Abstract
Although microglia activation plays an important role in the development of nerve injury-induced neuropathic pain, the molecular mechanisms of spinal cord microglia activation in nerve injury are not completely understood. Recently, two injured sensory neuron-derived molecules, colony stimulating factor-1 (CSF-1) and GT1b, were proposed to trigger spinal cord microglia activation, yet their relationship and relative contribution to microglia activation have not been addressed. In the present study, the role of GT1b and CSF-1 in microglia activation and proliferation was characterized. GT1b stimulation upregulated proinflammatory mediators such as IL-1β, TNF-α, and NADPH oxidase 2 (Nox2), without microglia proliferation. Conversely, CSF-1 stimulation induced microglia proliferation with minimal proinflammatory gene induction. Notably, neither GT1b nor CSF-1 induced mechanical hypersensitivity in female mice; however, they induced similar microglial proliferation in both male and female mice. Taken together, our data indicate that injured sensory neuron-derived GT1b and CSF-1 activate spinal cord microglia in concert through distinct activation pathways.
Keywords: Neuropathic pain, microglia, GT1b, CSF-1
Introduction
Neuropathic pain is a chronic pathological pain caused by damage or dysfunction of the nervous system, and it affects tens of millions of people worldwide. Clinical symptoms of this devastating disorder include allodynia, a pain sensation caused by non-noxious stimuli, and hyperalgesia, an exaggerated pain sensation caused by noxious stimuli.1 Such pain hypersensitivity is partly attributed to the enhanced excitability of pain-transmitting neurons at the spinal cord level, called central sensitization.2,3 Results of studies to date indicate that spinal cord microglia activation plays a critical role in the development of central sensitization; peripheral nerve injury induces microglia activation and proliferation in the spinal cord dorsal horn where the injured nerve innervates.4,5 Upon activation, spinal cord microglia express various pain-mediating proinflammatory molecules such as TNF-α, IL-1β, and BDNF.4,6 These pain mediators in turn enhance the excitability of pain-transmitting neurons in the spinal cord, leading to central sensitization.7–9 However, how peripheral nerve injury induces microglia activation in the spinal cord, which is anatomically remote from the injury site, remains unclear.
Various microglia-activating molecules, including ATP, fractalkine, cathepsin S (Ctss), colony stimulating factor-1 (CSF-1), and GT1b, have been implicated in nerve injury-induced spinal cord microglia activation in previous studies.10–15 Among these candidate molecules, only CSF-1 and GT1b were derived from the injured sensory afferents. Upon peripheral nerve injury, CSF-1 is induced in dorsal root ganglia (DRG) neurons that are transported to the spinal cord via afferent axons, and activates microglial CSF-1 receptor (CSF-1R), initiating DAP12-dependent microglia activation and proliferation.14 Similarly, GT1b, a ganglioside member, is upregulated in injured DRG neurons, and in turn is transported to the spinal cord and released, activating spinal cord microglia as a TLR2 endogenous agonist.15 In prior studies, GT1b and CSF-1 were posited to be two candidate signals derived from injured afferent axons that trigger spinal cord microglia activation, eventually leading to central sensitization. However, the relationship or relative contribution of these molecules to spinal cord microglia activation after peripheral nerve injury remains unclear.
In the present study, GT1b- and CSF-1-induced spinal cord microglia activation in vivo and in vitro were investigated and compared. CSF-1 stimulation contributes to microglia proliferation and GT1b transmits mainly inflammatory signals in microglia. The present study results show that GT1b and CSF-1 released from the injured primary afferents activate spinal cord microglia in concert via distinct activation pathways during nerve injury-induced neuropathic pain.
Materials and methods
Animals
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Seoul National University. Male and female C57BL/6J mice (approximately 8 – 10 weeks of age) were purchased from Daehan Biolink (DBL, Eumsung, Korea) and all animals were acclimatized to standard conditions with a 12-hr light/dark cycle in a specific pathogen-free environment and given access to chow and water ad libitum. All protocols were performed in accordance with the guidelines from the International Association for the Study of Pain.
