Abstract
Objective
The present meta-analysis aimed to determine the relationship between intra-abdominal hypertension (IAH) and an increased prevalence of acute kidney injury (AKI) and identify the associated risk factors in various patient populations, regardless of whether they were admitted to an intensive care unit.
Methods
We used three databases for the following search terms: “IAH,” “abdominal compartment syndrome,” “AKI,” “acute kidney failure,” and others. The articles retrieved were compared to identify appropriate studies published until 7 May 2020. The main outcome was AKI.
Results
Six studies with 344 individuals were included. The patients were divided into two main groups: the IAH and non-IAH groups. Compared with patients without IAH, patients with IAH had a higher risk of AKI (odds ratio = 2.57, 95% confidence interval: 1.55–4.26). In the subgroup and meta-regression analyses, body mass index, age, the presence or absence of burns, and cardiac surgery did not affect the risk of AKI.
Conclusion
IAH was associated with AKI risk, and this association was not influenced by age, body mass index, the presence or absence of burns, or cardiac surgery.
Keywords: Intra-abdominal hypertension, intra-abdominal pressure, acute kidney injury, meta-analysis, abdominal compartment syndrome, intensive care unit
Introduction
Intra-abdominal pressure (IAP) is an important clinical variable in critically ill patients after surgery.1 Intra-abdominal hypertension (IAH) is defined as an IAP ≥12 mmHg according to the World Society of Abdominal Compartment Syndrome (WSACS).2 An IAP of 20 mmHg is associated with abdominal compartment syndrome (ACS), which can lead to the failure of multiple organs, including the kidneys and lungs.3,4 However, the significance of IAH is often underappreciated by clinicians.3,5 A clinical survey indicated that less than 30% of doctors were aware of the definition of IAH, and only a few of them understood how to apply the WSACS guidelines for patients with increased IAP.5
A recent worldwide cohort study of 15 intensive care units (ICUs) that screened 491 patients found that IAH was present in 34% of patients on the day of ICU admission, and nearly 50% of individuals developed IAH during the observational period. This study also found that IAP was increased in almost 50% of all patients in ICUs, and the severity of IAP was found to independently increase the 28- and 90-day mortality rates.6 Indeed, a large number of patients with IAH develop organ failure, a complication that occurs even with moderate increases in IAP.7 For instance, higher levels of necrosis in the gut mucosa and respiratory diseases have been found among patients with IAH.4,8 Acute kidney injury (AKI) is gradually being recognized as a complication of IAH. Although the mechanism of IAH-induced AKI remains unclear, three main factors are suspected to be involved: (1) isolated elevation in renal vein pressure,9 (2) increased renal parenchymal pressure indirectly causing renal dysfunction via ischemic insult,10 and (3) elevation in catecholamine, renin, angiotensin, and inflammatory cytokine levels.11–13 However, another study reported that IAH did not predict the risk of AKI.14 Moreover, other events in critically ill patients may worsen renal perfusion, such as severe infection.
Thus, it remains unclear whether IAH increases the risk of AKI. Therefore, the present meta-analysis aimed to comprehensively summarize the available data on the relationship between increased IAP and the risk of AKI to raise the awareness of IAH and IAH-induced AKI among physicians and facilitate their timely detection and treatment.
Materials and methods
Literature search
The present systematic review and meta-analysis was performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.15 The PubMed, Cochrane Library, and EMBASE databases were searched to identify appropriate studies published until 7 May 2020. A combination of free terms and Medical Subject Heading terms was used to select the following search terms: (intra-abdominal pressure OR intra-abdominal hypertension OR abdominal compartment syndrome) and (acute kidney disease OR kidney diseases OR renal insufficiency OR acute kidney injury OR acute kidney failure OR AKI OR AKF). Ethical approval and informed consent were not applicable because this study was a meta-analysis of published data.
Study selection
Two reviewers independently selected relevant studies based on the selection criteria. Studies were included in this meta-analysis if they met the following criteria: (a) individuals with IAH, (b) IAH definition based on the WSACS recommendations, (c) acute kidney disease, and (d) observational studies. Studies were excluded if the participants had acute kidney disease before admission or if all participants were under 18 years of age. Duplicated articles were removed using the Endnote X9 software package (Clarivate, Philadelphia, PA, USA), and potentially eligible studies were identified by screening the titles and abstracts of the remaining articles. The full texts of the studies were subjected to a thorough review to identify appropriate trials for inclusion in this meta-analysis. Any disagreements were resolved by consultation with a third reviewer. If the odds ratio (OR) for IAH and AKI was provided in a particular study, we used the value directly. If not, we used Review Manager (RevMan) [computer program] Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) to calculate the OR for the IAH group vs. the non-IAH group.
