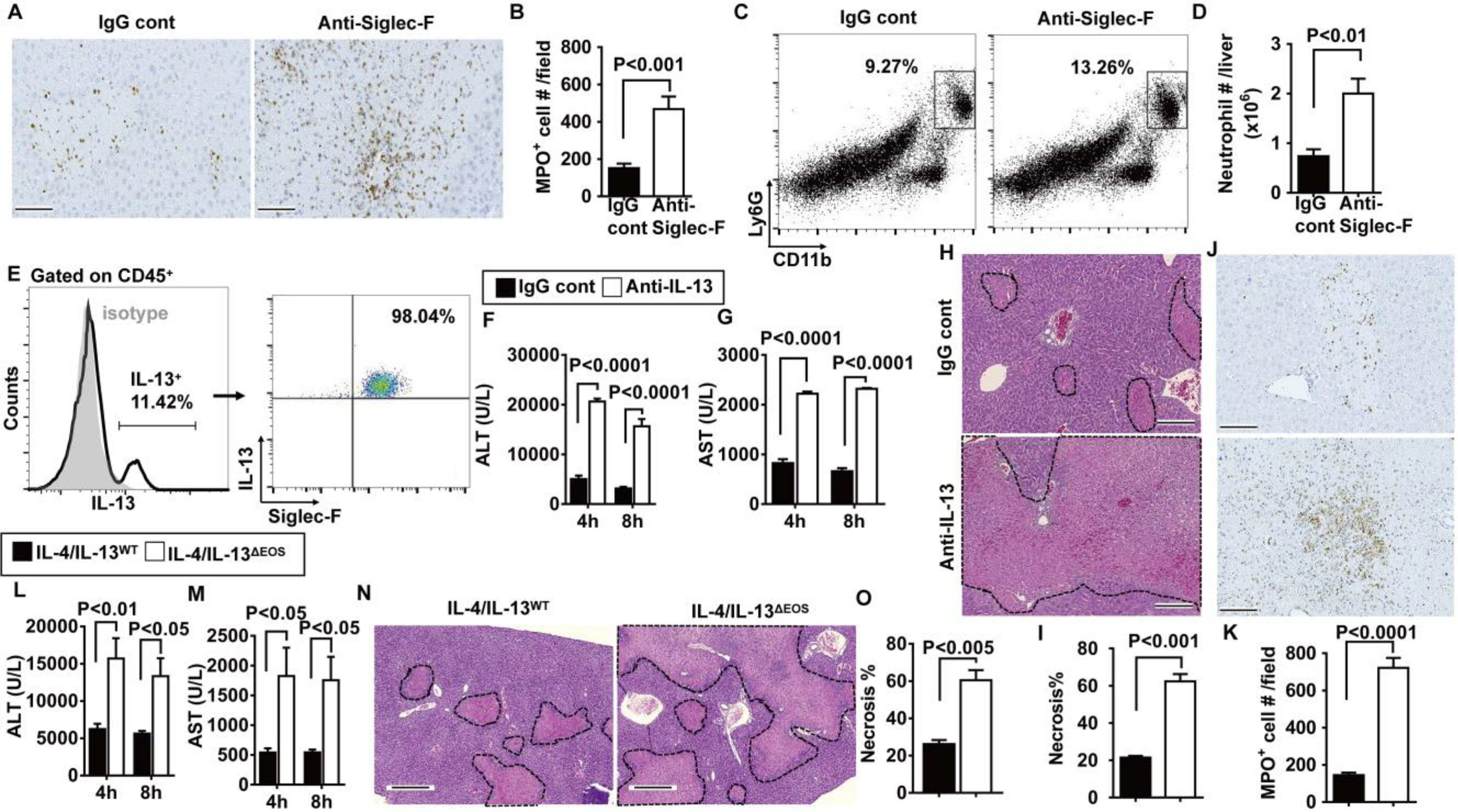
(A) Eosinophils were depleted by anti–Siglec-F antibody in male C57BL/6 mice at 24 hours before hepatic IR surgery, and neutrophils were stained for MPO (brown; scale bars, 200 μm) and (B) quantified in the liver (n = 3 mice per group). (C) Representative flow cytometry plots and (D) quantification of Ly6G+CD11b+ cells at 24 hours after reperfusion are shown (n = 4 mice per group). (E) Liver nonparenchymal cells were isolated from WT mice at 4 hours after reperfusion and stained for intracellular IL-13. IL-13–positive cells were gated, and the proportions of eosinophils (Siglec-F+) that express IL-13 among total IL-13+ cells are shown (n = 4 mice per group). (F to K) WT mice were injected with anti–IL-13–neutralizing antibody or IgG control 1 hour before IR surgery (n = 3 mice per group). Serum concentrations of ALT (F) and AST (G) were measured at 4 and 8 hours after reperfusion. Liver necrosis (scale bars, 200 μm) was evaluated (H) and quantified (I). Neutrophil accumulation was examined at 24 hours after reperfusion by myeloperoxidase (MPO) staining (brown; scale bars, 200 μm) (J) and quantified (K). (L to N) IL-4/IL-13ΔEOS mice and IL-4/IL-13WT littermates were subjected to IR surgery (n = 3 mice per group). Serum concentrations of ALT (L) and AST (M) were measured at 4 and 8 hours after reperfusion. Liver necrosis (scale bars, 200 μm) was examined (N) and quantified (O) at 24 hours after reperfusion. A two-tailed unpaired Student’s t test with Welch’s correction was performed in (B), (D), (I), (K), and (O). A two-way ANOVA was performed in (F), (G), (L), and (M).
