Abstract
Human papillomavirus (HPV)-associated anal and oropharyngeal cancer incidence has increased in recent years among US women. However, trends in incidence and burden (annual number of cases) of noncervical HPV-associated cancers relative to cervical cancer remain unclear. Using the 2001-2017 US cancer statistics dataset, we evaluated contemporary incidence trends and burden (annual number of cases) of HPV-associated cancers among women by anatomic site, race or ethnicity, and age. Overall, cervical cancer incidence plateaued among White women but continued to decline among Black and Hispanic women. Anal cancer incidence surpassed cervical cancer incidence among White women aged 65-74 years of age (8.6 and 8.2 per 100 000 in 2015) and 75 years or older (6.2 and 6.0 per 100 000 in 2014). The noncervical cancer burden (n = 11 871) surpassed the cervical cancer burden (n = 11 527) in 2013. Development of efficacious screening strategies for noncervical cancers and continued improvement in cervical cancer prevention are needed to combat HPV-associated cancers among women.
Human papillomavirus (HPV) primarily causes 5 (cervical, anal, oropharyngeal, vaginal, and vulvar) squamous epithelial cancers among women. While widely implemented screening programs have led to a decline in cervical cancer incidence (1), a marked increase in anal and oropharyngeal cancer incidence occurred in the last 2 decades in the United States, particularly among White women and those aged 50 years and older (2,3). As a result, the incidence and the number of new cancers (ie, burden) of these noncervical cancers may have surpassed cervical cancer in certain age and race or ethnic groups; however, such evidence is currently lacking.
We analyzed the 2001-2017 US Cancer Statistics dataset (4). HPV-associated cancers were identified based on International Classification of Diseases for Oncology-3 site and histology codes (5,6). SEER*Stat version 8.3.5 was used to calculate incidence rates. JoinPoint software version 4.7.0 was used to estimate piecewise–log-linear trends and derive annual and average annual percentage changes (APCs and AAPCs) (7). P values were estimated using the permutation distribution of the test statistic (statistical significance at P < .05, 2-sided). Cancer burden (ie, incident cases) was a function of population size and composition. Detailed methods are available in the Supplementary Methods (available online).
During 2001-2017, 378 629 HPV-associated cancer cases were diagnosed among women nationally. Case distribution and incidence rates by age, race or ethnicity, and year of diagnosis are presented in Supplementary Table 1 (available online).
Overall, during 2001-2012, cervical cancer incidence declined (APC = −1.5%, 95% confidence interval [CI] = −1.9% to −1.1%) and then plateaued during 2012-2017 (APC = 0.4%, 95% CI = −1.0% to 1.8%). In contrast, anal cancer incidence continued to rise (APC2001-2009 = 4.1%, 95% CI = 3.2% to 5.0%; APC2009-2017 = 2.0%, 95% CI = 1.3% to 2.7%), whereas vulvar cancer incidence stabilized after an initial increase (APC2001-2012 = 1.7%, 95% CI = 1.4% to 2.0%; APC2012-2017 = 0%, 95% CI = −0.9% to 0.9%) (Supplementary Figure 1, A; Supplementary Table 2, available online). After an initial increase in oropharyngeal cancer, incidence declined albeit the change was not statistically significant (APC2001-2015 = 1.0%, 95% CI = 0.7% to 1.3%; APC2015-2017 = −3.3%, 95% CI = −9.2% to 2.9%); no statistically significant change occurred in vaginal cancer incidence over time. The noncervical HPV-associated cancer burden (n = 11 871) surpassed the cervical cancer burden (n = 11 527) in 2013 (Supplementary Table 1; Supplementary Figure 2, B available online).
