Dimethyl fumarate (DMF), approved for relapsing-remitting multiple sclerosis (RRMS), exerts immune-mediated mechanisms crucial for T-cell survival and migration, preferentially reducing CD8+ T cells.1 Although baseline absolute lymphocyte count (ALC) is considered the most critical predictor of developing lymphopenia,2 it was recently concluded that lymphocyte subset monitoring is not required for safety vigilance because T-cell subset reduction does not increase risks for serious infections.3
We present 2 young patients with RRMS, under DMF treatment, negative for HIV and SARS-CoV-2 (by RT-PCR in nasal swab) and with normal follow-up white blood cell (WBC)/ALC counts, who developed severe herpes zoster (HZ) infection with normal ALC but low CD8+ and high CD56bright natural killer (NK) cells, and discuss the potential significance of T-cell immunophenotyping in HZ manifestation.
Patient 1
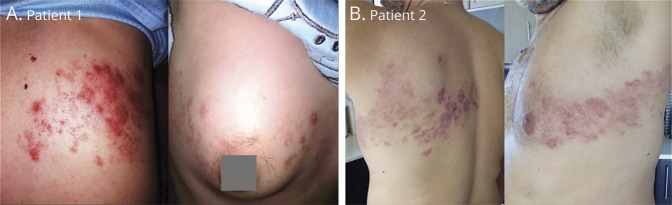
A 23-year-old woman was seen at age 16 years with acute cerebellar ataxia and trigeminal neuralgia. MRI showed nonenhancing T2-hyperintense periventricular and subtentorial lesions and CSF oligoclonal bands. Anti-AQP4 and anti-MOG antibodies were negative. After 6 months, new T2-hyperintense, enhancing, periventricular lesions developed and was started on interferon beta-1a with excellent response. Because of new enhancing cervical and thoracic demyelinating MRI lesions, she was started on DMF 240 mg BID 2 years ago. In March, during the first peak of COVID-19, she presented with an aggressive, blistering rash extending from the right side of back to the chest (figure), typical of HZ with positive anti-varicella-zoster virus (VZV) antibodies. She reported chickenpox in childhood. DMF was discontinued, and 1-week treatment with brivudine (bromovinyldeoxyuridine) began followed by valaciclovir 1,000 mg TID for 20 days, tapered to 500 mg daily. Her WBC and ALC counts were normal but had low CD3+, very low CD8+, low-normal CD4+ T cells, and very high NK cells (table). After 2 months, the rash improved. Repeated MRI of the brain and cervical spine was stable. She was started on glatiramer acetate 40 mg 3 times/wk. After 3 months, CD3+, CD4+, and CD8+ T subsets remain still low (table). Although SARS-CoV-2-PCR was negative and always asymptomatic, she had antibodies to SARS-CoV-2-spike protein when tested 10 months after the HZ manifestation.
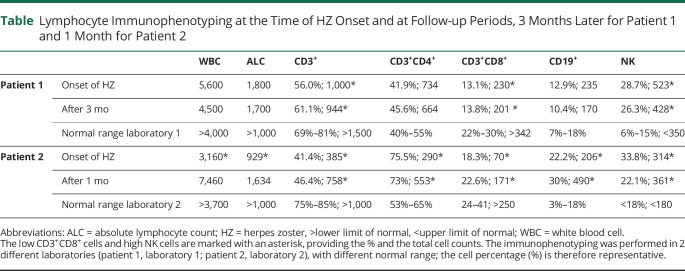
Figure. Herpes Zoster-Rash.
Extensive, blistering, herpes zoster (HZ) rash extending from the right back to the anterior chest in (A) patient 1 and from the left back to the anterior chest in (B) patient 2.
Table.