It is known that eosinophils produce IL-4 and IL-13 (28, 29). Therefore, we investigated whether these cytokines may confer the hepatoprotective effects of eosinophils during IR injury. Our data demonstrated that eosinophils account for more than 90% of IL-13–expressing nonparenchymal cells after liver IR injury (Fig. 5E). Eosinophils also express IL-4 but are not the main cellular source during liver IR injury (fig. S8A). IL-4 has been implicated in liver regeneration (18); however, our data showed that neutralizing IL-4 did not affect hepatic IR injury (fig. S8, B to E). In contrast, neutralizing IL-13 increased serum ALT and AST concentrations (P < 0.0001; Fig. 5, F and G), hepatocyte necrosis (P < 0.001; Fig. 5, H and I), and the number of neutrophils in the liver compared to IgG-treated control mice (P < 0.0001; Fig. 5, J and K). On the contrary, injection of recombinant mouse IL-13 to WT mice reduced hepatic IR injury (fig. S9). To determine whether IL-13 produced by eosinophils, rather than other cells, plays a critical protective role, we made use of a mouse with an eosinophil-specific deletion of IL-4 and IL-13 Δ (IL-4/IL-13 EOS, IL-4/IL-13fl/fl × eoCre+/−). The data demonstrated that hepatic IR injury was increased in these mice compared with WT littermates (IL-4/IL-13WT, IL-4/IL-13fl/fl × eoCre−/−), as measured by increased serum ALT (P < 0.01 and P < 0.05 at 4 and 8 hours after reperfusion, respectively; Fig. 5L) and AST (P < 0.05; Fig. 5M) concentrations and hepatocyte necrosis (P < 0.005; Fig. 5, N and O).
IL-33 stimulates eosinophils to produce IL-13 through ST2
The findings that eosinophils are the predominant source of IL-13, IL-13 mediates the protective effects of eosinophils, and the IL-33/ST2 axis is necessary in promoting the protective function of eosinophils led to our hypothesis that IL-33, acting through ST2, triggers IL-13 production by eosinophils. To examine this hypothesis, we stimulated bmEos generated from WT and ST2−/− mice with IL-33. The data showed that IL-33 induced the up-regulation of both mRNA and protein of IL-13 in WT-bmEos (P < 0.05; Fig. 6B), but not in ST2−/− bmEos (Fig. 6, A and B). Similarly, eosinophils freshly isolated from the bone marrow of WT mice, but not ST2−/− mice, responded to IL-33 stimulation to produce IL-13 (P < 0.0001; Fig. 6, C and D). Consistently, after IR injury, hepatic IL-13 concentrations were lower in eosinophil-depleted mice compared with IgG-treated control mice (P < 0.0001; Fig. 6E). Moreover, ST2−/− and IL-33−/− mice had reduced liver IL-13 concentrations as compared to WT mice (P < 0.01; Fig. 6F). Collectively, these data demonstrate that the hepatoprotective function of eosinophils is mediated by IL-33/ST2 signaling, which triggers IL-13 production and suppresses neutrophils during liver IR injury (fig. S10).
Fig. 6. -mediated IL-33 signaling stimulates eosinophils to produce IL-13.
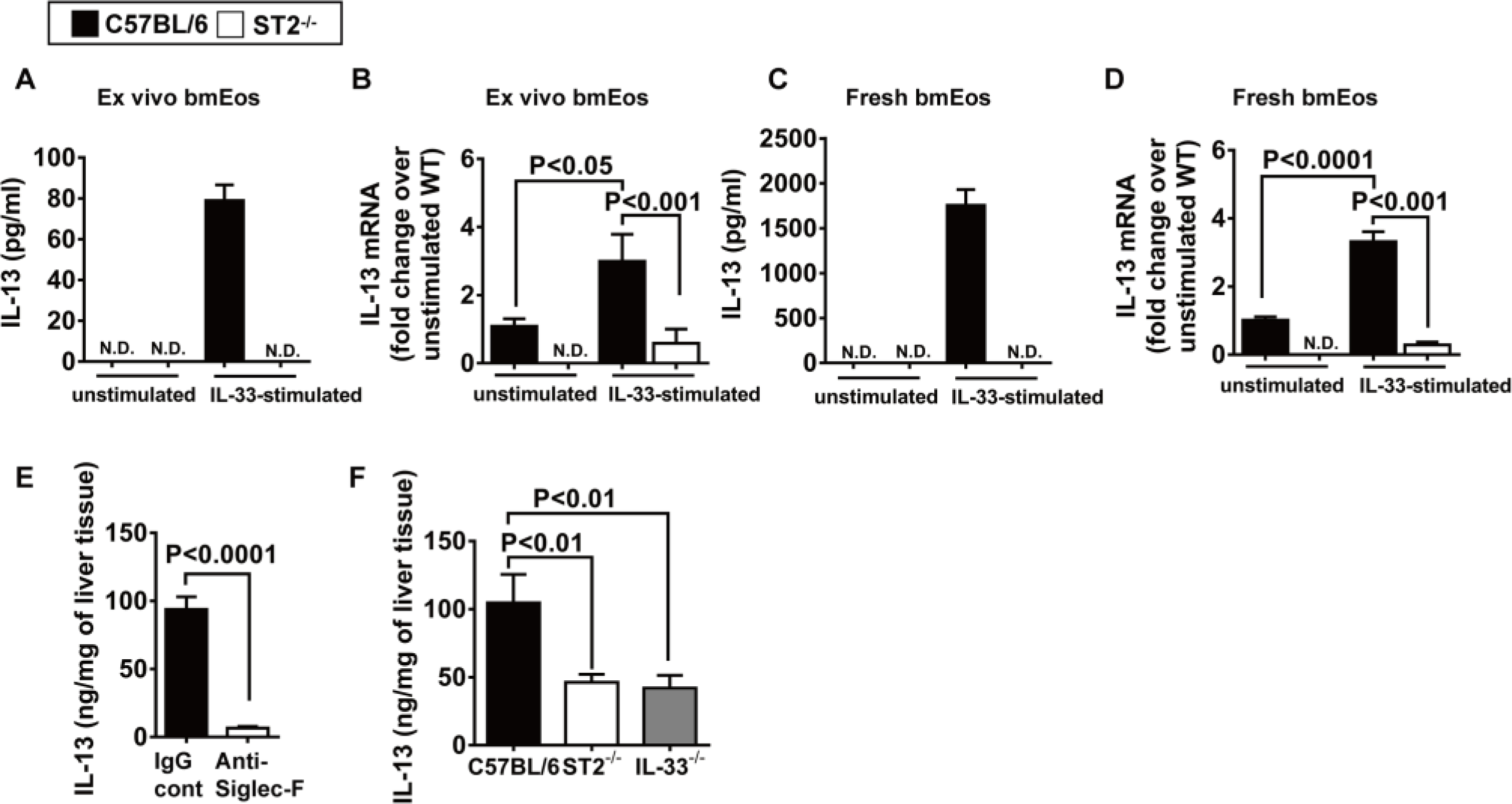
