Abstract
Epithelioid angiosarcoma is a rare and highly aggressive soft tissue angiosarcoma most commonly arising in the deep soft tissues. Given that abundant vascular cavities anastomose with each other, most angiosarcomas prone to metastasis recur quickly, and the overall prognosis is poor. We report a 25-year-old woman at 24 weeks’ gestation who presented with a 1-month history of abdominal distension. Ultrasonography suggested a mass in the right adnexa, and she underwent two operations owing to uncontrolled intraperitoneal bleeding with progressive anemia. The right ovarian tumor and right adnexa were removed successively. Biopsy yielded a diagnosis of primary epithelioid angiosarcoma with mature cystic teratoma. The patient died from uncontrolled progressive bleeding 1 week after the second operation. This case revealed that epithelial angiosarcoma is a highly malignant endothelial cell tumor. The results of surgery and chemoradiotherapy tend to be poor, and the recurrence rate is high. The purpose of this study is to raise clinical awareness of epithelial angiosarcoma and its adverse events and to provide new ideas for the treatment of these adverse events. Immunohistochemical staining of pathological specimens can facilitate diagnosis. Pregnancy with malignant tumors may lead to rapid disease progression, extensive lesions, and a poor prognosis.
Keywords: Epithelial angiosarcoma, ovary, tumor, pregnancy, case report, adnexa
Introduction
Angiosarcoma is a rare malignant soft tissue tumor originating from the vascular endothelium that accounts for approximately 2% of soft tissue sarcomas.1 The majority of angiosarcomas arise from the deep soft tissues of the extremities, but they can also occur in the skin, adrenal glands, breast, thyroid gland, small intestine, uterus, heart, liver, gallbladder, spleen, bone, lung, and peritoneum.2–12 Studies have shown that epithelioid angiosarcoma originates in endothelial cell lines of blood vessels and lymph nodes.13 The diversity of primary sites is because of the presence of blood vessels and lymphatic vessels throughout the body. Radiation therapy, environmental toxins, anabolic steroids, and synthetic estrogens are risk factors for angiosarcoma;1,14–16 however, the causes of this tumor are unknown. Angiosarcomas of the female reproductive tract are rare, most commonly occurring in the uterine body, and they are extremely rare in the ovaries.17 No more than 40 cases of primary ovarian angiosarcoma are documented in MEDLINE.18 Primary epithelial ovarian angiosarcoma complicating pregnancy has not been reported. We report a case of epithelial ovarian angiosarcoma with mature cystic teratoma that occurred during pregnancy and resulted in death owing to uncontrolled hemorrhage from progressive, metastatic tumor sites.
Case presentation
The patient was a 25-year-old pregnant woman at 24 weeks gestation, from Changsha, China. She was admitted to our hospital on 8 August 2019 owing to a 1-month history of abdominal distension. There were no abnormalities in her early pregnancy examinations. She developed abdominal distension in early July 2019, which was accompanied by constipation and tenesmus as well as occasional abdominal pain. Ultrasonography suggested a mass measuring approximately 10 cm in the right adnexa, which was considered a teratoma and was not treated. On 1 August 2019, owing to progressive worsening of the abdominal distension and severe anemia, ultrasonography suggested that the mass had increased to 14 cm in size. At 22:00 on 4 August 2019, she underwent emergency laparotomy in the First Hospital of Changsha, and the right ovarian tumor and appendix were removed. Upon entering the abdomen, 1500 mL of dark red bloody ascites was encountered; however, no obvious active bleeding was found. Exploratory laparotomy revealed a 16-cm × 11-cm right ovarian tumor adherent to the posterior uterus, broad ligament, Douglas’ pouch, and anterior sacrum. The right ovarian tumor was cystic and solid, with sebum, hair, blood clots, and focal necrosis. The surface of the tumor was covered by reddish, necrotic, and fragile tissue, and the capsule was thickened and hard without an obvious breach. The right fallopian tube was swollen, and partial necrosis of the appendix was noted. No abnormal nodules were observed elsewhere. The tumor and appendix were removed, and pathology yielded a diagnosis of primary ovarian epithelioid angiosarcoma with mature cystic teratoma. The immunohistochemical results were: GATA-3 (−), ETS-related gene (ERG) (++), cluster of differentiation (CD)31 (+++), friend leukemia integration-1 (FLI-1 (++), epithelial membrane antigen (EMA) (−), Sal-like protein 4 (SALL-4) (−), placental alkaline phosphatase (PLAP) (−), human chorionic gonadotropin (HCG) (−), melanoma-specific antibody (HMB45) (−), Ki67 (+, 30%), and CK-P (+) (Figures 1–4). Postoperatively, the volume of peritoneal bloody drainage continued to increase to approximately 100 mL to 200 mL/60 minutes. The symptoms did not improve after treatment with blood transfusions and fluid rehydration. Additionally, the total drainage volume within 24 hours after surgery (20:00, 7 August 2019) was approximately 3778 mL. The patient was then transferred to our hospital.
