Abstract
Dynamic cellular heterogeneity underlies melanoma progression and therapy resistance. Advances in single-cell technologies have revealed an increasing number of tumor and microenvironment cell states in melanoma, but little is understood about their function in vivo. Zebrafish models are a powerful system for discovery, live imaging, and functional investigation of cell states throughout melanoma progression and treatment. By capturing dynamic melanoma states in living animals, zebrafish have the potential to resolve the complexity of melanoma heterogeneity from a single cell through disease processes within the context of the whole body, revealing novel cancer biology and therapeutic targets.
Abbreviations: MEK, MAPK/extracellular signal‒regulated kinase kinase; MRD, minimal residual disease; PDX, patient-derived xenograft; scRNA-seq, single-cell RNA sequencing
Melanoma ranks among the highest of all cancers for intratumor heterogeneity and clonogenic potential—features associated with poor prognoses (Andor et al., 2016; Hachey and Boiko, 2016). Heterogeneity emerges from a combination of genetic mutations acquired during disease progression and from variations in nongenetic factors, such as gene transcription and metabolism (Lawrence et al., 2013; Rambow et al., 2019; Varum et al., 2019; Wolf et al., 2019). Melanoma transcriptional states can reflect the developmental trajectory of the neural crest, melanoblasts, or pigmented melanocytes as well as lineage independent states such as stress or starvation states (Arozarena and Wellbrock, 2019; Rambow et al., 2019). A further layer of heterogeneity stems from differences in cell cycle dynamics within tumors, with some populations actively cycling, while others are slow cycling or dormant, which can additionally exhibit stem cell properties (Perego et al., 2018; Roesch et al., 2010). Adding to the complexity, melanoma cell states show plasticity and can transition from one state to another. Understanding how transcriptional states contribute to melanoma progression in individual patients is critical for drug development and is an important step toward developing personalized therapeutic regimens.
Recent advances in single-cell RNA sequencing (scRNA-seq) technologies provide a powerful approach to explore the extent of intratumor heterogeneity. Using scRNA-seq analysis, Tirosh et al. (2016) demonstrated that transcriptomes of melanoma tumor cells aligned along a bipolar expression pattern, with treatment-naive tumors predominantly expressing MITF program genes at one end and BRAF/MAPK/extracellular signal‒regulated kinase kinase (MEK) inhibitor‒treated melanoma cells showing enrichment of AXL programs at the other. This finding supports a rheostat model of phenotypic switching whereby MITF gene expression programs direct differentiated (MITF-high), proliferative (MITF-moderate) and invasive (MITF-low) cell states, and MITF-low and/or AXL-high cell states show drug resistance (Arozarena and Wellbrock, 2019; Bittner et al., 2000; Hoek and Goding, 2010; Hoek et al., 2008, 2006; Müller et al., 2014; Rambow et al., 2019). scRNA-seq studies from short-term patient-derived cultures further validated these programs and increased the diversity to include stromal and oxidative phosphorylation signatures (Gerber et al., 2017; Kunz et al., 2018). It is now becoming evident that in addition to the extreme melanocytic and mesenchymal cell states, melanoma cell states can be found in a dynamic spectrum along the trajectory, transiting through one or more intermediate cell states, and even transdifferentiating toward the endothelial lineage (Ennen et al., 2015; Li et al., 2020; Rambow et al., 2018; Tsoi et al., 2018; Wouters et al., 2020).
