Abstract
Poorly differentiated gastric adenocarcinoma is commonly associated with lymph node metastasis, peritoneal spread, and liver metastasis but rarely with intraintestinal metastasis. Most patients with metastatic gastric carcinoma are unable to undergo surgical treatment and have a poor prognosis. A 42-year-old man with hunger-related abdominal pain was diagnosed as having gastric cancer. After the first surgery (distal partial gastrectomy) and the second surgery (gastric stump carcinoma (GSC) resection), the patient suffered repeated multiple intracolonic metastases and underwent three additional resection operations. The patient survived for 154 months after the first operation. In patients with gastric carcinoma that metastasizes to the colonic lumen, radical resection, if possible, can extend survival. Once patients develop extensive extraintestinal metastasis, radical resection cannot be performed, and patients often exhibit a poor prognosis.
Keywords: Gastric carcinoma, intracolonic metastasis, multiple surgeries, resection, long-term survival, case report, radical resection
Introduction
Gastric carcinoma is a relatively high-grade malignant tumor with a 5-year survival rate of 20% to 40%.1 Surgical treatment is the preferred therapy for early-stage gastric carcinoma and can improve patient survival.2 Distant metastasis of gastric carcinoma requires comprehensive treatment centered on chemotherapy and is usually associated with a poor prognosis. In this paper, we report that multi-surgical resections extended survival time in a patient with gastric stump carcinoma (GSC) and multiple intracolonic metastases after gastrectomy.
Case report
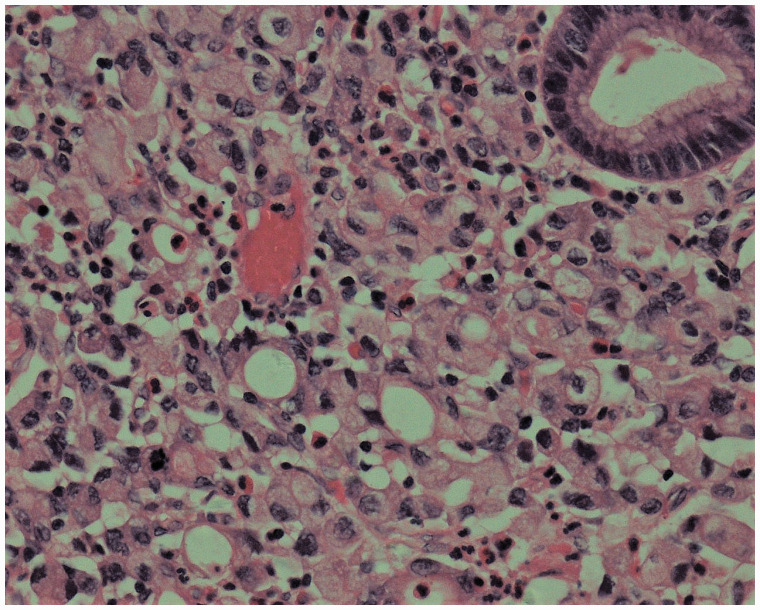
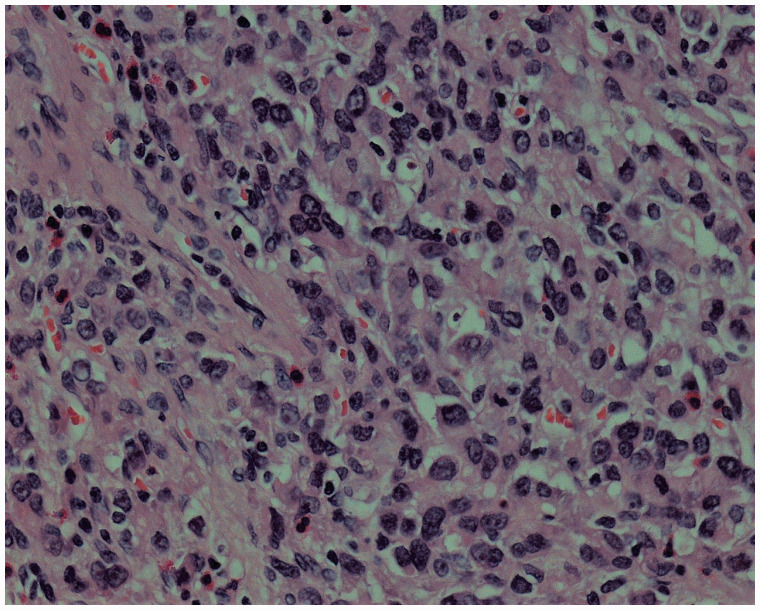
The male patient was first hospitalized at the age of 42 years, and radical distal gastrectomy was performed. Postoperative pathology [Figure 1] showed invasive poorly differentiated adenocarcinoma with lymph node metastatic cancer (6/21), categorized as tumor-node-metastasis stage T3N2M0 according to American Joint Committee on Cancer (AJCC) classification, 7th edition. Chemotherapy was initiated 16 days after the surgery (oxaliplatin (L-OHP), folinic acid (CF), and 5 fluorouracil (5-FU)) for six cycles and was completed in 6 months.