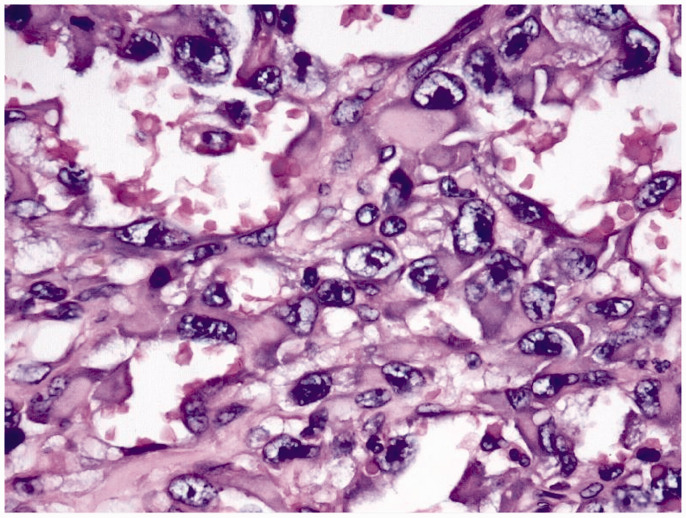
Figure 1.
Hematoxylin and eosin (H&E)-stained section showing formation of sinusoidal spaces and epithelioid cells (×10 magnification).
Figure 2.
Hematoxylin and eosin (H&E)-stained section showing formation of sinusoidal spaces and epithelioid cells (×60 magnification).
Figure 3.
Immunostaining of epithelioid angiosarcoma showing that cluster of differentiation (CD)31 demonstrates intense membranous and cytoplasmic staining (×10 magnification).
Figure 4.
Tumor cells stained diffusely with ETS-related gene (ERG) immunostain (×20 magnification).
The patient had a temperature of 37.3°C, blood pressure of 104/72 mmHg, and pulse rate of 120 beats/minute on admission to our hospital. The fetal heart rate was 160 beats/minute. Dark red bloody fluid was draining from the abdominal drainage tube at approximately 100 mL/30 minutes. Laboratory studies revealed a red blood cell count of 2.13 × 1012 cells/L, white blood count of 7.1 × 109 cells/L, hemoglobin level of 66 g/L, hematocrit level of 19.2%, and platelet count of 98 × 109 cells/L. The patient underwent computed tomography angiography, which revealed hemoperitoneum with peritoneal thickening, and that the distal branches of bilateral internal iliac arteries were dilated and tortuous, with patchy hyperchromatic areas.
Bilateral internal iliac artery embolization was performed, during which the patient delivered a dead infant of approximately 904 g at 01:31 on 8 August 2019, with hemorrhage of 100 mL. The total drainage volume from the abdominal cavity was approximately 800 mL within 24 hours and 2850 mL within 48 hours after arterial embolization. Therefore, a second exploratory laparotomy was performed, and right adnexectomy was performed. Upon entering the abdomen, 2500 mL of dark red bloody ascites was encountered. A serosal defect in the posterior uterus with blood clots was also encountered.
The right ovary was congested and slightly larger, whereas the right fallopian tube was swollen; the left ovary and fallopian tube were normal. Blood effused from the peritoneal surface of the anterior sacral and Douglas’ pouches, with blood clots weighing approximately 300 g. The bowel was swollen. Multiple subcentimeter dark red nodules were excised from the surface of the omentum and transverse colon for examination, and flash-frozen pathologic examination showed that the transverse colon was invaded. Large dark red ecchymotic hematomas were found in the infracolic omentum, splenic area, lower margin of the liver, paracolic sulcus, and other areas. Additionally, active bleeding was found in the retroperitoneal area of the pancreatic tail.
