Abstract
It has been widely accepted that mesenchymal stem cells (MSCs) can evade the immune surveillance of the recipient. However, emerging research cast doubt on whether MSCs are intrinsically immune-privileged. Previously, we observed that the transplantation of human MSCs (hMSCs) into the mouse parenchyma attracted a high infiltration of leukocytes into the injection tract. Thus, in order to reduce the immune responses generated by hMSCs, the aim of this study was to assess which immunosuppressant condition (dexamethasone only, tacrolimus only, or dexamethasone and tacrolimus together) would not only reduce the overall immune response but also enhance the persistence of MSCs engrafted into the caudate putamen of wild-type C57BL/6 mice. According to immunohistochemical analysis, compared to the hMSC only group, the administration of immunosuppressants (for all three conditions) reduced the infiltration of CD45-positive leukocytes and neutrophils at the site of injection. The highest hMSC persistence was detected from the group that received combinatorial administrations of dexamethasone and tacrolimus. Moreover, compared to the immunocompetent WT mouse, higher MSC engraftment was observed from the immunodeficient BALB/c mice. The results of this study support the use of immunosuppressants to tackle MSC-mediated immune responses and to possibly prolong the engraftment of transplanted MSCs.
Keywords: mesenchymal stem cell, immunosuppressive agents, immunologic surveillance, transplants
Introduction
Easily accessible and proposed to possess wide ranging capabilities via their paracrine activities, mesenchymal stem cells (MSCs) have gained favorable attention as a promising regenerative therapy for neurodegenerative disorders1–4. However, there are many issues that need to be addressed for MSCs to be used for clinical purpose. Major issues include the route of administration that can overcome the limitation of the blood brain barrier5–7, poor engraftment, and rapid clearance of MSCs following transplantation8,9.
To be used for clinical purposes, another major issue for MSCs is immunogenicity10,11. To date MSCs have been described to be positive for the major histocompatibility complex (MHC) class I, but negative for MHC class II12. MSCs have been considered immune-privileged since they do not express MHC class II or human leukocyte antigen (HLA)-DR and thus are able to evade the immune surveillance of the recipient. Recently, however, this prevailing dogma has been challenged by reports suggesting that MSCs are not immune-privileged or hypo-immunogenic but immune evasive13. According to a study that we reported recently14, minimal infiltration of CD45-positive leukocytes was observed at the injection site from the vehicle group where only minimal essential medium alpha 1x was administered into the left caudate putamen (CPu) of wild-type C57BL/6 mice. This was a striking contrast to the xenogeneic group, where the injection site was densely populated by CD45-positive leukocytes following administration of human mesenchymal stem cells (hMSCs). This activation of innate immune responses exhibited via infiltration of macrophages and neutrophils at the injection site of human MSCs was also demonstrated from past studies conducted in rats and mice13. Furthermore, following transplantation of MSCs from 3 different sources: xenogeneic, allogeneic, and syngeneic, the highest immune response was observed from the xenogeneic followed by the allogeneic and lastly by the syngeneic group. Such results not only reinforced the proposition that MSCs are immunogenic, but also raised concerns on the immunologic problems of xenotransplantation.
Surmounting evidence of MSC immunogenicity has implications in animal studies, let alone clinical trials for human subjects. First, the safety and efficacy of hMSCs must be evaluated in animal models to be utilized in clinical trials. Thus, performing xenotransplantation is inevitable. However, the immune responses exerted by hMSCs transplanted into the mouse parenchyma impose hurdles on the clinical translation of MSC therapy. Immune responses that are generated following transplantation of hMSCs in mice may not only affect the therapeutic efficacy of hMSCs in the recipient's environment but also the duration of their survival. This may act as a possible hindrance in accurately assessing the safety and efficacy of hMSCs in vivo. Second, for clinical trials, allogeneic MSCs are preferred over autologous MSCs. While issues such as immune rejection or HLA mismatching does not need to be considered for autologous cells, it is both challenging and time consuming to acquire and expand cells from patients with comorbidities15,16. Allogeneic cells, on the other hand, are readily available as “off-the-shelf” therapeutic products15 and cells can be obtained from young, healthy donors17 but there is a risk of immune rejection18. Results from our recently reported study14 add to the growing evidence19,20 that the potential for immunogenicity cannot be ruled out following transplantation of allogeneic MSCs.
To address this imminent and compelling need to ameliorate immune responses that arise via xenotransplantation, here, we transplanted hMSCs into the parenchyma of wild-type mice and also co-administered immunosuppressants. Various immunosuppressants (dexamethasone, tacrolimus, and combination of dexamethasone and tacrolimus) were assessed under several conditions to explore which immunosuppressant regimen (s) can reduce immune responses exerted following hMSCs transplantation and also enhance the engraftment of hMSCs in the mouse parenchyma.
Materials and Methods
Ethical Statement
This study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Samsung Biomedical Research Institute (SBRI) at Samsung Medical Center (SMC). SBRI abides by the Institute of Laboratory Animal Resources (ILAR) guide and is an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) accredited facility.
Experimental Animals
All mice were fed ad libitum and were maintained in a 12-hour (hr) light/12-hr dark cycle. Female C57BL/6 (7-9 week of age) and BALB/c nude mice (7-9 week of age) were both purchased from Orientbio (Seongnam, Republic of Korea). Out of the 54 C57BL/6 mice, brain tissue samples harvested from 26 mice (hMSC only: n = 6, dexamethasone (Dexa): n = 7, tacrolimus (Tac): n = 6, dexamethasone + tacrolimus (DexaTac): n = 7) were used to assess persistence of human MSCs via ALU quantitative polymerase chain reaction (qPCR) and immunohistochemistry (IHC) was carried out using the brain tissue samples harvested from the remaining 25 mice to evaluate the expressions of immune and inflammatory markers at the site of hMSC injection (hMSC only: n = 7, Dexa: n = 6, Tac: n = 5, DexaTac: n = 7). To examine differences in MSC persistence at 7 days-post transplantation, a 0 hr (positive control) group (n = 3) was included in the study. These mice were sacrificed immediately after receiving transplantations of hMSCs. An additional experiment was performed to investigate on differences in hMSC persistence and immune responses between C57BL/6 (n = 9) and BALB/c mice (n = 12). A total of 11 mice were used to carry out immunohistochemical analysis (C57BL/6: n = 4, BALB/c: n = 7) and an additional 10 mice were used to evaluate hMSC persistence via ALU qPCR (C57BL/6: n = 5, BALB/c: n = 5).