ST2 (A and B) bmEos were generated ex vivo from C57BL/6 and ST2−/− mice (n = 5 mice per group). IL-13 concentrations in culture supernatants (5 × 106 cells per well) (A) and mRNA expression (B) were measured after stimulation with recombinant mouse IL-33 for 24 hours by ELISA and qPCR, respectively. N.D. indicates not detectable. (C and D) Eosinophils (1 × 106 cells per well) were freshly isolated from the bone marrow of C57BL/6 and ST2−/− mice (n = 4 mice per group) and stimulated with IL-33 for 24 hours. IL-13 protein in culture supernatants (C) and mRNA (D) expression were measured by ELISA and qPCR, respectively. (E) WT C57BL/6 mice were treated with anti–Siglec-F antibody to deplete eosinophils or IgG as control at 24 hours before hepatic IR surgery (n = 4 mice per group). IL-13 concentration in the livers was measured at 4 hours after reperfusion by ELISA. (F) C57BL/6, ST2−/−, and IL-33−/− mice were subjected to IR surgery (n = 5 in C57BL/6, n = 7 in ST2−/−, and n = 5 IL-33−/− mice). IL-13 concentrations in the livers were measured at 4 hours after reperfusion by ELISA. A two-tailed unpaired Student’s t test with Welch’s correction was performed in (E). A one-way ANOVA was performed in (F). A two-way ANOVA was performed in (A), (B), (C), and (D).
DISCUSSION
The current studies demonstrate that eosinophils accumulate rapidly in the liver after orthotopic liver transplantation or upon hepatic IR. Eosinophil accumulation in the liver has been observed in patients with chronic hepatitis C infection and those with drug-induced liver injury (30–32), but the implications of this observation are not known. The role of eosinophils in liver injury was examined in very few studies, which yielded opposite results (16–18). A study of concanavalin A–induced hepatitis in mice demonstrated that eosinophil depletion by an anti-CCR3 antibody reduced liver injury, suggesting a pathologic role of eosinophils in this experimental model of immune-mediated hepatitis (16). Another study using eosinophil-deficient ΔdblGata1 mice showed that these cells contribute to halothane-induced liver injury in mice (17). However, this finding is contradictory to the correlation of hepatic eosinophils accumulation with a better prognosis of patients with drug-induced liver injury (31, 32). A more recent study using murine models of partial hepatectomy and CCl4-induced liver injury demonstrated a critical protective role of eosinophils in promoting liver regeneration through the secretion of IL-4 (18). Our experiments using genetic models of eosinophil deficiency (PHIL or ΔdblGata1 mice) or antibody-mediated eosinophil depletion revealed exacerbated injury after hepatic IR. The data provided compelling evidence to support a critical hepatoprotective function of eosinophils during liver IR injury.
A limitation of the present study is that our mouse model of hepatic IR injury only simulates warm IR-induced injury during liver transplantation. Although this model has been widely used to understand the contribution of leukocytes, recruited during warm reperfusion, it does not take into consideration the impact of cold storage on organ damage. We also recognize that the number of tissue samples from patients undergoing orthotopic liver transplantation is relatively small. Future studies with a larger sample size, which allows more detailed analyses, are warranted.