Owing to the poor effect of sutures and hemostasis at the bleeding site, we performed abdominal packing. Biopsy yielded a diagnosis of primary ovarian epithelioid angiosarcoma of the right ovary and tumor invasion of the transverse colon. The abdominal drainage fluid volume was 2250 mL on the first postoperative day and 2750 mL on the second postoperative day, indicating uncontrolled intra-abdominal bleeding. Disseminated intravascular coagulation was not indicated according to the patient's coagulation function during her hospitalization. After informing the patient of the poor prognosis of her rapidly progressing cancer and uncontrolled bleeding, the patient elected to go home and receive further transfusions there, and died 1 week postoperatively secondary to hemorrhage from cancer. The reporting of this study conforms to the CARE guidelines.19
Discussion
Angiosarcoma is a rare malignant tumor of endothelial cell origin that can be divided into cutaneous, visceral, and soft tissue subtypes. Epithelioid angiosarcoma is a rare soft tissue angiosarcoma with special morphology that was first reported by Weiss et al.20 Ovarian angiosarcoma can occur in all age groups, although it is more common in women of child-bearing age (<40 years of age). The clinical symptoms of ovarian angiosarcoma are similar to those of ovarian cancer but lack specificity. This disease is very difficult to identify in the early stage, and when symptoms appear, most patients have extensive pelvic abdominal metastasis and a poor prognosis. During the first operation in our patient, the tumor invaded only the appendix. During the second operation, extensive peritoneal, omental, and intestinal metastases were found, indicating that the growth and spread of epithelioid angiosarcoma was extremely fast and easy, and pregnancy may have accelerated the tumor spread.
The incidence of ovarian malignancies during pregnancy is 1/10,000 to 1/100,000.21 Pregnancy with ovarian angiosarcoma is extremely rare. Sedgely et al.22 reported a case of angiosarcoma of the breast that spread to the ovary during pregnancy 6 years after the initial diagnosis. Angiosarcoma was reactivated by the pregnancy, suggesting that pregnancy altered the biological behavior of the tumor and promoted the growth of malignant tumors.23 We report the first case of pregnancy with ovarian primary epithelioid angiosarcoma. In this case, the patient had a rapid course of disease and a poor prognosis, which may have been related to pregnancy. During pregnancy, the uterus enlarges, and changes in the position of the pelvic and abdominal organs mask the tumor.
Pelvic hyperemia, abundant blood supply, and lymphatic drainage during pregnancy can create favorable conditions for the rapid growth, seeding, and dissemination of malignant tumor cells. Some scholars have proposed that during pregnancy, sex hormones secreted by the placenta increase, which can affect the biological behavior of tumor tissues and cause malignant tumors to grow rapidly.24 Maternal immune responses and immune function change during pregnancy, and the placenta releases immunosuppressive factors and progesterone, which inhibits lymphocyte activity and immune response as well as the T-cell response in pregnant women. In addition, the presence of serum blocking antibodies during pregnancy enables malignant tumor cells to grow without immune monitoring. For these reasons, malignant tumors in pregnancy tend to be associated with a high degree of malignancy, rapid progression, and a poor prognosis.
Ovarian angiosarcoma may metastasize from other sites, but the primary tumor can usually be found elsewhere.25–27 Most primary ovarian angiosarcomas are unilateral.28,29 In addition, ovarian angiosarcoma can infiltrate and metastasize to adjacent tissues and organs, resulting in serious complications, such as massive abdominal bleeding. Bradford et al. reported a case of primary angiosarcoma of the ovary complicated by hemoperitoneum. The patient died 3 weeks postoperatively secondary to hemorrhage from progressive, metastatic tumor sites.30
Histologic features of epithelioid angiosarcoma include the presence of interlacing vascular spaces lined by endothelial cells showing nuclear pleomorphism and mitotic activity. The tumor cells are large, and most or all are epithelioid, with abundant eosinophilic cytoplasm. The nucleus is larger in the center or is deviated and vacuolated, with obvious nucleoli. Immunohistochemistry is of great value in the diagnosis of epithelioid angiosarcoma. Common vascular endothelial cell markers, namely CD31, CD34, and FLI-1, supplemented with epithelial markers, can be used to diagnose epithelial angiosarcoma. CD31 is approximately 90% positive for any type of angiosarcoma, with a relatively high specificity and sensitivity.31–35
Conclusion
Angiosarcoma is a malignant tumor derived from vascular endothelial cells or mesenchymal cells. Epithelioid angiosarcoma is a unique morphologic subtype of angiosarcoma in which malignant endothelial cells have a predominantly epithelioid appearance and are highly aggressive. Because the tumor has abundant vascular cavities that are anastomosed with each other, most ovarian angiosarcomas are prone to bleeding and metastasis and have a poor prognosis. Pregnancy with epithelioid angiosarcoma can accelerate disease progression. Current treatments constitute surgery, postoperative adjuvant chemotherapy, and combination therapy. It has been reported that ovarian angiosarcoma can be treated with doxorubicin combined with ifosfamide;36,37 however, there is no known optimal chemotherapy regimen. The mortality rate within 1 year of diagnosis is >50%, whereas the overall 5-year survival rate is approximately 10% to 40%.38–40
Footnotes
Ethics statement: The study protocol was approved by the Medical Ethics Committee, Xiangya Hospital, Central South University. The patient described in this report provided informed consent for publication of the details of her case. A copy of the signed informed consent form has been provided to the journal.
