Abstract
Objective
To determine the association between serum visfatin levels and psoriasis and to evaluate the correlation between serum visfatin levels and the severity of psoriasis.
Methods
The electronic databases PubMed®, Embase® and the Cochrane Library were searched for articles published from inception to 1 May 2020. Data were extracted and then standard mean differences (SMDs) and 95% confidence intervals (CIs) were calculated for pooled estimates.
Results
A total of 11 studies met the inclusion criteria and were included (448 patients diagnosed with psoriasis and 377 controls). This meta-analysis demonstrated that patients with psoriasis had significantly higher levels of visfatin than the controls (SMD = 0.90, 95% CI 0.52, 1.28). Subgroup analyses showed that differences in serum visfatin levels between the patient group and the control group were associated with ethnicity, Psoriasis Area and Severity Index (PASI) and body mass index. Additionally, a meta-analysis of correlations showed that visfatin levels in patients with psoriasis were positively correlated with PASI (r = 0.51, 95% CI 0.14, 0.75).
Conclusions
This meta-analysis showed that serum visfatin levels in patients with psoriasis were significantly higher than those in the controls and a positive correlation between serum visfatin levels and psoriasis severity was observed.
Keywords: Serum, visfatin, psoriasis, disease severity, meta-analysis
Introduction
Psoriasis is a common chronic relapsing inflammatory skin disease.1 The economic burden of psoriasis is significant worldwide. For example, in 2013, the annual cost of psoriasis in the United States was approximately $112 billion.2 The published prevalence of psoriasis in various regions ranges from 0.09% to 11.43%.3,4 Psoriatic skin symptoms, complex triggering factors and serious complications are important global healthcare problems.1 Although great advancements in the treatment of psoriasis have recently been made,5 there is still no cure that can completely address it, partially due to its exact mechanism remaining unknown.1 Currently, it is generally believed that the occurrence and development of psoriasis are related to the interactions of multiple systems because an increased frequency of some non-skin disorders, including Crohn’s disease, cardiovascular diseases, metabolic syndrome and even psychological diseases, has been epidemiologically observed in psoriasis patients.6 The study of the relationship between psoriasis and metabolic syndrome (MetS) has attracted increasing attention from researchers in recent years. MetS is a group of metabolic disorders, including obesity, atherogenic dyslipidaemia, dysglycaemia, hypertension, type 2 diabetes and insulin resistance.7 Studies conducted in different countries have noted that the prevalence of MetS in psoriasis patients is significantly higher than that in controls.8–10 Understanding the potential relationship between MetS and psoriasis may be critical for the diagnosis and treatment of this disease. Obesity has been reported as an independent risk factor for the occurrence and severity of psoriasis in recent studies.11 The exact link between obesity and psoriasis is unclear, but studies have shown that adipose tissue can produce some cytokines, such as visfatin, which may make a significant contribution to the development of psoriasis.12
Visfatin, a protein initially described as pre-B cell colony-enhancing factor (PBEF), was identified as a new adipocytokine in 2005.13 However, that article was retracted because of controversy about the conclusion that visfatin mimics the effects of insulin.14 In recent years, the effects of visfatin in many chronic inflammatory diseases, metabolic conditions and cancers have been widely studied.15–17 However, the effects of visfatin on the psoriatic process remain controversial. On the one hand, evidence has shown that serum visfatin levels in psoriasis patients are higher than those in controls.18,19 Moreover, a study indicated that visfatin could induce the production of Vascular endothelial growth factor and matrix metalloproteinase-2/9 through the mitogen-activated protein kinase and phosphatidylinositol 3-kinase-/protein kinase B signalling pathways, leading to angiogenesis.20 A previous study found that visfatin could modulate the production of proinflammatory mediators via the nuclear factor kappa-B pathway.21 Additionally, visfatin plays a regulatory role in cell proliferation and apoptosis.22 All of these factors may make important contributions to the process of psoriasis. In addition, a positive correlation between the expression of serum visfatin and psoriasis severity was found in some studies.18,19 On the other hand, studies have shown that the serum visfatin levels in psoriasis patients are not significantly different from those in controls.23,24 Therefore, a meta-analysis was conducted to obtain an overview of the association between psoriasis and visfatin.
Materials and methods
Literature search
The electronic databases PubMed®, Embase® and the Cochrane Library were searched for articles published from inception to 1 May 2020. In addition, related articles were manually searched to identify other potentially eligible studies. The search terms (all fields) were as follows: “visfatin” AND “psoriasis”. This meta-analysis was conducted in accordance with the PRISMA Guidelines (registration no. CRD42020206344).