Immunosuppressant Administration and MSC Transplantation
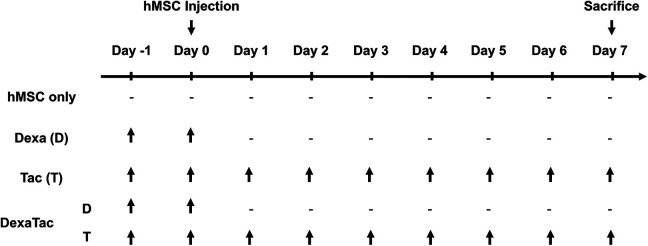
The experiment started at Day -1 and mice were sacrificed at Day 7 (total of 9 days) (Fig. 1). The dosage and administration routes for tacrolimus and dexamethasone were determined by referring to previous reports21–24. Tacrolimus (PROGRAF ®) was purchased from Astellas Pharma (Tokyo, Japan). It was diluted using an isotonic sodium chloride solution (Dai Han Pharm, Republic of Korea) and was administered at 3 mg/kg/day (total volume: 100 µL) via the intraperitoneal (I.P.) route (Fig. 1). Tacrolimus (Tac; T) was administered starting a day before the cell injection (Day -1) and was continually administered once a day, daily, up to the sacrifice time point (Day 7). Water soluble dexamethasone (Dexa; D; Sigma Aldrich, St Louis, MO, USA) was administered for 2 days (Day -1 and Day 0: 1 day before cell injection and the day of MSC transplantation) at 1 mg/kg/day (total volume: 100 µL) by oral gavage (P.O.). The DexaTac group received co-administrations of both dexamethasone (P.O.) and tacrolimus (I.P.) by applying the equivalent dosage and administration routes used for the Dexa and Tac groups, respectively.
Figure 1.
Timeline of experiment. C57BL/6 mice are randomly allocated into four different groups: hMSC only, dexamethasone (Dexa; D) only, tacrolimus (Tac; T) only, and a combination of dexamethasone and tacrolimus (DexaTac). Human mesenchymal stem cells (hMSCs) are transplanted into the left CPu of wild-type C57BL/6 mice at Day 0. Mice are sacrificed at Day 7. Dexamethasone is administered at 1 mg/kg/day via oral gavage at Day -1 and Day 0. Tacrolimus is administered at 3 mg/kg/day via the intraperitoneal route daily starting from Day -1 up to the termination time point (Day 7). The DexaTac group is given administrations of both dexamethasone and tacrolimus via the respective administration routes and dosage. Note the hyphen indicates that no immunosuppressants have been administered.
Human mesenchymal stem cells (hMSCs) were prepared as described previously14,24. Human MSCs were expanded in media containing minimal essential alpha 1x medium (MEMα1x; Gibco, Waltham, MA, USA), 10% fetal bovine serum (FBS; Biowest, Riverside, MO, USA), and 0.5% gentamicin (Gibco, Waltham, MA, USA) at 37°C in a 5% CO2 incubator. As reported previously25, we applied a preconditioning protocol by treating hMSCs with ethionamide prior to transplantation to enhance the overall therapeutic efficacy of hMSCs. To prepare cells for transplantation, preconditioned hMSCs were harvested using 0.25% Trypsin-Ethylenediaminetetraacetic acid (EDTA) (Gibco, Waltham, MA, USA). Cells were then suspended in serum-free phenol red-free MEMα1x (Gibco, Waltham, MA, USA) at a concentration of 2 × 105/ 5 µL14. At Day 0, 2 × 105 hMSCs suspended in 5 µL of serum-free phenol red-free MEMα1x were injected into the left caudate putamen (CPu) of mice at the following coordinates: -0.5 mm anterior to bregma (A/P), -1.7 mm from the midline (M/L), and 3.3 mm ventral from the surface of the skull (D/V). A 25 µL Hamilton syringe (Hamilton Company, Reno, NV, USA) was used to deliver the cells and cells were injected at a rate of 1 µL per min. Following the completion of cell transplantation, a 5-minute delay was carried out before retracting the syringe to reduce backflow of cells. BALB/c nude mice did not receive any administrations of immunosuppressants. Equivalent to the C57BL/6 mice that received transplantations of hMSCs only, hMSCs were transplanted to the left CPu of BALB/c nude mice and the mice were subsequently sacrificed at Day 7.
Quantification of Human DNA Using Real-Time Quantitative PCR
The persistence of hMSCs in the mouse parenchyma was assessed by targeting the human ALU element via quantitative real-time PCR (qPCR). Genomic DNA isolation and qPCR were carried out as described previously14. At the day of sacrifice, brain tissue samples were harvested (left and right hemispheres separated) from both C57BL/6 and BALB/c nude mice and were then immediately frozen in liquid nitrogen. Genomic DNA was extracted only from the left hemisphere where the injection was performed (Gentra Puregene Tissue Kit; Qiagen, Hilden, Germany). ALU primers were synthesized by referring to previous reports26,27: Forward (5′-CAT GGT GAA ACC CCG TCT CTA-3′) and reverse (5′–GCC TCA GCC TCC CGA GTA G-3′). PCR was carried out on a QuantStudio 6 system (Applied Biosystems, Foster City, CA, USA) by using a mixture containing 20 ng of genomic DNA, ALU primers (forward and reverse), and the SYBR Green Master Mix probe (Thermo Fisher Scientific, Waltham, MA, USA). The PCR conditions were as follows: a total of 40 cycles starting with 95°C for 10 min, 95°C for 15 sec, 68°C for 30 sec, and lastly 72°C for 30 sec. The standard curve was generated by using the threshold cycle (CT) values acquired from varying number of hMSCs (10 2 , 103, 104, 105, 106). The persistence of hMSCs in the mouse parenchyma was quantitated by fitting the CT values of the respective samples to the standard curve.
Immunohistochemical Staining and Analysis
The expressions of immune and inflammatory markers and engraftment of hMSCs were evaluated by performing immunohistochemical (IHC) staining. IHC was carried out by referring to studies reported previously14,24. On Day 7, mice that were randomly allocated to undergo IHC analysis was sacrificed via cardiac perfusion. Harvested brain tissue samples were fixated in 4% paraformaldehyde prior to embedding the tissue samples in paraffin blocks. 4 µm thick coronal sections were deparaffinized in xylene, varying percentages of ethanol, and distilled water. Sodium citrate buffer (1×, pH 6.0 (Dako, Glostrup, Denmark) was used to perform heat-induced epitope retrieval. Slides were incubated in primary antibodies overnight at 4°C and secondary antibodies for 1 hr at room temperature. Primary antibodies used in this study were as follows: anti-rat CD45 (1:200; Biolegend, San Diego, CA, USA), anti-rabbit Iba-1 (1:250; Wako Chemicals, Osaka, Japan), anti-rat neutrophil (1:200; Abcam, Cambridge, UK), anti-rabbit CD68 (1:200; Abcam, Cambridge, UK), anti-mouse STEM121 (1:500; Cellartis, Japan), anti-rabbit CD4 (1:1000; Abcam, Cambridge, UK), and anti-rabbit CD8α (1:100; Cell Signaling, Danvers, MA, USA). Secondary antibodies used in this study were as follows: Alexa Fluor 633-conjugated donkey anti-rat (1:400; Life Technologies, Carlsbad, CA, USA), Alexa Fluor 546-conjugated donkey anti-rabbit (1:400), and Dako EnVision + System-HRP Labelled Polymer anti-mouse and anti-rabbit (Dako, Carpinteria, CA, USA). By referring to the manufacturer’s (Dako, USA) instructions, further dilutions were not performed for each of the HRP labeled polymers. Images of slides that underwent immunofluorescent staining were acquired using a confocal microscope (Carl Zeiss AG, Jena, Germany). Slides that underwent 3,3′-Diaminobenzidine (DAB) staining were scanned using the Scanscope AT scanner (Leica Biosystems, Wetzlar, Germany). Images of all IHC-stained slides were re-acquired using the Vectra® Automated Imaging System (version 2.4.1, PerkinElmer Applied Biosystems, Waltham, MA, USA) and quantification was performed subsequently via the InForm 2.4.1 image analysis software.