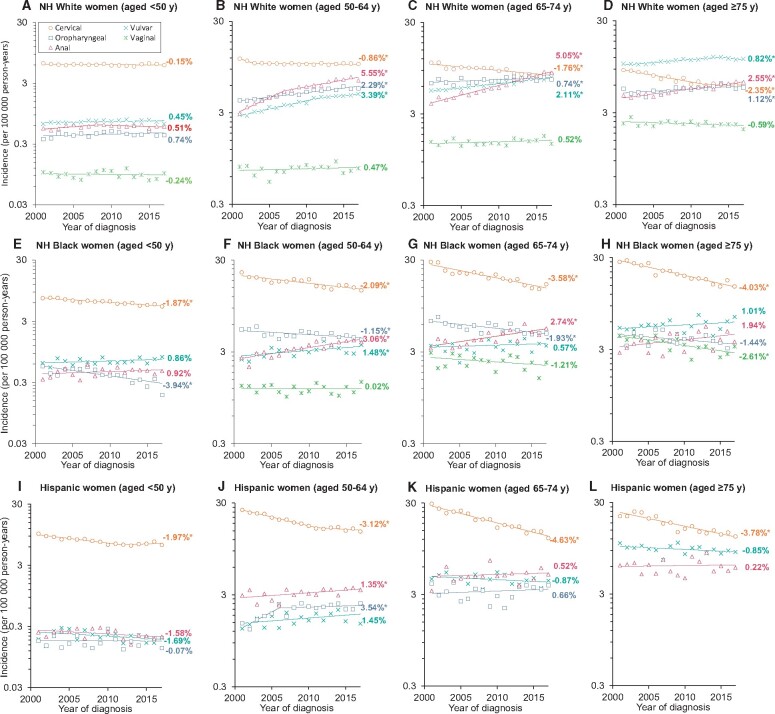
Among White women younger than 50 years, no statistically significant change in cervical cancer incidence occurred from 2001 to 2017 (AAPC = −0.2%, 95% CI = −0.5% to 0.2%). Among those aged 50-64 years, after an initial decline (APC2001-2003 = −6.0%, 95% CI = −10.3% to −1.5%), cervical cancer incidence stabilized in recent years (APC2003-2017 = −0.1%, 95% CI = −0.3% to 0.1%) (Figure 1, A). Among age groups 50-64 years, 65-74 years, and 75 years and older, a statistically significant increase in anal, oropharyngeal, and vulvar cancer incidence occurred (Figure 1, B-D). Notably, with an annual increase of 5.1% (95% CI = 4.6% to 5.5%) in those aged 65-74 years and 2.6% (95% CI = 2.1% to 3.0%) among those aged 75 years and older, anal cancer incidence (8.6 and 6.2 per 100 000) surpassed cervical cancer incidence (8.2 and 6.0 per 100 000) in 2016 and 2014, respectively. Across 2001-2017, vulvar cancer incidence was higher than other HPV-associated cancers in White women aged 75 years and older. Although a statistically significant decline in cervical cancer incidence across all age groups (<50 years, 50-64 years, 65-74 years, ≥75 years) occurred in Black (AAPCs = −1.9%, −2.1%, −3.6%, and −4.0%, respectively) and Hispanic (AAPCs = −2.0%, −3.1%, −4.6%, and −3.8%, respectively) women, cervical cancer incidence continued to remain higher than other HPV-associated cancers (Figure 1, E-L). Detailed findings, including APCs and AAPCs, are shown in Supplementary Table 2 (available online).
Figure 1.
Trends in the annual incidence rates of cervical, anal, vulvar, vaginal, and oropharyngeal cancers among women according to age at diagnosis and race/ethnicity: National Program of Cancer Registries and Surveillance Epidemiology, and End Results program (2001-2017). Data markers represent the observed incidence rates (cases per 100 000 person-years). Panels A, B, C, and D show cervical, anal, vulvar, vaginal, and oropharyngeal cancer incidence trends among White women younger than 50 years, aged 50-64 years, 65-74 years, and 75 years and older, respectively. Panels E, F, G, and H show cervical, anal, vulvar, vaginal, and oropharyngeal cancer incidence trends among Black women younger than 50 years, aged 50-64 years, 65-74 years, and 75 years and older, respectively. Panels I, J, K, and L show cervical, anal, vulvar, vaginal, and oropharyngeal cancer incidence trends among Hispanic women younger than 50 years, aged 50-64 years, 65-74 years, and 75 years and older, respectively. Rates are age-adjusted to the 2000 US standard population. NH = non-Hispanic. *P < .05.