Eosinophils were thought to be cytotoxic cells involved in host defense against parasitic infections and in the pathogenesis of allergic diseases such as asthma (9, 10). However, emerging evidence supports a paradigm-shifting hypothesis on the biological functions of eosinophils, which proposes that rather than a destructive end-stage effector cells, eosinophils are an important regulator of local immunity and remodeling/repair (33). For example, eosinophils can function in vivo as antigen-presenting cells. It is reported that airway eosinophils are able to process inhaled antigens, migrate to the draining lymph nodes, and stimulate CD4+ T cell responses (12). Eosinophils tend to suppress TH1 responses either directly or indirectly through regulating dendritic cells (13, 34). Tissue infiltration of eosinophils has been observed in various disease models, and the functions of eosinophils in these conditions are beginning to be understood (14, 15, 35). In zymosan-induced peritonitis, eosinophil-derived protectin D1 plays an indispensable role in promoting the resolution of acute inflammation (14). A study of muscle damage demonstrated a rapid recruitment of eosinophils, which were required for muscle regeneration through activating resident fibro/adipocyte progenitor cells (15). In a mouse model of diet-induced obesity, it was shown that eosinophils migrated to white adipose tissues and promoted alternative action of macrophages, thereby contributing to the maintenance of glucose homeostasis (35). In the current study, we observed an accumulation of eosinophils in the liver after IR injury. Our data demonstrated that eosinophils, through releasing IL-13 and inhibiting neutrophils, attenuated hepatic IR injury.
We found that IL-33 was released during liver IR injury and its receptor, ST2 was expressed by eosinophils. Moreover, the IL-33/ST2 signaling in eosinophils was critical in mediating the hepatoprotective effects of these cells. Although well known as a link to allergic diseases, emerging evidence supports an important function of the IL-33/ST2 axis in limiting tissue inflammation and injury (36–39). For example, IL-33/ST2 plays a critical role in the expansion and activation of regulatory T cells, thereby attenuating intestinal inflammation (36), preventing obesity-associated inflammation (37), inhibiting graft-versus-host disease (40), and promoting tissue repair of the lung, skeletal muscle, kidney, liver, and heart (38, 39, 41–43). In terms of the role of IL-33/ST2 in hepatic IR injury, there are currently two publications. The first study (44) demonstrated that the administration of recombinant IL-33 reduced but anti-ST2 worsened hepatic IR injury, indicating a protective function of ST2. In contrast, the second study (45) showed that recombinant IL-33 exacerbated hepatic IR injury and that IL-33 or ST2 deletion attenuated liver injury, suggesting a pathological function of ST2. Our finding of a protective role of ST2 is in agreement with the first study but contradictory to the second study. The difference in background strains of the ST2−/− mice used in the second study (Balb/c background) and ours (C57BL/6 background) could contribute to the discrepancy of the results. The second study shows that ST2 was expressed by neutrophils and triggered neutrophil extracellular traps, thereby causing liver IR injury (45). However, we could not detect ST2 expression on neutrophils. Other studies have also reported that neutrophils do not express ST2 (46, 47). Regarding how eosinophils protect against hepatic IR injury, our data revealed a previously unrecognized interplay between eosinophils and neutrophils. When eosinophils are absent or reduced (in PHIL, ΔdblGata1, IL-33−/−, ST2−/−, and anti–Siglec-F–treated mice), the number of neutrophils in the liver is profoundly increased, coinciding with an aggravated hepatic IR injury. The in vitro and in vivo experiments demonstrate that through ST2, IL-33 stimulates eosinophil production of IL-13, which suppresses neutrophils.
The present study uncovered a previously unrecognized hepatoprotective function of eosinophils and implicated the IL-33/ST2–dependent IL-13 production in mediating the protective effect. The findings support further exploration of eosinophils and IL-33/ST2 signaling as candidate therapeutic targets to improve the outcomes of liver surgery and transplantation.
MATERIALS AND METHODS
Study design
This study was designed to determine the functional role of eosinophils in liver IR injury in liver transplant patient samples and three different mouse models of eosinophil deficiency. All patient sample collection and use were approved by the Committee for the Protection of Human Subjects (CPHS) at the University of Texas Health Science Center at Houston (UTHealth; CPHS protocol HSC-MS-12–0652). For in vivo studies, study protocols were approved by the Center for Laboratory Animal Medicine and Care at UTHealth. For patient samples, we included all that were available to us. Sample size for animal studies was determined by statistical analysis of variance and on the basis our experience with similar studies. The sample size (n) for each experimental group is indicated in the figure legends and is between three and eight mice per group. All of the in vivo experiments were replicated three or more times by two experimentalists independently. All histological and IHC samples were blinded before the images were taken and quantified.
Human liver tissues
Liver samples were obtained from patients undergoing orthotopic liver transplantation (tables S1 and S2). Liver biopsies were taken at the conclusion of cold ischemia time during back table preparation of the cadaveric liver allograft (Fig. 1A, pre-IR). A second biopsy was taken immediately before closure of the abdomen after drain placement (warm ischemia time) (Fig. 1A, post-IR). Total reperfusion time is defined as the time from portal vein perfusion to abdominal closure at the conclusion of the procedure. Liver histopathology, including the degrees of macrosteatosis, was evaluated and reported independent of the present study by trained liver pathologists.
Animals and hepatic IR surgery
Balb/c and ΔdblGata1 mice were purchased from the Jackson laboratory. Breeders of IL-33−/− and ST2−/− mice were obtained from J. Brown (University of Colorado Anschutz Medical Campus) and S. Akira (Osaka University) via material transfer agreements (MTAs). Both IL-33−/− and ST2−/− mice were on a C57BL/6N genetic background. Therefore, we used C57BL/6N mice as WT controls, and breeding pairs were purchased from Taconic Biosciences. Both WT and knockout mouse colonies have been maintained in UTHealth animal facility for the past 3 years. Breeders of PHIL (23) and IL-4/IL-13fl/fl × (48) eoCre (backcrossed to C57BL/6J) were obtained from E. Jacobsen (Mayo Clinic Arizona) via MTAs. All colonies were established and maintained at the UTHealth animal facility. All experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee at UTHealth.
