Abstract
Background
Long-term effects of assisted reproductive technology (ART) on ovarian tumor risk are unknown.
Methods
This nationwide cohort study comprises 30 625 women who received ovarian stimulation for ART in 1983-2000 and 9988 subfertile women not treated with ART. Incident invasive and borderline ovarian tumors were ascertained through linkage with the Netherlands Cancer Registry and the Dutch Pathology Registry until July 2018. Ovarian tumor risk in ART-treated women was compared with risks in the general population and the subfertile non-ART group. Statistical tests were 2-sided.
Results
After a median follow-up of 24 years, 158 invasive and 100 borderline ovarian tumors were observed. Ovarian cancer risk in the ART group was increased compared with the general population (standardized incidence ratio [SIR] = 1.43, 95% confidence interval [CI] = 1.18 to 1.71) but not when compared with the non-ART group (age- and parity-adjusted hazard ratio [HR] = 1.02, 95% CI = 0.70 to 1.50). Risk decreased with higher parity and with a larger number of successful ART cycles (resulting in childbirth, Ptrend = .001) but was not associated with the number of unsuccessful ART cycles. Borderline ovarian tumor risk was increased in ART-treated women compared with the general population (SIR = 2.20, 95% CI = 1.66 to 2.86) and with non-ART women (HR = 1.84, 95% CI = 1.08 to 3.14). Risk did not increase with more ART cycles or longer follow-up time.
Conclusions
Increased ovarian cancer risk in ART-treated women compared with the general population is likely explained by nulliparity rather than ART treatment. The increased risk of borderline ovarian tumors after ART must be interpreted with caution because no dose-response relationship was observed.
Since the introduction of assisted reproductive technology (ART), such as in vitro fertilization (IVF), 4 decades ago, concerns have been raised that ART might increase the risk of ovarian tumors. Suggested mechanisms include strong increases of gonadotrophin levels and/or multiple punctures disrupting the ovarian epithelium (1). Because of the worldwide increase in the use of ART and the poor prognosis of ovarian cancer, it is important from a public health perspective to examine the association between ART and long-term ovarian tumor incidence. Consequently, several epidemiological studies have investigated the association between ART and risks of ovarian tumors, with inconsistent results (2–13). In 2013, 2 meta-analyses were published (2,6) showing that ART-treated women had an increased risk for ovarian cancer compared with the general population [relative risk (RR) = 1.35, 95% confidence interval (CI) = 0.99 to 1.84 (2) and RR = 1.50, 95% CI = 1.17 to 1.92 (6)]. This risk increase, however, might be due to higher prevalence of nulliparity and/or subfertility in ART-treated women. One meta-analysis concluded, based on only 2 studies, that risk of ovarian cancer was not increased compared with subfertile women not treated with ART (RR = 1.26, 95% CI = 0.62 to 2.55), emphasizing the importance of a subfertile comparison group (6). However, most recent studies (published after the meta-analyses) also lacked an appropriate comparison group (3,5,7,10–12,14); the few studies that did have an appropriate comparison group (8,9,13) remained inconclusive because of relatively short follow-up and few ovarian tumors (n ≤ 25) in ART-treated women. Therefore, the aim of the current study was to determine long-term ovarian tumor risk in a large nationwide cohort of women treated with ART in 1983-2000 compared with women in the Dutch general population and subfertile women not treated with ART. In addition, because complete information on parity was available, we also examined whether ART cycles leading to childbirth have a different effect on ovarian tumor risk than unsuccessful ART cycles, hypothesizing that a full-term pregnancy from ART might counteract any ART-associated risk increase of ovarian tumors, if present.
Methods
Study Population
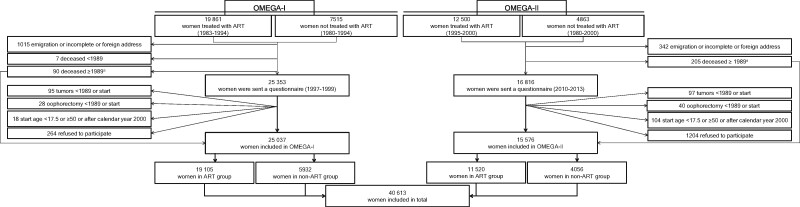
A nationwide retrospective cohort study with prospective follow-up was conducted to investigate long-term health after ovarian stimulation for ART, covering ART treatments applied in 1983-2000. In 1995-1996, the OvariuMstimulatie En Gynecologische Aandoeningen-I (OMEGA-I) cohort was identified, comprising 19 861 women who started ovarian stimulation for ART in 1 of the 12 in vitro fertilization (IVF) clinics operating in the Netherlands in 1983-1994 (ART group) and a comparison group of 7515 women diagnosed with subfertility (ie, failure to achieve a clinical pregnancy after ≥12 months of regular unprotected sexual intercourse) but not treated with ART in 4 of the 12 clinics and diagnosed in 1980-1994 (non-ART group). The OMEGA-I study cohort and data collection methods have been extensively described (4,15).