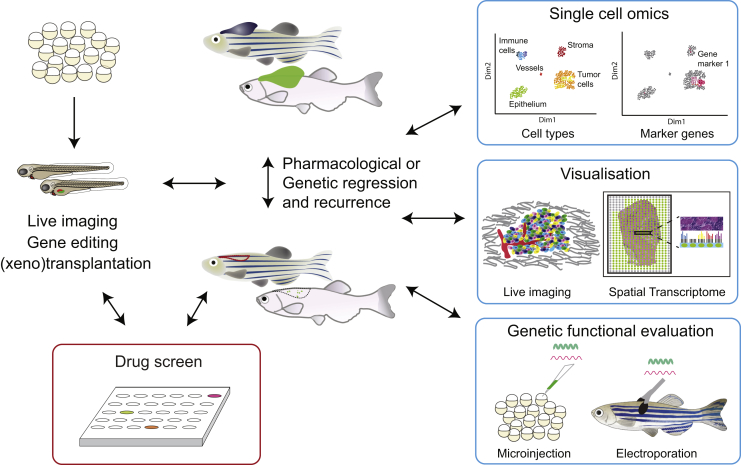
From our perspective, the zebrafish (Danio rerio) is a powerful lens through which we can resolve the complexity of melanoma intratumor heterogeneity (Figure 1). Zebrafish are vertebrates and share over 82% conservation with known disease-related genes (Howe et al., 2013). Similar to human skin, zebrafish melanocytes are found at the basal layer of the epidermis in zebrafish (they do not have hair follicles), and much of the developmental biology of the melanocytes is shared between human and zebrafish (Mort et al., 2015). Transgenic and gene-editing technologies are highly developed in zebrafish, enabling comparative oncogenomics, advanced imaging, and functional manipulation of biological processes at the level of the single cell (Frantz and Ceol, 2020; Venkatesan et al., 2018). Genetic engineering and transplantation-based zebrafish cancer avatars are developing clinically relevant models of cutaneous melanoma as well as rare melanoma subtypes such as uveal and mucosal melanomas (Fazio et al., 2020). Zebrafish have emerged as a highly successful model for phenotypic drug screening, in particular by screening melanocyte developmental lineages (Johansson et al., 2020; MacRae and Peterson, 2015; Santoriello et al., 2020; Sarvi et al., 2018; White et al., 2011; Zhou et al., 2012).
Figure 1.
Zebrafish tools to deconvolve melanoma cell states. Zebrafish represents a powerful model to tackle melanoma heterogeneity and plasticity. A parallel study of developmental lineages (left) and melanoma initiation and residual disease modeling (right) allows simultaneously screening and detailed single-cell and functional analysis of melanoma within a complex microenvironment. Examples of the individual technologies to evaluate the varied aspects of melanoma cell states, plasticity, and microenvironmental factors are presented.
scRNA-seq in zebrafish melanoma
scRNA-seq of zebrafish melanoma models are revealing cancer cell states that are specific to the neural crest‒melanocyte lineage and those that extend beyond this and are shared by other cancer types. For example, we have recently generated BRAF-mutant and BRAF-independent melanoma models characterized by MITF-low and MITF-high transcriptional signatures (Travnickova et al., 2019). The MITF-low zebrafish melanomas shared transcriptional states with MITF-low melanomas in patients, a melanoma subtype that is characterized by poor outcomes (Lauss et al., 2016). Through scRNA-seq analysis, we validated the transcriptomic classification of the melanomas and thereby revealed that states at the single-cell level can reflect the signatures of bulk tumor samples. Furthermore, Baron et al. (2020) recently reported that BRAFV600Ep53-mutant melanomas have three transcriptional states: melanocytic, neural crest, and stress-like states, with the latter emerging as a pan-cancer cell state. They added spatial information to the single-cell transcriptomic data by using barcoding to refer the transcriptional signature back to the position within the tissue. This step provided two-dimensional insight into tumor heterogeneity at the histology level and validated the stress-like signature in patients, which had been previously thought to be an artifact of cell dissociation (Baron et al., 2020).