Surgical induction of hepatic IR or sham surgery was performed in 10- to 12-week-old male mice as previously reported (49). Briefly, mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg) and placed on their back on a temperature-controlled (set at 37°C) surgery table to keep the body temperature of the mice at 33°C. A midline laparotomy was performed and an atraumatic clip was used to interrupt blood supply to the left lateral and median lobes of the liver. After 60 min of partial hepatic ischemia, the clip was removed to initiate hepatic reperfusion. Sham control mice underwent the same protocol without vascular occlusion. During the process, mice were kept well hydrated with warm saline. Mice were euthanized at indicated time points after reperfusion to collect blood and the ischemic portions of the liver tissues for further analysis.
For eosinophil depletion, mice were injected intraperitoneally with anti-mouse Siglec-F antibody (10 μg per mouse; clone E50–2440, BD Pharmingen) or control IgG at 24 hours before ischemia. For IL-13 neutralization, mice were injected intravenously with anti-mouse IL-13 antibody (1 μg per mouse; AF-413-NA, R&D Systems) or control IgG at 1 hour before ischemia. For IL-4 neutralization, mice were intravenously injected with anti-mouse IL-4 antibody (10 μg per mouse; clone: 11B11, BioLegend) or control IgG at 1 hour before ischemia. For exogenous IL-13 treatment, mice were injected intravenously with recombinant mouse IL-13 (2 μg per mouse; PeproTech) at 1 hour before ischemia.
For adoptive transfer of eosinophils, WT-bmEos were prepared as described below and intravenously injected into mice (2 × 107) at 24 hours before IR surgery. In some experiments, the cells were labeled with 20 μM carboxyfluorescein diacetate succinimidyl ester (Invitrogen) for 8 min at 37°C or treated with IL-33 (10 ng/ml; PeproTech) for 24 hours before adoptive transfer.
Measurements of liver injury and isolation of liver nonparenchymal cells
Serum concentrations of ALT and AST were detected using the diagnostic assay kits from Teco Diagnostics following the manufacturer’s protocols. Tissue sections from the median and left lobes of the liver were collected. Tissues were fixed in 10% formalin overnight and embedded in paraffin, cut in 4-μm sections, and stained with hematoxylin and eosin.
Liver nonparenchymal cells were isolated following a previously established method (50). Briefly, liver tissues were perfused in situ with a perfusion buffer [1× Hank’s balanced salt solution (HBSS)], followed by digestion buffer (1× HBSS supplemented with 0.04% collagenase type I; Sigma-Aldrich). After digestion, the liver was disrupted in anticoagulant-citrate-dextrose solution, and the cells passed through a 70-μm cell strainer. Subsequently, the cells were fractionated using 30% (w/v) Nycodenz (Axis Shield Poc AS) at 1.155 g/ml to yield liver nonparenchymal cells, which were further purified using 35% Percoll (Sigma-Aldrich) at 1.04 g/ml. Red blood cells were lysed in ACK buffer (150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM Na2EDTA, at pH 7.2 to 7.4).
Flow cytometry analysis
Cultured or isolated cells were stained with antibodies for 30 min on ice and analyzed using a CytoFLEX flow cytometer (Beckman Coulter). Data were analyzed with FlowJo v10.7 according to the manufacturers’ protocols. Dead cells were excluded by staining with blue fluorescent reactive dye (1:100; Invitrogen, #2176884). Anti-mouse F4/80 (1:100; BM8), anti-mouse IL-4 (1:200; 11B11), anti-mouse CD193 (1:100; CCR3, J073E5), anti-mouse CD125 (1:100; IL-5Ra, DIH37), and anti-mouse CD11c (1:100; N418) were from BioLegend. Anti-mouse CD19 (1:100; ebio1D3), anti-mouse NK1.1 (1:100; PK136), anti-mouse IL-13 (1:200; eBio13A), and anti-mouse CD45 (1:100; 30-F11) were from eBioscience (Thermo Fisher Scientific). Anti-mouse CD3 (1:100; 145–2C11), anti-mouse Ly6G (1:100; 1A8), anti-mouse CD11b (1:100; M1/70), anti-mouse Siglec-F (1:100; E50–2440), and anti-mouse CD146 (1:100; ME-9F1) were from BD Pharmingen. For intracellular staining, cells were treated with brefeldin A (BioLegend) and were stained and analyzed by using the Fixation and Permeabilization Kit (Invitrogen) following the manufacturer’s recommended protocols.
IHC staining and enzyme-linked immunosorbent assay
IHC staining was performed using paraffin-embedded sections to determine the expression of MPO and HOCl-protein adducts in the liver. Briefly, endogenous peroxidases were inactivated by 3% hydrogen peroxide. Nonspecific signals were blocked using 2.5% goat serum. Rabbit anti-human/mouse MPO antibody (SKU:023, Biocare Medical) and a monoclonal antibody that detects HOCl-protein adducts (clone: 2D10G9, gift from E. Malle, Medical University Graz) were used. After overnight incubation, the slides were washed and incubated with secondary antibody (horseradish peroxidase polymer, Vector Laboratories) for 30 min at room temperature. The slides were washed three times and stained with 3,3′-diaminobenzidine substrate (Vector Laboratories). The slides were counterstained with hematoxylin and then washed and mounted with mounting medium. Mouse anti-human EPX (clone: MM25 82.2.2) and rat anti-mouse MBP (clone: MT2 14.7.3) from E. Jacobsen (Mayo Clinic Arizona) were provided via MTAs, and staining were performed as previously described (51, 52). Serum concentrations of IL-33 and the concentration of IL-13 in cell culture supernatants and in liver tissues were measured using enzyme-linked immunosorbent assay (ELISA) kits from R&D systems according to the manufacturers’ protocols.
