Abstract
Background:
Little is known on the clinical manifestations of coconut allergy. Our knowledge to date is mainly based on case reports.
Objective:
To characterize the allergic reactions to coconut and suggest diagnostic cutoffs for specific immunoglobulin E (sIgE) and skin prick testing (SPT) to predict clinically reactive coconut allergy.
Methods:
Methods include retrospective chart review at an urban tertiary care center of patients with positive testing result for coconut. Probability curves were computed by logistic regression for SPT and coconut sIgE.
Results:
Of 275 records reviewed, 69 patients reported coconut reactions and 206 were sensitized only or nonallergic. The reactions occurred with breastfeeding (n = 2), contact (n = 10), or oral ingestion (n = 57). Approximately 50% of oral ingestion reactions were associated with mild/moderate anaphylaxis. Clinical reactivity vs sensitization was more common in topical coconut users (2-fold) (P = .02). Although not statistically significant, there was a trend toward more coconut allergy vs sensitization in Asian and African American patients. The probability of allergy with positive SPT result was approximately 50% and with sIgE was approximately 60%. At an SPT of 9 mm wheal or sIgE of 58 kU of allergen/L, there is a 95% probability of reaction. Cosensitization with tree nuts, legumes, and seeds was common. Macadamia nut had the strongest correlation with coconut (r = 0.81, P < .001, n = 101).
Conclusion:
Although the rate of reactivity to coconut in sensitized individuals is low, half of the reactions from consumption met the criteria for anaphylaxis. Clinicians should be aware of the spectrum of reactions and diagnostic use of sIgE and SPT.
Introduction
Coconut allergy is becoming a more common concern among parents of children with food allergy, in part owing to the required labeling on packaged foods.1 In addition, coconut has increasingly become part of the US diet and is a nutritional alternative beverage for children with cow’s milk allergy. It is striking that coconut is the most common food allergen present in commercially available skin care products, with 1 study revealing approximately 75% of shampoos and body soaps contain coconut.2 Furthermore, coconut has been popularized as a natural moisturizer for babies, particularly children with atopic dermatitis at high risk for food allergy.3 In infants, applying coconut to inflamed skin and not engaging in oral consumption is a concerning set-up for percutaneous sensitization and ultimately food allergy, not oral tolerance.4–6
Importantly, coconut (Cocos nucifera) is a fruit and not a tree nut, a misconception perpetuated by the Food Allergen Labeling and Consumer Protection Act which requires coconut labeling on packaged foods.7 We have observed that coconut is often tested in evaluation of tree nut allergies likely because it is included in tree nut allergen panels. In a study of children with allergy to tree nut, coconut sensitization was reported to be approximately 30% in 298 children7 and approximately 20% in another study of 191 children with sesame and tree nut allergy, with a 25% patient-reported rate of allergic reactions.1 Although reaction characteristics have been described in published case reports and case series,8–14 diagnostic cutoffs associated with reactions on specific immunoglobulin E (sIgE) and skin prick testing (SPT) have not been established.
Given our institutional experience and the paucity of literature on this topic, we sought to: (1) characterize the spectrum of reactions to coconut from a US cohort and (2) assess for possible diagnostic cutoffs for sIgE and SPT that may correlate with clinically relevant coconut allergy.
Methods
Chart Review
After the institutional review board approval, we retrospectively identified patients who were evaluated in the allergy clinic at Ann & Robert H. Lurie Children’s Hospital of Chicago between January 1, 2002, and August 1, 2017, and had sensitization (≥0.1 kU of allergen/L [kUa/L] for sIgE or ≥3 mm wheal to bifurcated needle on SPT) to coconut. Patients were excluded if they had no clinical notes in the medical record. Medical records including clinical notes, demographic information, and laboratory data were reviewed. Demographic information, such as insurance type, race or ethnicity, and age, was captured from the initial visit. The International Classification of Diseases–diagnosed asthma, allergic rhinitis, or atopic dermatitis was captured from the initial visit for coconut allergy evaluation. Results of SPT to coconut extract (Greer) and coconut sIgE (Phadia ImmunoCAP) testing performed at the initial evaluation for coconut and subsequent visits if available were included. Detailed manual chart review of physician/nurse practitioner notes was conducted to determine the characteristics of initial reactions to coconut and subsequent reactions if they occurred. Use of coconut-containing products for topical application was noted when documented in the chart. Dates of reactions or oral food challenges were coded when available.
Statistical Analyses
Patient characteristics were compared between sensitized patients who had no history of reaction (or no history of ingestion) and those with self- or parent-reported history of reaction, using χ2 for frequency data and t test to compare means. Descriptive statistics were used to compare reaction characteristics. Cosensitization to other tree nuts, soy, and common food allergens was compared using Pearson correlation coefficient. Receiver operating characteristic curves and probability curves (from logistic regression) were computed to develop diagnostic predictors to differentiate sensitized vs patients with allergy. We used the Statistical Package for Social Sciences (version 25; International Business Machines Corporation, Chicago, Illinois). A P value less than .05 was considered statistically significant.