It is important to capture melanoma vulnerabilities at the point of therapeutic shift and disease regression. The Marine laboratory and our own used scRNA-seq to address the melanoma cell dynamics at the minimal residual disease (MRD) stage‒that decisive moment where persistent cells are present in otherwise disease-free tissue while they predispose or acquire properties for tumor recurrence (Rambow et al., 2018; Travnickova et al., 2019). These studies reveal an unanticipated complexity of cells at the MRD site, suggesting that several cell states coexist. Notably, an invasive cell state was shared between persister cells generated by BRAF/MEK-targeted therapy in patient-derived xenograft (PDX) models and by MITF lineage dependency in zebrafish, demonstrating that shared dedifferentiation cell states emerge during melanoma regression and bulk tumor collapse. Our models further identified a G0-like cell population in the residual disease that is extant in the primary tumor, reminiscent of G0-like populations identified by others in melanoma and in additional cancer types. (Chang et al., 20201; Facompre et al., 2016; Roesch et al., 2010). Slow-cycling cell populations, some of which express epigenetic modifiers, including jarid1b, suggest that epigenetic control of transcriptional mechanisms underlie persistent cancer cell states (Roesch et al., 2013, 2010; Shen et al., 2020). Functional analysis shows that slow cycling cell populations are required for continued cell growth, emerge upon drug treatment, and that even within this state some cells are highly invasive (Perego et al., 2018; Roesch et al., 2013, 2010). However, how these cells contribute to melanoma recurrence is yet to be discovered. Altogether, these models provide strong evidence that the cell states identified in zebrafish are shared with human cancer states, including G0-like, invasive, starvation, and pigmentation states.
Imaging cell subpopulations in vivo
Dynamic visualization of cell subpopulations and their relationship with the surrounding microenvironment is critical to understand tumor evolution and the phenotypic switching from one cell state to another. Zebrafish embryos are transparent, enabling live imaging of development, oncogenic transformation, and interaction with neighboring cells at a single-cell resolution over extended time periods. The zebrafish immune system is conserved with humans (Renshaw and Trede, 2012), and melanoma studies in zebrafish are beginning to incorporate components of the immune response. For example, Feng et al. (2010) were the first to use live imaging of zebrafish embryos to discover that the immune system can recognize transformed RAS-mutant melanocytes at the earliest stages of melanoma development.
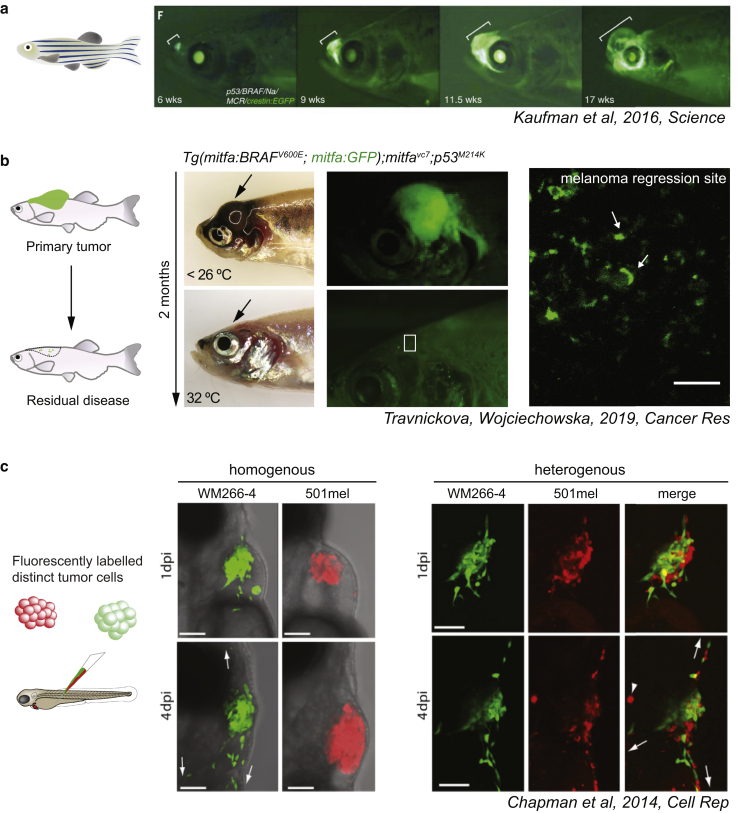
The parallel study of developmental lineages and melanoma modeling in zebrafish has powered forward our understanding of lineage dysregulation in melanoma. Live imaging has been central to these studies. White, et al. (2011) revealed that a neural crest expression signature re-emerges during melanoma initiation and this led to the first clinical trial targeting transcriptional elongation in melanoma cells. Using live imaging, Kaufman, et al. (2016) further employed a neural crest‒specific lineage transgene (crestin:EGFP) to demonstrate that within a cancerized field (comprising a nevus harboring an oncogenic BRAFV600E mutation), only cells that reacquire their early neural crest identity evolve into a malignant population (Figure 2a). Employing transparent adult zebrafish (White et al., 2008), some of the earliest metastatic melanoma cell populations have been captured in vivo (Heilmann et al., 2015). Live imaging of adult fish was also critical to capture a subpopulation of melanoma cells at the site of residual disease in situ, providing some of the first images of live persister cells within an endogenous MRD site (Travnickova et al., 2019) (Figure 2b). These studies illustrate one of the particular strengths of the zebrafish system: the ability to relate scRNA-seq cell state information captured from a fixed time point to the dynamics of melanoma cell states within their native microenvironment over time using live imaging.