In 2010-2012, the OMEGA-I cohort was expanded with 12 500 women who started ART treatment in 1995-2000 in 1 of the 12 IVF clinics and a comparison group of 4863 non-ART women diagnosed in 1980-2000 (OMEGA-II) (see Figure 1). Non-ART women were identified in 6 of the 12 IVF clinics and 2 regional hospitals (for expansion of the non-ART group). A more detailed description of the ART and non-ART groups is given in the Supplementary Methods (available online). The institutional ethics committees of all participating hospitals approved the study procedures (4,16).
Figure 1.
Flow chart of OMEGA-I and OMEGA-II cohort. aWomen in this category contributed person-time from the first assisted reproductive technology (ART) treatment or first gynecological visit until date of death.
Collection of Subfertility Treatment Data and Potential Confounders
For the OMEGA-I cohort, trained abstractors registered subfertility causes, fertility-improving surgical procedures, and for each intrauterine insemination and ART cycle, date, dosage, and type of fertility drug, and outcome from medical records. For the OMEGA-II cohort, electronic medical record data were obtained for all centers. Furthermore, 42 169 women alive at study invitation were invited to complete a risk factor questionnaire and informed consent form for future linkages with disease registries (the OMEGA-I cohort was invited in 1997-1999 and OMEGA-II in 2010-2013). The questionnaire ascertained information on reproductive histories, fertility treatments, hormone use, lifestyle, and family history of cancer; 61% of women completed the questionnaire.
In 2013, all cohort members except women who refused linkages, (94.1% are the women who did not refuse participation) were linked with the Dutch Municipal Personal Records Database, yielding nearly complete information on parity and age at first birth until August 2013.
Ascertainment of Outcome Data
Cancer incidence from 1989 to July 2018 was ascertained through the population-based Netherlands Cancer Registry (NCR) (4,17) for the entire cohort except 5.9% of women who refused linkage (17). Cancer diagnoses included all invasive malignancies and borderline ovarian tumors except for nonmelanoma skin cancer (18). Information on each malignancy included date of diagnosis, topography, and morphology. Because the NCR only registered borderline ovarian tumors from 2001, we used data from the Dutch Pathology Registry (PALGA) (19), with nationwide coverage from 1991 onward, to identify additional borderline ovarian tumors. The Dutch Municipal Personal Records Database provided vital status for all women.
Statistical Analysis
Eligible women (ie, women responding and not responding to the questionnaire as well as women who were deceased at study invitation) entered the cohort on the date of first ART treatment (ART group, 1983-2000) or first clinic visit for subfertility evaluation (non-ART group, 1980-2000). Because the NCR did not have nationwide coverage until 1989, the observation time for each participant started on January 1, 1989, the date of first ART treatment, or the date of first clinic visit for subfertility evaluation (non-ART group), whichever came last. Women who developed cancer before start of subfertility treatment or evaluation were excluded from analysis (including 25 ovarian tumors), as well as women who developed cancer (including 13 ovarian tumors) or died (n = 7) after start of subfertility treatment or evaluation but before 1989 (see Figure 1). To exclude tumors diagnosed during evaluation or treatment of subfertility, time at risk was calculated from 1 year after the first ART treatment or visit to gynecologist until the date NCR follow-up ended (July 1, 2018), date of diagnosis of any first malignancy (including borderline ovarian tumors), date of bilateral oophorectomy, or date of death, whichever came first. For women who refused linkage with disease registries, follow-up ended at date of questionnaire completion.
First, ovarian tumor incidence in the ART and non-ART groups was compared with incidence in the general population. Standardized incidence ratios (SIRs) were calculated as the ratio of the observed and expected numbers of tumors in the cohort. Expected numbers were based on sex-, age-, and calendar year–specific incidence rates from the NCR (18). Because expected numbers were based on NCR incidence data, only borderline tumors retrieved from NCR were included in these analyses. Second, Cox proportional hazards models, with number of ART cycles and births as time-dependent variables and age (in years) as time scale, were used to compare the risks of invasive and borderline tumors (from NCR and PALGA) between the ART and non-ART group. All other covariates were included as fixed variables. The proportional hazards assumption was not violated when evaluating log-minus-log plots.