Results
Patient Characteristics
We identified 275 patients with positive coconut allergen testing result either by means of SPT or sIgE who had been evaluated in our allergy clinic. In reviewing the chart of the initial encounter for coconut allergy evaluation, we found that 69 (25%) had a history of contact/ingestion with reaction, 9 (3%) had ingested without reaction, and 197 (72%) were sensitized with no known history of ingestion. Average SPT wheal size between these groups ± SD was 7.67 ± 3.92 (n = 30), 4.75 ± 2.87 (n = 4), and 4.38 ± 2.64 (n = 52), respectively (P < .01). Coconut sIgE means ± SD were 13.83 ± 18.48 kUa/L (n = 62), 2.95 ± 4.03 kUa/L (n = 8), and 7.06 ± 13.92 kUa/L (n = 168), respectively (P < .01). The characteristics of patients sensitized with no known exposure or no history of reaction are compared with those with sensitization and history of reaction in Table 1. Coconut sIgE and SPT wheal and flare sizes were significantly different between the groups. Furthermore, although not statistically significant, there was a trend for racial demographic differences between the group with allergy vs the sensitized-only group. Asian children had a 2-fold increased rate of allergy vs sensitization (17.4% vs 8.7%), and African American children had a 1.5-fold increased rate (13.0% vs 8.7%). Moreover, although not many charts documented whether or not coconut oil had been applied topically (n = 57), 22% of patients with allergy vs 10% of sensitized patients reported use of such products.
Table 1.
Characteristics of Sensitized vs Children With Allergy to Coconut in Children on Initial Presentation to our Practice
| Variable | Sensitized with no history of exposure or no history of reaction (n = 206) | Allergic, sensitized with history of reaction (n = 69) | P value |
|---|---|---|---|
| Male, n (%) | 127 (61.6) | 44 (63.7) | .75 |
| Age (at first coconut test), mean (SD) | 6.18 (4.07) | 5.57 (4.28) | .29 |
| Race or ethnicity, n (%) | |||
| White | 136 (66.0) | 33 (47.8) | |
| Black or African American | 18 (8.7) | 9 (13.0) | |
| Hispanic or Latino | 29 (14.1) | 11 (15.9) | |
| Asian | 18 (8.7) | 12 (17.4) | |
| Other or unknown | 5 (2.4) | 4 (5.8) | |
| Insurance, n (%) | .72 | ||
| Private | 153 (74.3) | 54 (78.2) | |
| Public | 51 (24.8) | 14 (20.3) | |
| Government or other | 2 (1.0) | 1 (1.4) | |
| History of asthma (at time of first coconut test), n (%) | 78 (37.9) | 27 (39.1) | .85 |
| History of atopic dermatitis (at time of first coconut test), n (%) | 123 (59.7) | 44 (63.8) | .55 |
| History of allergic rhinitis (at time of first coconut test), n (%) | 111 (53.9) | 39 (56.5) | .70 |
| Other food allergies, n (%) | 206 (100) | 67 (97.1) | .01 |
| ImmunoCAP (n = 238) | 6.87 (13.65), range: 0.24–78.9 | 13.83 (18.48); range, 0.38–69.0 | <.01 |
| Skin test wheal (n = 86) | 4.41 (2.63), range: 0–13.0 | 7.67 (3.93); range, 0–17.0 | <.01 |
| Skin test flare (n = 83) | 11.11 (6.27), range: 0–26.0 | 17.41 (7.41); range, 0–30.0 | <.01 |
| History of initial reaction (n = 69) | |||
| At first exposure | 9 (13.0) | ||
| Previously tolerating | 5 (7.2) | ||
| Not documented if first exposure | 55 (79.7) | ||
| Initial source of reaction, n (%) | |||
| Ingestion | 57 (82.6) | ||
| Skin contact | 10 (14.5) | ||
| Breastfed | 2 (2.9) | ||
| Age at initial reaction (n = 58), mean (SD) | 5.0 (4.4) | ||
| History of topical coconut application, n (%) | .02 | ||
| Yes | 21 (10.2) | 15 (21.7) | |
| No | 14 (6.8) | 7 (10.1) | |
| Not documented | 171 (83.0) | 47 (68.1) |
Bolded values are significant (P < .05).