Figure 2.
Selected examples of imaging melanoma cell states in zebrafish. (a) Capturing melanoma initiation in zebrafish. Melanoma-prone zebrafish expressing the neural crest transgene crestin:EGFP fish are tracked over time to follow melanoma initiation through tumor growth. Image was adapted from Kaufman et al. (2016). (b) Visualizing MRD. Brightfield and fluorescent images of zebrafish with primary melanoma and MRD. This model depends on a temperature-sensitive allele for MITF activity whereby Mitfa protein is depleted at the regression site while cells maintain GFP expression from the mitfa promoter. Turning MITF activity off by shifting the fish to the nonpermissive temperature leads to melanoma regression and evidence of MRD. Even in zebrafish with no overt evidence of disease, live GFP-positive persister cells are detected at the regression site (boxed region). Image was adapted from Travnickova et al. (2019).(c) Phenotypic cooperation between cell states. Homogenous xenografts of two fluorescent melanoma cell lines after transplantation into zebrafish embryos. Heterogeneous xenografts show cooperative migration. Images were adapted from Chapman et al. (2014) with permission from the American Association for the Advancement of Science (AAAS). MRD, minimal residual disease.
Functional analysis of melanoma subpopulations and the microenvironment
New cell populations and cell states need to be functionally validated and tested for their contribution to melanoma outcomes. Individual cell states as well as components of the tumor microenvironment can be experimentally altered and tracked by using fluorescently labeled marker genes. Zebrafish-based platforms that enable tissue-specific and rapid gene overexpression (Ceol et al., 2011), editing (Ablain et al., 2018), or electroporation (transgene electroporation in adult zebrafish) (Callahan et al., 2018) allow to spatially and temporally control gene expression.
Zebrafish can provide a tractable model for (xeno)transplantation at embryonic and adult stages, especially when combined with genetics, drug treatments, and live imaging (Fazio et al., 2020). Similar to the mouse PDX models, human or zebrafish melanoma cells can be transplanted into immunocompetent zebrafish embryos with functional innate immunity or to an immunocompromised transparent adult zebrafish and traced over time (Tang et al., 2016; White et al., 2008; Yan et al., 2019). Chapman et al., (2014) employed xenotransplantation to discover co-operation between phenotypically different cells to promote melanoma metastasis, a phenomenon called cooperative invasion (Figure 2c). Furthermore, Baron et al., (2020) demonstrated a direct link between cell state transcriptional signatures derived from scRNA-seq analyses and their biological function by transplanting melanoma cells into the early zebrafish embryo. They showed that the melanoma subpopulation enriched for a stress-like response was the most robust in generating new tumors and in providing BRAF/MEK inhibitor resistance.
Cancer-associated cell populations in the microenvironment have a profound impact on melanoma growth and can be functionally validated and mechanistically explored in zebrafish models. For example, using exogenous EDN3 peptide and edn3b mutants, Kim et al. (2017) showed that EDN3 microenvironment cues control phenotypic switching toward a proliferative and differentiated state in melanoma. Zhang et al. (2018) demonstrated that cancer-associated adipocytes directly transfer lipids to zebrafish melanomas to promote growth. Finally, Ferreira et al. developed tert mutant chimeric zebrafish to demonstrate that noncancer cells with short telomeres promote senescence and inflammation, thereby promoting melanoma in a noncell autonomous fashion (Lex et al., 2020). Such functional assays, combined with reporter lines for imaging and gene-editing platforms, provide the tools to reveal the new biology of cancer-associated cell populations within the complex microenvironment of a living animal.