Exposure variables (ART, intrauterine insemination, and fertility drug use) and confounding factors were primarily based on medical record data and supplemented with data from questionnaires if missing. Age at start of treatment or first gynecologist visit, age at menarche, parity, multiple birth, age at first birth, subfertility cause, intrauterine insemination, endometriosis, oral contraceptive use, and body mass index were tested as confounders and retained in the analysis if they changed the hazard ratio (HR) for receipt of ART by 10.0% or more.
Two sensitivity analyses were performed: 1) with ovarian cancer and borderline tumors as a combined outcome variable, including the first year of follow-up; 2) in women who started fertility treatment after 1988, to eliminate the effect of left censoring. Furthermore, we examined whether successful ART cycles (leading to childbirth) had a different effect on ovarian tumor risk than unsuccessful ART cycles, incorporating numbers of successful and unsuccessful cycles time dependently in 1 model. Statistical tests were 2-sided, and a P value less than .05 was considered statistically significant. All analyses were performed with STATA 13 (20).
Results
Population Characteristics
The study cohort comprised 30 625 ART-treated women and 9988 non-ART–treated women (Table 1 and Figure 1). Women in the non-ART group had a slightly longer follow-up duration than women in the ART group (25.7 vs 23.4 years) and were also older at end of follow-up (median age 57.0 vs 55.9 years). The mean number of ART cycles was 3.3. More women in the ART group remained nulliparous (35.4% vs 25.2%).
Table 1.
Population characteristics by ART exposure status
| Characteristics | ART group | Non-ART group | Entire cohort |
|---|---|---|---|
| (n = 30 625) | (n = 9988) | (n = 40 613) | |
| No. (%) | No. (%) | No. | |
| Year of birth | |||
| <1955 | 3597 (11.8) | 2245 (22.5) | 5842 |
| 1955-1959 | 8622 (28.2) | 2617 (26.2) | 11 239 |
| 1960-1964 | 10 468 (34.2) | 2759 (27.6) | 13 227 |
| ≥1965 | 7938 (25.9) | 2367 (23.7) | 10 305 |
| Major subfertility diagnosisa | |||
| Male factor | 8205 (26.8) | 1646 (16.5) | 9851 |
| Tubal factor | 7896 (25.8) | 2672 (26.8) | 10 568 |
| Unexplained or other factorb | 10 147 (33.1) | 2816 (28.2) | 12 963 |
| Missing | 4377 (14.3) | 2854 (28.6) | 7231 |
| Age at 1st ART treatment or 1st visit to the gynecologist, yc | |||
| <27 | 2457 (8.0) | 1878 (18.8) | 4335 |
| 27-29 | 4920 (16.1) | 2157 (21.6) | 7077 |
| 30-32 | 7941 (25.9) | 2295 (23.0) | 10 236 |
| 33-35 | 7448 (24.3) | 1908 (19.1) | 9356 |
| ≥36 | 7859 (25.7) | 1750 (17.5) | 9609 |
| Year of 1st ART treatment or 1st visit to the gynecologistc | |||
| <1989 | 1818 (5.9) | 3040 (30.4) | 4858 |
| 1989-1992 | 8731 (28.5) | 2977 (29.8) | 11 708 |
| 1993-1996 | 11 633 (38.0) | 2473 (24.8) | 14 106 |
| 1997-2000 | 8443 (27.6) | 1498 (15.0) | 9941 |
| Total No. of ART cycles | |||
| 0 | 0 (0.0) | 9988 (100.0) | 9988 |
| 1-2 | 11 520 (37.6) | 0 (0.0) | 11 520 |
| 3-4 | 10 695 (34.9) | 0 (0.0) | 10 695 |
| 5-6 | 3733 (12.2) | 0 (0.0) | 3733 |
| ≥7 | 2080 (6.8) | 0 (0.0) | 2080 |
| Missing | 2597 (8.5) | 0 (0.0) | 2597 |
| Total No. of IUI and ART cycles | |||
| 0 | 0 (0.0) | 4584 (45.9) | 4584 |
| 1-2 | 8103 (26.5) | 891 (8.9) | 8994 |
| 3-4 | 7,909 (25.8) | 736 (7.4) | 8645 |
| 5-6 | 4009 (13.1) | 503 (5.0) | 4512 |
| ≥7 | 8054 (26.3) | 383 (3.8) | 8437 |
| Missing | 2550 (8.3) | 2891 (28.9) | 5441 |
| Time since 1st treatment, yc | |||