Isolation and ex vivo culturing of mouse bmEos
The ex vivo culturing of mouse bmEos was performed as reported previously (53). Briefly, bone marrow cells were collected from the femurs of mice and cultured at 5 × 106/ml in RPMI 1640 (Corning Cellgro) containing 20% fetal bovine serum (Corning), penicillin/streptomycin 100× (Corning Cellgro), 2 mM glutamine (Invitrogen), 25 mM Hepes, 1× nonessential amino acids, 1 mM sodium pyruvate (Gibco), and 50 μM β-mercaptoethanol (Sigma-Aldrich) and supplemented with stem cell factor (100 ng/ml; PeproTech), and fms like tyrosine kinase 3 (FLT3) ligand (100 ng/ml; PeproTech) from day 0 to day 4. On day 4, the cells were washed and cultured in fresh medium containing recombinant mouse IL-5 (10 ng/ml; R&D Systems) for an additional 8 days. On day 12, the cells were collected and analyzed by flow cytometry and imaged by light microscopy.
Quantitative real-time PCR and microarray analyses
Total RNA was isolated from liver nonparenchymal cells for the measurement of TNF-α, IL-1β, ST2, and MPO expression and for microarray analysis. RNA was isolated from cultured cells and mouse liver tissues for IL-13 mRNA measurements using the Qiagen RNeasy Mini Kit (#74104, Qiagen) according to the manufacturer’s instructions. Total RNA was reverse transcribed to complementary DNA (cDNA), followed by amplification using quantitative real-time PCR (qRT-PCR). A reaction mixture in a total volume of 10 μl contained cDNA, the One-Step RT-ddPCR Advanced Kit (#1864021, Bio-Rad), and primers at final concentrations of 1 μM. qRT-PCR was performed using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The relative amount of target mRNA was determined using the comparative threshold (Ct) method by normalizing target mRNA Ct values to those of 18S. For microarray analysis, total RNA was isolated from liver nonparenchymal cells obtained from mice treated with IgG or anti–Siglec-F antibody at 24 hours before liver ischemia. The microarray analysis was performed by the Genomics and Microarray Core Anschutz Medical Campus, University of Colorado.
Statistical analysis
All statistical analyses and graphing were conducted with GraphPad Prism version 7.0 (GraphPad Software Inc.). Results were presented as means ± SEM. For Fig. 1C, a two-tailed paired Student’s t test with Welch’s correction was performed. For all other comparisons in which there were two groups of values, a two-tailed unpaired Student’s t test with Welch’s correction was performed after demonstrating data, following a normal distribution by Shapiro-Wilk normality test. To compare values obtained from three or more groups with one independent variable, a one-way analysis of variance (ANOVA) was used followed by Tukey’s test. To compare groups with two independent variables, a two-way ANOVA was used followed by Tukey’s test. Differences in values were considered significant at P < 0.05. All experiments were repeated a minimum of three times.
Supplementary Material
Table S1. Donor characteristics.
Table S2. Recipient characteristics.
Fig. S1. Correlation between hepatic eosinophil frequency and ALT concentration after orthotopic liver transplantation.
Fig. S2. Anti–Siglec-F antibody effectively depletes eosinophils from the bone marrow, blood, and liver.
Fig. S3. Eosinophils from IR-injured livers of WT mice can attenuate IR injury in eosinophil-deficient PHIL mice.
Fig. S4. ΔdblGata-1 mice develop exacerbated liver IR injury.
Fig. S5. Hepatic eosinophils express ST2 during IR injury.
Fig. S6. Adoptively transferred eosinophils accumulate in the liver.
Fig. S7. Increase in hepatic neutrophil accumulation in the absence of eosinophils.
Fig. S8. IL-4 is not required for the hepatoprotective function of eosinophils.
Fig. S9. Recombinant mouse IL-13 reduces hepatic IR injury.
Fig. S10. Schematic summary of the main findings.
Data file S3. Microarray raw data for fig. S5A.
Acknowledgments:
We thank E. Malle (Institute of Molecular Biology and Biochemistry, Medical University Graz, Austria) for providing the antibody to detect HOCl-protein adducts in the liver. We also thank the Multimedia Scriptorium at UTHealth for the schematic summary.
Funding:
This work was supported by the NIH (DK121330, DK109574, DK122708, DK122796, AA021723, Ertan Research, and Educational Fund to C.J.; ES019311 to J.M.B.; DK121330 to W.D.; DK097075, HL114457, HL109233, DK109574, HL119837, and HL133900 to H.K.E.; AI132840-01A, HL065228, HL124165, and AI145108 to E.A.J.), the Mayo Foundation grant (to E.A.J.), and the University of Texas System Translational STARs award (to C.J.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are in the paper or Supplementary Materials.
SUPPLEMENTARY MATERIALS
View/request a protocol for this paper from Bio-protocol.