Future directions and conclusions
Drugs that target melanoma cell states and subpopulations are already emerging (Boshuizen et al., 2018; Rambow et al., 2018; Roesch et al., 2013; Sáez-Ayala et al., 2013; Sarvi et al., 2018; Smith et al., 2016; Viswanathan et al., 2017). We advocate that zebrafish is a powerful model system to reconstruct melanoma tumors in vivo and a unique platform for phenotypic drug screens that target melanoma cell states and follow the fate of these cells during treatment. With the expansion of single-cell omic technologies (such as RNA, DNA, methylated DNA, or open chromatin position sequencing) that span space and time, zebrafish are poised to catalog the mutational and transcriptional dynamics of melanoma progression and treatment throughout a whole organism. The first examples of combining scRNA-seq with spatial transcriptomics have already identified drug-resistant cell populations within the morphological context of the tumor (Baron et al., 2020). Combined with live, in vivo imaging, these techniques will be a powerful approach to capture how tumor cell populations interact with each other and the microenvironment within the architecture of the tumor over time and after treatment.
Despite the potential of the zebrafish system to study cellular heterogeneity, there remain some challenges. Imaging live adult zebrafish can still be limited by resolution and feasibility, and lineage tracing in adult zebrafish and site-specific gene-editing techniques are not yet routine. However, looking forward, we will be able to combine scRNA-seq with DNA mutation‒based lineage tracing to follow clonal evolution and incorporate proteomic and metabolomic profiles (Alemany et al., 2018; Raj et al., 2018; Wagner and Klein, 2020). Gene-editing and tissue-specific cell ablation techniques will further help interrogate the function of cell states and types and to validate drug targets. Ultimately, our collective goal is to better predict patient outcomes and discover new therapies that best match the needs of patients. Zebrafish are powerful allies in this endeavor.
ORCIDs
Jana Travnickova: http://orcid.org/0000-0002-8339-9162
E. Elizabeth Patton: http://orcid.org/0000-0002-2570-0834
Conflict of Interest
The authors state no conflict of interest.
Acknowledgments
This work was supported by the MRC Human Genetics Unit Programme (MC_UU_00007/9), the European Research Council (ZF-MEL-CHEMBIO-648489), and the Anna-Maria and Stephen Kellen Foundation-Melanoma Research Alliance Team Science Award (#687306).
Author Contributions
Conceptualization: JT, EEP; Funding Acquisition: EEP; Writing - Original Draft Preparation: JT, EEP; Writing - Review and Editing: JT, EEP
accepted 16 December 2020; corrected proof published online 31 March 2021
Footnotes
Chang CA, Jiang S, Sayad A, Brown KR, Nixon A, Dhabaria A, et al. Ontogeny and Vulnerabilities of Lapatinib Drug-Tolerant Persisters in HER2+ Breast Cancer. bioRxiv 2020.