Intrathecal administration
Mice were anesthetized with isoflurane in O2 carrier (induction at 2% and maintenance at 1.5%), and GT1b (Matreya LLC, State College, PA, USA) or MCSF-1 (ThermoFisher, Waltham, MA, USA) in saline solution was administrated using a 10-µl Hamilton syringe (Hamilton Company, Reno, NV, USA) with a 30-G needle as previously descrived.16 Success of intrathecal injection was assessed by monitoring for a slight tail-flick when the needle penetrated the subarachnoid space.
Allodynia test
Mechanical sensitivity of the right hind paw was assessed using a calibrated series of von Frey hairs (0.02–6 g, Stoelting, Wood Dale, IL, USA), following an up-down method.17 Tests were performed after at least three habituations at 24-hr intervals. Assessments were made 1 day before surgery for baseline, and 1, 3, and 7 days after injection. Rapid paw withdrawal, licking, and flinching were interpreted as a pain response. All behavioral tests were performed blinded.
In vitro primary microglia culture
Primary mixed glia cultures were prepared from 1–2-day-old mice, as previously described.18 In brief, brain glial cells were cultured in DMEM, high-glucose formula supplemented with 10% FBS, 10 mM HEPES, 2 mM L-glutamine, 1x penicillin/streptomycin, and 1x nonessential amino acid mixture at 37 °C in a 5% CO2 incubator. After 2 weeks, primary microglia were harvested from mixed glial cultures with mechanical dislodgment and plated in 6-well plates at a density of 5 × 105 cells per well for real-time RT-PCR and in 15-mm glass bottom dishes at a density of 5 × 104 cells for immunohistochemistry.
Immunohistochemistry
Mice were transcardially perfused with 0.1 M phosphate buffer followed by 4% paraformaldehyde, and the L5 spinal cord was removed and post-fixed in the same solution at 4 °as removed. Spinal cord samples were transferred to 30% sucrose for at least 36 hr and cut coronally to 14-µm thickness using a cryostat (CM1860, Leica, Wetzlar, Germany). For in vitro staining, pure microglia were fixed with 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 10 min at room temperature. The spinal cord section and pure microglia were blocked in a solution containing 5% normal goat serum, 2.5% BSA, and 0.2% Triton X-100 for 1 hr at room temperature, and then incubated with rabbit anti-Iba-1 (1:1,000, Wako, Osaka, Japan) and mouse anti-Ki67 (1:500, Invitrogen, Carlsbad, CA, USA) antibodies. After rinsing five times with 0.1 M PBS, samples were incubated with FITC- or CY3-conjugated secondary antibodies (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1.5 hr at room temperature. The samples were mounted on glass slides with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) and examined using an LSM800 confocal Laser scanning microscope (Carl Zeiss, Oberkochen, Germany). The Ki67 and DAPI double-positive cells and intensity of Iba-1 were quantified using ImageJ software (NIH, https://imagej.net).
Real-time RT-PCR
Real-time RT-PCR experiments were performed using the StepOnePlus Real-Time PCR system (Applied Biosystem, Foster City, CA, USA) following the 2-ΔΔCt method.19 Total RNA from L5 spinal cord tissue and primary microglia were extracted with TRIzol reagent (Invitrogen) and reverse transcribed using TOPscript RT DryMIX (Enzynomics, Daejeon, Korea). All ΔCt values were normalized to the value of corresponding GAPDH and represented as fold induction. The following PCR primers were used: Gapdh forward: 5′-AGT ATG ACT CCA CTC ACG GCA A-3′, Gapdh reverse: 5′-TCT CGC TCC TGG AAG ATG GT-5′, Csf-1 forward: 5′-ACA CGC ACG GCC ACC ATG AA-3′, Csf-1 reverse: 5′-GCA TGG ACC GTG AGG ATG AGG C-3′, Tnf-α forward: 5′-AGC AAA CCA CCA AGT GGA GGA-3′, Tnf-α reverse: 5′-GCT GGC ACC ACT AGT TGG TTG T-3′, Nox2 forward: 5′-GAC CCA GAT GCA GGA AAG GAA-3′, Nox2 reverse: 5′-TCA TGG TGC ACA GCA AAG TGA T-3′, Irf5 forward: 5′-TGG GGA CAA CAC CAT CTT CA-3′, Irf5 reverse: 5′-CTG GAA GTC ACG GCT TTT GT-3′.