Inclusion and exclusion criteria
Studies meeting the following criteria were included: (i) case–control or cross-sectional studies; (ii) studies reporting a comparison of serum visfatin levels between patients with psoriasis and controls; (iii) studies in which all patients were clinically or pathologically diagnosed with psoriasis. The major exclusion criteria were as follows: (i) duplicate or overlapping publications; (ii) review articles, conference abstracts, letters, case reports or animal studies; (iii) studies with incomplete data; (iv) articles not published in English.
Data extraction
All included studies were imported into Endnote, which facilitated the identification and removal of duplicate data. All relevant records after screening were assessed by two independent reviewers (J.W.S. and Y.T.G.). Any disagreements were resolved by a third investigator (H.J.S.) and an agreement was reached after discussion.
Quality assessment
The quality of the selected studies was analysed in compliance with the requirements of the Newcastle–Ottawa scale (NOS),25 including three parts: selection of study participants (4 scores), comparability of groups (2 scores) and measurement of exposures and outcome (3 scores). A study with a score of 7 points or above was considered to be a high-quality study.
Statistical analyses
Standardized mean differences (SMDs) and their 95% confidence intervals (CIs) were calculated for the current study. The heterogeneity among studies was assessed using Cochran's Q test. A random-effects model was utilized when heterogeneity was greater than 50%; otherwise, a fixed-effects model was used. Subgroup analysis and meta-regression analysis were performed to assess the source of heterogeneity. Moreover, sensitivity analysis was conducted by excluding one study at a time to determine its impact on the obtained outcomes; and funnel plots and Egger’s regression test were used to examine publication bias in the literature. A P-value < 0.05 was considered to be statistically significant. Statistical meta-analyses were performed with STATA® version 12.0 software (STATA Corp., College Station, TX, USA).
Results
As shown in Figure 1, a total of 11 case–control studies were eligible to be included in the meta-analysis, with 448 patients with psoriasis and 377 controls.18,19,23,24,26–32 These studies were published from 2012 to 2020 and were undertaken in Asia, Africa and Europe. In the study quality assessment, 10 studies had a NOS score ≥7. The basic features of the included studies are shown in Table 1.18,19,23,24,26–32
Figure 1.
Flow chart of eligible studies showing the number of citations identified, retrieved and included in the final meta-analysis.
Table 1.
Characteristics of eligible studies included in this meta-analysis to evaluate the relationship between psoriasis and serum visfatin levels.18,19,23,24,26–32
| Author | Ethnicity |
Sample size |
Serum level of visfatin(mean ± SD, ng/ml) |
Correlation coefficient with PASI (r) | Matchedvariables | Sample type | Measurementmethod | NOS | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||||||
| Badran et al. 201418 | African | 40 | 40 | 26.60 ± 10.21 | 15.60 ± 3.38 | 0.951 | Age, sex, BMI | Serum | EIA | 7 |
| Chyl-Surdacka et al., 202019 | European | 102 | 40 | 5.19 ± 3.31 | 0.93 ± 0.62 | 0.059 | Age | Serum | ELISA | 6 |
| Capo et al., 202023 | European | 19 | 17 | 4.94 ± 1.28 | 4.62 ± 0.89 | 0.062 | Age, sex, BMI | Serum | ELISA | 7 |
| Zu-Elfakkar et al., 201726 | African | 20 | 20 | 28.90 ± 19.47 | 16.77 ± 6.57 | 0.619 | Age, sex | Serum | NA | 6 |
| Coban et al., 201627 | Asian | 35 | 50 | 8.37 ± 1.41 | 12.89 ± 27.26 | 0.168 | Age, sex, BMI | Serum | ELISA | 7 |
| Elgarhy et al., 201628 | African | 25 | 15 | 78.60 ± 48.10 | 9.70 ± 4.90 | 0.869 | Age, sex, BMI | Serum | ELISA | 7 |
| Okan et al., 201629 | Asian | 45 | 45 | 20.78 ±11.36 | 12.83 ± 7.87 | 0.396 | Age, sex, BMI | Serum | ELISA | 7 |
| Sereflican et al., 201624 | Asian | 42 | 42 | 6.94 ± 2.29 | 6.01 ± 2.35 | –0.021 | Age, sex, BMI | Serum | EIA | 7 |
| Campanati et al., 201530 | European | 47 | 39 | 128.70 ± 53.36 | 72.53 ± 17.02 | NA | Age, sex, BMI | Serum | EIA | 7 |
| Lora et al., 201331 | European | 27 | 27 | 3.80 ± 2.10 | 3.10 ± 1.30 | NA | Age, sex, BMI | Serum | ELISA | 7 |
| Ismail et al., 201232 | African | 46 | 42 | 62.20 ± 39.40 | 21.30 ± 15.30 | 0.440 | Age, sex, BMI | Serum | ELISA | 7 |
PASI, Psoriasis Area and Severity Index; NOS, Newcastle–Ottawa scale; BMI, body mass index; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; NA, not available.