Quality assessment
The Newcastle–Ottawa Scale was used to determine the quality of the included trials. Two reviewers independently graded the included trials according to the following categories: ascertainment of outcomes, comparability of study groups, and selection of participants. The items in the above categories were awarded a maximum of four stars, and studies with 7 to 9 stars were deemed to be of high quality.
Statistical analysis
All statistical analyses were conducted using the STATA software package version 11.0 (Stata Corp., College Station, TX, USA). We compared the risk of AKI between the IAH and non-IAH groups. The results were expressed as combined ORs. When the I2 statistic was <50%, a fixed-effects model was applied; otherwise, a random-effects model was used. Visual inspection of funnel plots was used to detect publication bias. Funnel plot asymmetry was assessed using the Egger test and Begg test (P < 0.1). P values less than 0.05 were considered statistically significant.
Results
Characteristics of included studies
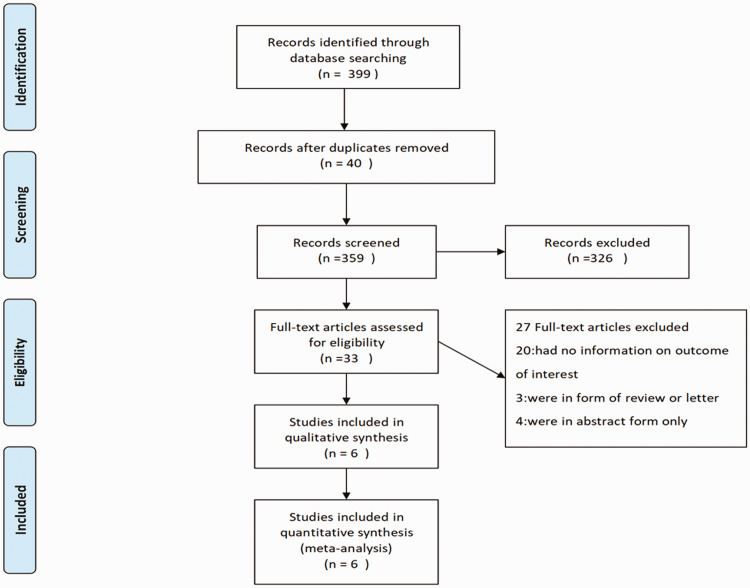
Of 359 screened studies, 6 were included in this meta-analysis (Figure 1). These six studies enrolled a total of 344 patients, including patients admitted to an ICU and patients receiving tertiary care. Most patients were European. The main characteristics and quality of the six included studies are shown in Tables 1 and 2, respectively.
Figure 1.
PRISMA flowchart of study selection
Table 1.