Statistical Analysis
The GraphPad Prism 8.0 software (version 8, Graphpad; San Diego, CA, USA) was utilized to conduct statistical analysis. All values are presented as mean ± standard error of mean (S.E.M). One-way ANOVA or a t-test (unpaired, two-tailed) was used to assess significance and a P-value ≤ 0.05 was considered statistically significant. One-way ANOVA was utilized to compare the Dexa, Tac, and DexaTac groups to the hMSC group and the t-test was used to compare the BALB/c nude mice to the C57BL/6 mice.
Results
Immunosuppressant Administration Drastically Attenuated the Infiltration of Leukocytes and Neutrophils at the hMSC Injection Site
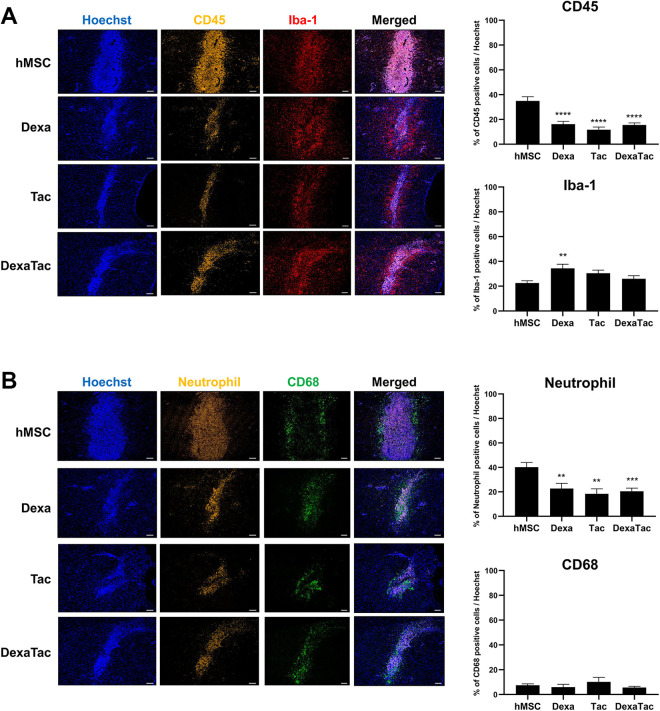
Co-immunostaining was conducted to compare the distribution of CD45-positive leukocytes and Iba-1 positive microglia cells at the site of injection among the 4 groups (hMSC, Dexa, Tac, and DexaTac) (Fig. 2A). CD45-positive leukocytes were identified mostly at the site of injection while the presence of Iba-1 positive microglia cells was discernible not only at the injection site but also in the vicinity of the graft. The highest CD45-positive leukocyte population was detected from the hMSC group (34.97% ± 3.41%). Compared to the hMSC group, administration of immunosuppressants dramatically reduced the expression of CD45-positive leukocytes in the following order (highest to lowest): Dexa (16.18% ± 2.28%), DexaTac (15.57% ± 1.65%), and Tac (11.68% ± 2.20%) (Fig. 2A). The difference in expression of CD45-positive leukocytes for all three immunosuppressant conditions was statistically significant when compared to that of the hMSC group (****P < 0.0001). Contrary to the expression of CD45-positive leukocytes, a decrease in expression of Iba-1 positive microglia was not discovered following administration of immunosuppressants (Dexa, Tac, and DexaTac) (Fig 2A). The expressions of Iba-1 positive microglia cells were as follows for all four groups: hMSC (22.54% ± 1.84%), Dexa (34.36% ± 3.33%), Tac (30.47% ± 2.45%), and DexaTac (25.95% ± 2.45%). The percentage of Iba-1 microglia cells was greater by 1.5-fold when comparing the results of the Dexa group to that of the hMSC group and the difference was also statistically significant (**P < 0.01). Although an increase in Iba-1 microglia cells was evident, no statistically significant differences were noted when comparing the results of DexaTac (P = 0.5705) and Tac (P = 0.1434), respectively, to that of the hMSC group (Fig. 2A).
Figure 2.
Administration of immunosuppressants reduces the expression levels of CD45-positive leukocytes and neutrophils at the hMSC injection site. (A) Compared to the Dexa (n = 6), Tac (n = 5), and DexaTac (n = 7) groups, severe infiltration of CD45-positive leukocytes (indicated in golden yellow) is visualized from the injection site of the hMSC group (n = 7) via IHC staining. For all 4 groups (hMSC, Dexa, Tac, and DexaTac), Iba-1 microglia cells (indicated in red) are randomly dispersed at the injection site and the region surrounding the cell graft. (B) The hMSC group shows the highest infiltration of neutrophils (indicated in golden yellow) at the injection site. The expression of neutrophils is significantly reduced for the Dexa (n = 6), Tac (n = 5), and DexaTac (n = 7) groups. CD68-positive macrophages (indicated in green) are not as densely populated as neutrophils. Macrophages are identified at and the region bordering the engraftment site. Statistical significance is defined as ** P < 0.01, *** P < 0.001, **** P < 0.0001 vs. hMSC; mean ± SEM (One-way ANOVA). Scale bar = 100 µm.
The expression levels of immune and inflammatory cells at the injection sites were further examined by carrying out additional IHC staining using neutrophil and CD68 markers. Neutrophils are known to be the most common type of leukocytes14,28,29 and thus a neutrophil marker was used to confirm the CD45 IHC results. Although widely known as a marker for microglia, several studies report that Iba-1 is expressed by both microglia and macrophages30,31. CD68, a common marker used to identify macrophages32, was used to evaluate whether similar expression levels could be observed in comparison to that of the Iba-1 marker. Like the distribution of CD45-positive leukocytes, a dense population of neutrophils was concentrated at the site of hMSC injection (Fig. 2B). Compared to the hMSC group (40.25% ± 3.80%), administration of immunosuppressants reduced the expression of neutrophils strikingly in the following order (highest to lowest): Dexa (22.69% ± 4.31%), DexaTac (20.47% ± 2.56%), and Tac (18.41% ± 4.03%) (Fig. 2B). The difference in expression of neutrophils for all three immunosuppressant conditions was statistically significant when compared to that of the hMSC group (Dexa, Tac: **P < 0.01, DexaTac: *** P < 0.001). The expression pattern of CD68-positive macrophages resembled that of Iba-1-positive microglial cells. CD68-positive macrophages were distributed at the vicinity or border of the hMSC engraftment site (Fig. 2B). Compared to the hMSC group, the Tac group did show a slight increase in expression of CD68-positive macrophages, but the difference was not statistically significant (P = 0.6362). Likewise, statistically significant differences were not observed from the Dexa (P = 0.8682) and DexaTac (P = 0.6733) groups, respectively, when compared to the hMSC group.