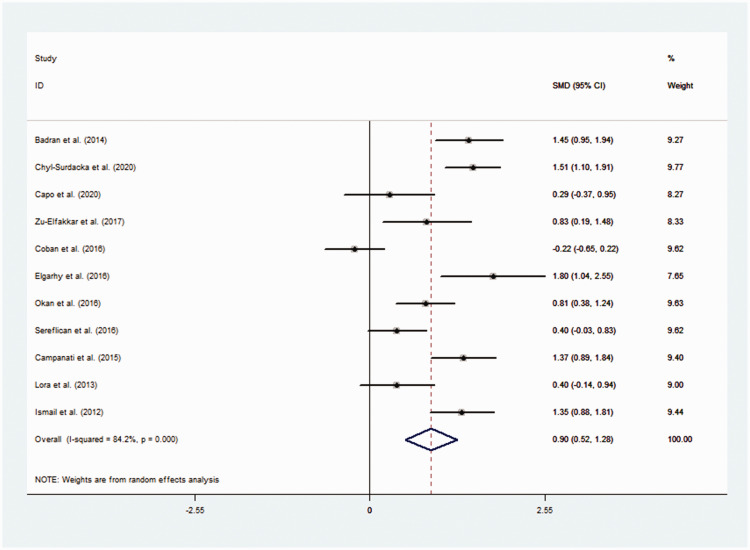
The pooled results demonstrated that visfatin levels were significantly higher in the psoriasis group than in the control group (SMD = 0.90, 95% CI 0.52, 1.28, P < 0.001) (Figure 2).18,19,23,24,26–32 Cochran's Q test showed significant heterogeneity among these included studies (I2 = 84.2%, P < 0.001). Subgroup analyses were performed due to the existence of heterogeneity. The subgroup analysis based on ethnicity revealed that serum visfatin levels were significantly higher in psoriasis patients in Africa and Europe (Africa: SMD = 1.34, 95% CI 1.02, 1.67, P < 0.001; Europe: SMD = 0.92, 95% CI 0.31, 1.53, P = 0.003) but not in Asian patients. A subgroup analysis by Psoriasis Area and Severity Index (PASI) showed significantly higher visfatin levels in psoriasis patients with higher PASI scores (PASI ≥ 10) (SMD = 1.17, 95% CI 0.80, 1.53, P < 0.001). Stratification by body mass index (BMI) revealed significantly higher visfatin levels in psoriasis patients with greater BMI values (BMI ≥25 kg/m2) (SMD = 0.83, 95% CI 0.33, 1.33, P = 0.001). Subgroup analyses to identify possible sources of heterogeneity evaluated sample size, matching of age, sex and BMI, measurement method and ELISA kits but found no statistically significant association in these subgroups (Table 2). Meta-regression analysis was used to further identify sources of heterogeneity. Covariates included publication year, sample size, BMI and PASI. The results showed that PASI could explain 82.78% of the heterogeneity (Table 3). However, none of the remaining covariates changed the correlation between visfatin levels and psoriasis. Meta-analysis of correlation coefficients showed a significant positive correlation between serum visfatin levels and PASI (r = 0.51, 95% CI 0.14, 0.75, P = 0.009) (Table 4).
Figure 2.
Forest plot of a meta-analysis to evaluate the relationship between psoriasis and serum visfatin levels.18,19,23,24,26–32
Table 2.