Statistical analysis
Data were analyzed using the Student’s t-test for comparison between groups. One-way analysis of variance (ANOVA) with Bonferroni’s post-hoc test was used for statistical analysis of multiple comparisons. All data are presented as the mean ± standard error of the mean (SEM) and differences were considered statistically significant when the p-value was <0.05.
Results
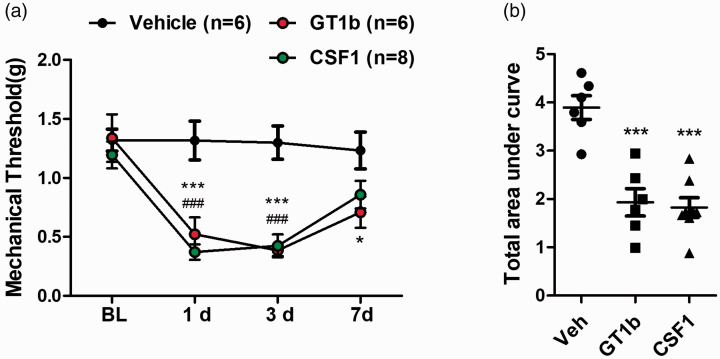
In previous studies, both GT1b and CSF-1 were shown to activate spinal cord microglia and lead to central sensitization in nerve injury-induced neuropathic pain. To confirm these prior study results, the effects of intrathecal GT1b or CSF-1 administration on pain hypersensitivity was investigated (Figure 1(a)). A significant decrease in mechanical threshold was observed in GT1b- and CSF-1-injected male mice 1 day post-injection, which lasted up to 7 days (Figure 1(a)). To quantify the intensity of mechanical hypersensitivity, the area under the threshold line that showed comparable pain induction in GT1b- and CSF-1-injected male mice was measured (Figure 1(b)).
Figure 1.
Intrathecal GT1b or CSF-1 administration induces mechanical hypersensitivity. (a) Mechanical thresholds were measured for a week following intrathecal injection of GT1b (25 µg/5 µl) or CSF-1 (100 ng/5 µl) in male mice (n = 6 for vehicle, n = 6 for GT1b, n = 8 for CSF-1). (b) The area under the threshold line was calculated. All data are presented as mean ± SEM (one-way ANOVA, ***p < 0.001, p < 0.05, GT1b vs. vehicle, ###p < 0.001, #p < 0.05, CSF-1 vs. vehicle at each time point).
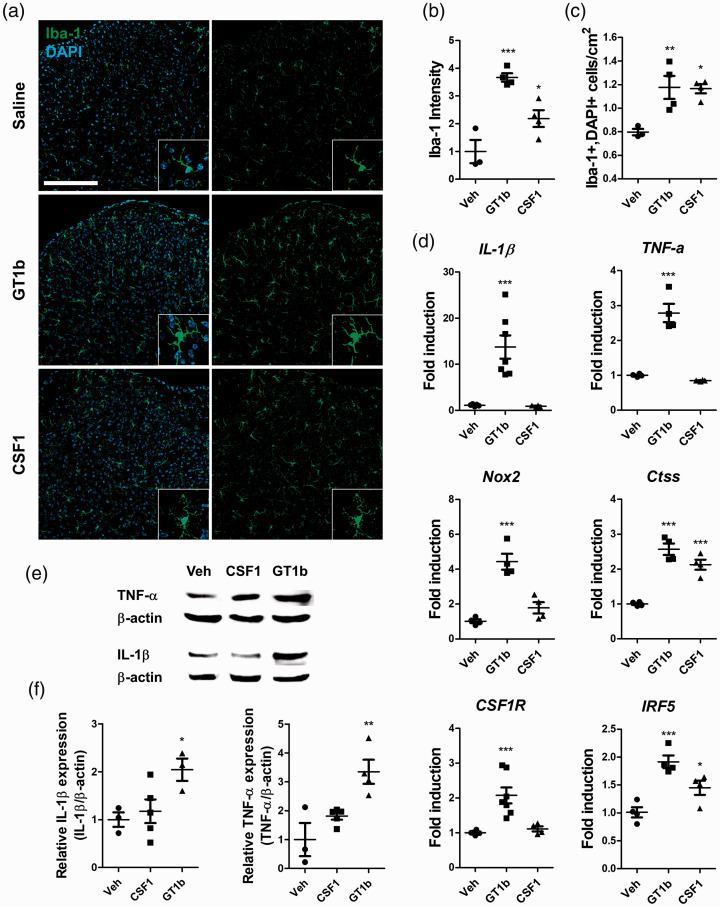
Next, the in vivo effects of intrathecal GT1b and CSF-1 administration on spinal cord microglia activation were compared. In GT1b-injected mice, microglia in the spinal cord manifested typical morphological features of activated microglia with an enlarged cell body (Figure 2(a)) and enhanced Iba-1 immunoreactivity (Figure 2(b)). CSF-1 injection also significantly increased Iba-1 immunoreactivity in the spinal cord, but with less intensity compared with GT1b (Figure 2(a) and (b)). However, the number of Iba-1-positive microglia increased at a comparable level in both GT1b- and CSF-1-injected mice (Figure 2(c)).