Immunosuppressant Administration Increased the Persistence of hMSCs in the Mouse Brain Parenchyma
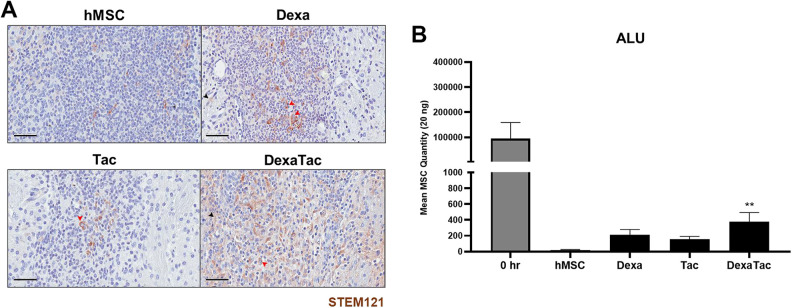
According to IHC-DAB staining results, STEM121 positive human cells were identified as brown-colored signals surrounding the nuclei in the cytoplasm (Fig. 3A; solid red arrowhead). Small, circular, pyknotic cells were also identified at the injection sites of all 4 groups (hMSC, Dexa, Tac, DexaTac). While a high distribution of these pyknotic cells was identified from all of the groups, more STEM121 positive cells was identified from the DexaTac group. Apart from the clearly visible STEM121 positive signals which have been indicated as red arrowheads, there were non-specific signals that were discernible at and in the vicinity of the injection site (Fig. 3A; solid black arrowhead). Thus, hMSC persistence was quantitated separately via ALU qPCR (Fig. 3B). Compared to the 0 hr (positive control) group, a marked reduction in hMSC persistence was observed at 7 days-post transplantation (with or without the administration of immunosuppressants). However, compared to the hMSC group, administrations of Dexa only, Tac only, and DexaTac increased the persistence of hMSCs in the mouse parenchyma by 10.7, 7.8, and 19.1-fold, respectively (Fig. 3B). While statistically significant differences were not noted when comparing the Dexa (P = 0.1918) and Tac (P = 0.5829) groups to the hMSC group, respectively, statistically significant differences were noted when comparing the DexaTac group to the hMSC group (**P < 0.01) (Fig. 3B).
Figure 3.
Residual hMSCs remaining in the mouse parenchyma following immunosuppressant administration at Day 7. (A) IHC results of representative tissue sections from the hMSC (n = 7), Dexa (n = 6), Tac (n = 5), and DexaTac (n = 7) groups, respectively, show STEM121 positive cells that are discernible as brown signals surrounding the nuclei of the cells (solid red arrowhead). Non-specific brown signals, not enclosing the nuclei of cells, can also be identified in the surrounding tissue regions of the injection site (solid black arrowhead). (B) The number of residual hMSCs present in the parenchyma of the 0 hr (n = 3), hMSC (n = 6), Dexa (n = 7), Tac (n = 6), and DexaTac (n = 7) groups, respectively, is quantitated via ALU qPCR. 0 hr is the positive control group where unlike the other experimental groups, this group was sacrificed not at Day 7 but immediately following transplantation of hMSCs. Out of the immunosuppressant groups, the highest number of persisting hMSCs is observed from the DexaTac group. Statistical significance is defined as ** P < 0.01 vs. hMSC; mean ± SEM (One-way ANOVA). Scale bar = 50 µm.
T Cell Reactivity Is Not Pronounced at the hMSC Engraftment Site
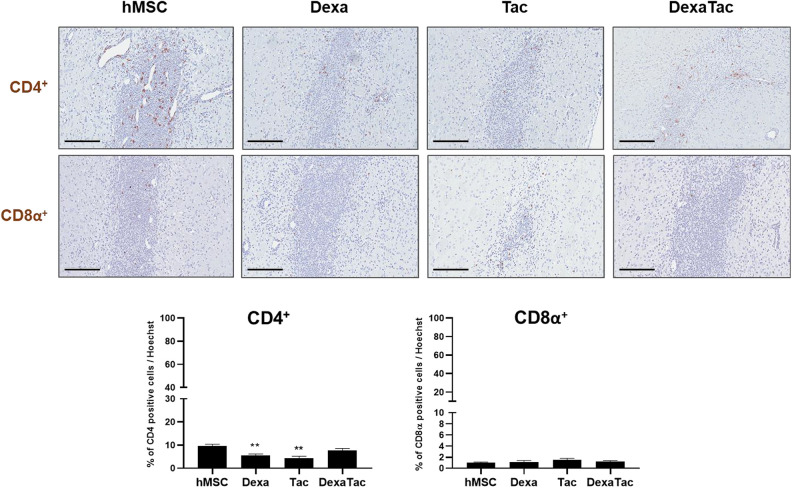
In addition to examining the expressions of various immune and inflammatory cells, IHC staining was carried out to assess changes in CD4+ and CD8α+ T cell proliferation following administrations of Dexa, Tac, and DexaTac, respectively. Overall, the proliferation of both CD4+ and CD8α+ T cells was relatively low and sparsely distributed (Fig. 4) in comparison to the densely populated leukocyte and neutrophil populations that were observed following hMSC injection (Fig. 3). For all four groups (hMSC, Dexa, Tac, and DexaTac), the population of CD4+ T cells was greater than the population of CD8α+ T cells. When compared to that of the hMSC only (no immunosuppressant) group (9.65% ± 0.73%), the percentage of CD4+ T cells at the implantation site was reduced for each of the immunosuppressant conditions (Dexa: 5.57% ± 0.62%, Tac: 4.35% ± 0.86%, and DexaTac: 7.59% ± 1.00%). Statistical significance was observed between the hMSC group and the Dexa (**P < 0.01) and Tac (**P < 0.01) groups, respectively. Unexpectedly, however, the DexaTac group did not differ from the hMSC group (P = 0.1581) in terms of CD4+ T cell expression. Contrary to the CD4+ IHC results, little differences were noted when comparing the CD8α+ expression of the hMSC group (0.99% ± 0.12%) to that of the Dexa (1.10% ± 0.31%; P = 0.9744), Tac (1.48% ± 0.31%; P = 0.4389), and DexaTac (1.20% ± 0.19%; P = 0.7175) groups, respectively (Fig. 4).
Figure 4.
Administering immunosuppressants decreases the proliferation of CD4+ T cells at the hMSC engraftment site. The expressions of CD4+ (top row) and CD8α+ (bottom row) T cells at the site of hMSC injection are assessed via IHC staining for the hMSC (n = 7), Dexa (n = 6), Tac (n = 5), and DexaTac (n = 7) groups, respectively. T cells are identified as dark brown signals colocalized to the nuclei of cells. CD4+ T cells are sparsely distributed and CD8α+ T cells are barely identified at the site where hMSCs have been implanted. Statistical significance is defined as ** P < 0.01 vs. hMSC; mean ± SEM (One-way ANOVA). Scale bar = 200 µm.