| <10 | 1627 (5.3) | 331 (3.3) | 1958 |
| 10-14 | 866 (2.8) | 270 (2.7) | 1136 |
| 15-19 | 5687 (18.6) | 1240 (12.4) | 6927 |
| 20-24 | 12 433 (40.6) | 2664 (26.7) | 15 097 |
| 25-29 | 8884 (29.0) | 3205 (32.1) | 12 089 |
| ≥30 | 1128 (3.7) | 2278 (22.8) | 3406 |
| Age at end of follow-up, y | |||
| <45 | 2121 (6.9) | 605 (6.1) | 2726 |
| 45-49 | 3444 (11.3) | 1140 (11.4) | 4584 |
| 50-54 | 7902 (25.8) | 2210 (22.1) | 10 112 |
| 55-59 | 9575 (31.3) | 2718 (27.2) | 12 293 |
| ≥60 | 7583 (24.8) | 3315 (33.2) | 10 898 |
| No. of births | |||
| 0 | 10 832 (35.4) | 2517 (25.2) | 13 349 |
| 1 | 9766 (31.9) | 2486 (24.9) | 12 252 |
| ≥2 | 9913 (32.4) | 4844 (48.5) | 14 757 |
| Missing | 114 (0.4) | 141 (1.4) | 255 |
| Age at 1st birth, yd | |||
| <30 | 6390 (20.9) | 3534 (35.4) | 9924 |
| 30-34 | 7526 (24.6) | 2368 (23.7) | 9894 |
| ≥35 | 5720 (18.7) | 1410 (14.1) | 7130 |
| Missing | 43 (0.1) | 18 (0.2) | 61 |
Based on information from medical records if available and on information from questionnaires if no medical record information was available. If several diagnoses had been registered, without mention of the main diagnosis, the following order was applied: male factor, tubal factor, unexplained or other factor (including hormonal factor) for main diagnosis. ART = assisted reproductive technology; IUI = intrauterine insemination.
Other factors include endometriosis and cervical factors and hormonal factors such as ovulation disorders, polycystic ovary syndrome, and premature menopause.
First treatment indicates start of ART treatment for ART group and first visit at gynecologist for the non-ART group.
Only among 27 009 parous women (19 679 ART and 7330 non-ART).
Risk of Ovarian Cancer
In total, 158 ovarian cancers were observed: 53.2% were serous, 10.8% mucinous, 7.0% clear cell, 14.6% endometrioid, and 14.5% other or not otherwise specified. Median age at diagnosis was 50.2 years.
Comparisons With External Reference Rates
Compared with ovarian cancer incidence in the Dutch population, ovarian cancer risk was increased in the ART group (SIR = 1.43, 95% CI = 1.18 to 1.71) but not in the non-ART group (SIR = 1.15, 95% CI = 0.81 to 1.59; Pdifference = .25) (Table 2). Risk of ovarian cancer was statistically significantly increased in women who received 1-2 cycles (SIR = 1.58, 95% CI = 1.17 to 2.08) or 7 or more cycles (SIR = 1.92, 95% CI = 1.05 to 3.22), but no trend was observed with more ART treatment cycles (Ptrend = .83). After 15 or more years of follow-up, risk in the ART group was higher than in the general population (SIR = 1.62, 95% CI = 1.27 to 2.05), but no trend was observed of increasing risk with longer follow-up (Ptrend = .25).
Table 2.
Incidence of invasive ovarian cancer and borderline ovarian tumor in ART-treated women compared with the general population, excluding the first year of follow-up
| Fertility treatment characteristics | Invasive ovarian cancera |
Borderline ovarian tumorb |
||||||
|---|---|---|---|---|---|---|---|---|
| Person-years | Observed/expected | SIR (95% CI) | P c | Person-years | Observed/expected | SIR (95% CI) | P c | |
| Entire cohort | 884 415 | 152/112.7 | 1.35 (1.14 to 1.58) | 883 714 | 72/34.1 | 2.11 (1.65 to 2.66) | ||
| ART exposure | ||||||||
| Non-ART | 236 907 | 37/32.1 | 1.15 (0.81 to 1.59) | 236 721 | 16/8.7 | 1.84 (1.05 to 2.99) | ||
| ART | 647 507 | 115/80.6 | 1.43 (1.18 to 1.71) | .25 | 646 994 | 56/25.4 | 2.20 (1.66 to 2.86) | .52 |
| No. of ART cycles | ||||||||
| 1-2 | 258 309 | 49/31.1 | 1.58 (1.17 to 2.08) | 258 122 | 21/10.2 | 2.07 (1.28 to 3.16) | ||