Lymphocyte Immunophenotyping at the Time of HZ Onset and at Follow-up Periods, 3 Months Later for Patient 1 and 1 Month for Patient 2
Patient 2
A 42-year-old man was admitted at age 28 years, with acute right arm numbness and dysuria. He had 2 other acute episodes 2 years earlier. MRI revealed multiple T2-hyperintense periventricular, subcortical, and cervical lesions. He was given glatiramer acetate but because of 2 subsequent relapses was started on DMF 240 mg BID 2 years ago. In October, during the COVID-19 peak, he developed an aggressive blistering rash, extending from his left back to the chest, typical of HZ with positive anti-VZV antibodies (figure). DMF was discontinued and was treated with 2-week brivudine followed by valaciclovir 1,000 mg TID for 30 days, tapered to 500 mg daily. WBC and ALC were slightly below normal (grade-1 lymphocytopenia), but immunophenotyping showed low CD3, very low CD8+, and very high NK cells with increased CD4+ and CD19+ T cells (table). After 1 month, the rash improved, but his CD3+ and CD8-T cells remain low and NK cells high (table). MRI showed a new enhancing demyelinating cervical lesion, treated with corticosteroids. He was SARS-CoV-2-antibody-negative.
Discussion
VZV is a lymphotropic virus, which in immunosuppressed patients can be reactivated leading to HZ infection. Our patients are probably the first young, not immunodeficient patients with normal ALC to manifest severe HZ while on DMF, without any new CNS-clinical signs to warrant CSF examination. The study of their T-cell immunophenotyping at onset and after the infection revealed low CD8+ T cells and increased CD56bright NK cells that may be implicated in extensive HZ manifestation.
VZV acts on immune cells through cell-surface proteins, like programmed cell death protein 1 (PD-1), eliciting an immunoinhibitory function and reducing T-cell activation and cytokine secretion. DMF causes reduction of CD8+ T-cells, and CD8-cell depletion is associated with increased VZV reactivation,4,5 but whether in both our patients, the noted CD8+ T-cell reduction was the only culprit is unclear.
DMF also upregulates CD56bright NK cells, which, in turn, inhibits CD4+ and CD8+ IFN γ-producing T-cell populations, promoting an anti-inflammatory state and enhancing its beneficial effects in DMF-treated patients.6 VZV can however cause a productive infection of human CD56bright NK cells and upregulates surface expression of chemokine receptors associated with trafficking to the skin where highly infectious lesions develop.7 The high number of CD56bright NK cells noted in both our patients at the onset of skin rash might have facilitated the spread of VZV to the skin, producing such aggressive lesions unusual for young nonimmunosuppressed patients, requiring 6 weeks of antiviral therapy before resolution of skin lesions began. It is likely that the low CD8+ cells triggered HZ reactivation, but the presence of increased NK cells facilitated such an extensive manifestation. Our cases highlight that T-cell subset monitoring may still have a role during DMF therapy and that the combination of low CD8/high NK requires attention. Although rare, the cases raise the question of pharmacovigilance and consideration for VZV vaccination in certain DMF-receiving patients.
Appendix. Authors

Contributor Information
Georgios Velonakis, Email: giorvelonakis@gmail.com.
Marinos C. Dalakas, Email: mdalakas@med.uoa.gr.
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Spencer MC, Crabtree-Hartman EC, Lehmann-Horn K, Cree B, Zamvil SS. Reduction of CD8+ T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de la Maza SS, Medina S, Villarrubia N, et al. Factors associated with dimethyl fumarate-induced lymphopenia. J Neurol Sci. 2019;398:4-8. [DOI] [PubMed] [Google Scholar]
- 3.Mehta D, Miller C, Arnold DL, et al. Effect of dimethyl fumarate on lymphocytes in RRMS: implications for clinical practice. Neurology. 2019;92(15):e1724-e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones D, Como CN, Jing L, et al. Varicella zoster virus productively infects human peripheral blood mononuclear cells to modulate expression of immunoinhibitory proteins and blocking PD-L1 enhances virus specific CD8+ T cell effector function. PLoS Pathog. 2019;15(3):e1007650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341-357. [DOI] [PubMed] [Google Scholar]
- 6.Al-Ani M, Elemam NM, Hundt JE, Maghazachi AA. Drugs for multiple sclerosis activate natural killer cells: do they protect against COVID-19 infection? Infect Drug Resist. 2020;13:3243-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell TM, McSharry BP, Steain M, Ashhurst TM, Slobedman B, Abendroth A. Varicella zoster virus productively infects human natural killer cells and manipulates phenotype. PLoS Pathog. 2018;14(4):e1006999. [DOI] [PMC free article] [PubMed] [Google Scholar]