Author contributions: Xiaotong Peng: Project development, data collection, manuscript writing
Zhi Duan: Provision of figures
Hongling Yin: Data collection
Furong Dai: Data collection
Huining Liu: Supervision, manuscript writing
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Huining Liu https://orcid.org/0000-0002-5151-6176
References
- 1.Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma: a report of 67 patients and a review of the literature. Cancer 1996; 77: 2400–2406. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CDM, Beham A, Bekir S, et al. Epithelioid angiosarcoma of deep soft tissue: a distinctive tumor readily mistaken for an epithelial neoplasm. Am J Surg Pathol 1991; 15: 915–924. [DOI] [PubMed] [Google Scholar]
- 3.Suster S, Wong TY. On the discriminatory value of anti-HPCA-1 (CD34) in the differential diagnosis of benign and malignant cutaneous vascular proliferations. Am J Dermatopathol 1994; 16: 35–63. [DOI] [PubMed] [Google Scholar]
- 4.Goh SG, Chuah KL, Goh HK, et al. Two cases of epithelioid angiosarcoma involving the thyroid and a brief review of non-alpine epithelioid angiosarcoma of the thyroid. Arch Pathol Lab Med 2003; 127: 70–73. [DOI] [PubMed] [Google Scholar]
- 5.Wenig BM, Abbondanzo SL, Heffess CS. Epithelioid angiosarcoma of the adrenal glands. A clinicopathologic study of nine cases with a discussion of the implications of finding “epithelial specific” markers. Am J Surg Pathol 1994; 18: 62–73. [PubMed] [Google Scholar]
- 6.Weiss SW, Ishak KG, Dail DH, et al . Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol 1986; 3: 259–287. [PubMed] [Google Scholar]
- 7.Tallini G, Price FV, Carcangiu ML. Epithelioid angiosarcoma arising in uterine leiomyomas. Am J Clin Pathol 1993; 100: 514–518. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande V, Rosenberg AE, O’Connell JX, et al. Epithelioid angiosarcoma of the bone a series of 10 cases. Am J Surg Pathol 2003; 27: 709–716. [DOI] [PubMed] [Google Scholar]
- 9.Val-Bernal JF, Figols J, Arce FP, et al. Cardiac epithelioid angiosarcoma presenting as cutaneous metastases. J Cutan Pathol 2001; 28: 265–270. [DOI] [PubMed] [Google Scholar]
- 10.Delvaux V, Sciot R, Neuville B, et al. Multifocal epithelioid angiosarcoma of the small intestine. Virchows Arch 2000; 437: 90–94. [DOI] [PubMed] [Google Scholar]
- 11.White J, Chan YF. Epithelioid angiosarcoma of the gallbladder. Histopathology 1994; 24: 269–271. [DOI] [PubMed] [Google Scholar]
- 12.Olawaiye AB, Morgan JA, Goodman A, et al. Epithelioid angiosarcoma of the uterus: a review of management. Arch Gynecol Obstet 2008; 278: 401–404. [DOI] [PubMed] [Google Scholar]
- 13.Fukunaga M. Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology 2005; 46: 396–402. [DOI] [PubMed] [Google Scholar]
- 14.Falk H, Thomas LB, Popper H, et al. Hepatic angiosarcoma associated with androgenic anabolic steroids. Lancet 1979; 2: 1120–1122. [DOI] [PubMed] [Google Scholar]
- 15.Edeiken S, Russo DP, Knecht J, et al. Angiosarcoma after tylectomy and radiation therapy for carcinoma of the breast. Cancer 1992; 70: 644–647. [DOI] [PubMed] [Google Scholar]
- 16.Popper H, Thomas LB, Telles NC, et al. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol 1978; 92: 349–369. [PMC free article] [PubMed] [Google Scholar]
- 17.Kruse AJ, Sep S, Slangen BF, et al. Angiosarcomas of primary gynecologic origin: a clinicopathologic review and quantitative analysis of survival. Int J Gynecol Cancer 2014; 24: 4–12. [DOI] [PubMed] [Google Scholar]
- 18.Bosmuller H, Gruber C, Haitchi-Petnehazy S, et al. Primary angiosarcoma of the ovary with prominent fibrosis of the ovarian stroma. Case report of an 81-year old patient. Diagn Pathol 2011; 6: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 20.Cai JT, Liu HN, Zhang Y. Preparation and characterization of magnetic nanoparticles containing Fe(3)O(4)-dextran-anti-beta-human chorionic gonadotropin, a new generation choriocarcinoma-specific gene vector. Int J Nanomedicine 2011; 6: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlidis NA. Coexistence of pregnancy and malignancy. Oncologist 2002; 7: 279–287. [PubMed] [Google Scholar]