Figure 2.
GT1b and CSF-1 differentially activate spinal cord microglia in vivo. L5 spinal cord tissues were isolated 3 days after intrathecal injection of GT1b (25 µg/5 µl) or CSF-1 (100 ng/5 µl). (a) L5 spinal cord sections were immunostained with Iba-1 antibody and representative images are shown (n = 3 for control, n = 4 for GT1b and CSF-1; scale bar, 200 µm). (b) The fluorescent intensity of Iba-1 was measured and (c) Iba-1+/DAPI+ cells were counted. (d) Transcript levels of IL-1β, TNF-α, Nox2, CSF-1R, Ctss, and IRF5 in the spinal cord were measured. (e) IL-1β and TNF-α expression in the spinal cord with or without CSF-1 or GT1b injection was measured by western blot assay. A representative image (e) and quantified data are shown (f). Data are presented as mean ± SEM (Student’s t-test, ***p < 0.001, **p < 0.01, p < 0.05 vs. vehicle).
Nerve injury-induced spinal cord microglia activation is accompanied by IRF-5 and Ctss upregulation.11,20 In addition, inflammatory gene expression from activated microglia, such as IL-1β, TNF-α and Nox2, is implicated in the development of central sensitization.21,22 To test the relative contribution of GT1b and CSF-1 to the microglia activation phenotype, the transcript expression of the above-mentioned genes was measured in GT1b- or CSF-1-injected spinal cord tissue. Transcripts of IRF-5 and Ctss were upregulated in both GT1b- and CSF-1-injected mice (Figure 2(d)). Upregulation of proinflammatory cytokines (IL-1β and TNF-α) and Nox2 was observed in GT1b-injected mice, but not in CSF-1-injected mice. Similarly, protein expression of proinflammatory cytokines was upregulated only in GT1b-injected mice (Figure 2(e) and (f)). These data indicate that the microglia activation phenotype in terms of pain-relevant gene expression profile after GT1b and CSF-1 stimulation is distinct, although both stimuli increase microglia number and morphological activation in vivo.