Increase in hMSC Persistence and Reduction of Immune Responses Are Discernible from BALB/c Nude Mice
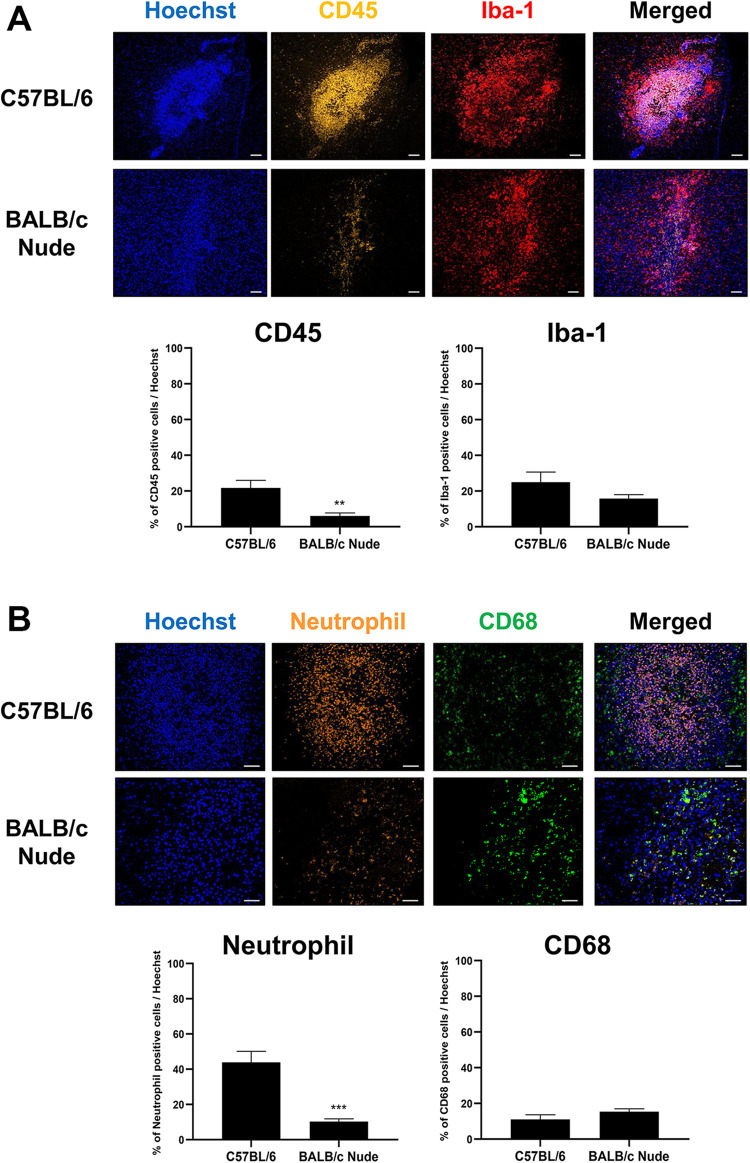
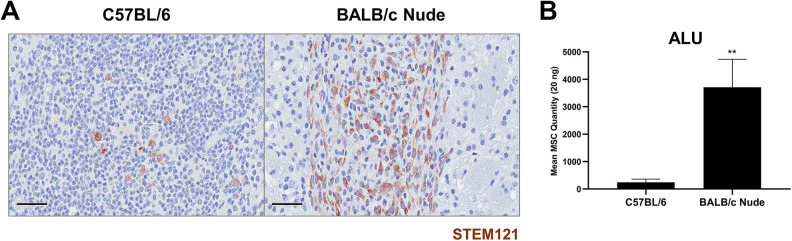
As a proof-of-principle we subsequently carried out an experiment to evaluate immune responses exerted following transplantation of hMSCs in an immunodeficient mouse model. By applying the same experimental design as that performed using C57BL/6 mice, hMSCs were transplanted into the left CPu of BALB/c nude mice but without the co-administration of immunosuppressants. Expressions of immune and inflammatory responses generated following hMSC transplantation were explored via IHC staining (Fig. 5). The expression of CD45-positive leukocytes was significantly reduced in BALB/c nude mice (6.02% ± 1.67%) in comparison to that of C57BL/6 mice (21.68% ± 4.26%) (Fig. 5A). Like C57BL/6 mice, Iba-1 positive microglia cells were observed at but mostly surrounding the border of the hMSC engraftment site (Fig. 5A). Compared to the C57BL/6 mice (23.16% ± 5.06%), a reduction in expression of Iba-1 positive microglial cells was discernible from the BALB/c nude mice (13.98% ± 2.28%) but the difference was not statistically significant (P = 0.1153). Along with CD45-positive leukocytes, an extremely high population of neutrophils was identified in the injection site of C57BL/6 mice. While there was a 3.6-fold difference in expression of CD45-positive leukocytes, there was a 4.2-fold difference in expression of neutrophils between the C57BL/6 (43.85% ± 6.30%) and BALB/c nude mice (10.33% ± 1.48%). This difference was statistically significant (***P < 0.001) (Fig. 5B). Contrasting results were observed when expression levels of CD68-positive macrophages were assessed. Macrophages were present at and near the bordering regions of the hMSC engraftment site (Fig. 5B). Overall, the percentage of Iba-1 positive microglia cells was higher in both C57BL/6 and BALB/c nude mice in comparison to the percentages of CD68-positive macrophages that were present from both groups. Compared to the C57BL/6 mice (11.03% ± 2.61%), a slight increase in percentage of CD68-positive macrophages was apparent from the BALB/c nude mice (15.34% ± 1.66%) but the difference was not statistically significant (P = 0.1844). Based on IHC staining, a striking increase in number of STEM121-positively stained cells was detected from BALB/C nude mice in comparison to wild-type C57BL/6 mice (Fig. 6A). When quantitated via ALU qPCR, there was a dramatic and significant increase in number of residual hMSCs from the BALB/c nude mice (**P < 0.01) in comparison to that of C57BL/6 mice (Fig. 6B).
Figure 5.
Infiltration of leukocytes and neutrophils are attenuated at the injection site of BALB/c nude mice. (A) Based on IHC staining, the percentage of CD45-positive leukocytes (indicated in golden yellow) is greatly reduced in the injection site of BALB/c nude mice (n = 7) compared to that of C57BL/6 mice (n = 4). Notable and significant differences are not observed when comparing the percentages of Iba-1 positive microglia cells (indicated in red) from both groups. (B) Contrary to C57BL/6 mice (n = 4), severe infiltration of neutrophils (indicated in orange) is not identified from the injection site of BALB/c nude mice (n = 7). The percentage of CD68-positive macrophages was slightly higher in the C57BL/6 group in comparison to that of BALB/c nude mice but there was no significant difference between the groups. Statistical significance is defined as **P < 0.01, *** P < 0.001 vs. C57BL/6 mice; mean ± SEM (t-test). Scale bars: (A) 100 µm, (B) 50 µm.
Figure 6.
Higher number of residual hMSCs is quantitated from the parenchyma of BALB/c nude mice at Day 7. (A) Via IHC staining, STEM121-positive cells are identified as brown signals from representative tissue sections of both C57BL/6 (n = 5) and BALB/c nude mice (n = 5). (B) The number of residual hMSCs persisting in the parenchyma of both C57BL/6 (n = 5) and BALB/c nude mice (n = 5) is assessed via ALU qPCR. MSC persistence is higher in BALB/c nude mice. Statistical significance is defined as **P < 0.01 vs. C57BL/6 mice; mean ± SEM (t-test). Scale bar = 50 µm.