Figure 1.
Tissue pathology of a specimen obtained during the first surgery, hematoxylin and eosin (H & E) staining, ×400.
Seventy months after the initial surgery, gastroscopy showed a cauliflower-like mass at the gastrointestinal anastomosis site. Radical GSC resection and jejunal esophageal anastomosis were performed. Postoperative pathology revealed diffuse invasive and poorly differentiated adenocarcinoma, including signet ring cell carcinoma, of the residual stomach, with a large number of vascular tumor emboli. Lymph node metastatic carcinoma was identified (1/9). Chemotherapy was initiated 15 days after the second surgery (six cycles of paclitaxel, CF, 5-FU, and recombinant human endostatin (YH-16;3 Medgenn Bio-Pharmaceutical Co., Ltd., Yantai, China)) and was completed in 5 months.
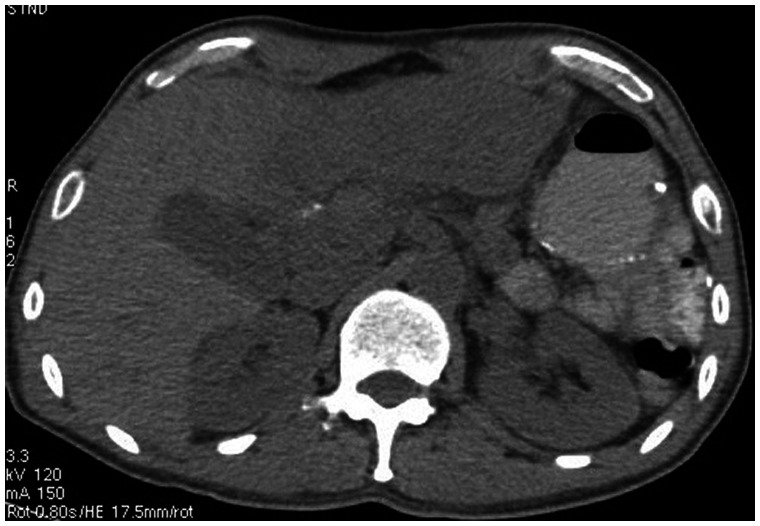
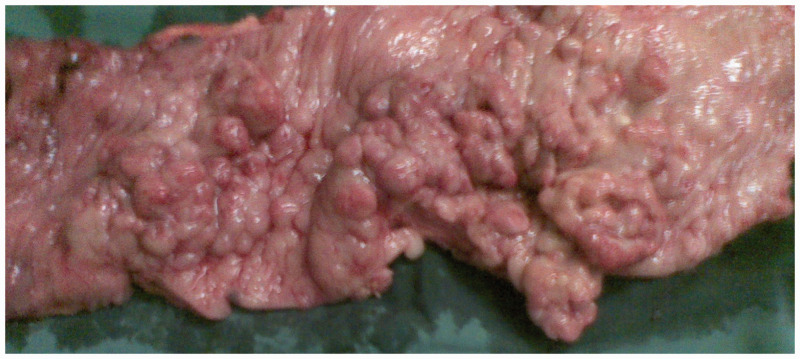
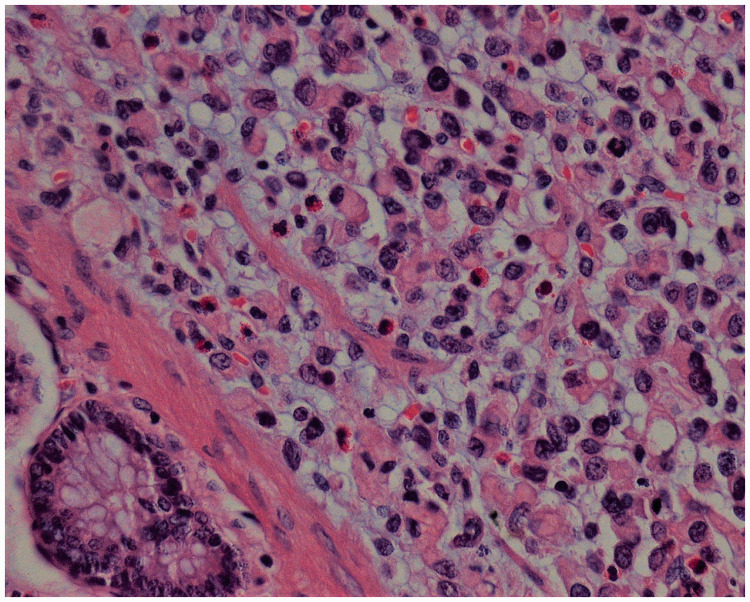
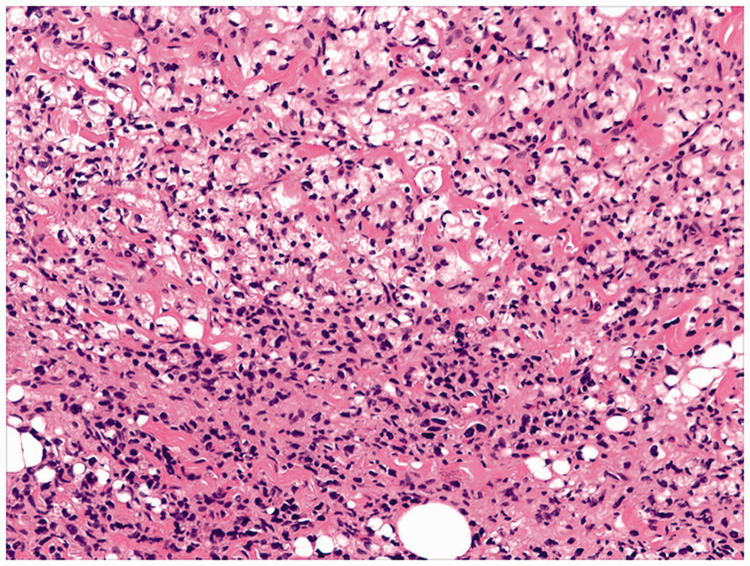
Beginning 110 months after the first surgery, the patient developed diarrhea 4 to 5 times/day, and lost 5 kg of weight within 5 months. Computed tomography (CT) revealed circumferential colonic wall thickening [Figure 2]. Colonoscopy demonstrated a concentric stenotic lesion 60 cm from the anal verge and another lesion measuring approximately 1.8 cm in the sigmoid colon; the third operation was performed. The tumors were located within the colon and manifested as multiple polypoid lesions [Figure 3]. In addition, local lesions penetrated the intestinal wall, invaded the peritoneal surface of the upper abdominal wall and part of the rectus abdominis posterior sheath, and adhered firmly to the falciform ligament. The colonic liver flexure, transverse colon, sigmoid colon, and partial abdominal wall tissue were resected. Postoperative pathology [Figure 4] revealed poorly differentiated adenocarcinoma (partially signet ring cell carcinoma). Lymph node metastatic cancer was also identified (7/26). After this surgery, the chemotherapy regimen constituted epirubicin hydrochloride (Famaxin; Pfizer Inc., New York, NY, USA), L-OHP, and capecitabine (Xeloda; Genentech, Inc., South San Francisco, CA) for eight cycles, and cetuximab was added from the fourth cycle to the eighth cycle;4 the therapy lasted for 4 months.