Reaction Characteristics
Of the 69 patients with known coconut reactions, 2 reacted owing to breastfeeding exposure, 10 reacted with skin contact, and 57 reacted after ingestion. Of the 57 patients who had oral ingestion, 4 had a mixed allergen ingestion that included another known allergen, making it unclear whether coconut was definitively implicated (details provided in eResults). Figure 1 characterizes the ingestion reactions of the 53 patients with reactions clearly to coconut only. Regarding the reactions, 17% had mild mouth/tongue pruritus or throat symptoms, 34% had rash, urticaria, pruritus or angioedema, 25% had nasal congestion, vomiting/diarrhea, or mild anaphylaxis, and 25% had moderate anaphylaxis (ie, respiratory symptoms present or more than 1 system affected). In the 9 patients with oral or throat-only reactions, 7 were coincidentally evaluated by our standard aeroallergen panel. Of the pollens tested, 4 patients had allergy to ragweed (short or long ragweed), 5 to grass (timothy or orchard), and 5 to tree (ash, oak, elm, birch, or maple/box elder). There were no anaphylactic reactions with contact-only exposure; all were characterized by cutaneous reactions; urticaria (n = 3), diffuse pruritus (n = 1), or nonurticarial rash (n = 6). Of these patients, 6 had localized reactions immediately after application and the remaining 4 charts reported nonurticarial rash but did not have specific timing or details. Breastfeeding reactions in 2 patients were recorded as rash or eczema. In both of these patients, the reaction occurred at under 6 months of age. The sIgE was drawn shortly after the reaction in 1 patient (0.57 kUa/L) and several years later in the other patient (4.16 kUa/L); no SPT was performed. Only 1 patient continues to avoid and has not directly consumed coconut, and follow-up of the other child is not available.
Figure 1.
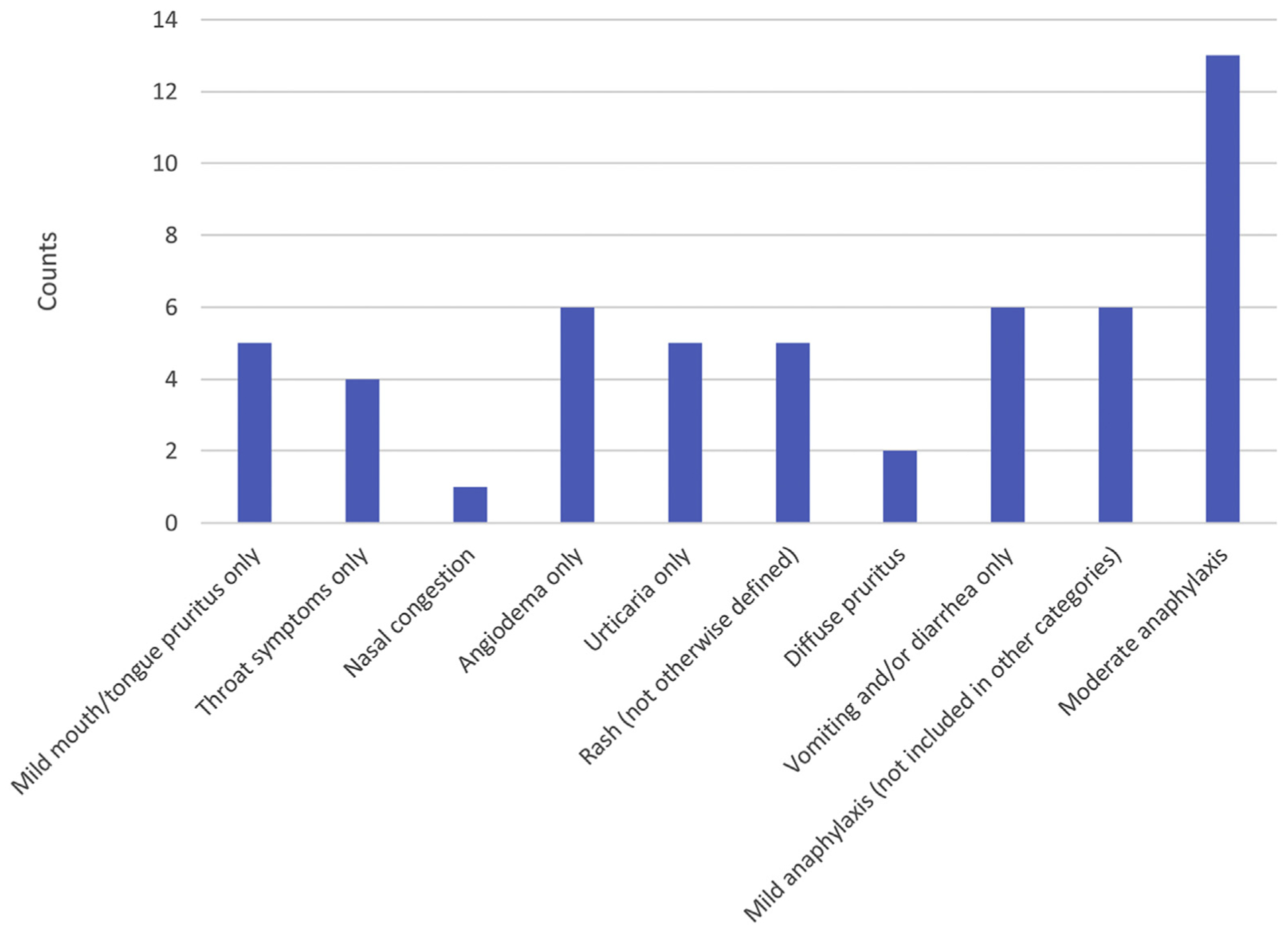
Reaction characteristics with oral ingestion of coconut (n = 53) derived from parent report at initial evaluation for coconut allergy at our clinic, excluding those with mixed allergen reactions.