Proliferation of T Cells Is Reduced at the hMSC Engraftment Site of BALB/c Nude Mice
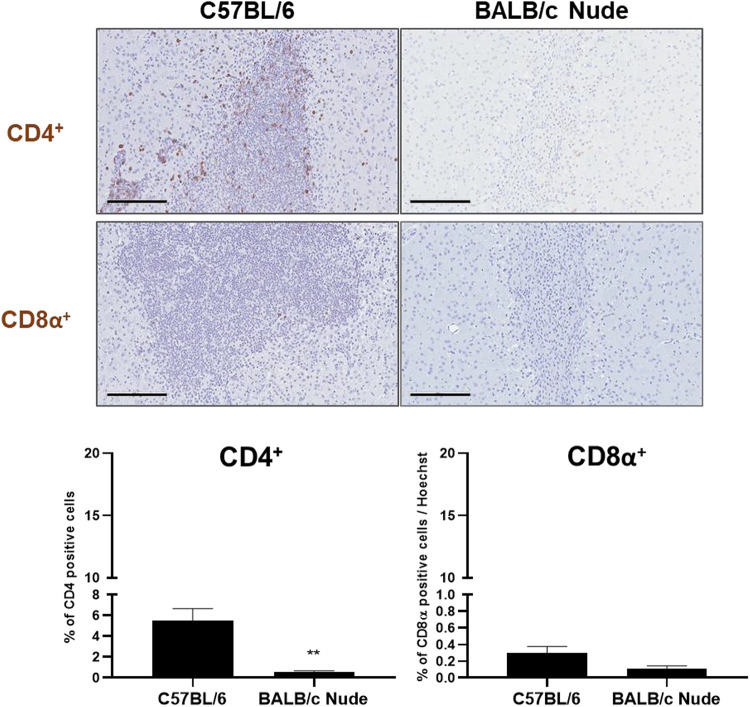
In addition to the analysis of immune and inflammatory cell infiltration at the hMSC injection site, the distribution of T cells was closely examined via IHC staining. A remarkable difference was noted in the expression of CD4+ T cells between the C57BL/6 (5.52% ± 1.12%) and BALB/c nude mice (0.57% ± 0.09%) (Fig. 7). Brown signals representative of CD4+ T cells were clearly identified from IHC-stained tissue sections of C57BL/6 mice while only few were discernible from the stained slides of BALB/c nude mice. Close to an 8.6-fold decrease in CD4+ T cells was observed from the hMSC injection site of BALB/c nude mice. The difference between the CD4+ T cell expression levels of C57BL/6 and BALB/c nude mice was statistically significant (**P < 0.01). CD8α+ T cells, on the other hand, were barely visible at the injection sites of both C57BL/6 and BALB/c nude mice (Fig. 7). Non-specific signals were detected but very few positive signals co-localized in the nuclei of cells. Compared to C57BL/6 mice (0.30% ± 0.08%), although a decrease in CD8α+ T cell expression was noted from BALB/c nude mice (0.11% ± 0.03%), the difference was not statistically significant (P = 0.0546).
Figure 7.
T cell reactivity is attenuated at the hMSC engraftment site of BALB/c nude mice. The proliferation of CD4+ (top row) and CD8α+ (bottom row) T cells at the site of hMSC injection in C57BL/6 (n = 4) and BALB/c nude mice (n = 7) is evaluated via IHC staining. Dark brown signals, indicating the presence of T cells, can be clearly identified from the representative tissue section of C57BL/6 mice stained with the CD4 antibody while positive signals are barely detectable from representative tissue sections of BALB/c nude mice. Statistical significance is defined as **P < 0.01 vs. C57BL/6 mice; mean ± SEM (t-test). Scale bar = 200 µm.
Discussion
This study demonstrates the feasibility of ameliorating immune responses generated following transplantation of hMSCs into the parenchyma of WT C57BL/6 mice by co-administering the following immunosuppressants: dexamethasone only, tacrolimus only, or a combination of dexamethasone and tacrolimus. First, we hypothesized that administration of immunosuppressants would reduce expression levels of immune (CD45 and neutrophil) cells. As expected, administration of immunosuppressants did lower the expression levels of both CD45-positive leukocytes and neutrophils at the site of hMSC transplantation. A limited number of studies have investigated on the effects of immunosuppressive agents such as dexamethasone, a potent glucocorticoid, and tacrolimus (FK506), a calcineurin inhibitor, on the accumulation of immune cells at the site of hMSC engraftment in the central nervous system (CNS). It has been suggested that dexamethasone has the ability to enhance the apoptosis of monocytes and also decrease the infiltration of inflammatory cells33,34. Glucocorticoids have also been reported to reduce the infiltration of polymorphonuclear leukocyte, or neutrophils, in inflamed tissues35. In a rat model of transient retinal ischemia, compared to the vehicle-treated rats, intramuscular administration of tacrolimus decreased the accumulation of leukocytes in the retina36.
We also hypothesized that administration of immunosuppressants would reduce inflammatory (Iba-1 and CD68) cells. Contrary to our expectation, significant differences were not discernible in expression levels of both Iba-1 microglial cells and CD68-positive macrophages. Interestingly, regardless of whether or not immunosuppressants were administered, a high percentage of Iba-1 positive microglial cells were observed surrounding the border of the engraftment site. These results are in concordance with past findings where the presence of Iba-1 positive microglial cells was evident in the border of the engraftment site a week following transplantation of autologous MSCs into the parenchyma of FVB/NCrl mice37. The same group also detected the localization of Iba-1 positive microglial cells in the border region where C57BL/6 bone marrow-derived blue fluorescent protein expressing MSCs were implanted into the parenchyma of C57BL/6 CX3CR1+/- mice38. The authors proposed that the pro-inflammatory environment generated following transplantation of MSCs may account for the accumulation of microglial cells surrounding the engraftment site37. Overall, the expression of CD68 positive cells or macrophages for all experimental groups was not as strong when compared to that of Iba-1 positive microglial cells, although the macrophages and microglial cells accumulated at similar regions (at and surrounding the graft). The localization of microglia and macrophages at or surrounding the engraftment site possibly indicates the presence of apoptotic and necrotic cells39 which may have triggered the recruitment of macrophages and resident microglia in the mouse parenchyma. These apoptotic and necrotic bodies could have originated from both human MSCs and also the immune / inflammatory cells of the mouse or recipient. As indicated from our ALU-based real-time PCR assay results, the presence of apoptotic and or necrotic cells of human origin at the engraftment site is highly possible considering that residual human cells were barely remaining in the mouse parenchyma at Day 7 for the hMSC group (no immunosuppressant treatment group) compared to that of the 0 hr (positive control) group. These results replicate our previous findings where at the same endpoint, the persistence of human adipose-derived MSCs in the parenchyma of C57BL/6 was lower than expected14.
It is noteworthy that in addition to reducing the percentage of infiltrating immune cells, the administration of immunosuppressants enhanced the persistence of hMSCs in the parenchyma of C57BL/6 mice. Although the exact mechanism has not been elucidated, such results suggest that mitigating immune responses may enhance the engraftment of xenogeneic MSCs in the mouse parenchyma. It has previously been demonstrated that administering tacrolimus via the I.P. route in a rat model of spinal cord injury enhanced the graft survival of allogeneic MSCs21,40. The positive effects of tacrolimus on graft survival may not be surprising considering that the immunosuppressive drug has been used widely in renal transplantations. Use of the tacrolimus-based regimen not only reduced the risk of acute rejection but also promoted long-term graft survival in kidney transplant patients41,42. It has been suggested that utilizing multiple immunosuppressants with different targets may enhance the overall graft survival21. In the current study, the highest MSC persistence was also observed from the DexaTac group that received a combination of both dexamethasone and tacrolimus.