REFERENCES AND NOTES
- 1.Hilmi I, Horton CN, Planinsic RM, Sakai T, Nicolau-Raducu R, Damian D, Gligor S, Marcos A, The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver. Transpl 14, 504–508 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Watt KDS, Lyden ER, Gulizia JM, McCashland TM, Recurrent hepatitis C posttransplant: Early preservation injury may predict poor outcome. Liver Transpl. 12, 134–139 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Busuttil RW, Tanaka K, The utility of marginal donors in liver transplantation. Liver Transpl. 9, 651–663 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Kageyama S, Nakamura K, Fujii T, Ke B, Sosa RA, Reed EF, Datta N, Zarrinpar A, Busuttil RW, Kupiec-Weglinski JW, Recombinant relaxin protects liver transplants from ischemia damage by hepatocyte glucocorticoid receptor: From bench-to-bedside. Hepatology 68, 258–273 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Ji H, Zhang Y, Shen X, Gao F, He X, Li GA, Busuttil RW, Kuchroo VK, Kupiec-Weglinski JW, Recipient T cell TIM-3 and hepatocyte galectin-9 signalling protects mouse liver transplants against ischemia-reperfusion injury. J. Hepatol 62, 563–572 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Lu T, Zhang C, Xu J, Xue Z, Busuttil RW, Xu N, Xia Q, Kupiec-Weglinski JW, Ji H, Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J. Hepatol 71, 719–730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura K, Kageyama S, Ito T, Hirao H, Kadono K, Aziz A, Dery KJ, Everly MJ, Taura K, Uemoto S, Farmer DG, Kaldas FM, Busuttil RW, Kupiec-Weglinski JW, Antibiotic pretreatment alleviates liver transplant damage in mice and humans. J. Clin. Invest 129, 3420–3434 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Jin Y, Wei S, Sun Y, Jiang L, Zhu Q, Farmer DG, Busuttil RW, Kupiec-Weglinski JW, Ke B, Hippo signaling controls NLR family pyrin domain containing 3 activation and governs immunoregulation of mesenchymal stem cells in mouse liver injury. Hepatology 70, 1714–1731 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klion AD, Nutman TB, The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol 113, 30–37 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Gleich GJ, Mechanisms of eosinophil-associated inflammation. J. Allergy Clin. Immunol 105, 651–663 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ, Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J. Exp. Med 205, 699–710 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF, Lymph node trafficking and antigen presentation by endobronchial eosinophils. J. Clin. Invest 105, 945–953 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ, Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J. Immunol 187, 6059–6068 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H, Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J. 25, 561–568 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A, Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis H, le Moine A, Flamand V, Nagy N, Quertinmont E, Paulart F, Abramowicz D, le Moine O, Goldman M, Devière J, Critical role of interleukin 5 and eosinophils in concanavalin A-induced hepatitis in mice. Gastroenterology 122, 2001–2010 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Proctor WR, Chakraborty M, Chea LS, Morrison JC, Berkson JD, Semple K, Bourdi M, Pohl LR, Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology 57, 2026–2036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goh YP, Henderson NC, Heredia JE, Eagle AR, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, Chawla A, Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. U.S.A 110, 9914–9919 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kono H, Fujii H, Ogiku M, Hosomura N, Amemiya H, Tsuchiya M, Hara M, Role of IL-17A in neutrophil recruitment and hepatic injury after warm ischemia-reperfusion mice. J. Immunol 187, 4818–4825 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Inoue Y, Shirasuna K, Kimura H, Usui F, Kawashima A, Karasawa T, Tago K, Dezaki K, Nishimura S, Sagara J, Noda T, Iwakura Y, Tsutsui H, S.’. Taniguchi, K. Yanagisawa, T. Yada, Y. Yasuda, M. Takahashi, NLRP3 regulates neutrophil functions and contributes to hepatic ischemia-reperfusion injury independently of inflammasomes. J. Immunol 192, 4342–4351 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Liu P, McGuire GM, Fisher MA, Farhood A, Smith CW, Jaeschke H, Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock 3, 56–62 (1995). [PubMed] [Google Scholar]
- 22.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS, Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 63, 1156–1163 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry M, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA, Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305, 1773–1776 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH, Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med 195, 1387–1395 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drube S, Heink S, Walter S, Löhn T, Grusser M, Gerbaulet A, Berod L, Schons J, Dudeck A, Freitag J, Grotha S, Reich D, Rudeschko O, Norgauer J, Hartmann K, Roers A, Kamradt T, The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood 115, 3899–3906 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H, A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol 121, 1484–1490 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaeschke H, Ho YS, Fisher MA, Lawson JA, Farhood A, Glutathione peroxidase-deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: Importance of an intracellular oxidant stress. Hepatology 29, 443–450 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Diny NL, Baldeviano GC, Talor MV, Barin JG, Ong SF, Bedja D, Hays AG, Gilotra NA, Coppens I, Rose NR, Čiháková D, Eosinophil-derived IL-4 drives progression of myocarditis to inflammatory dilated cardiomyopathy. J. Exp. Med 214, 943–957 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woerly G, Lacy P, Younes AB, Roger N, Loiseau S, Moqbel R, Capron M, Human eosinophils express and release IL-13 following CD28-dependent activation. J. Leukoc. Biol 72, 769–779 (2002). [PubMed] [Google Scholar]