- 22.Sedgely MG, Ostör AG, Fortune DW. Angiosarcoma of breast metastatic to the ovary and placenta. Aust N Z J Obstet Gynaecol 1985; 25: 299–302. [DOI] [PubMed] [Google Scholar]
- 23.Van Calsteren K, Heyns L, De Smet F, et al. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J Clin Oncol 2010; 28: 683–689. [DOI] [PubMed] [Google Scholar]
- 24.Hermans RH, Fischer DC, Putten HW, et al . Adnexal masses in pregnancy. Onkologie 2003; 26: 167–172. [DOI] [PubMed] [Google Scholar]
- 25.Hermann GG, Fogh J, Græm N, et al. Primary hemangiosarcoma of the spleen with angioscintigraphic demonstration of metastases. Cancer 1984; 53: 1682–1685. [DOI] [PubMed] [Google Scholar]
- 26.Young RH, Scully RE. Sarcomas metastatic to the ovary. A report of 21 cases. Int J Gynecol Pathol 1990; 9: 231–252. [DOI] [PubMed] [Google Scholar]
- 27.Contreras AL, Malpica A. Angiosarcoma arising in mature cystic teratoma of the ovary: a case report and review of the literature. Int J Gynecol Pathol 2009; 28: 453–457. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen GP, Young RH, Prat J, et al. Primary angiosarcoma of the ovary: a report of seven cases and review of the literature. Int J Gynecol Pathol 1997; 16: 378–382. [DOI] [PubMed] [Google Scholar]
- 29.Kudela E, Nachajova M, Biringer K, et al. Bilateral ovarian angiosarcoma arising from the mature cystic teratomas: a case report and review of the literature. Int J Surg Case Rep 2018; 42: 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford L, Swartz K, Rose S. Primary angiosarcoma of the ovary complicated by hemoperitoneum: a case report and review of the literature. Arch Gynecol Obstet 2010; 281: 145–150. [DOI] [PubMed] [Google Scholar]
- 31.Zhang SH, Zheng YY, Liu HW, et al. Primary epithelioid angiosarcoma of the pleura: a case report and review of literature. Int J Clin Exp Pathol 2015; 8: 2153–2158. [PMC free article] [PubMed] [Google Scholar]
- 32.Folpe AL, Chand EM, Goldblum JR, et al. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol 2001; 25: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 33.Verbeke SL, Bertoni F, Bacchini P, et al. Distinct histological features characterize primary angiosarcoma of bone. Histopathology 2011; 58: 254–264. [DOI] [PubMed] [Google Scholar]
- 34.Hendry S, Forrest C. Epithelioid angiosarcoma arising in an adrenal cortical adenoma: a case report and review of the literature. Int J Surg Pathol 2014; 22: 744–748. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Zhao M, Huang J, et al. Primary epithelioid angiosarcoma of right hip joint: a case report and literature review. Medicine (Baltimore) 2018; 97: e0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham MJ, Brooks JS, Noumaoff JS. Treatment of primary ovarian angiosarcoma with ifosfamide and doxorubicin. Gynaecol Oncol 1994; 53: 265–268. [DOI] [PubMed] [Google Scholar]
- 37.Jha S, Chan KK, Poole CJ, et al. Pregnancy following recurrent angiosarcoma of the ovary: a case report and review of literature. Gynecol Oncol 2005; 97: 935–937. [DOI] [PubMed] [Google Scholar]
- 38.Karpeh MS, Jr, Caldwell C, Gaynor JJ, et al. Vascular soft-tissue sarcomas: an analysis of tumor related mortality. Arch Surg 1991; 126: 1474–1481. [DOI] [PubMed] [Google Scholar]
- 39.Iannaci G, Crispino M, Cifarelli P, et al. Epithelioid angiosarcoma arising in schwannoma of the kidney: report of the first case and review of the literature. World J Surg Oncol 2016; 14: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Huang X, Peng C, et al. Primary pulmonary epithelioid angiosarcoma: a case report and literature review. J Cancer Res Ther 2018; 14: S533–S535. [DOI] [PubMed] [Google Scholar]