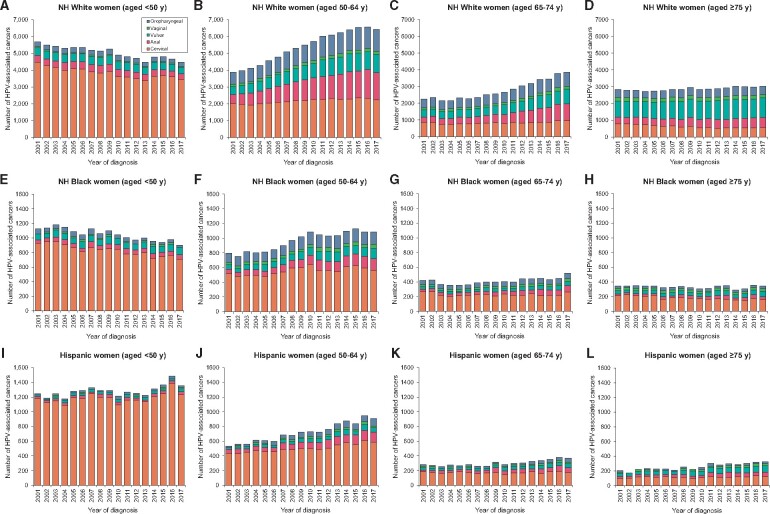
Cervical cancer burden increased in White women aged 50-74 years, Black women aged 50-64 years, and across all ages among Hispanic women (Figure 2). The annual number of anal, oropharyngeal, vaginal, and vulvar cancer cases increased in all age and racial or ethnic groups. Notably, anal cancer burden surpassed cervical cancer burden in White women aged 65-74 years and 75 years and older. Vulvar cancer continued to remain the most common HPV-associated cancer among White women aged 75 years and older. Among other race or ethnic groups, cervical cancer continues to remain the leading HPV-associated cancer.
Figure 2.
Burden (annual number of cases) of cervical, anal, vulvar, vaginal, and oropharyngeal cancers among women according to age at diagnosis at race or ethnicity: National Program of Cancer Registries and Surveillance Epidemiology, and End Results program (2001-2017). Vertical bars represent the burden (annual number of cases). Panels A, B, C, and D show cervical, anal, vulvar, vaginal, and oropharyngeal cancer burden among White women younger than 50 years, aged 50-64 years, 65-74 years, and 75 years and older, respectively. Panels E, F, G, and H show cervical, anal, vulvar, vaginal, and oropharyngeal cancer burden among Black women younger than 50 years, aged 50-64 years, 65-74 years, and 75 years and older, respectively. Panels I, J, K, and L show cervical, anal, vulvar, vaginal, and oropharyngeal cancer incidence burden among Hispanic women younger than 50 years, aged 50-64 years, 65-74 years, and 75 years and older, respectively. HPV = human papillomavirus; NH = non-Hispanic.
The collective burden of HPV-associated cancers has increased among US women. This finding reflects recent stabilization in cervical cancer and concurrent increases in oropharyngeal, anal, and vulvar cancer incidence, particularly among non-Hispanic White women.
Plateauing of cervical cancer incidence may have resulted from the recent decline in cervical cancer screening rates (8-10). The rise in anal and oropharyngeal cancers is possibly due to the increased acquisition of HPV infection attributable to changes in sexual behaviors (2,11,12). However, the role of HPV infection may be limited in the rising rates of vulvar cancer given the low observed prevalence of HPV DNA in vulvar cancer tissues among elderly women (13). Although these factors explain the changes in incidence, the increasing burden may be attributable to the population growth of women aged 50 years and older (14).