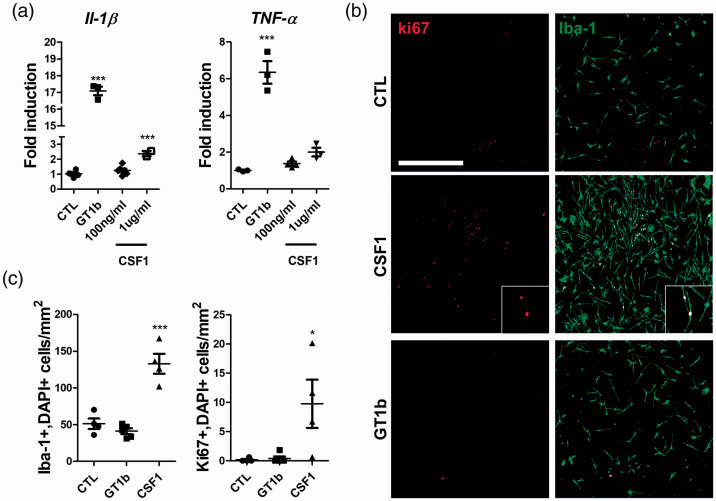
To confirm the results in vitro, proinflammatory gene expression was measured in primary cultured microglia. GT1b stimulation upregulated IL-1β and TNF-α transcripts 17- and 6-fold, respectively (Figure 3(a)). CSF-1 treatment up to 1 µg/ml failed to induce TNF-α transcript expression in primary microglia; however, the IL-1β transcript was induced 2-fold.
Figure 3.
GT1b upregulates proinflammatory cytokines and CSF-1 induces microglia proliferation in vitro. Cultured pure microglia were stimulated with GT1b (10 µg/ml) or CSF-1 (100 ng/ml and 1 µg/ml) for 3 hr. (a) IL-1β and TNF-α gene expression levels were measured using real-time RT-PCR. Microglia were stained with Iba-1 (green) and Ki67 (red) antibodies 4 days after GT1b or CSF-1 treatment. (b) Representative images are shown. (c) The number of Iba-1+/DAPI+ and Ki67+/DAPI+ cells were counted (scale bar, 500 µm). Data are presented as mean ± SEM (Student’s t-test, ***p < 0.001, *p < 0.05 vs. vehicle).
Next, the relative contribution of GT1b and CSF-1 to microglia proliferation was assessed. CSF-1 treatment significantly increased Ki67-positive proliferating microglia, and the microglia cell number increased 3-fold (Figure 3(b) and (c)). However, GT1b treatment could not induce Ki67-positive microglia or increase cell number. Taken together, these data indicate that CSF-1 exerts mainly microglia-proliferating effects and has minimal effect on proinflammatory cytokine induction. In contrast, GT1b induces pain-relevant inflammatory gene expression without inducing microglia proliferation in vitro.
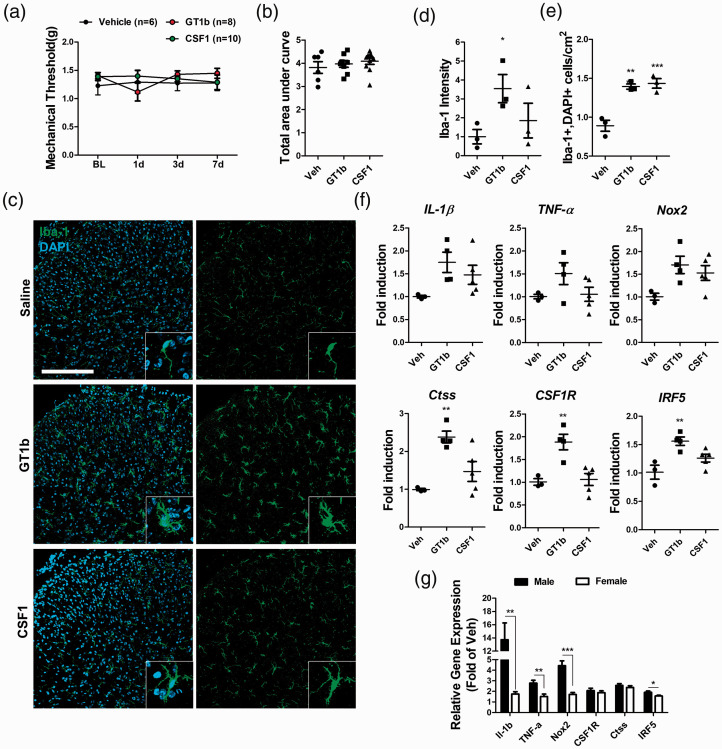
Increasing evidence has indicated that central sensitization mechanisms for nerve injury-induced neuropathic pain are sexually dimorphic.23,24 To determine whether GT1b- and CSF-1-induced mechanical hypersensitivity differs in terms of sex, pain behaviors in female mice were examined (Figure 4). GT1b- and CSF-1-induced mechanical hypersensitivity was completely abrogated in female mice, indicating sexual dimorphism (Figure 4(a) and (b)), even though both stimuli induced spinal cord microglia proliferation in female mice (Figure 4(c) to (e)). In the meanwhile, GT1b-stimulated proinflammatory cytokines and Nox2 gene induction was completely abrogated in female mice (Figure 4(f) and (g)), while IRF5 gene induction was slightly attenuated in female mice compared to male mice (Figure 4(f) and (g)). These data suggest that female mice are relatively resistant to GT1b-induced proinflammatory gene induction in the spinal cord, which might explain the observed sexual dimorphism in GT1b-induced pain. Taken together, our data suggest that GT1b and CSF-1, two injured sensory neuron-derived molecules, activate spinal cord microglia in concert via distinct pathways, but induce central sensitization only in male mice.