- 30.Tarantino G, Cabibi D, Cammà C, Alessi N, Donatelli M, Petta S, Craxì A, di Marco V, Liver eosinophilic infiltrate is a significant finding in patients with chronic hepatitis C. J. Viral Hepat 15, 523–530 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Bjornsson E, Kalaitzakis E, Olsson R, The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment. Pharmacol. Ther 25, 1411–1421 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, Hayashi PH, Davern TJ, Navarro V, Reddy R, Talwalkar JA, Stolz A, Gu J, Barnhart H, Hoofnagle JH; Drug‐Induced Liver Injury Network (DILIN), Hepatic histological findings in suspected drug-induced liver injury: Systematic evaluation and clinical associations. Hepatology 59, 661–670 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA, Eosinophils in health and disease: The LIAR hypothesis. Clin. Exp. Allergy 40, 563–575 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold IC, Artola-Borán M, Tallón de Lara P, Kyburz A, Taube C, Ottemann K, van den Broek M, Yousefi S, Simon HU, Müller A, Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med 215, 2055–2072 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM, Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Löhning M, Belkaid Y, Fallon PG, Powrie F, The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, Nakae S, Saito H, Wentworth JM, Li P, Liao W, Leonard WJ, Smyth GK, Shi W, Nutt SL, Koyasu S, Kallies A, The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol 16, 276–285 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY, A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D, Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 44, 355–367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matta BM, Reichenbach DK, Zhang X, Mathews L, Koehn BH, Dwyer GK, Lott JM, Uhl FM, Pfeifer D, Feser CJ, Smith MJ, Liu Q, Zeiser R, Blazar BR, Turnquist HR, Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD. Blood 128, 427–439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stremska ME, Jose S, Sabapathy V, Huang L, Bajwa A, Kinsey GR, Sharma PR, Mohammad S, Rosin DL, Okusa MD, Sharma R, IL233, A novel IL-2 and IL-33 hybrid cytokine, ameliorates renal injury. J. Am. Soc. Nephrol 28, 2681–2693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popovic B, Golemac M, Podlech J, Zeleznjak J, Bilic-Zulle L, Lukic ML, Cicin-Sain L, Reddehase MJ, Sparwasser T, Krmpotic A, Jonjic S, IL-33/ST2 pathway drives regulatory T cell dependent suppression of liver damage upon cytomegalovirus infection. PLOS Pathog. 13, e1006345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Beer Stolz D, Zheng XX, Demetris AJ, Liew FY, Wood KJ, Thomson AW, IL-33 expands suppressive CD11b+ Gr-1int and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J. Immunol 187, 4598–4610 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakai N, van Sweringen HL, Quillin RC, Schuster R, Blanchard J, Burns JM, Tevar AD, Edwards MJ, Lentsch AB, Interleukin-33 is hepatoprotective during liver ischemia/reperfusion in mice. Hepatology 56, 1468–1478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yazdani HO, Chen H-W, Tohme S, Tai S, van der Windt DJ, Loughran P, Rosborough BR, Sud V, Beer-Stolz D, Turnquist HR, Tsung A, Huang H, IL-33 exacerbates liver sterile inflammation by amplifying neutrophil extracellular trap formation. J. Hepatol, S0168–8278(17)32291–2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, Berdnikovs S, Bryce PJ, IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J. Immunol 197, 3445–3453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu J, Guardado J, Hoffman R, Xu H, Namas R, Vodovotz Y, Xu L, Ramadan M, Brown J, Turnquist HR, Billiar TR, IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model. PLOS Med. 14, e1002365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doyle AD, Mukherjee M, LeSuer WE, Bittner TB, Pasha SM, Frere JJ, Neely JL, Kloeber JA, Shim KP, Ochkur SI, Ho T, Svenningsen S, Wright BL, Rank MA, Lee JJ, Nair P, Jacobsen EA, Eosinophil-derived IL-13 promotes emphysema. Eur. Respir. J 53, 1801291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abe Y, Hines IN, Zibari G, Pavlick K, Gray L, Kitagawa Y, Grisham MB, Mouse model of liver ischemia and reperfusion injury: Method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic. Biol. Med 46, 1–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M, You Q, Lor K, Chen F, Gao B, Ju C, Chronic alcohol ingestion modulates hepatic macrophage populations and functions in mice. J. Leukoc. Biol 96, 657–665 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O’Neill KR, Colbert DC, Colby TV, Shen H, Blackburn MR, Irvin CC, Lee JJ, Lee NA, Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J. Immunol 178, 7879–7889 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Ochkur SI, Protheroe CA, Li W, Colbert DC, Zellner KR, Shen HH, Luster AD, Irvin CG, Lee JJ, Lee NA, Cys-leukotrienes promote fibrosis in a mouse model of eosinophil-mediated respiratory inflammation. Am. J. Respir. Cell Mol. Biol 49, 1074–1084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF, Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol 181, 4004–4009 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Donor characteristics.
Table S2. Recipient characteristics.
Fig. S1. Correlation between hepatic eosinophil frequency and ALT concentration after orthotopic liver transplantation.
Fig. S2. Anti–Siglec-F antibody effectively depletes eosinophils from the bone marrow, blood, and liver.
Fig. S3. Eosinophils from IR-injured livers of WT mice can attenuate IR injury in eosinophil-deficient PHIL mice.
Fig. S4. ΔdblGata-1 mice develop exacerbated liver IR injury.
Fig. S5. Hepatic eosinophils express ST2 during IR injury.
Fig. S6. Adoptively transferred eosinophils accumulate in the liver.
Fig. S7. Increase in hepatic neutrophil accumulation in the absence of eosinophils.
Fig. S8. IL-4 is not required for the hepatoprotective function of eosinophils.
Fig. S9. Recombinant mouse IL-13 reduces hepatic IR injury.
Fig. S10. Schematic summary of the main findings.
Data file S3. Microarray raw data for fig. S5A.