References
- Ablain J., Xu M., Rothschild H., Jordan R.C., Mito J.K., Daniels B.H. Human tumor genomics and zebrafish modeling identify SPRED1 loss as a driver of mucosal melanoma. Science. 2018;362:1055–1060. doi: 10.1126/science.aau6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany A., Florescu M., Baron C.S., Peterson-Maduro J., van Oudenaarden A. Whole-organism clone tracing using single-cell sequencing. Nature. 2018;556:108–112. doi: 10.1038/nature25969. [DOI] [PubMed] [Google Scholar]
- Andor N., Graham T.A., Jansen M., Xia L.C., Aktipis C.A., Petritsch C. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med. 2016;22:105–113. doi: 10.1038/nm.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arozarena I., Wellbrock C. Phenotype plasticity as enabler of melanoma progression and therapy resistance. Nat Rev Cancer. 2019;19:377–391. doi: 10.1038/s41568-019-0154-4. [DOI] [PubMed] [Google Scholar]
- Baron M., Tagore M., Hunter M.V., Kim I.S., Moncada R., Yan Y. The stress-like cancer cell state is a consistent component of tumorigenesis. Cell Syst. 2020;11:536–546.e7. doi: 10.1016/j.cels.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M., Meltzer P., Chen Y., Jiang Y., Seftor E., Hendrix M. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Boshuizen J., Koopman L.A., Krijgsman O., Shahrabi A., van den Heuvel E.G., Ligtenberg M.A. Cooperative targeting of melanoma heterogeneity with an AXL antibody-drug conjugate and BRAF/MEK inhibitors. Nat Med. 2018;24:203–212. doi: 10.1038/nm.4472. [DOI] [PubMed] [Google Scholar]
- Callahan S.J., Tepan S., Zhang Y.M., Lindsay H., Burger A., Campbell N.R. Cancer modeling by Transgene Electroporation in Adult Zebrafish (TEAZ) Dis Model Mech. 2018;11 doi: 10.1242/dmm.034561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceol C.J., Houvras Y., Jane-Valbuena J., Bilodeau S., Orlando D.A., Battisti V. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471:513–517. doi: 10.1038/nature09806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A., Fernandez del Ama L., Ferguson J., Kamarashev J., Wellbrock C., Hurlstone A. Heterogeneous tumor subpopulations cooperate to drive invasion. Cell Rep. 2014;8:688–695. doi: 10.1016/j.celrep.2014.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennen M., Keime C., Kobi D., Mengus G., Lipsker D., Thibault-Carpentier C. Single-cell gene expression signatures reveal melanoma cell heterogeneity. Oncogene. 2015;34:3251–3263. doi: 10.1038/onc.2014.262. [DOI] [PubMed] [Google Scholar]
- Facompre N.D., Harmeyer K.M., Sole X., Kabraji S., Belden Z., Sahu V. JARID1B enables transit between distinct states of the stem-like cell population in oral cancers [published correction appears in Cancer Res 2017;77:7136. Cancer Res. 2016;76:5538–5549. doi: 10.1158/0008-5472.CAN-15-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio M., Ablain J., Chuan Y., Langenau D.M., Zon L.I. Zebrafish patient avatars in cancer biology and precision cancer therapy. Nat Rev Cancer. 2020;20:263–273. doi: 10.1038/s41568-020-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Santoriello C., Mione M., Hurlstone A., Martin P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz W.T., Ceol C.J. From tank to treatment: modeling melanoma in zebrafish. Cells. 2020;9:1289. doi: 10.3390/cells9051289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber T., Willscher E., Loeffler-Wirth H., Hopp L., Schadendorf D., Schartl M. Mapping heterogeneity in patient-derived melanoma cultures by single-cell RNA-seq. Oncotarget. 2017;8:846–862. doi: 10.18632/oncotarget.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachey S.J., Boiko A.D. Therapeutic implications of melanoma heterogeneity. Exp Dermatol. 2016;25:497–500. doi: 10.1111/exd.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann S., Ratnakumar K., Langdon E., Kansler E., Kim I., Campbell N.R. A quantitative system for studying metastasis using transparent zebrafish. Cancer Res. 2015;75:4272–4282. doi: 10.1158/0008-5472.CAN-14-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek K.S., Eichhoff O.M., Schlegel N.C., Döbbeling U., Kobert N., Schaerer L. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- Hoek K.S., Goding C.R. Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res. 2010;23:746–759. doi: 10.1111/j.1755-148X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- Hoek K.S., Schlegel N.C., Brafford P., Sucker A., Ugurel S., Kumar R. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 2006;19:290–302. doi: 10.1111/j.1600-0749.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M. The zebrafish reference genome sequence and its relationship to the human genome [published correction appears in Nature 2014;505:248] Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J.A., Marie K.L., Lu Y., Brombin A., Santoriello C., Zeng Z. PRL3-DDX21 transcriptional control of endolysosomal genes restricts melanocyte stem cell differentiation. Dev Cell. 2020;54:317–332.e9. doi: 10.1016/j.devcel.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman C.K., Mosimann C., Fan Z.P., Yang S., Thomas A.J., Ablain J. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science. 2016;351:aad2197. doi: 10.1126/science.aad2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I.S., Heilmann S., Kansler E.R., Zhang Y., Zimmer M., Ratnakumar K. Microenvironment-derived factors driving metastatic plasticity in melanoma. Nat Commun. 2017;8:14343. doi: 10.1038/ncomms14343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M., Löffler-Wirth H., Dannemann M., Willscher E., Doose G., Kelso J. RNA-seq analysis identifies different transcriptomic types and developmental trajectories of primary melanomas. Oncogene. 2018;37:6136–6151. doi: 10.1038/s41388-018-0385-y. [DOI] [PubMed] [Google Scholar]
- Lauss M., Nsengimana J., Staaf J., Newton-Bishop J., Jönsson G. Consensus of melanoma gene expression subtypes converges on biological entities. J Invest Dermatol. 2016;136:2502–2505. doi: 10.1016/j.jid.2016.05.119. [DOI] [PubMed] [Google Scholar]
- Lawrence M.S., Stojanov P., Polak P., Kryukov G.V., Cibulskis K., Sivachenko A. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex K., Maia Gil M., Lopes-Bastos B., Figueira M., Marzullo M., Giannetti K. Telomere shortening produces an inflammatory environment that increases tumor incidence in zebrafish. Proc Natl Acad Sci USA. 2020;117:15066–15074. doi: 10.1073/pnas.1920049117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Karras P., Torres R., Rambow F., van den Oord J., Marine J.C. Disseminated melanoma cells transdifferentiate into endothelial cells in intravascular niches at metastatic sites. Cell Rep. 2020;31:107765. doi: 10.1016/j.celrep.2020.107765. [DOI] [PubMed] [Google Scholar]
- MacRae C.A., Peterson R.T. Zebrafish as tools for drug discovery. Nat Rev Drug Discov. 2015;14:721–731. doi: 10.1038/nrd4627. [DOI] [PubMed] [Google Scholar]
- Mort R.L., Jackson I.J., Patton E.E. The melanocyte lineage in development and disease [published correction appears in Development 2015;142:1387] Development. 2015;142:620–632. doi: 10.1242/dev.106567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Krijgsman O., Tsoi J., Robert L., Hugo W., Song C. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat Commun. 2014;5:5712. doi: 10.1038/ncomms6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M., Maurer M., Wang J.X., Shaffer S., Müller A.C., Parapatics K. A slow-cycling subpopulation of melanoma cells with highly invasive properties. Oncogene. 2018;37:302–312. doi: 10.1038/onc.2017.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj B., Wagner D.E., McKenna A., Pandey S., Klein A.M., Shendure J. Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat Biotechnol. 2018;36:442–450. doi: 10.1038/nbt.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambow F., Marine J.C., Goding C.R. Melanoma plasticity and phenotypic diversity: therapeutic barriers and opportunities. Genes Dev. 2019;33:1295–1318. doi: 10.1101/gad.329771.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambow F., Rogiers A., Marin-Bejar O., Aibar S., Femel J., Dewaele M. Toward minimal residual disease-directed therapy in melanoma. Cell. 2018;174:843–855.e19. doi: 10.1016/j.cell.2018.06.025. [DOI] [PubMed] [Google Scholar]