Both tacrolimus and dexamethasone are known to induce immunosuppression by impairing and inhibiting the proliferation of T cells21,23,43. This could possibly explain why a statistically significant reduction was observed when comparing the CD4+ T cell expression levels of the hMSC group to that of the Dexa only or Tac only group. It is unclear, however, as to why a slight decrease was noted but a statistically significant difference was not observed when comparing the CD4+ T cell expression levels of the hMSC group to that of the DexaTac group. Interestingly, while the presence of CD4+ T cells was easily identifiable from the 4 groups (hMSC, Dexa, Tac, and DexaTac), CD8α+ T cells was barely discernible via IHC staining and statistically significant differences did not exist when comparing the hMSC group to the Dexa, Tac, and DexaTac groups, respectively. Innate immune responses involving immune cells such as neutrophils, monocytes, and macrophages occur first and rapidly within hours, while adaptive immune responses take longer, possibly days, to activate multiple lymphocyte subpopulations including helper (CD4+) and cytotoxic (CD8α+) T cells44. This could partly explain why differences were noted in the expressions of CD4+ and CD8α+ T cells at the site of hMSC injection for all 4 groups. Unlike CD4+ T cells, CD8α+ T cells might not have been fully activated at Day 7. If sacrificed at a time point past 7 days, it is possible that a higher percentage of CD8α+ T cells may have been identified. As suggested previously45, it can also be speculated that T cells do not play a significant role in exerting immune responses against xenogeneic MSCs. For example, the presence of T cells infiltrating the MSC graft or the periphery of the graft was limited, or absent45, which was also demonstrated from the current study.
Immune reactions exerted in response to transplantation of hMSCs were assessed further using BALB/c nude mice as a proof-of principle. BALB/c nude mice are known for their lack of T cell production due to an absence of a thymus gland46. As expected, compared to C57BL/6 mice, immune responses observed at the injection sites of BALB/c nude mice were highly attenuated. Moreover, a striking increase in number of persisting hMSCs was also observed from the BALB/c nude mice. The difference between the C57BL/6 and BALB/c nude mice was close to 15-fold. These results further supported the notion that there may be a strong association between immune response and graft survival and that the use of immunosuppressants may be a favorable and effective approach to tackle both the high immune response and low graft survival rate observed following transplantation of xenogeneic MSCs.
This study has several limitations. First, the results of this current study highlight how reducing immune responses exerted by the recipient can improve the persistence of engrafted hMSCs. However, the underlying mechanism has not been delved deeply in this study. Second, whether similar or equivalent results will be replicated when hMSCs are administered via alternative routes such as the intracerebroventricular (ICV) route has also not been explored. Third, how various dosages, especially dosages used in the clinical settings, and different administration routes may affect the overall immune response and persistence of hMSCs have not been tested. Lastly, while recent studies have reported that including females does not necessarily increase the variability of small animal studies47–49, both genders were not represented in our study. Thus, we cannot rule out the possibility that gender may affect the host immune response and overall persistence of hMSCs in the mouse parenchyma, and further studies are warranted.
Conclusion
Altogether, we have presented findings that encourage the use of immunosuppressants when performing transplantations of human MSCs. Higher MSC persistence was observed with administration of immunosuppressants. While varying results were observed in expressions of representative immune and inflammatory markers for each of the immunosuppressant regimen, overall, administration of immunosuppressants consistently reduced expression of CD45-positive leukocytes and neutrophils. Whether the use of immunosuppressants will produce beneficial results when applied to varying disease models warrants further investigation. Nevertheless, the results of this current study put into perspective of what must be considered when performing transplantations of xenogeneic MSCs in the CNS.
Footnotes
Availability of Data and Materials: All data generated and/or analyzed during this study are included in this published article.
Author Contributions: Jung Won Hwang and Su Hyeon Myeong: collection and assembly of data, data analysis and interpretation, manuscript writing; Na Kyung Lee: conception and design, financial support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Na-Hee Lee and Hyeongseop Kim: collection and assembly of data; Hyo Jin Son and Jong Wook Chang: Provision of study material; Duk L. Na: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript. All of the authors have read and approved the final manuscript. Both Jung Won Hwang and Su Hyeon Myeong contributed equally to this work.
Ethical Approval: This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Samsung Biomedical Research Institute (SBRI) at Samsung Medical Center (SMC) (Approval Number: 20191007002, Date: 7 October 2019).
Statement of Human and Animal Rights: All the experimental procedures involving animals were performed in accordance with animal experiment guidelines issued by the Institutional Animal Care and Use Committee (IACUC) of the Samsung Biomedical Research Institute (SBRI) at Samsung Medical Center (SMC)
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants received from the Samsung Medical Center (grant number: SMX1200851), the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Republic of Korea (grant number: NRF-2020R1I1A1A01073533), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers: HI14C3484 and HI18C0560).
ORCID iD: Na Kyung Lee  https://orcid.org/0000-0001-6116-2562
https://orcid.org/0000-0001-6116-2562
References
- 1. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park SE, Lee NK, Lee J, Hwang JW, Choi SJ, Hwang H, Hyung B, Chang JW, Na DL. Distribution of human umbilical cord blood-derived mesenchymal stem cells in the Alzheimer’s disease transgenic mouse after a single intravenous injection. Neuroreport. 2016;27(4):235–241. [DOI] [PubMed] [Google Scholar]
- 6. Lee NK, Yang J, Chang EH, Park SE, Lee J, Choi SJ, Oh W, Chang JW, Na DL. Intra-arterially delivered mesenchymal stem cells are not detected in the brain parenchyma in an Alzheimer’s disease mouse model. PLoS One. 2016;11(5):e0155912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim H, Na DL, Lee NK, Kim AR, Lee S, Jang H. Intrathecal injection in a rat model: a potential route to deliver human Wharton’s jelly-derived mesenchymal stem cells into the brain. Int J Mol Sci. 2020;21(4):1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee S, Choi E, Cha MJ, Hwang KC. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid Med Cell Longev. 2015;2015:632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mao AS, Ozkale B, Shah NJ, Vining KH, Descombes T, Zhang L, Tringides CM, Wong SW, Shin JW, Scadden DT, Weitz DA, et al. Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc Natl Acad Sci U S A. 2019;116(31):15392–15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Huang J, Gong L, Yu D, An C, Bunpetch V, Dai J, Huang H, Zou X, Ouyang H, Liu H. The plasticity of mesenchymal stem cells in regulating surface HLA-I. iScience. 2019;15:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang XF, Chen T, Ren LW, Yang L, Qi H, Li FR. Immunogenicity of insulin-producing cells derived from human umbilical cord mesenchymal stem cells. Exp Ther Med. 2017;13(4):1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hwang JW, Lee NK, Yang JH, Son HJ, Bang SI, Chang JW, Na DL. A comparison of immune responses exerted following syngeneic, allogeneic, and xenogeneic transplantation of mesenchymal stem cells into the mouse brain. Int J Mol Sci. 2020;21(9):3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kot M, Baj-Krzyworzeka M, Szatanek R, Musial-Wysocka A, Suda-Szczurek M, Majka M. The importance of HLA assessment in “off-the-shelf” allogeneic mesenchymal stem cells based-therapies. Int J Mol Sci. 2019;20(22):5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Tian M, Wang F, Heng BC, Zhou J, Cai Z, Liu H. Understanding the immunological mechanisms of mesenchymal stem cells in allogeneic transplantation: from the aspect of major histocompatibility complex class I. Stem Cells Dev. 2019;28(17):1141–1150. [DOI] [PubMed] [Google Scholar]