Subgroup analyses of the relationship between visfatin levels and psoriasis.
| Stratification group | n | SMD (95% CI) | z | P-value |
Heterogeneity test |
||
|---|---|---|---|---|---|---|---|
| Q | I2 (%) | P-value | |||||
| Ethnicity | |||||||
| African | 4 | 1.34 (1.02, 1.67) | 8.12 | P < 0.001 | 3.92 | 23.4 | NS |
| Asian | 3 | 0.33 (–0.25, 0.92) | 1.11 | NS | 11.05 | 81.9 | P = 0.004 |
| European | 4 | 0.92 (0.31, 1.53) | 2.97 | P = 0.003 | 17.28 | 82.6 | P = 0.001 |
| Combined | 11 | 0.90 (0.52, 1.28) | 4.65 | P < 0.001 | 63.30 | 84.2 | P < 0.001 |
| Sample size | |||||||
| <30 | 4 | 0.80 (0.18, 1.41) | 2.55 | P = 0.011 | 10.90 | 72.5 | P = 0.012 |
| ≥30 | 7 | 0.95 (0.46, 1.44) | 3.78 | P < 0.001 | 51.18 | 88.3 | P < 0.001 |
| Combined | 11 | 0.90 (0.52, 1.28) | 4.65 | P < 0.001 | 63.30 | 84.2 | P < 0.001 |
| PASI | |||||||
| <10 | 3 | 0.14 (–0.27, 0.55) | 0.67 | NS | 4.17 | 52.1 | NS |
| ≥10 | 6 | 1.17 (0.80, 1.53) | 6.24 | P < 0.001 | 15.45 | 67.6 | P = 0.009 |
| Combined | 9 | 0.83 (0.40, 1.26) | 3.81 | P < 0.001 | 52.89 | 84.9 | P < 0.001 |
| BMI | |||||||
| <25 | 2 | 0.85 (–0.20, 1.91) | 1.58 | NS | 6.82 | 85.3 | P = 0.009 |
| ≥25 | 7 | 0.83 (0.33, 1.33) | 3.22 | P = 0.001 | 44.59 | 86.5 | P < 0.001 |
| Combined | 9 | 0.84 (0.41, 1.26) | 3.81 | P < 0.001 | 52.89 | 84.9 | P < 0.001 |
| Matching | |||||||
| Age, sex BMI all Matched | 9 | 0.84 (0.41, 1.26) | 3.81 | P < 0.001 | 52.89 | 84.9 | P < 0.001 |
| Not all Matched | 2 | 1.22 (0.57, 1.87) | 3.68 | P < 0.001 | 2.95 | 66.2 | NS |
| Combined | 11 | 0.90 (0.52, 1.28) | 4.65 | P < 0.001 | 63.30 | 84.2 | P < 0.001 |
| Measurement method | |||||||
| ELISA | 7 | 0.84 (0.30, 1.38) | 3.02 | P = 0.002 | 49.06 | 87.8 | P < 0.001 |
| EIA | 3 | 1.06 (0.38, 1.74) | 3.06 | P = 0.002 | 12.82 | 84.4 | P = 0.002 |
| Combined | 10 | 0.91 (0.50, 1.32) | 4.33 | P < 0.001 | 63.28 | 85.8 | P < 0.001 |
| ELISA kits | |||||||
| AdipoGen | 1 | 1.51 (1.10, 1.91) | 7.27 | P < 0.001 | 0.00 | NA | NA |
| Phoenix | 3 | 0.70 (0.00, 1.40) | 1.97 | P = 0.049 | 9.75 | 79.5 | P = 0.008 |
| RayBiotech | 1 | 1.80 (1.04, 2.55) | 4.65 | P < 0.001 | 0.00 | NA | NA |
| Shanghai Yehua | 1 | 0.81 (0.38, 1.24) | 3.71 | P < 0.001 | 0.00 | NA | NA |
| Combined | 6 | 1.02 (0.58, 1.47) | 4.51 | P < 0.001 | 22.02 | 77.3 | P = 0.001 |
SMD, standardized mean difference; CI, confidence interval; PASI, Psoriasis Area and Severity Index; BMI, body mass index; ELISA, enzyme-linked immunosorbent assay; EIA, enzyme immunoassay; NA, not available; NS, no significant association (P ≥ 0.05).
Table 3.
Univariate meta-regression analysis of heterogeneity caused by patient variables across studies.
| Variables | Coefficient | Standard error | 95% CI | t | P-value |
|---|---|---|---|---|---|
| Publication year | 0.44 | 0.08 | –0.14, 0.23 | 0.53 | P = 0.606 |
| Sample size | 0.11 | 0.01 | –0.01, 0.03 | 1.33 | P = 0.216 |
| BMI | 0.09 | 0.11 | –0.17, 0.35 | 0.82 | P = 0.441 |
| PASI | 0.08 | 0.02 | 0.04, 0.13 | 4.44 | P = 0.003 |
CI, confidence interval; BMI, body mass index; PASI, Psoriasis Area and Severity Index.