Figure 2.
Abdominal computed tomography (CT).
Note the circumferential thickening of the colonic wall.
Figure 3.
Macroscopic appearance of the resected colonic specimen showing multiple polypoid lesions.
Figure 4.
Tissue pathology of a specimen obtained during the third surgery, hematoxylin and eosin (H & E) staining, ×400.
Colonoscopy was performed 135 months after the first surgery. Biopsy showed poorly differentiated adenocarcinoma in the sigmoid colon. Positron emission tomography (PET)/CT and magnetic resonance imaging (MRI) showed para-gallbladder nodes, which were considered metastases. After a discussion with the multidisciplinary team (MDT), ultrasound-guided radiofrequency ablation was performed to ablate a 1.9-cm mass in the liver. The fourth surgery was performed 138 months after the first surgery. During the fourth surgery, multiple masses were observed in the sigmoid colon, which caused local intestinal stenosis (full circumference). Postoperative pathologic analysis revealed [Figure 5] multiple protruded-type poorly differentiated adenocarcinomas that had partially invaded the entire layer of the intestinal wall to the extra-serosal adipose tissue and to the serosal muscular layer of the adjacent small intestinal wall. Lymph node metastatic cancer was identified (3/6). On the 26th day after the procedure, colonic leakage from the abdominal incision was confirmed. After treatment, the leakage resolved, and full enteric nutrition was gradually restored. During this period, sunitinib and S-1 were given orally5 (irregularly).
Figure 5.
Tissue pathology of a specimen obtained during the fourth surgery, hematoxylin and eosin (H & E) staining, ×400.
One year after the fourth surgery, the patient developed dull lower abdominal pain and loose stool (4–5 times a day). Colonoscopy showed lesions in the colon 35 cm from the anal verge, and pathologic analysis revealed cancer cells. CT revealed an upper and middle abdominal mass that was considered a metastatic tumor. The fifth surgery was performed 151 months after the first surgery, which involved subtotal colectomy and ileal-rectal end-to-side anastomosis. The mass in the upper and middle abdomen was unresectable. Postoperative pathology [Figure 6] revealed poorly differentiated adenocarcinoma (partially signet ring cell carcinoma) in the sigmoid colon, invasion of the entire intestinal wall, tumor emboli in the vasculature, and cancer metastasis in the intestinal lymph nodes (2/13).
Figure 6.
Tissue pathology of a specimen obtained during the fifth surgery, hematoxylin and eosin (H & E) staining, ×200.
Subsequently, the mid-upper abdominal mass enlarged further around the pancreas, where it surrounded the blood vessels and gradually involved the porta hepatis; this enlargement was followed by high bile duct obstruction, combined with a liver abscess and obstructive jaundice. The patient died after multiple treatments. The survival time after the first surgery was 154 months.
Discussion
After tumor recurrence and metastasis, physicians should consider whether radical resection can be performed, if surgical treatment is an option, and the degree to which patients can benefit from the surgery; i.e., whether surgery can prolong survival or improve quality of life. Generally, only radical resection can prolong survival [Table 1]. Therefore, a thorough assessment should be performed before surgery, including the location of the metastatic tumor, its relationship with adjacent organs, and the patient’s expected quality of life after resecting the lesion. For patients undergoing multiple abdominal surgeries, multiple previous procedures will inevitably increase the difficulty of subsequent surgery owing to adhesions and changes in anatomical relationships. Thus, the risk of adverse injuries, such as intestinal leakage, bleeding, and pancreatic and ureteral injuries will increase. Tissue healing and organ function is affected after chemotherapy, and the patient’s tolerance to surgery may be reduced. In the current case, multiple operations were performed, and multiple courses of chemotherapy were administered after gastric cancer surgery, increasing the patient’s survival time.3–5 This result was related to the biological characteristics of the tumor itself, as well as to careful treatment design.