Diagnostic Predictors of Coconut Allergy
We sought to evaluate for associations between the results of sIgE or SPT and clinical reactivity or tolerance to coconut. We analyzed a subset of patients in whom ingestion or oral food challenge occurred within 12 months of testing. Table 2 details the characteristics of those patients. Only 4 patients had subsequent consumption and reaction documented after the initial visit. Of these patients, 2 were offered oral food challenges that have not been performed to date. Only 1 patient in our cohort had an oral food challenge to determine the development of natural tolerance. That patient had an initial reaction of gagging and throat discomfort with an sIgE of 0.74 kUa/L. After 1 year, the sIgE was 0.26 kUa/L, and a coconut oral food challenge was conducted without adverse reaction. Table 2 also describes the 23 sensitized patients who had no reaction on oral food challenge or at-home ingestion.
Table 2.
Subset of Patients With Oral Food Challenge or Home Ingestion Within 12 Months of Coconut Skin Prick or sIgE Testing
| Patient no. | Age of initial reaction | Age of first test | ImmunoCAP | Skin test size | Age of challenge/ingestion | ImmunoCAPa | OFC | Reaction | |
|---|---|---|---|---|---|---|---|---|---|
| With allergy | 1 | 5 | 7 | 17.6 | 9 | 7 | Y | Oral pruritus, perioral urticaria | |
| 2 | 3 | 3 | 5.06 | 9 | 3 | N | Rubbed eyes, periorbital edema | ||
| 3 | 8 | 5.59 | 11 | 11.20 | N | Ears became red, hot, and a bit itchy | |||
| 4 | 5 | 2.84 | 11 | 3.36 | N | Lip edema | |||
| Naturally tolerant | 5 | 3 | 3 | 0.74 | 8 | 4 | 0.26 | Y | No reaction |
| Without allergy | 6 | 5 | 12.6 | 5 | 5 | N | No reaction | ||
| 7 | 5 | 12.0 | 5 | N | No reaction | ||||
| 8 | 11 | 8.58 | 12 | 7.46 | Y | No reaction | |||
| 9 | 5 | 4.01 | 6 | Y | No reaction | ||||
| 10 | 2 | 3.07 | 6 | 4 | 1.14 | Y | No reaction | ||
| 11 | 8 | 2.36 | 10 | 1.60 | Y | No reaction | |||
| 12 | 6 | 2.35 | 6 | 1.16 | N | No reaction | |||
| 13 | 9 | 1.77 | 3 | 9 | N | No reaction | |||
| 14 | 18 | 1.21 | 20 | 0.72 | N | No reaction | |||
| 15 | 1 | 1.17 | 1 | N | No reaction | ||||
| 16 | 7 | 1.16 | 7 | Y | No reaction | ||||
| 17 | 12 | 1.07 | 0 | 14 | N | No reaction | |||
| 18 | 1 | 1.03 | 1 | Y | No reaction | ||||
| 19 | 6 | 0.99 | 7 | Y | No reaction | ||||
| 20 | 4 | 0.97 | 4 | 9 | 0.83 | N | No reaction | ||
| 21 | 10 | 0.95 | 10 | Y | No reaction | ||||
| 22 | 4 | 0.88 | 0.5 | 5 | Y | No reaction | |||
| 23 | 9 | 0.86 | 9 | Y | No reaction | ||||
| 24 | 4 | 0.54 | 0 | 6 | N | No reaction | |||
| 25 | 15 | 0.42 | 15 | N | No reaction | ||||
| 26 | 3 | 0.36 | 6 | 0.55 | Y | No reaction | |||
| 27 | 4 | 3 | 5 | 0.20 | Y | No reaction | |||
| 28 | 1 | 4 | 1 | N | No reaction |
Abbreviations: N, no; OFC, oral food challenge; sIgE, specific immunoglobulin E; Y, yes.
ImmunoCAP within 12 months of challenge/ingestion.
Given the small number of food challenge-proven reactions, we created the probability of reaction models on the basis of home contact/ingestion or food challenge-proven allergy. Patients who were sensitized without history of ingestion were excluded, and those with mixed allergen ingestions were excluded. The cohort of patients with allergy used for this analysis is depicted in Table 1 (in addition to patients with mixed allergens) including patients 3 and 4 with allergy who had reactions at challenge but were initially sensitized only defined in Table 2. Patients without allergy are defined in Table 2. We created receiver operating characteristic curves to analyze the sensitivity and specificity of coconut sIgE and SPT associations with reactions (eFig 1). Although not all subjects had both SPT and sIgE, we found that SPT (n = 27 with allergy and 9 without allergy) had better performance characteristics than sIgE (n = 60 with allergy and 21 without allergy) with an area under the curve of 0.89 vs 0.74. We constructed a probability curve of SPT and sIgE values with this patient cohort, revealed in Figure 2A and Figure 2B, respectively. Given the many patients with allergy (reactive) with low sIgE values, we found that the probability of allergy within those with coconut sIgE greater than 0.1 kUa/L is approximately 60%. SPT of 3 mm wheal or greater is associated with a 50% probability of allergy. A total of 95% probability of allergy was found at a SPT value of 9 mm wheal and sIgE of 58 kUa/L. We also constructed a probably curve on the basis of the small subset of patients who reported moderate anaphylaxis at initial visit and had sIgE testing available (n = 12). Sensitization was associated with an approximately 30% rate of moderate anaphylaxis.