Figure 4.
GT1b and CSF-1 do not induce mechanical hypersensitivity in female mice. (a) The mechanical threshold was measured for 1 week following intrathecal injection of GT1b (25 µg/5 µl) or CSF-1 (100 ng/5 µl) in female mice (n = 6 for vehicle, n = 8 for GT1b, n = 10 for CSF-1) and (b) the areas under the threshold line were calculated. Female L5 spinal cord tissues were isolated 3 days after intrathecal injection of GT1b (25 µg/5 µl) or CSF-1 (100 ng/5 µl). (c) Coronal section of L5 spinal cord was immunostained with Iba-1 antibody and representative images are shown (n = 3 for each group; scale bar, 200 µm). (d) The fluorescent intensity of Iba-1 was measured and (e) Iba-1+/DAPI+ cells were counted. (f) The transcript levels of IL-1β, TNF-α, Nox2, CSF-1R, Ctss, and IRF5 were measured. Data are presented as mean ± SEM (one-way ANOVA, *** p < 0.0001, ** p < 0.001, p < 0.005 vs. vehicle at each time point). (g) The fold induction levels of each transcript in GT1b-injected male and female mice were compared. Data are presented as mean ± SEM (Student’s t-test, ***p < 0.001, **p < 0.01, p < 0.05).
Discussion
In the present study, the function of GT1b and CSF-1 in nerve injury-induced spinal cord microglia activation was compared. Although both molecules are released from injured sensory neurons to activate spinal cord microglia and induce central sensitization in vivo, GT1b transmits mainly inflammatory signals in the microglia without affecting cell proliferation, while CSF-1 induces microglia cell proliferation with minimal effect on proinflammatory gene expression. Therefore, these two injured sensory neuron-derived molecules may activate spinal cord microglia in concert via distinct activation pathways in nerve injury-induced neuropathic pain (Figure 5). However, in the present study, microglia were stimulated with different concentrations of GT1b and CSF-1 (10 µg/ml of GT1b and up to 1 µg/ml of CSF-1). Therefore, the different effects of these two molecules could potentially be due to the dosage difference. Although the exact concentration of CSF-1 used was 10-fold lower than GT1b, the concentration of CSF-1 (1 µg/ml) used was significantly higher than the KD of CSF-1 (approximately 0.23 nM). In addition, the cell-proliferating effects of GT1b were not observed even with a significantly higher concentration (up to 100 µg/ml; data not shown). Therefore, it is unlikely the different effects of these two molecules on microglia in vitro are merely due to dosage differences.
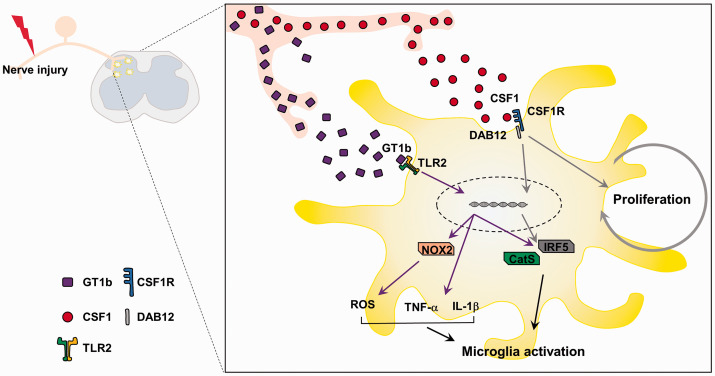
Figure 5.