Table 1.
Previous studies of metachronous colorectal recurrence from gastric cancer after radical gastrectomy.
| No: | Year | First author | Age (years) | Sex | Primary tumor | Stage of GC* | Chemotherapy after gastrectomy | Disease-free interval | Recurrent lesion | Operation for colorectal lesion(s) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2014 | Noji et al6 | 61 | M | Por, Mod | IIA | No | 110 M | T, D, S | Lht, Tsc | 125 M, alive |
| 2 | 2014 | Noji et al6 | 46 | F | Por | IIIA | Yes | 106 M | R | LAR | 144 M, alive |
| 3 | 2018 | Su et al7 | 78 | M | Por | IIIB | Yes | 18 M | T | Tsc | 24 M, died |
| 4 | 2016 | Fujimoto et al8 | 58 | F | Por, Sig | IA | Yes | 28 M | S | Sdt, | 59 M, alive |
| 5 | 2018 | Uemura et al9 | 60 | M | Well | IA | No | 24 M | R | LAR | 79 M, alive |
| 6 | 2020 | Yang et al10 | 57 | M | Por, Sig Muc | IIIA | Yes | 30 M | A, C | Rht | 39 M, died |
| 7 | 2021 | Our case | 42 | F | Por, Sig | IIIA | Yes | 30 M | A, T, S | Rht, Sdt | 154 M, died |
*According to the 7th edition of the American Joint Committee on Cancer (AJCC).
Sex (M/F: male/female); GC, gastric cancer; C: cecum; A: ascending colon; T: transverse colon; D: descending colon; S: sigmoid colon; R: rectum; Por: poorly differentiated adenocarcinoma; Sig: signet ring cell adenocarcinoma; Muc: mucinous adenocarcinoma; Mod: moderately differentiated adenocarcinoma; Well: well-differentiated adenocarcinoma; LAR: low anterior resection; Rht: right hemicolectomy; Lht: left hemicolectomy; Tsc: transverse colectomy; Sdt: sigmoidectomy; M: month.
Colonic metastasis of gastric cancer is rare and has been reported only occasionally in the literature [Table 1].6–10 The characteristics of the case in this study were as follows: 1. The pathological diagnosis of specimens from the five surgeries was poorly differentiated adenocarcinoma containing components of signet ring cell carcinoma. 2. Lymph node metastasis was seen in all five surgeries. 3. Intestinal metastases included multiple, scattered polyp-like lesions, which were similar to those reported in the literature.11,12 4. Tumor marker concentrations, namely for carcinoembryonic antigen (CEA), carbohydrate antigen (CA)19-9, and CA724, were within normal ranges consistently over time.
We believe that the intestinal lesions in our patient originated in the stomach for the following reasons: First, regarding the timing, gastric adenocarcinoma occurred first, and colon cancer occurred later. Second, the morphology was similar; adenocarcinomas in the colon were similar to the first gastric adenocarcinomas. These tumors were diffusely invasive and poorly differentiated adenocarcinomas. No typical intestinal adenocarcinoma morphology and necrosis were observed, and no precancerous lesions were seen around the colonic carcinoma. Colonic adenocarcinoma lesions were mainly located in the serosal layer to the submucosa, and a small amount of cancer tissue was observed in the mucosal layer. Third, the immunophenotypes of the colonic tumors were similar to that of the gastric adenocarcinoma observed in the first surgery; i.e., cytokeratin (CK)7+/CK20−. This outcome suggests that the stomach was likely the primary site.
Intestinal metastasis of gastric cancer may be asymptomatic when the metastatic lesions are small. Metastases develop to a certain extent and can then manifest as diarrhea, abdominal pain, bloating, difficulty defecating, hematochezia, weight loss, and even intestinal obstruction. Owing to the lack of symptom specificity, intestinal metastasis is often difficult to clearly diagnose. If the patient has undergone surgical treatment for gastric cancer, surgery-related complications, especially adhesive intestinal obstruction, are the first consideration. If symptoms occur a long time after surgery, recurrence and metastasis are considered.