Table 4.
Meta-analysis of the correlation coefficients between visfatin levels and psoriasis severity.
| Variable | Comparison | No. of studies | CC | 95% CI | P-value |
Heterogeneity |
||
|---|---|---|---|---|---|---|---|---|
| Model | P | I2 (%) | ||||||
| Visfatin | PASI | 9 | 0.51 | 0.14, 0.75 | P = 0.009 | Random | P < 0.001 | 93.3 |
CC, correlation coefficient; CI, confidence interval; PASI, Psoriasis Area and Severity Index.
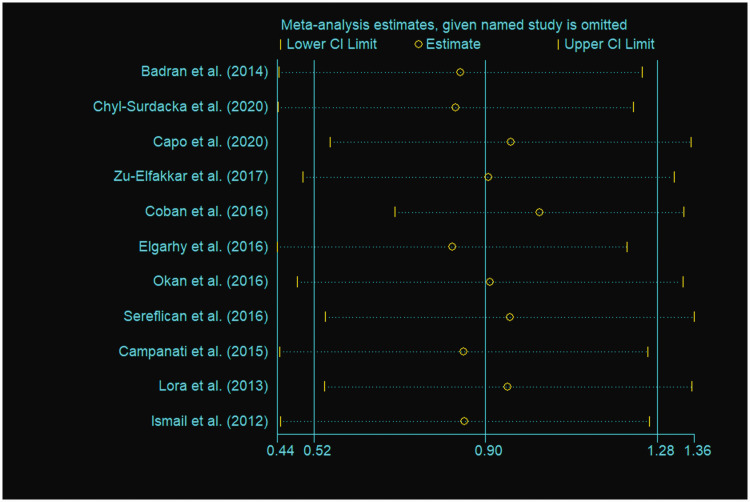
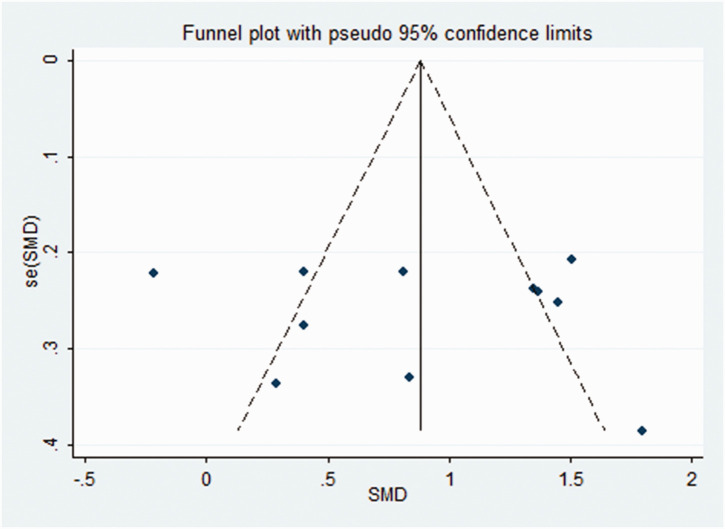
Sequential removal of each study did not influence the obtained outcomes, indicating the stability and reliability of the meta-analysis (Figure 3). A funnel plot of all eligible studies showed approximate symmetry (Figure 4) and Egger's test showed that there was no obvious publication bias (P = 0.813) in this meta-analysis.
Figure 3.
Sensitivity analysis of studies included in a meta-analysis to evaluate the relationship between psoriasis and serum visfatin levels.18,19,23,24,26–32
Figure 4.