GT1b and CSF-1 activate spinal cord microglia in concert in nerve injury-induced neuropathic pain. Peripheral nerve injury induces GT1b and CSF-1 in DRG neurons, which is then transported to and released in the spinal cord dorsal horn. GT1b activates microglial TLR2 and induces pain-relevant inflammatory mediators without affecting cell proliferation. CSF-1 activates CSF receptor and induces microglia proliferation. Therefore, GT1b and CSF-1 activate spinal cord microglia in concert with distinct activation pathways during nerve injury-induced neuropathic pain.
Notably, although GT1b failed to induce microglia proliferation in vitro, a significant increase in microglia cell number was observed in the spinal cord when injected in vivo. In addition to proinflammatory genes such as IL-1β and TNF-α, the CSF-1 receptor was significantly upregulated in microglia by GT1b stimulation (Figure 2(d)). Therefore, the microglia-proliferating effects of GT1b injection in vivo may be indirectly due to the CSF-1-induced microglia proliferation promoted by an increase in CSF-1 receptors. Likewise, a slight increase in proinflammatory cytokines in CSF-1-injected mice observed in a prior report 14 and in the present in vitro study (Figure 3(a)) might be due to microglia proliferation and a subsequent increase in basal-level cytokine expression.
In addition to GT1b and CSF-1, other molecules have been implicated in spinal cord microglia activation during nerve injury-induced neuropathic pain. For instance, fractalkine, a member of the CX3C class of chemokines, is cleaved from primary afferent fibers to activate microglia via CX3CR1.25 To interact with CX3CR1, however, fractalkine needs to be cleaved by Ctss, a cysteine protease released from activated microglia, therefore requiring a prior microglia-activating signal.11,13 In addition, it has been proposed that ATP is released from damaged afferent neurons to activate spinal cord microglia via P2X4.26,27 However, in a subsequent study, the source of spinal cord ATP was shown to be the vesicular nucleotide transporter-expressing inhibitory interneurons rather than damaged afferent neurons.10 Therefore, GT1b and CSF-1 are the most likely molecules that trigger spinal cord microglia activation in nerve injury-induced neuropathic pain.
Regarding pain-relevant inflammatory gene induction, transcripts of IL-1β, TNF-α, and Nox2 were upregulated by GT1b stimulation in vivo and in vitro. IL-1β increases excitatory synaptic transmission through phosphorylation of the NMDA receptor and may enhance nociceptive signals resulting in mechanical hypersensitivity.28 Similarly, TNF-α can affect neuronal excitability, specifically increasing LTP at C-fiber synapses.7,9,29 Reactive oxygen species, which are regulated by Nox2, are also required for expression of proinflammatory cytokines and central sensitization.22 Because these pain-relevant genes were upregulated by GT1b stimulation but not by CSF-1, we hypothesize that proinflammatory microglia activation is mainly mediated by GT1b signals, and CSF-1 likely contributes to central sensitization by amplifying microglia cell number (Figure 5).
Notably, when GT1b or CSF-1 was intrathecally injected into female mice, neither GT1b nor CSF-1 induced mechanical hypersensitivity. These data suggest that the pain-inducing effects of GT1b and CSF-1 are sexually dimorphic. Furthermore, spinal cord microglia still proliferated after GT1b or CSF-1 injection, similar to male mice. These data indicate that spinal cord microglia proliferation is insufficient to induce central sensitization in female mice. Of interest, the GT1b-induced proinflammatory cytokine and Nox2 gene induction observed in male mice was absent from female mice. Considering the pivotal role of these genes in spinal cord central sensitization,4,22,29 differential gene induction by GT1b in male versus female mice may underlie the sexual dimorphism of GT1b-induced pain. However, further research is needed to confirm this.