- 17. Zhang J, Huang X, Wang H, Liu X, Zhang T, Wang Y, Hu D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. 2015;6:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yasuhara T, Kawauchi S, Kin K, Morimoto J, Kameda M, Sasaki T, Bonsack B, Kingsbury C, Tajiri N, Borlongan CV, Date I. Cell therapy for central nervous system disorders: current obstacles to progress. CNS Neurosci Ther. 2020;26(6):595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schu S, Nosov M, O’Flynn L, Shaw G, Treacy O, Barry F, Murphy M, O’Brien T, Ritter T. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16(9):2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Isakova IA, Lanclos C, Bruhn J, Kuroda MJ, Baker KC, Krishnappa V, Phinney DG. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS One. 2014;9(1):e87238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Antonios JP, Farah GJ, Cleary DR, Martin JR, Ciacci JD, Pham MH. Immunosuppressive mechanisms for stem cell transplant survival in spinal cord injury. Neurosurg Focus. 2019;46(3):E9. [DOI] [PubMed] [Google Scholar]
- 22. McGinley LM, Kashlan ON, Chen KS, Bruno ES, Hayes JM, Backus C, Feldman S, Kashlan BN, Johe K, Feldman EL. Human neural stem cell transplantation into the corpus callosum of Alzheimer’s mice. Ann Clin Transl Neurol. 2017;4(10):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giles AJ, Hutchinson MND, Sonnemann HM, Jung J, Fecci PE, Ratnam NM, Zhang W, Song H, Bailey R, Davis D, Reid CM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee NK, Kim H, Chang JW, Jang H, Kim H, Yang J, Kim J, Son JP, Na DL. Exploring the potential of mesenchymal stem cell-based therapy in mouse models of vascular cognitive impairment. Int J Mol Sci. 2020;21(15):5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee NH, Myeong SH, Son HJ, Hwang JW, Lee NK, Chang JW, Na DL. Ethionamide preconditioning enhances the proliferation and migration of human Wharton’s jelly-derived mesenchymal stem cells. Int J Mol Sci. 2020;21(19):7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real-time PCR. Cytotherapy. 2003;5(1):7–18. [DOI] [PubMed] [Google Scholar]
- 27. Funakoshi K, Bagheri M, Zhou M, Suzuki R, Abe H, Akashi H. Highly sensitive and specific Alu-based quantification of human cells among rodent cells. Sci Rep. 2017;7(1):13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chan YK, Tsai MH, Huang DC, Zheng ZH, Hung KD. Leukocyte nucleus segmentation and nucleus lobe counting. BMC Bioinformatics. 2010;11:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, Ariel A, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10(4):562–573. [DOI] [PubMed] [Google Scholar]
- 30. Nakamura R, Nishimura T, Ochiai T, Nakada S, Nagatani M, Ogasawara H. Availability of a microglia and macrophage marker, iba-1, for differential diagnosis of spontaneous malignant reticuloses from astrocytomas in rats. J Toxicol Pathol. 2013;26(1):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amici SA, Dong J, Guerau-de-Arellano M. Molecular mechanisms modulating the phenotype of macrophages and microglia. Front Immunol. 2017;8:1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hopperton KE, Mohammad D, Trepanier MO, Giuliano V, Bazinet RP. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol Psychiatry. 2018;23(2):177–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55(7):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt M, Pauels HG, Lugering N, Lugering A, Domschke W, Kucharzik T. Glucocorticoids induce apoptosis in human monocytes: potential role of IL-1 beta. J Immunol. 1999;163(6):3484–3490. [PubMed] [Google Scholar]
- 35. Nakagawa M, Terashima T, D’Yachkova Y, Bondy GP, Hogg JC, van Eeden SF. Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98(21):2307–2313. [DOI] [PubMed] [Google Scholar]
- 36. Tsujikawa A, Ogura Y, Hiroshiba N, Miyamoto K, Kiryu J, Honda Y. Tacrolimus (FK506) attenuates leukocyte accumulation after transient retinal ischemia. Stroke. 1998;29(7):1431–1437; discussion 1437-8. [DOI] [PubMed] [Google Scholar]
- 37. De Vocht N, Lin D, Praet J, Hoornaert C, Reekmans K, Le Blon D, Daans J, Pauwels P, Goossens H, Hens N, Berneman Z, et al. Quantitative and phenotypic analysis of mesenchymal stromal cell graft survival and recognition by microglia and astrocytes in mouse brain. Immunobiology. 2013;218(5):696–705. [DOI] [PubMed] [Google Scholar]
- 38. Le Blon D, Hoornaert C, Daans J, Santermans E, Hens N, Goossens H, Berneman Z, Ponsaerts P. Distinct spatial distribution of microglia and macrophages following mesenchymal stem cell implantation in mouse brain. Immunol Cell Biol. 2014;92(8):650–658. [DOI] [PubMed] [Google Scholar]
- 39. Xia Z, Ye H, Choong C, Ferguson DJ, Platt N, Cui Z, Triffitt JT. Macrophagic response to human mesenchymal stem cell and poly(epsilon-caprolactone) implantation in nonobese diabetic/severe combined immunodeficient mice. J Biomed Mater Res A. 2004;71(3):538–548. [DOI] [PubMed] [Google Scholar]
- 40. Torres-Espin A, Redondo-Castro E, Hernandez J, Navarro X. Immunosuppression of allogenic mesenchymal stem cells transplantation after spinal cord injury improves graft survival and beneficial outcomes. J Neurotrauma. 2015;32(6):367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kramer BK, Montagnino G, Del Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, Kruger B, Ortuno J, Kohler H, Kunzendorf U, Stummvoll HK, et al. Efficacy and safety of tacrolimus compared with cyclosporin a microemulsion in renal transplantation: 2 year follow-up results. Nephrol Dial Transplant. 2005;20(5):968–973. [DOI] [PubMed] [Google Scholar]
- 42. Kamel M, Kadian M, Srinivas T, Taber D, Posadas Salas MA. Tacrolimus confers lower acute rejection rates and better renal allograft survival compared to cyclosporine. World J Transplant. 2016;6(4):697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Orlikowsky TW, Dannecker GE, Spring B, Eichner M, Hoffmann MK, Poets CF. Effect of dexamethasone on B7 regulation and T cell activation in neonates and adults. Pediatr Res. 2005;57(5 Pt 1):656–661. [DOI] [PubMed] [Google Scholar]
- 44. Peterfalvi A, Miko E, Nagy T, Reger B, Simon D, Miseta A, Czeh B, Szereday L. Much more than a pleasant scent: a review on essential oils supporting the immune system. Molecules. 2019;24(24):4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoornaert CJ, Le Blon D, Quarta A, Daans J, Goossens H, Berneman Z, Ponsaerts P. Concise review: innate and adaptive immune recognition of allogeneic and xenogeneic cell transplants in the central nervous system. Stem Cells Transl Med. 2017;6(5):1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Okada S, Vaeteewoottacharn K, Kariya R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells. 2019;8(8):889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beery AK. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci. 2018;23:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smarr B, Rowland NE, Zucker I. Male and female mice show equal variability in food intake across 4-day spans that encompass estrous cycles. PLoS One. 2019;14(7):e0218935. [DOI] [PMC free article] [PubMed] [Google Scholar]