Funnel plot of studies included in a meta-analysis to evaluate the relationship between psoriasis and serum visfatin levels. SMD, standard mean difference.18,19,23,24,26–32
Discussion
It has been reported that obesity and related metabolic conditions are associated with chronic inflammation, characterized by increased expression of proinflammatory factors.33 Patients with psoriasis often have concomitant MetS and this association may be mediated by adipokines.18 Visfatin is considered to be a proinflammatory cytokine widely and differently expressed in various tissues, but adipose tissue is the most important source.34 A previous study demonstrated that visfatin may induce the infiltration of type 1 or type 17 helper T cells or neutrophils into the skin through chemokine induction in human keratinocytes, thus linking MetS to psoriasis.35
Visfatin, also known as nicotinamide phosphoribosyltransferase, is a unique adipokine with proinflammatory effects because of its enzymatic activity.36 As a rate-limiting step in the rescue pathway of nicotinamide adenine dinucleotide (NAD) biosynthesis, visfatin can regulate the level of NAD in cells, affecting cellular energy and NAD-dependent enzymes.36 Additionally, visfatin can promote the production of inflammatory cytokines.18 PBEF is upregulated in the context of several chronic inflammatory diseases,37 and it is involved in delaying neutrophil apoptosis in the context of inflammation.38 Moreover, research has found that brown adipose tissue (BAT) is a new target for the treatment of human obesity.39 Uncoupling protein 1 (UCP-1) is the only uncoupling protein expressed in BAT.40 UCP-1 participates in the thermal regulation and energy metabolism of BAT to maintain the balance of the body.41 High concentrations of visfatin cause a sharp drop in the level of UCP-1 protein, which promotes increased consumption of BAT.42 This may partially explain the role of visfatin in obesity. The possible effects of visfatin on psoriasis are presented in Figure 5.
Figure 5.
Possible biological effects of visfatin on psoriasis. MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; VEGF, vascular endothelial growth factor; MMP ,matrix metalloproteinase; NF-κB, nuclear factor kappa-B; UCP-1, uncoupling protein 1.
Although there have been several studies on the relationship between serum visfatin levels and psoriasis, the conclusions have not been consistent.18,19,23,24 Some research suggests that the serum visfatin levels were higher in psoriasis patients and was positively correlated with PASI,18 whereas other research suggests that the serum visfatin level in psoriasis patients was not significantly different from that in controls.23 In this current meta-analysis, the results showed that the serum level of visfatin was significantly higher in psoriasis patients than in controls, indicating that visfatin might be related to psoriasis. Moreover, serum visfatin levels showed a positive correlation with PASI, suggesting that visfatin levels might reflect the severity of psoriasis. In addition, subgroup analysis also revealed that this association was stronger in patients with higher PASI scores. A previous meta-analysis that included four studies demonstrated no significant differences in the levels of serum visfatin expression between psoriasis patients and controls.43 A possible explanation for the disparity between this previous meta-analysis and the current findings is that this current meta-analysis included more studies (11 studies), so it might provide more accurate results.
In the current subgroup analysis, it must be noted that African and European psoriasis patients, psoriasis patients with BMI ≥ 25 kg/m2 and psoriasis patients with PASI ≥10 had higher serum visfatin levels than controls. Several studies found a positive but nonsignificant correlation between the serum level of visfatin and BMI, revealing a possible trend of higher serum lipid levels in psoriasis patients with greater BMI values (BMI ≥ 25 kg/m2).18,23,26 Likewise, multiple studies (Table 1) have demonstrated that serum visfatin levels were significantly positively correlated with the severity of psoriasis. Other potential causes may be the fact that the number of studies on Asian populations, psoriasis patients with BMI < 25 kg/m2 and patients with PASI < 10 were relatively small. Thus, it will be necessary to conduct a larger-scale study of patients by region, severity of psoriasis and BMI to further determine the correlations.
This current meta-analysis had several limitations. First, there were only 448 patients included in the study and the sample size was relatively small. Secondly, the included studies were limited to those published in English, while studies in other languages were eliminated. Thirdly, underlying biases cannot be completely ruled out, although no obvious publication biases were found in this study. More importantly, great heterogeneity between the included studies was observed, and meta-regression analysis suggested that differences in the PASI of patients with psoriasis may be one of the sources of heterogeneity. The sources of heterogeneity in statistics are very complex. For patients with psoriasis, the patient's disease course, medication history, age of onset, may all contribute to the heterogeneity of the results. Therefore, more research in the future is required to determine the sources of heterogeneity.
In conclusion, this current meta-analysis demonstrated that serum visfatin levels were significantly upregulated in psoriasis patients compared with those in the controls. This finding suggests that visfatin may contribute to the pathogenesis and progression of psoriasis, which may be helpful for the diagnosis and treatment of this disease. Moreover, a positive correlation was found between serum visfatin levels and psoriasis severity, suggesting that visfatin may be a potential biomarker for psoriasis. It will be necessary to conduct further research to verify the results and clarify the mechanism through which this may occur.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This study was supported by the Key Research and Development Project of Ningxia (key project; no. 2019BFG02008) and the Construction of Talent Platform Project of Ningxia (Innovation Team for Skin disease diagnosis and treatment technology & drug discovery and development; no. NXKJT2019012).