- Renshaw S.A., Trede N.S. A model 450 million years in the making: zebrafish and vertebrate immunity. Dis Model Mech. 2012;5:38–47. doi: 10.1242/dmm.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A., Fukunaga-Kalabis M., Schmidt E.C., Zabierowski S.E., Brafford P.A., Vultur A. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A., Vultur A., Bogeski I., Wang H., Zimmermann K.M., Speicher D. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez-Ayala M., Montenegro M.F., Sánchez-Del-Campo L., Fernández-Pérez M.P., Chazarra S., Freter R. Directed phenotype switching as an effective antimelanoma strategy. Cancer Cell. 2013;24:105–119. doi: 10.1016/j.ccr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Santoriello C., Sporrij A., Yang S., Flynn R.A., Henriques T., Dorjsuren B. RNA helicase DDX21 mediates nucleotide stress responses in neural crest and melanoma cells. Nat Cell Biol. 2020;22:372–379. doi: 10.1038/s41556-020-0493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvi S., Crispin R., Lu Y., Zeng L., Hurley T.D., Houston D.R. ALDH1 bio-activates nifuroxazide to eradicate ALDHhigh melanoma-initiating cells. Cell Chem Biol. 2018;25:1456–1469.e6. doi: 10.1016/j.chembiol.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Vagner S., Robert C. Persistent cancer cells: the deadly survivors. Cell. 2020;183:860–874. doi: 10.1016/j.cell.2020.10.027. [DOI] [PubMed] [Google Scholar]
- Smith M.P., Brunton H., Rowling E.J., Ferguson J., Arozarena I., Miskolczi Z. Inhibiting drivers of non-mutational drug tolerance is a salvage strategy for targeted melanoma therapy. Cancer Cell. 2016;29:270–284. doi: 10.1016/j.ccell.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Moore J.C., Ignatius M.S., Tenente I.M., Hayes M.N., Garcia E.G. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat Commun. 2016;7:10358. doi: 10.1038/ncomms10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh I., Izar B., Prakadan S.M., Wadsworth M.H., 2nd, Treacy D., Trombetta J.J. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travnickova J., Wojciechowska S., Khamseh A., Gautier P., Brown D.V., Lefevre T. Zebrafish MITF-low melanoma subtype models reveal transcriptional subclusters and MITF-independent residual disease. Cancer Res. 2019;79:5769–5784. doi: 10.1158/0008-5472.CAN-19-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi J., Robert L., Paraiso K., Galvan C., Sheu K.M., Lay J. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell. 2018;33:890–904.e5. doi: 10.1016/j.ccell.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varum S., Baggiolini A., Zurkirchen L., Atak Z.K., Cantù C., Marzorati E. Yin Yang 1 orchestrates a metabolic program required for both neural crest development and melanoma formation. Cell Stem Cell. 2019;24:637–653.e9. doi: 10.1016/j.stem.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Venkatesan A.M., Vyas R., Gramann A.K., Dresser K., Gujja S., Bhatnagar S. Ligand-activated BMP signaling inhibits cell differentiation and death to promote melanoma. J Clin Invest. 2018;128:294–308. doi: 10.1172/JCI92513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V.S., Ryan M.J., Dhruv H.D., Gill S., Eichhoff O.M., Seashore-Ludlow B. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.E., Klein A.M. Lineage tracing meets single-cell omics: opportunities and challenges. Nat Rev Genet. 2020;21:410–427. doi: 10.1038/s41576-020-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.M., Cech J., Ratanasirintrawoot S., Lin C.Y., Rahl P.B., Burke C.J. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature. 2011;471:518–522. doi: 10.1038/nature09882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.M., Sessa A., Burke C., Bowman T., LeBlanc J., Ceol C. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y., Bartok O., Patkar S., Eli G.B., Cohen S., Litchfield K. UVB-induced tumor heterogeneity diminishes immune response in melanoma. Cell. 2019;179:219–235.e21. doi: 10.1016/j.cell.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters J., Kalender-Atak Z., Minnoye L., Spanier K.I., De Waegeneer M., Bravo González-Blas C. Robust gene expression programs underlie recurrent cell states and phenotype switching in melanoma. Nat Cell Biol. 2020;22:986–998. doi: 10.1038/s41556-020-0547-3. [DOI] [PubMed] [Google Scholar]
- Yan C., Brunson D.C., Tang Q., Do D., Iftimia N.A., Moore J.C. Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell. 2019;177:1903–1914.e14. doi: 10.1016/j.cell.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Di Martino J.S., Bowman R.L., Campbell N.R., Baksh S.C., Simon-Vermot T. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov. 2018;8:1006–1025. doi: 10.1158/2159-8290.CD-17-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Ishizaki H., Spitzer M., Taylor K.L., Temperley N.D., Johnson S.L. ALDH2 mediates 5-nitrofuran activity in multiple species [published correction appears in Cell Chem Biol 2020;27:1452] Chem Biol. 2012;19:883–892. doi: 10.1016/j.chembiol.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]