Figure 2.
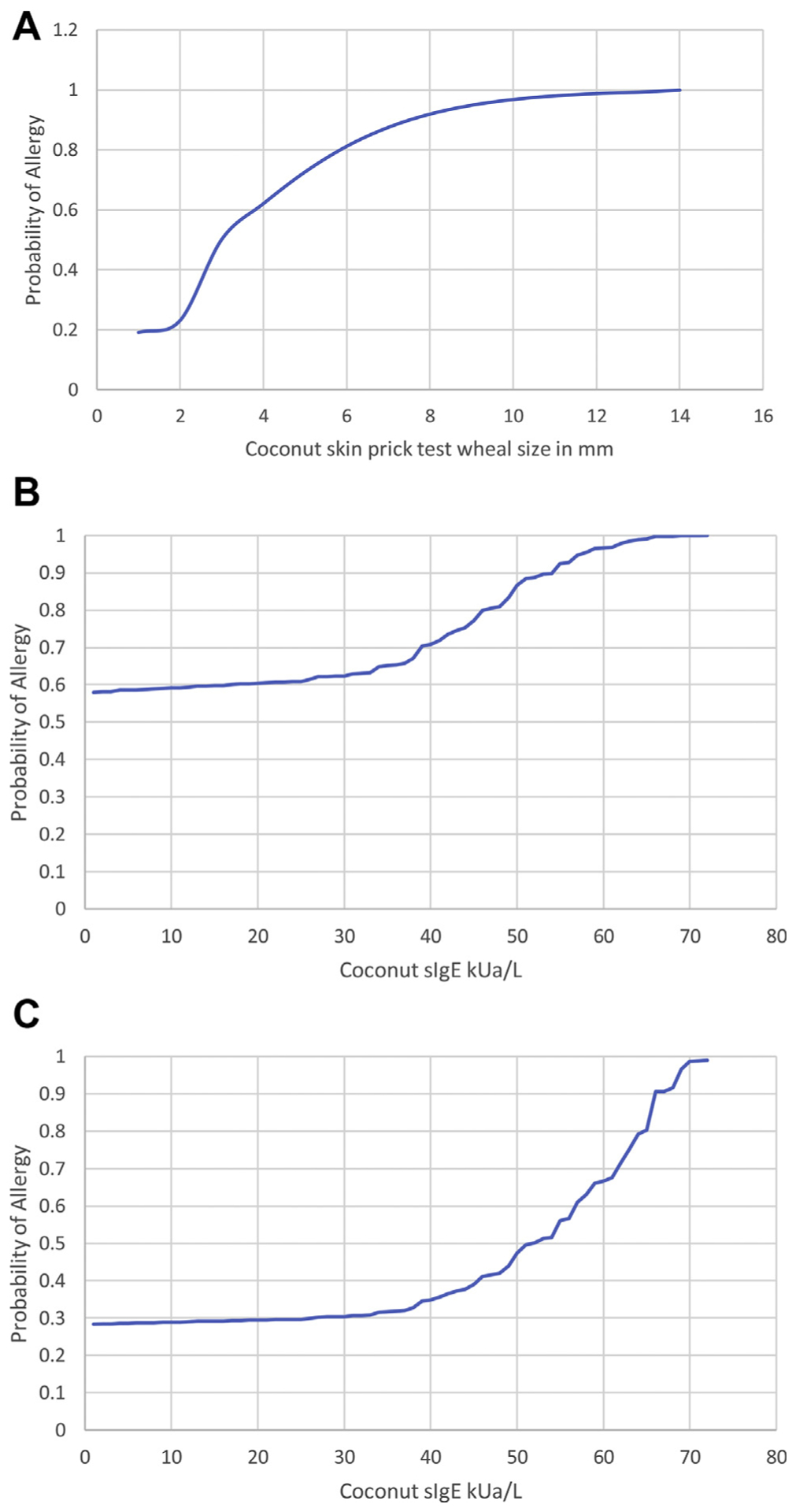
A, Estimated probability curve for allergy at a given coconut SPT wheal size in millimeters derived from logistic regression; n = 27 patients with allergy and n = 9 patients without allergy. B, Estimated probability curve for allergy at a given coconut sIgE antibody level derived from logistic regression; n = 60 patients with allergy and n = 21 patients without allergy. C, Estimated probability curve for moderate anaphylaxis by parent report at a given coconut sIgE antibody level derived from logistic regression; n = 12 patients with allergy and n = 21 patients without allergy. kUa/L, kU of allergen/L; sIgE, specific IgE; SPT, skin prick testing.