ORCID iDs: Qian Zou https://orcid.org/0000-0001-6562-7945
Jiawei Si https://orcid.org/0000-0002-9299-3657
Yatao Guo https://orcid.org/0000-0002-1264-6955
References
- 1.Lebwohl M. Psoriasis. Ann Intern Med 2018; 168: ITC49–ITC64. [DOI] [PubMed] [Google Scholar]
- 2.Brezinski EA, Dhillon JS, Armstrong AW. Economic Burden of Psoriasis in the United States: A Systematic Review. JAMA Dermatol 2015; 151: 651–658. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs S. Skin disease and socioeconomic conditions in rural Africa: Tanzania. Int J Dermatol 1996; 35: 633–639. [DOI] [PubMed] [Google Scholar]
- 4.Danielsen K, Olsen AO, Wilsgaard T, et al. Is the prevalence of psoriasis increasing? A 30-year follow-up of a population-based cohort. Br J Dermatol 2013; 168: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 5.Sonnenreich P. Advances in psoriasis treatment: experts comment on recent developments. P T 2013; 38: 776–779. [PMC free article] [PubMed] [Google Scholar]
- 6.Grozdev I, Korman N, Tsankov N. Psoriasis as a systemic disease. Clin Dermatol 2014; 32: 343–350. [DOI] [PubMed] [Google Scholar]
- 7.Corte CD, Alterio A, Nobili V. Metabolic syndrome. Ital J Pediatr 2015; 41: A26. [Google Scholar]
- 8.Milčić D, Janković S, Vesić S, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based cross-sectional study. An Bras Dermatol 2017; 92: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui XY, Yu XL, Jin HZ, et al. Prevalence of metabolic syndrome in Chinese psoriasis patients: A hospital‐based cross‐sectional study. J Diabetes Investig 2018; 9: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003–2006. Arch Dermatol 2011; 147: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunz M, Simon JC, Saalbach A. Psoriasis: Obesity and Fatty Acids. Front Immunol 2019; 10: 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu XL, Wu C, Jin HZ. Psoriasis, Cardiovascular Disease, and Adipokines. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2018; 40: 556–562 [Article in Chinese, English abstract]. [DOI] [PubMed] [Google Scholar]
- 13.Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005; 307: 426–430. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara A, Matsuda M, Nishizawa M, et al. Retraction. Science 2007; 318: 565. [DOI] [PubMed] [Google Scholar]
- 15.Neubauer K, Bednarz-Misa I, Walecka-Zacharska E, et al. Oversecretion and Overexpression of Nicotinamide Phosphoribosyltransferase/Pre-B Colony-Enhancing Factor/Visfatin in Inflammatory Bowel Disease Reflects the Disease Activity, Severity of Inflammatory Response and Hypoxia. Int J Mol Sci 2019; 20: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YH, Chang DM, Lin KC, et al. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev 2011; 27: 515–527. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi M, Mianabadi F, Mehrad-Majd H. Circulating visfatin levels and cancers risk: A systematic review and meta-analysis. J Cell Physiol 2019; 234: 5011–5022. [DOI] [PubMed] [Google Scholar]
- 18.Badran FK, Genedy RM, Swelem RS, et al. Evaluation of serum level of visfatin among psoriatic patients. Egypt J Dermatol Venerol 2014; 34: 107–113. [Google Scholar]
- 19.Chyl-Surdacka KM, Bartosińska J, Kowal M, et al. Assessment of visfatin concentrations in the serum of male psoriatic patients in relation to metabolic abnormalities. Adv Clin Exp Med 2020; 29: 79–84. [DOI] [PubMed] [Google Scholar]
- 20.Adya R, Tan BK, Punn A, et al. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res 2008; 78: 356–365. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Chen S, Gao H, et al. Visfatin induces the apoptosis of endothelial progenitor cells via the induction of pro-inflammatory mediators through the NF-κB pathway. Int J Mol Med 2017; 40: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD. Chronic stretching of amniotic epithelial cells increases pre-B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosis. Placenta 2008; 29: 255–265. [DOI] [PubMed] [Google Scholar]