Characteristics of observational trials included in the meta-analysis
| Study | Country | Men/Patients (n/n) | BMI (kg/m2) | Mean age (yrs) | Baseline comorbidities and ward/room | Mean IAP | How and when IAP was measured | IAH definition | Concomitant treatment | Outcome | Outcome definition |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Talizin et al.18, 2017 | Brazil | 33/46 | NR | 41 | Burns; in an ICU room | 15.0 mmHg | Measurement was within 3 hours of admission; the AbViser measurement system was used. | ≥12 mm Hg | Vasopressors, mechanical ventilation, glycopeptides, polymyxin | AKI | KDIGO (2012) |
| Ruiz-Castilla et al.7, 2014 | Spain | 21/25 | 24.5 | 42 | Cancer, hypertension, ischemic cardiopathy; in the Burns Unit | 13 mmHg | Measurement was every 6 hours during the 5-day study period; a closed measurement system (Abdo-Pressure™, ConvatecSL, Espana) was used. | ≥12 mm Hg | Mechanical ventilation, crystalloids | Renal failure | Plasma creatinine >1.5 mg/dL during first 5 days of admission |
| Dalfino et al.19, 2008 | Turkey | 78/123 | NR | 62 | Chronic cardiovascular disease, chronic respiratory disease, chronic renal disease, chronic liver disease; in a mixed ICU room. | 12 mmHg | Measurements were performed on admission and at least every 24 hours; a Foley bladder catheter was used. | ≥12 mm Hg | Diuretics. CRRT, crystalloids | RIFLE | ADQI (2004) |
| Mazzeffi et al.1, 2016 | USA | 29/42 | 29.1 ± 5.2 | 61 | Chronic lung disease, peripheral vascular disease, cerebrovascular disease; in the general ward | NR | Measurements were prior to surgery; immediately after surgery in the operating room; and 3, 6, 12, and 24 hours after surgery; bladder pressure was used. | ≥12 mm Hg | NR | RIFLE | ADQI (2004) |
| Demarchi et al.17, 2014 | USA | 35/60 | 26 ± 5.0 | 59 | NR; in an ICU room | NR | Measurements were at admission to the ICU; bladder pressure was used. | ≥12 mm Hg | NR | AKI | KDIGO (2012) |
| Tyagi et al.14, 2018 | India | 0/48 | 22.2 ± 3 | 25 | NR; in an ICU room | NR | Measurements were twice daily; a Foley bladder catheter was used. | ≥12 mm Hg | NR | AKI | KDIGO (2012) |
BMI, body mass index; IAP, IAH, intra-abdominal hypertension; ICU, intensive care unit; AKI, acute kidney injury; NR, not reported; KDIGO, Kidney Disease Improving Global Outcomes; ADQI, Acute Dialysis Quality Initiative; RIFLE, Risk, Injury, Failure, Loss of Kidney Function, and End-stage Kidney Disease.
Table 2.
Newcastle–Ottawa Scale quality assessment of included studies.
| Selection of participants | Comparability of study groups | Assessment of outcome/ ascertainment of exposure |
Total score* | |
|---|---|---|---|---|
| Talizin et al.18 | < > > |
< > > |
< > > |
7 |
| Ruiz-Castilla et al.7 | < > > |
< > > |
< > > |
7 |
| Dalfino et al.19 | < > > |
< > > |
< > > |
8 |
| Mazzeffi et al.1 | < > > |
< > > |
< > > |
7 |
| Demarchi et al.17 | < > > |
< > > |
< > > |
7 |
| Tyagi et al.14 | < > > |
< > > |
< > > |
7 |
*Studies with 7 to 9 stars were deemed to be of high quality.
All included studies compared the IAH group with the non-IAH group. ORs were used in all studies. AKI was not present at the time of admission in any of the patients.
IAH group vs. non-IAH group
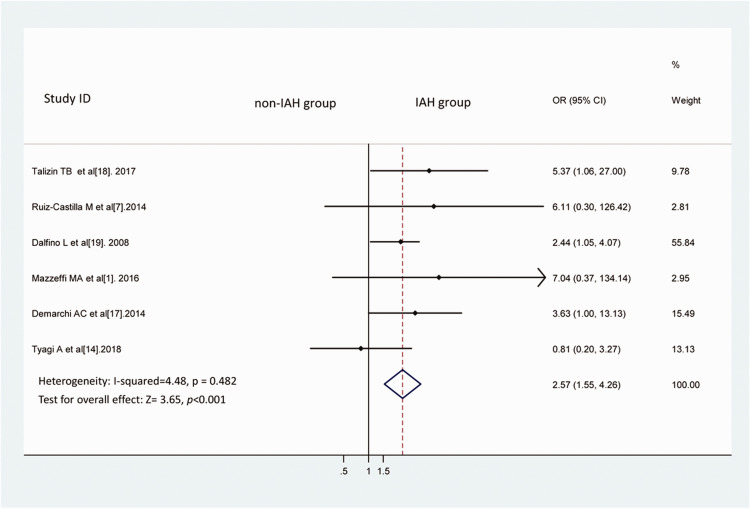
Compared with the non-IAH group, the IAH group showed a higher incidence of AKI (Figure 2), as determined using the fixed-effects model [I2 = 4.48, OR = 2.57, 95% confidence interval (CI): 1.55–4.26, P < 0.001]. No significant heterogeneity was present.
Figure 2.
IAH and the risk of AKI. We compared the risk of AKI between the IAH and non-IAH groups using a fixed-effects model. The results were expressed as combined ORs.