Cosensitization
Allergen sIgE in coconut-sensitized patients at initial presentation was compared across several groups of allergens. Tree nuts were most often tested with coconut, and all tree nuts consistently correlated with coconut levels. The strongest association was with macadamia nut (r = 0.81, P < .001, n = 101). Legumes (in addition to peanut) and seeds (in addition to sunflower) were also significantly associated. Fruits were only tested in a few patients, but they did not consistently correlate with coconut sIgE levels (Table 3).
Table 3.
Correlation Between Coconut sIgE and Other Food and Environmental Allergen sIgE With Initial Coconut Testing
| Tree nuts | Almond | Brazil nut | Cashew nut | Hazel nut | Macadamia | Pecan | Pine nut | Pistachio | Walnut |
| r = 0.77 | r = 0.70 | r = 0.45 | r = 0.61 | r = 0.81 | r = 0.64 | r = 0.36 | r = 0.43 | r = 0.49 | |
| P < .001 | P < .001 | P < .001 | P < .001 | P < .001 | P < .001 | P < .001 | P < .001 | P < .001 | |
| n = 202 | n = 142 | n = 190 | n = 170 | n = 101 | n = 172 | n = 103 | n = 148 | n = 185 | |
| Fruits | Apple | Avocado | Banana | Kiwi | Melon | Orange | Tomato | ||
| r = 0.17 | r = 0.90 | r = 0.29 | r = −0.09 | r = 0.71 | r = 0.27 | r = 0.80 | |||
| P = .575 | P < .001 | P = .202 | P = .818 | P = .032 | P = .487 | P = .002 | |||
| n = 13 | n = 11 | n = 21 | n = 9 | n = 9 | n = 9 | n = 12 | |||
| Seeds | Cacao | Flaxseed | Poppy seed | Pumpkin seed | Sesame seed | Sunflower seed | |||
| r = 0.84 | r = 0.57 | r = 0.94 | r = 0.58 | r = 0.64 | r = 0.21 | ||||
| P = .003 | P = .022 | P < .001 | P = .048 | P < .001 | P = .199 | ||||
| n = 10 | n = 16 | n = 16 | n = 12 | n = 86 | n = 40 | ||||
| Legumes | Chickpea | Green bean | Lentil | Pea | Peanut | Soybean | |||
| r = 0.86 | r = 0.53 | r = 0.49 | r = 0.12 | r = 0.12 | r = 0.64 | ||||
| P < .001 | P = .043 | P = .005 | P = .017 | P = .084 | P < .001 | ||||
| n = 30 | n = 15 | n = 31 | n = 195 | n = 195 | n = 69 | ||||
| Other common foods | Egg white | Milk | Wheat | ||||||
| r = 0.27 | r = 0.05 | r = 0.38 | |||||||
| P = .001 | P = .659 | P = .007 | |||||||
| n = 139 | n = 84 | n = 49 |
Abbreviation: sIgE, specific immunoglobulin E.
Bolded values are significant (P < .05).
Discussion
This is the most comprehensive report to date revealing the gamut of reactions to coconut reported at a pediatric tertiary care center that can occur by means of skin contact, breastfeeding, or ingestion. Reactions can present as atopic dermatitis flare, urticaria, mild oral symptoms, and mild/moderate anaphylaxis. No reactions by means of skin contact or breastfeeding resulted in anaphylaxis. Sensitization to coconut as determined by SPT and sIgE is associated with an approximately 50% and 60% chance of patient/parent report of clinical reactivity, respectively. This rate of reaction in sensitized individuals is similar to what has been reported for most other common food allergens.15 Of the ingestion reactions, approximately 50% were associated with mild/moderate anaphylaxis. Many patients were referred to our practice from another provider who had performed panel testing. We believe that this was the reason for the high positive rate of coconut sensitization and lack of history of reactivity. As is the case for other food allergens, this finding suggests that panel testing is a highly inaccurate approach.16
Although our study was limited in that we did not have food challenge-proven allergy, we are the first study to provide sIgE and SPT correlates with allergic reactions to coconut. Presented probability curves can be helpful for clinicians in determining whether to offer food challenge17 in coconut-sensitized individuals. Similar to other food allergens,15 we found a 95% probability of allergy to high numbers on SPT and sIgE, with SPT value of 9 mm wheal and sIgE of 58 kUa/L. Low specificity of coconut allergen testing is likely due to high rates of sensitization in patients with atopic dermatitis18 who might be using coconut-containing cosmetic products19 and owing to crossreactivity of coconut with other allergens (tree nuts, soy, etcetera).20
We found a trend for racial differences in those with allergy to coconut, specifically a higher risk for allergy vs sensitization in Asian and African American patients, at 2-fold and 1.5-fold, respectively. This might reflect more frequent inclusion of coconut in the diet21 or in topical application. Topical exposure to food allergens, such as peanut, is associated with increased risk of food allergy,22 which from our data also seems to be the case for coconut. Although history of topical application was not well documented in the charts we reviewed, we found higher rates of allergy in those who used coconut topically. Given the high prevalence of coconut in skin and hair care products,2 we suggest that patients are counseled on the potential of topical coconut to cause allergy, particularly in high-risk groups. Specifically, products with higher amount of allergen, such as pure coconut oil, should be the focus of counseling. There is only 1 case report suggesting ingestion of coconut might not be tolerogenic in someone applying coconut topically,10 but we believe that this case report reflects the need for frequent ingestion, several times per week, to maintain tolerance in high-risk individuals. Although none of our patients had documented allergic contact dermatitis to coconut, this and irritant dermatitis have been described as risks of topical coconut and possibly coconut derivatives.23,24 Future work is needed to determine the safety of topical coconut oil, and other coconut derivatives, in children with atopy and whether early and frequent oral ingestion of coconut can promote tolerance.