- 23.Capo A, Di Nicola M, Costantini E, et al. Circulating levels of Apelin-36 in patients with mild to moderate psoriasis. G Ital Dermatol Venereol 2020; 155: 646–651. [DOI] [PubMed] [Google Scholar]
- 24.Sereflican B, Goksugur N, Bugdayci G, et al. Serum Visfatin, Adiponectin, and Tumor Necrosis Factor Alpha (TNF-α) Levels in Patients with Psoriasis and their Correlation with Disease Severity. Acta Dermatovenerol Croat 2016; 24: 13–19. [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 26.Zu-Elfakkar NM, Asaad MK, Wahab HEAA, et al. Serum level of Visfatin in Psoriasis and its Relation to Disease Severity. Egypt J Hosp Med 2017; 69: 1558–1562. [Google Scholar]
- 27.Coban M, Tasli L, Turgut S, et al. Association of Adipokines, Insulin Resistance, Hypertension and Dyslipidemia in Patients with Psoriasis Vulgaris. Ann Dermatol 2016; 28: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elgarhy L, Abdelnabi N, Abdullatif A, et al. Circulating endothelial cells and serum visfatin are indicators of cardiovascular disease risk in psoriasis patients. Dermatologica Sinica 2016; 34: 20–25. [Google Scholar]
- 29.Okan G, Baki AM, Yorulmaz E, et al. Serum Visfatin, Fetuin-A, and Pentraxin 3 Levels in Patients With Psoriasis and Their Relation to Disease Severity. J Clin Lab Anal 2016; 30: 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campanati A, Ganzetti G, Giuliodori K, et al. Serum levels of adipocytokines in psoriasis patients receiving tumor necrosis factor-α inhibitors: results of a retrospective analysis. Int J Dermatol 2015; 54: 839–845. [DOI] [PubMed] [Google Scholar]
- 31.Lora V, Bonaguri C, Gisondi P, et al. Autoantibody induction and adipokine levels in patients with psoriasis treated with infliximab. Immunol Res 2013; 56: 382–389. [DOI] [PubMed] [Google Scholar]
- 32.Ismail SA, Mohamed SA. Serum levels of visfatin and omentin-1 in patients with psoriasis and their relation to disease severity. Br J Dermatol 2012; 167: 436–439. [DOI] [PubMed] [Google Scholar]
- 33.Lu H, Gauvreau D, Tom FQ, et al. Inflammatory markers and adipokines alter adipocyte-derived ASP production through direct and indirect immune interaction. Exp Clin Endocrinol Diabetes 2013; 121: 194–200. [DOI] [PubMed] [Google Scholar]
- 34.Franco-Trepat E, Guillán-Fresco M, Alonso-Pérez A, et al. Visfatin Connection: Present and Future in Osteoarthritis and Osteoporosis. J Clin Med 2019; 8: 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanda N, Hau CS, Tada Y, et al. Visfatin enhances CXCL8, CXCL10, and CCL20 production in human keratinocytes. Endocrinology 2011; 152: 3155–3164. [DOI] [PubMed] [Google Scholar]
- 36.Wang T, Zhang XB, Bheda P, et al. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat Struct Mol Biol 2006; 13: 661–662. [DOI] [PubMed] [Google Scholar]
- 37.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol 2008; 83: 804–816. [DOI] [PubMed] [Google Scholar]
- 38.Jia SH, Li Y, Parodo J, et al. P re-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest 2004; 113: 1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frühbeck G, Becerril S, Sáinz N, et al. BAT: a new target for human obesity? Trends Pharmacol Sci 2009; 30: 387–396. [DOI] [PubMed] [Google Scholar]
- 40.Jezek P. Possible physiological roles of mitochondrial uncoupling proteins – UCPn. Int J Biochem Cell Biol 2002; 34: 1190–1206. [DOI] [PubMed]
- 41.Kozak LP, Anunciado-Koza R. . UCP1: its involvement and utility in obesity. Int J Obes (Lond) 2009; 32(Suppl 7): S32–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimitriadis GK, Adya R, Tan BK, et al. Effects of visfatin on brown adipose tissue energy regulation using T37i cells. Cytokine 2019; 113: 248–255. [DOI] [PubMed] [Google Scholar]
- 43.Bai F, Zheng W, Dong Y, et al. Serum levels of adipokines and cytokines in psoriasis patients: a systematic review and meta-analysis. Oncotarget 2017; 9: 1266–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]