IAH, intra-abdominal hypertension; AKI, acute kidney injury; OR, odds ratio; CI, confidence interval.
Subgroup and meta-regression analysis
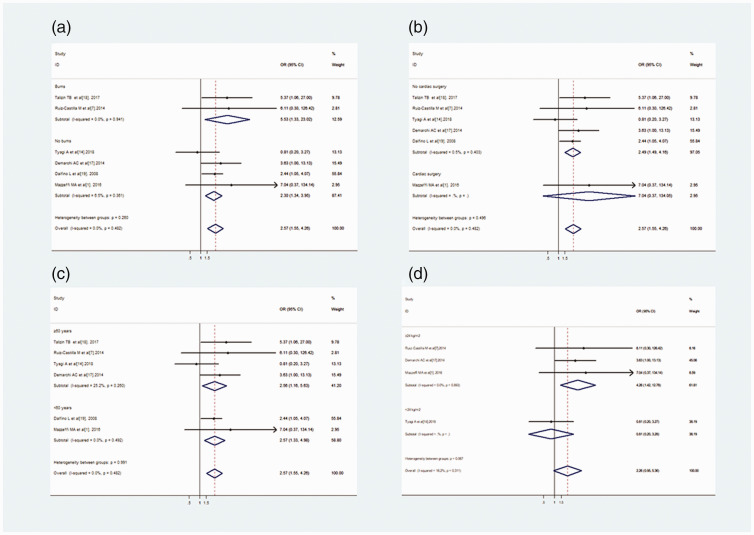
We conducted a subgroup analysis by dividing individuals into several groups based on the cause of hospital admission, whether or not they had undergone cardiac surgery, average age, and body mass index (BMI). The subgroup analysis indicated that IAH increased the risk of AKI regardless of the average age compared with patients without IAH (≥60 years: OR = 2.56, 95% CI: 1.16–5.63, P = 0.02; <60 years: OR = 2.57, 95% CI: 1.33–4.98, P = 0.005). The incidence of a BMI ≥24 significantly differed between the IAH and non-IAH groups (OR = 4.26, 95% CI: 1.42–12.78, P = 0.01; Figure 3). However, in the meta-regression models based on patient characteristics, BMI and average age were not associated with the risk of AKI. In addition, the presence or absence of burns or cardiac surgery did not affect the risk of AKI (Table 3).
Figure 3.
Subgroup analyses. Burns and No burns (a), Cardiac surgery and No cardiac surgery (b), and average age ≥60 years and average age <60 years between the IAH group and Non-IAH group (c). Subgroup analysis of BMI ≥24 kg/m2 and BMI <24 kg/m2.
IAH, intra-abdominal hypertension; BMI, body mass index; OR, odds ratio; CI, confidence interval.
Table 3.
Subgroup analysis and meta-regression models
| Subgroup | Studies (n) | P value* | P value** |
|---|---|---|---|
| Cause of hospital admission | |||
| Burns | 2 | 0.019 | 0.32 |
| No burns | 4 | 0.003 | |
| Cardiac surgery | 1 | NA | 0.53 |
| No cardiac surgery | 5 | 0.194 | |
| Average age | |||
| IAH vs. Non-IAH | |||
| ≥60 years | 4 | 0.02 | 0.96 |
| <60 years | 2 | 0.005 | |
| Body mass index | |||
| ≥24 kg/m2 | 3 | 0.01 | 0.21 |
| <24 kg/m2 | 1 | NA | |
IAH, intra-abdominal hypertension.
*P value represents test effect of subgroup analysis; **P value represents interaction assessed by meta-regression models.
Publication bias and sensitivity analysis
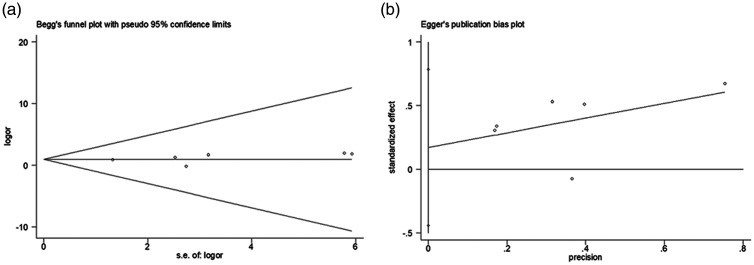
No significant funnel plot asymmetry (Figure 4) or publication bias was found. Sensitivity analysis could not be conducted owing to the limited number of trials.