CT and ultrasonography are commonly used in postoperative examinations of gastric carcinoma. However, CT and ultrasonography are not very sensitive for diagnosing intestinal metastases. PET/CT is a more sensitive examination method, and colonoscopy is needed to confirm the diagnosis. However, owing to the low incidence of intestinal metastasis in gastric carcinoma, it is difficult to attract the attention of doctors and patients to this condition. Moreover, colonoscopy requires intestinal preparation, and patient compliance is low. Patients rarely undergo colonoscopy after gastric carcinoma surgery. To detect simultaneous gastric carcinoma with intestinal metastasis, it is often necessary to perform concurrent gastroscopy and colonoscopy. According to the biopsy pathology, the diagnosis can be confirmed through comparison with the endoscopy findings. Metachronous intestinal metastasis of gastric carcinoma (as in this case) is often diagnosed by colonoscopy after the appearance of more obvious symptoms. In the current case, the patient’s symptoms were diarrhea and weight loss. However, if intestinal metastatic lesions involve the submucosa in patients with an intact mucosa, lesion tissue must be obtained for pathological examination, which is difficult using colonoscopy. These lesions can be misdiagnosed as intestinal polyps, primary intestinal tumors, or Crohn's disease. Therefore, intestinal metastasis of gastric cancer is often difficult to diagnose, which can result in delayed treatment. Patients who develop intestinal lesions after gastric carcinoma surgery should be aware of the possibility of intestinal metastasis. Colonoscopy combined with PET/CT is recommended to ensure a clear and timely diagnosis. For patients with early intestinal metastatic lesions, endoscopic mucosal resection (EMR) can be used as a timely treatment.13
Patients with colonic metastases of gastric carcinoma often have a poor prognosis, with a survival period of 1 to 10 months;7 however, the survival time for the patient in this report was 154 months, even though multiple relapses occurred. After the previous three surgeries, no residual tumors were seen on gross examination. Postoperative chemotherapy and targeted therapy effectively extended the time to recurrence and metastasis. After the fourth surgery, owing to intestinal leakage, subsequent treatment was delayed, and the tumor recurred 13 months after the surgery. The fifth surgery was not a radical surgery. The tumor quickly involved the porta hepatis and led to obstructive jaundice combined with biliary infection and a liver abscess. The patient died 3 months after this surgery. Therefore, in surgery for colonic metastasis of gastric cancer, all lesions must be removed to ensure negative margins; this can prolong patients’ survival.6
Our patient had multiple repeated colonic metastases of gastric carcinoma but no liver metastases, lung metastases, or bone metastases before the fourth surgery. If total colectomy had been performed during the fourth surgery, it would have been difficult to predict whether subsequent recurrence and metastasis could have been avoided; however, the survival time may have been longer. The patient experienced tumor metastases in the porta hepatis with jaundice and a liver abscess before death. For similar patients with multiple colonic metastases of gastric carcinoma, we recommend performing total colectomy, when appropriate. This patient underwent five complicated abdominal surgeries. As the number of surgeries increased, intra-abdominal adhesions and anatomical variations increased the difficulty of subsequent operations. Furthermore, the patient had received relatively large doses of chemotherapy after each of the first three surgeries, and many physicians would be reluctant to perform such an extensive range of surgeries. The first partial gastrectomy and the second gastrectomy for remnant gastric carcinoma caused adhesions and anatomical variations near the splenic hilum, porta hepatis, and pancreatic tail. During the third surgery, unclear splenic blood vessel anatomy caused concerns regarding uncontrollable bleeding, and intestinal loop adhesions at the gastroesophageal anastomosis caused concerns regarding proximal intestinal leakage; therefore, the surgery failed to free the splenic flexure of the colon or to resect the local intestinal segment. To provide maximum benefits for such patients, the anatomical layers should be clearly distinguished intraoperatively to achieve radical resection and avoid accompanying injuries. After the fourth surgery, intestinal leakage affected the patient’s ability to eat. Although he tolerated enteral nutritional therapy with an elemental diet, his physical strength decreased significantly. Physicians must fully evaluate patient conditions before surgery; e.g., age, general condition, weight loss before surgery, and albumin levels, which are all associated with risks of complications and mortality.14–16
Intestinal metastasis of gastric carcinoma is a rare phenomenon, but it has been reported in the literature. The primary lesions may be diagnosed at an early stage,8 and colonic metastases may occur long after treatment of the primary lesions.6 Metastatic lesions can manifest as multiple or recurrent lesions, as in the patient in this report. To that end, we must pay attention to this metastatic pattern of gastric cancer, and we cannot ignore colonic examinations during regular follow-up after gastric carcinoma treatment. If colonic metastasis occurs, as long as the metastasis is detected early and radical surgery is performed, patients can still achieve longer survival.