In conclusion, GT1b and CSF-1 activate microglia through distinct activation pathways transmitting proinflammatory and cell proliferation signals, respectively. Therefore, GT1b and CSF-1 can concertedly contribute to nerve injury-induced spinal cord microglia activation and subsequent central sensitization. The results of the present study will provide insight into the development of pathway-specific spinal cord microglia manipulation strategies for the treatment of nerve injury-induced neuropathic pain.
Footnotes
Author Contributions: HH and JL performed all experiments and analyzed the data. JL and SJL conceived and designed the study and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) have no potential conflicts of interest to declare with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1A2C2087493 and NRF-2016M3C7A1905074).
ORCID iD: Jaesung Lee https://orcid.org/0000-0001-5841-2946
References
- 1.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 2014; 13: 924–935. [DOI] [PubMed] [Google Scholar]
- 2.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuner R. Central mechanisms of pathological pain. Nat Med 2010; 16: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Zhang Y-Q, Qadri YJ, Serhan CN, Ji R-R. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018; 100: 1292–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018; 19: 138–152. [DOI] [PubMed] [Google Scholar]
- 6.Ji R-R, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain® 2013; 154: S10–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klapal L, Igelhorst BA, Dietzel-Meyer ID. Changes in neuronal excitability by activated microglia: differential Na+ current upregulation in pyramid-shaped and bipolar neurons by TNF-α and IL-18. Front Neurol 2016; 7: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, Hempstead BL, Littman DR, Gan W-B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013; 155: 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFα production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci U S A 2008; 105: 17151–17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuda T, Ozono Y, Mikuriya S, Kohro Y, Tozaki-Saitoh H, Iwatsuki K, Uneyama H, Ichikawa R, Salter MW, Tsuda M, Inoue K. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat Commun 2016; 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark AK, Malcangio M. Microglial signalling mechanisms: cathepsin S and fractalkine. Exp Neurol 2012; 234: 283–292. [DOI] [PubMed] [Google Scholar]
- 12.Clark AK, Wodarski R, Guida F, Sasso O, Malcangio M. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia 2010; 58: 1710–1726. [DOI] [PubMed] [Google Scholar]
- 13.Clark AK, Yip PK, Malcangio M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci 2009; 29: 6945–6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI. Injured sensory neuron-derived CSF-1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 2016; 19: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim H, Lee J, You B, Oh JH, Mok HJ, Kim YS, Yoon B-E, Kim BG, Back SK, Park J-S, Kim KP, Schnaar RL, Lee SJ. GT 1b functions as a novel endogenous agonist of toll‐like receptor 2 inducing neuropathic pain. Embo J 2020; 39: e102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980; 67: 313–316. [DOI] [PubMed] [Google Scholar]
- 17.Chaplan SR, Bach F, Pogrel J, Chung J, Yaksh T. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Zhou T, Choi C, Wang Z, Benveniste EN. Differential regulation and function of fas expression on glial cells. J Immunol 2000; 164: 1277–1285. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 20.Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, Mak TW, Tamura T, Tsuda M, Inoue K. Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun 2014; 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji R-R, Chamessian A, Zhang Y-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016; 354: 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D, You B, Jo E-K, Han S-K, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A 2010; 107: 14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III and Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009; 10: 447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mapplebeck JC, Beggs S, Salter MW. Sex differences in pain: a tale of two immune cells. Pain 2016; 157: S2–S6. [DOI] [PubMed] [Google Scholar]
- 25.Clark AK, Malcangio M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci 2014; 8: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toulme E, Tsuda M, Khakh BS, Inoue K. On the role of ATP-Gated P2X receptors in acute, inflammatory and neuropathic pain. Transl Pain Res Mouse Man 2010; 1–11. [PubMed] [Google Scholar]
- 27.Trang T, Salter MW. P2X4 purinoceptor signaling in chronic pain. Purinerg Signal 2012; 8: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the src family of kinases. J Neurosci 2003; 23: 8692–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruber-Schoffnegger D, Drdla-Schutting R, Honigsperger C, Wunderbaldinger G, Gassner M, Sandkuhler J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-α and IL-1β is mediated by glial cells. J Neurosci 2013; 33: 6540–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]