Figure 4.
Egger test (a) and Begg test (b) results of the 6 included studies. Visual inspection of funnel plots was performed to detect publication bias. Funnel plot asymmetry was assessed using the Egger test and Begg test (P < 0.1)
Discussion
IAH is frequently linked to ACS,16 which contributes to serial organ dysfunction. However, the impact of IAH is underestimated and remains controversial.3 This meta-analysis is the first to demonstrate that acute renal outcomes were linked to IAH in patients in ICUs and not in ICUs. In addition, we demonstrated that patient characteristics, such as age, BMI, the presence or absence of burns, or cardiac surgery, were not linked to the risk of IAH-induced AKI.
An increasing number of studies are now reporting that IAH may lead to AKI. Demarchi et al. reported that increased IAP was related to a higher incidence of AKI after abdominal surgery; the authors found that the IAP measured on the day of ICU admission significantly predicted the development of AKI in this population with a sensitivity of 87% and a specificity of 46%. In addition, IAH does not always occur after surgery.17 Mazzeffi et al. reported that among 42 patients undergoing cardiac surgery, 35 (83.3%) patients had at least one elevated IAP measurement during the perioperative period; the authors concluded that in patients undergoing cardiac surgery, IAH might increase the risk of AKI, which is consistent with our results. The above findings indicate that AKI might be related to IAH, regardless of the severity of illness and the operation type.1 However, one study reported that IAP did not correlate with early AKI in critically ill obstetric patients.14 Therefore, we conducted a meta-analysis and determined the following: (1) IAH was associated with a higher prevalence of AKI; (2) the pooled OR demonstrated a 2.57-fold increased risk of AKI; and (3) our meta-analysis included both burn and non-burn patients, patients who underwent cardiac surgery and those who underwent non-cardiac surgery,1,7,18,19 patients whose average age was ≥60 years and those whose average age was <60 years, and patients whose BMI was ≥24 kg/m2 and those whose BMI was <24 kg/m2. Nevertheless, there was no heterogeneity among the included studies.
Persistent IAH is theorized to lead to a higher incidence of AKI via three main mechanisms. First, the IAH-induced decrease in renal function may be a consequence of increased renal vein pressure. One study of a swine model reported that an elevation in renal vein pressure (0–30 mmHg above the baseline) in the experimental group resulted in a significant decrease in the glomerular filtration rate (from 26 mL/min to 8 mL/min) compared with the control group.9 These changes were partially or completely reversed by decreasing the renal vein pressure. Second, the elevation in renal parenchymal pressure in a porcine model was not associated with a significant change in the glomerular filtration rate compared with the control group;20 nevertheless, elevated renal parenchymal pressure might indirectly cause renal dysfunction via renal ischemia.
There are other factors in the included studies that may be responsible for AKI, such as mechanical ventilation (MV). A systematic review and meta-analysis showed that invasive MV is associated with a three-fold increase in the likelihood of developing AKI, and various tidal volume and positive end-expiratory pressure settings do not modify this risk.21 Of the six articles included in this study, four articles found no link between MV and AKI,7,14,17 and two articles provided no data regarding MV causing AKI.1,19 Only one study indicated a link between MV and AKI.18 Therefore, in the present study, we consider that the influence of MV was negligible.
Certain limitations of our study must be acknowledged. First, few clinical trials were included, and all had relatively small sample sizes. Second, the study design was not blinded, and the effect of confounding factors cannot be excluded. Third, the outcome definitions were different. In the future, large-scale studies involving a stratified analysis of the association between IAH and AKI are required.