No evidence-based screening recommendations exist for noncervical HPV-associated cancers. Because of the current inability to detect HPV-induced precancers and insufficient diagnostic technology to detect early cancers, oropharyngeal cancer screening is not possible (15). Similar to cervical cancer, anal and vulvar cancers have precancerous lesions that are amenable to screening using cytology or HPV testing, and diagnostic biopsies can also be obtained while performing high-resolution anoscopy or pelvic examination. Age is considered an important factor for defining cancer-screening thresholds for screening initiation and termination. Comparable anal and vulvar cancer incidence with cervical cancer incidence among certain groups, and, as recently described by Tota et al. (16), the satisfaction of all criteria set forth by the World Health Organization (Wilson and Jungner’s criteria) (17) for the implementation of cancer screening make these diseases suitable targets for age-based screening evaluation. However, given that population-wide screening may still not be cost-effective, further research is needed to identify groups at elevated risk for these cancers (18-24).
The HPV vaccine may reverse the rising HPV-associated cancer burden in the future (25,26). However, the lack of improvement in HPV vaccination coverage in recent years (27,28), the growing sentiment of HPV vaccine hesitancy and ignorance (29-31), and the anticipated population growth of women aged 65 years and older (14) imply that HPV-associated cancer burden will continue to increase for several decades. Under such circumstances, the implementation of effective screening algorithms for birth cohorts who may not benefit from HPV vaccination becomes increasingly crucial.
The strength of this study is the use of nationwide, high-quality data. A limitation is that cancers were classified based on histologic criteria and not the actual assessment of individual tumors for the presence of HPV DNA. Therefore, a large fraction of cancer cases with tumor sites with a low-HPV DNA detection rate (eg, vulva) may not be HPV associated.
Our findings highlight the need to evaluate optimal age-based screening algorithms for noncervical HPV-associated cancers. Continued improvement in cervical cancer prevention is also crucial.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA232888 and by the Intramural Research Program of the National Cancer Institute.
Notes
Role of the funders/support: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure: Dr Deshmukh received a consulting fee from Merck on unrelated projects.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions: AAD: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing. RS: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing. MS: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing. HD: Conceptualization; Formal analysis; Investigation; Visualization; Writing – original draft; Writing – review & editing. YL: Conceptualization; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing. ES: Conceptualization; Investigation; Writing – review & editing. AGN: Conceptualization; Investigation; Writing – review & editing. EYC: Conceptualization; Investigation; Writing – review & editing. GN: Conceptualization; Investigation; Writing – review & editing. JC: Conceptualization; Investigation; Writing – review & editing. KSm: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing. KSi: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing. KSo: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Data Availability
The US Cancer Statistics Databases (National Program of Cancer Registries and Surveillance Epidemiology and End Results) are publicly available at https://www.cdc.gov/cancer/uscs/public-use/about.htm. Documentation for data use is also available at https://www.cdc.gov/cancer/uscs/public-use/us/index.htm.
Supplementary Material
References
- 1. Islami F, Fedewa SA, Jemal A. Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Prev Med. 2019;123:316–323. [DOI] [PubMed] [Google Scholar]
- 2. Deshmukh AA, Suk R, Shiels MS, et al. Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001-2015. J Natl Cancer Inst. 2020;112(8):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tota JE, Best AF, Zumsteg ZS, et al. Evolution of the oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J Clin Oncol. 2019;37(18):1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics 2001–2017 Public Use Research Database, 2019 submission (2001–2017), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Published June 2020. www.cdc.gov/cancer/uscs/public-use. Accessed July 31, 2020.