Cosensitization was common in our study between coconut, all tree nuts, egg, wheat, and most seeds/legumes. The high rate of cosensitization might in part be explained by our atopic tertiary care center patient population, in which approximately 60% of the patients had atopic dermatitis.18 Mechanistically, previously published work on seed storage proteins suggests that this might also be an explanation for some cosensitizations with tree nuts and legumes.25,26 For example, soy, coconut, and walnut cosensitization is common owing to shared legumin group of seed storage protein between these foods.20 This suggests that sIgE testing to these foods might reveal cosensitization, but not necessarily clinical reactivity. Of the tree nuts, we found that macadamia nut had the stronger correlation with coconut (r = 0.81, P < .001), similar to another previously published study.7 Although the allergens in macadamia nut remain to be characterized, we speculate that there is likely homology between coconut and macadamia nut owing to seed storage protein homology.27 Although we did not specifically evaluate the clinical reactivity to other allergens in our study, previous studies suggest that cosensitization with coconut was not necessarily associated with clinical reactivity.1,20 We recommend that coconut sIgE and SPT are not included in tree nut panels and that these tests be performed in individuals with an indication specific for coconut allergen testing.
This study has several limitations, particularly the lack of oral food challenges for patients labeled to have allergy. Our study likely overestimates food allergy, and the true rate of allergy in sensitized individuals might even be lower, given our reliance of patient report for food allergy and lack of food challenge–confirmed patients. We tried to mitigate this effect by not including those with mixed allergen exposures in our probability models. Another limitation is the presence of selection bias; we only analyzed those with sensitization and did not include any patients who had negative skin and blood testing results. In addition, many sensitized patients with higher SPT or sIgE values were not challenged. To improve the specificity of our group without allergy for allergic probability models, we only included those with food challenge or parent report of no reaction after ingestion within 12 months of testing, not those whom were only sensitized but had never consumed. The age at which the initial reaction presented was not always clear or missing from the chart. In addition, we were not able to collect consistent data on timing of initial reaction with ingestion, so we are unable to fully report on that. Furthermore, the association with sensitization is not necessarily reflective of allergy to other foods, so we cannot conclude that homology between allergens merely an association is present.
In conclusion, coconut is associated with a wide range of allergic reactions in children. Topical, breastfeeding, and ingestion exposures are associated with the reactions. Coconut-based products might be a source of sensitization, and clinicians should consider the risk of use in children with atopic dermatitis. Sensitization to coconut in our pediatric tertiary care center cohort is associated with a 50% to 60% rate of patient- or parent-reported reaction, with no severe anaphylaxis. Testing for coconut allergy should not be performed in patients without an indication. SPT and sIgE testing can be used to help guide clinicians in determining the probability of reaction, with a very low threshold for challenge. Further work is needed to improve the clinical use of coconut allergy diagnostics in conjunction with oral food challenges.
Supplementary Material
Funding:
The authors have no funding sources to report.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.anai.2021.01.027.