Conclusion
The present meta-analysis found that IAH was linked to a higher prevalence of AKI. In addition, patient characteristics, such as burns, cardiac surgery, age, and BMI, did not influence the risk of IAH-induced AKI. Further trials are needed to verify the relationship between IAH and AKI in different populations and determine how IAH-induced AKI may be prevented.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jianxin Wei https://orcid.org/0000-0001-8469-5409
References
- 1.Mazzeffi MA, Stafford P, Wallace K, et al. Intra-abdominal Hypertension and Postoperative Kidney Dysfunction in Cardiac Surgery Patients. J Cardiothorac Vasc Anesth 2016; 30: 1571–1577. [DOI] [PubMed] [Google Scholar]
- 2.Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32: 1722–1732. [DOI] [PubMed] [Google Scholar]
- 3.Patel DM, Connor MJ., Jr. Intra-Abdominal Hypertension and Abdominal Compartment Syndrome: An Underappreciated Cause of Acute Kidney Injury. Adv Chronic Kidney Dis 2016; 23: 160–166. [DOI] [PubMed] [Google Scholar]
- 4.Tonetti T, Cavalli I, Ranieri VM, et al. Respiratory consequences of intra-abdominal hypertension. Minerva Anestesiol 2020; 86: 877–883. [DOI] [PubMed] [Google Scholar]
- 5.Wise R, Roberts DJ, Vandervelden S, et al. Awareness and knowledge of intra-abdominal hypertension and abdominal compartment syndrome: results of an international survey. Anaesthesiol Intensive Ther 2015; 47: 14–29. [DOI] [PubMed] [Google Scholar]
- 6.Reintam Blaser A, Regli A, De Keulenaer B, et al. Incidence, Risk Factors, and Outcomes of Intra-Abdominal Hypertension in Critically Ill Patients-A Prospective Multicenter Study (IROI Study). Crit Care Med 2019; 47: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz-Castilla M, Barret JP, Sanz D, et al. Analysis of intra-abdominal hypertension in severe burned patients: the Vall d'Hebron experience. Burns 2014; 40: 719–724. [DOI] [PubMed] [Google Scholar]
- 8.Cheng J, Wei Z, Liu X, et al. The role of intestinal mucosa injury induced by intra-abdominal hypertension in the development of abdominal compartment syndrome and multiple organ dysfunction syndrome. Crit Care 2013; 17: R283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doty JM, Saggi BH, Sugerman HJ, et al . Effect of increased renal venous pressure on renal function. J Trauma 1999; 47: 1000–1003. [DOI] [PubMed] [Google Scholar]
- 10.Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol 2011; 22: 615–621. [DOI] [PubMed] [Google Scholar]
- 11.Oda J, Ivatury RR, Blocher CR, et al. Amplified cytokine response and lung injury by sequential hemorrhagic shock and abdominal compartment syndrome in a laboratory model of ischemia-reperfusion. J Trauma 2002; 52: 625–631; discussion 632. [DOI] [PubMed] [Google Scholar]
- 12.Mikami O, Fujise K, Matsumoto S, et al. High intra-abdominal pressure increases plasma catecholamine concentrations during pneumoperitoneum for laparoscopic procedures. Arch Surg 1998; 133: 39–43. [DOI] [PubMed] [Google Scholar]
- 13.Rezende-Neto JB, Moore EE, Melo De Andrade MV, et al . Systemic inflammatory response secondary to abdominal compartment syndrome: stage for multiple organ failure. J Trauma 2002; 53: 1121–1128. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi A, Lahan S, Verma G, et al. Role of Intra-abdominal Pressure in Early Acute Kidney Injury: A Prospective Cohort Study in Critically Ill Obstetric Patients. Indian J Crit Care Med 2018; 22: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers WK, Garcia L. Intraabdominal Hypertension, Abdominal Compartment Syndrome, and the Open Abdomen. Chest 2018; 153: 238–250. [DOI] [PubMed] [Google Scholar]
- 17.Demarchi AC, De Almeida CT, Ponce D, et al. Intra-abdominal pressure as a predictor of acute kidney injury in postoperative abdominal surgery. Ren Fail 2014; 36: 557–561. [DOI] [PubMed] [Google Scholar]
- 18.Talizin TB, Tsuda MS, Tanita MT, et al . Acute kidney injury and intra-abdominal hypertension in burn patients in intensive care. Rev Bras Ter Intensiva 2018; 30: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalfino L, Tullo L, Donadio I, et al. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med 2008; 34: 707–713. [DOI] [PubMed] [Google Scholar]
- 20.Doty JM, Saggi BH, Blocher CR, et al . Effects of increased renal parenchymal pressure on renal function. J Trauma 2000; 48: 874–877. [DOI] [PubMed] [Google Scholar]
- 21.Van Den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care 2013; 17: R98. [DOI] [PMC free article] [PubMed] [Google Scholar]