- 5. Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers—United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67(33):918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Senkomago V, Henley SJ, Thomas CC, et al. Human papillomavirus-attributable cancers—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2019;68(33):724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 8. Watson M, Benard V, King J, et al. National assessment of HPV and pap tests: changes in cervical cancer screening, National Health Interview Survey. Prev Med. 2017;100:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacLaughlin KL, Jacobson RM, Radecki Breitkopf C, et al. Trends over time in Pap and Pap-HPV cotesting for cervical cancer screening. J Womens Health (Larchmt). 2019;28(2):244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watson M, Benard V, Flagg EW. Assessment of trends in cervical cancer screening rates using healthcare claims data: United States, 2003-2014. Prev Med Rep. 2018;9:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167(10):714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vespa J, Armstrong DM, Medina L. Demographic Turning Points for the United States: Population Projections for 2020 to 2060: Population Estimates and Projections. Washington, DC: Department of Commerce Economics and Statistics Administration; 2018:25–1144. [Google Scholar]
- 15. Kreimer AR, Shiels MS, Fakhry C, et al. Screening for human papillomavirus-driven oropharyngeal cancer: considerations for feasibility and strategies for research. Cancer. 2018;124(9):1859–1866. [DOI] [PubMed] [Google Scholar]
- 16. Tota JE, Isidean SD, Franco EL. Defining benchmarks for tolerable risk thresholds in cancer screening: impact of HPV vaccination on the future of cervical cancer screening. Int J Cancer. 2020;doi:10.1002/ijc.33178. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. Geneva, Switzerland: World Health Organization; 1968:281–393. [Google Scholar]
- 18. Fokom Domgue J, Messick C, Milbourne A, et al. Prevalence of high-grade anal dysplasia among women with high-grade lower genital tract dysplasia or cancer: results of a pilot study. Gynecol Oncol. 2019;153(2):266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin C, Slama J, Gonzalez P, et al. Cervical determinants of anal HPV infection and high-grade anal lesions in women: a collaborative pooled analysis. Lancet Infect Dis. 2019;19(8):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deshmukh AA, Chiao EY, Cantor SB, et al. Management of precancerous anal intraepithelial lesions in human immunodeficiency virus-positive men who have sex with men: clinical effectiveness and cost-effectiveness. Cancer. 2017;123(23):4709–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deshmukh AA, Cantor SB, Fenwick E, et al. Adjuvant HPV vaccination for anal cancer prevention in HIV-positive men who have sex with men: the time is now. Vaccine. 2017;35(38):5102–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deshmukh AA, Chhatwal J, Chiao EY, et al. Long-term outcomes of adding HPV vaccine to the anal intraepithelial neoplasia treatment regimen in HIV-positive men who have sex with men. Clin Infect Dis. 2015;61(10):1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suk R, Mahale P, Sonawane K, et al. Trends in risks for second primary cancers associated with index human papillomavirus-associated cancers. JAMA Netw Open. 2018;1(5):e181999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stier EA, Lensing SY, Darragh TM, et al. Prevalence of and risk factors for anal high-grade squamous intraepithelial lesions in women living with human immunodeficiency virus. Clin Infect Dis. 2020;70(8):1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sonawane K, Nyitray AG, Nemutlu GS, et al. Prevalence of human papillomavirus infection by number of vaccine doses among US women. JAMA Netw Open. 2019;2(12):e1918571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burger EA, Smith MA, Killen J, et al. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. Lancet Public Health. 2020;5(4):e213–e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. NCHS Data Brief: Human Papillomavirus Vaccination Among Adults Aged 18−26, 2013−2018. https://www.cdc.gov/nchs/data/databriefs/db354-h.pdf. Accessed March 2020. [PubMed]
- 29. Sonawane K, Zhu Y, Montealegre JR, et al. Parental intent to initiate and complete the human papillomavirus vaccine series in the USA: a nationwide, cross-sectional survey. Lancet Public Health. 2020;doi:10.1016/S2468-2667(20)30139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suk R, Montealegre JR, Nemutlu GS, et al. Public knowledge of human papillomavirus and receipt of vaccination recommendations. JAMA Pediatr. 2019;173(11):1099–1001. /jamapediatrics.2019.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chido-Amajuoyi OG, Jackson I, Yu R, et al. Declining awareness of HPV and HPV vaccine within the general US population. Hum Vaccin Immunother. 2020;doi:10.1080/21645515.2020.1783952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The US Cancer Statistics Databases (National Program of Cancer Registries and Surveillance Epidemiology and End Results) are publicly available at https://www.cdc.gov/cancer/uscs/public-use/about.htm. Documentation for data use is also available at https://www.cdc.gov/cancer/uscs/public-use/us/index.htm.