References
- 1.Stutius LM, Sheehan WJ, Rangsithienchai P, et al. Characterizing the relationship between sesame, coconut, and nut allergy in children. Pediatr Allergy Immunol. 2010;21(8):1114–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newhall KK, Amoruso LS, Sinacore JM, Pongracic JA. Presence of common food allergens in commercially available pediatric skin care products. J Allergy Clin Immunol. 2004;113(2):S235. [Google Scholar]
- 3.Silverberg NB. Selected active naturals for atopic dermatitis: atopic dermatitis part 1. Clin Dermatol. 2017;35(4):383–386. [DOI] [PubMed] [Google Scholar]
- 4.Du Toit G, Sampson HA, Plaut M, Burks AW, Akdis CA, Lack G. Food allergy: update on prevention and tolerance. J Allergy Clin Immunol. 2018;141(1):30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker MT, Green JE, Ferrie RP, Queener AM, Kaplan MH, Cook-Mills JM. Mechanism for initiation of food allergy: dependence on skin barrier mutations and environmental allergen costimulation. J Allergy Clin Immunol. 2018;141(5): 1711–1725.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brough HA, Nadeau KC, Sindher SB, et al. Epicutaneous sensitization in the development of food allergy: what is the evidence and how can this be prevented? Allergy. 2020;75(9):2185–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polk BI, Dinakarpandian D, Nanda M, Barnes C, Dinakar C. Association of tree nut and coconut sensitizations. Ann Allergy Asthma Immunol. 2016;117(4): 412–416. [DOI] [PubMed] [Google Scholar]
- 8.Tella R, Gaig P, Lombardero M, Paniagua MJ, García-Ortega P, Richart C. A case of coconut allergy. Allergy. 2003;58(8):825–826. [DOI] [PubMed] [Google Scholar]
- 9.Michavila Gomez A, Amat Bou M, Gonzalez Cortés MV, Segura Navas L, Moreno Palanques MA, Bartolomé B. Coconut anaphylaxis: case report and review. Allergol Immunopathol (Madr). 2015;43(2):219–220. [DOI] [PubMed] [Google Scholar]
- 10.Anagnostou K. Coconut allergy revisited. Children (Basel). 2017;4(10):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernández Santana GL, Rodríguez Plata E, González Colino CE, et al. Induction of oral tolerance in a case of severe allergy to coconut. J Investig Allergol Clin Immunol. 2019;29(5):380–381. [DOI] [PubMed] [Google Scholar]
- 12.Benito C, González-Mancebo E, de Durana MD, Tolón RM, Fernández-Rivas M. Identification of a 7S globulin as a novel coconut allergen. Ann Allergy Asthma Immunol. 2007;98(6):580–584. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen SA, More DR, Whisman BA, Hagan LL. Cross-reactivity between coconut and hazelnut proteins in a patient with coconut anaphylaxis. Ann Allergy Asthma Immunol. 2004;92(2):281–284. [DOI] [PubMed] [Google Scholar]
- 14.Pathmanandavel K, Kaur N, Joshi P, Ford LS. Anaphylaxis and allergy to coconut: an Australian pediatric case series. J Allergy Clin Immunol Pract. 2020; 8(10):3657–3659. [DOI] [PubMed] [Google Scholar]
- 15.Fishbein AB, Makhija MM, Pongracic JA. Anaphylaxis to food. Immunol Allergy Clin North Am. 2015;35(2):231–245. [DOI] [PubMed] [Google Scholar]
- 16.Kraft MT, Wilson J, Leber AL, Stukus DR, Scherzer R. Review of ordering practices for single allergen and serum IgE panel tests for food allergy. Ann Allergy Asthma Immunol. 2020;125(3):343–344. [DOI] [PubMed] [Google Scholar]
- 17.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891–896. [DOI] [PubMed] [Google Scholar]
- 18.Spergel JM, Boguniewicz M, Schneider L, Hanifin JM, Paller AS, Eichenfield LF. Food allergy in infants with atopic dermatitis: limitations of food-specific IgE measurements. Pediatrics. 2015;136(6):e1530–e1538. [DOI] [PubMed] [Google Scholar]
- 19.Karagounis TK, Gittler JK, Rotemberg V, Morel KD. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36(1):9–15. [DOI] [PubMed] [Google Scholar]
- 20.Teuber SS, Peterson WR. Systemic allergic reaction to coconut (Cocos nucifera) in 2 subjects with hypersensitivity to tree nut and demonstration of cross-reactivity to legumin-like seed storage proteins: new coconut and walnut food allergens. J Allergy Clin Immunol. 1999;103(6):1180–1185. [DOI] [PubMed] [Google Scholar]
- 21.Suraiya C, Shaikh WE. Food allergies. Indian Journal of Clinical Practice, Allergy and asthma: a clinical primer. 1999:94–98. [Google Scholar]
- 22.Lack G, Fox D, Northstone K, Golding J. Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348(11):977–985. [DOI] [PubMed] [Google Scholar]
- 23.Shaffer KK, Jaimes JP, Hordinsky MK, Zielke GR, Warshaw EM. Allergenicity and cross-reactivity of coconut oil derivatives: a double-blind randomized controlled pilot study. Dermatitis. 2006;17(2):71–76. [PubMed] [Google Scholar]
- 24.Pinola A, Estlander T, Jolanki R, Tarvainen K, Kanerva L. Occupational allergic contact dermatitis due to coconut diethanolamide (cocamide DEA). Contact Dermatitis. 1993;29(5):262–265. [DOI] [PubMed] [Google Scholar]
- 25.Garcia RN, Arocena RV, Laurena AC, Tecson-Mendoza EM. 11S and 7S globulins of coconut (Cocos nucifera L.): purification and characterization. J Agric Food Chem. 2005;53(5):1734–1739. [DOI] [PubMed] [Google Scholar]
- 26.Balasundaresan D, Sugadev R, Ponnuswamy MN. Purification and crystallization of coconut globulin cocosin from Cocos nucifera. Biochim biophys Acta. 2002;1601(1):121–122. [DOI] [PubMed] [Google Scholar]
- 27.Geiselhart S, Hoffmann-Sommergruber K, Bublin M. Tree nut allergens. Mol Immunol. 2018;100:71–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