Footnotes
Ethics statement: The case report was reviewed and approved by the Ethical Committee and Institutional Review Board of the Cancer Hospital, Chinese Academy of Medical Sciences. Patient details were de-identified; therefore, consent for publication by the patient was not required by our institution.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Qian Liu https://orcid.org/0000-0002-6319-5410
References
- 1.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37513025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer 2018; 21: 144–154. [DOI] [PubMed] [Google Scholar]
- 3.Zhang DW, Li HL, Yao Q, et al. The synergistic effect of recombinant human endostatin (YH-16) combined with oxaliplatin on human colorectal carcinoma. J Int Med Res 2010; 38: 111–126. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Guo W, Zhang W, et al. A multi-center phase II study and biomarker analysis of combined cetuximab and modified FOLFIRI as second-line treatment in patients with metastatic gastric cancer. BMC Cancer 2017; 17: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boku N, Muro K, Machida N, et al. Phase I study of sunitinib plus S-1 and cisplatin in Japanese patients with advanced or metastatic gastric cancer. Invest New Drugs 2014; 32: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noji T, Yamamura Y, Muto J, et al. Surgical resection of colorectal recurrence of gastric cancer more than 5 years after primary resection. Int J Surg Case Rep 2014; 5: 954–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su WC, Tsai HL, Wu CC, et al. Two rare cases of synchronous and metachronous colonic metastases in patients with advanced gastric cancer. World J Surg Oncol 2018; 16: 21. doi: 10.1186/s12957-018-1323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto D, Hirono Y, Goi T, et al. Sigmoid colonic metastasis by lymphatic spread occurring with unilateral Krukenberg tumor considered to be caused by stage IA early gastric cancer: a case report. Oncol Lett 2016; 11: 668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uemura N, Kurashige J, Kosumi K, et al. Early gastric cancer metastasizing to the rectum, possibly via a hematogenous route: a case report and review of literature. Surg Case Rep 2016; 2: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S, Liu XL, Guo XL, et al. Solitary metastasis to the skin and colon from gastric cancer after curative gastrectomy and chemotherapy: a case report. Medicine (Baltimore) 2020; 99: e21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao B, Xue X, Tai W, et al. Polypoid colonic metastases from gastric stump carcinoma: a case report. Oncol Lett 2014; 8: 1119–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonoda H, Kawai K, Yamaguchi H, et al. Lymphogenous metastasis to the transverse colon that originated from signet-ring cell gastric cancer: a case report and review of the literature. Clin Res Hepatol Gastroenterol 2017; 41: e81–e86. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H, Fu K, Fukui H, et al. A solitary colonic metastasis from gastric cancer detected at an early stage. Gastrointest Endosc 2008; 67: 1000–1004. [DOI] [PubMed] [Google Scholar]
- 14.Liu G, Xu M, Gao T, et al. Surgical compliance and outcomes in gastric cancer: a population-based cohort study. J Cancer 2019; 10: 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aahlin EK, Tranø G, Johns N, et al. Risk factors, complications and survival after upper abdominal surgery: a prospective cohort study. BMC Surg 2015; 15: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moghadamyeghaneh Z, Hanna MH, Hwang G, et al. Outcomes of colon resection in patients with metastatic colon cancer. Am J Surg 2016; 212: 264–271. [DOI] [PubMed] [Google Scholar]