| 3-4 | 245 263 | 40/30.5 | 1.31 (0.94 to 1.79) | 245 109 | 23/9.7 | 2.38 (1.51 to 3.58) | ||
| 5-6 | 90 920 | 12/11.7 | 1.03 (0.53 to 1.79) | 90 847 | 6/3.5 | 1.70 (0.62 to 3.69) | ||
| ≥7 | 53 018 | 14/7.3 | 1.92 (1.05 to 3.22) | .34 | 52 920 | 6/2.1 | 2.90 (1.07 to 6.32) | .78 |
| Age at 1st ART cycle, y | ||||||||
| <30 | 158 058 | 7/11.6 | 0.61 (0.24 to 1.25) | 15 885 | 16/4.9 | 3.26 (1.86 to 5.30) | ||
| 30-32 | 169 644 | 28/17.7 | 1.59 (1.05 to 2.29) | 169 500 | 15/6.2 | 2.42 (1.35 to 3.99) | ||
| 33-35 | 157 439 | 28/21.2 | 1.32 (0.88 to 1.91) | 157 364 | 13/6.5 | 2.00 (1.07 to 3.42) | ||
| ≥36 | 162 315 | 52/30.2 | 1.73 (1.29 to 2.26) | .03 | 162 194 | 12/7.8 | 1.54 (0.80 to 2.69) | .24 |
| Time since 1st ART cycle, yd | ||||||||
| 1-4 | 120 009 | 10/8.1 | 1.24 (0.60 to 2.28) | 120 082 | 2/1.7 | 1.20 (0.15 to 4.32) | ||
| 5-9 | 147 016 | 11/12.4 | 0.89 (0.44 to 1.59) | 146 977 | 9/5.5 | 1.63 (0.75 to 3.10) | ||
| 10-14 | 142 927 | 23/16.3 | 1.41 (0.90 to 2.12) | 142 842 | 12/8.5 | 1.42 (0.73 to 2.48) | ||
| 15-19 | 132 370 | 40/20.7 | 1.94 (1.38 to 2.64) | 132 217 | 20/7.2 | 2.78 (1.70 to 4.30) | ||
| ≥20 | 104 558 | 31/23.1 | 1.34 (0.91 to 1.90) | .16 | 104 332 | 13/2.6 | 5.04 (2.69 to 8.63) | .01 |
| Parity (in ART group) | ||||||||
| Nulliparous | 228 000 | 61/31.0 | 1.97 (1.51 to 2.53) | 227 726 | 24/9.2 | 2.60 (1.67 to 3.87) | ||
| Parous | 416 870 | 54/49.2 | 1.10 (0.83 to 1.43) | .002 | 416 631 | 32/16.1 | 1.99 (1.36 to 2.81) | .06 |
| Subfertility diagnosis (in ART group) | ||||||||
| Male factor | 193 075 | 33/21.7 | 1.52 (1.05 to 2.13) | 193 018 | 9/7.4 | 1.21 (0.55 to 2.30) | ||
| Tubal factor | 198 707 | 38/26.8 | 1.42 (1.01 to 1.95) | 198 409 | 29/7.8 | 3.69 (2.47 to 5.30) | ||
| Unexplained or other factor | 255 725 | 44/32.1 | 1.37 (1.00 to 1.84) | .01 | 255 567 | 18/10.1 | 1.78 (1.06 to 2.81) | .003 |
Women with a first invasive ovarian cancer or ovarian cancer diagnosed within 3 months after another invasive cancer in the abdominal area (n = 6) or women who developed invasive ovarian cancer following borderline ovarian cancer (n = 1) are included in the analyses. Time at risk ends at date of diagnosis of invasive ovarian cancer. ART = assisted reproductive technology; CI = confidence interval; SIR = standardized incidence ratio.
Only first borderline ovarian tumors are included in the analyses. Subsequent invasive ovarian cancers after a borderline ovarian tumor (n = 1) are ignored as events and in calculating the follow-up duration.
P value of Likelihood ratio test.
The follow-up category to which women were allocated was calculated from start of first ART treatment or gynecologist visit until censoring date; also for women whose observation time started after 1989.
Nulliparous women had a statistically significantly 2-fold increased risk of ovarian cancer, whereas parous women (irrespective of ART) had no increased risk compared with the general population (Table 2; Supplementary Table 1, available online). Each subfertility diagnosis (male, tubal, and unexplained or other) was associated with statistically significantly increased risk of ovarian cancer in ART-treated women but not in non-ART–treated women.
A sensitivity analysis with time at risk starting at date of first ART treatment or subfertility evaluation showed similar results (ie, SIR = 1.44, 95% CI = 1.19 to 1.72) for the ART group and 1.23 (95% CI = 0.88 to 1.68) for the non-ART group.
Within Cohort Comparisons
When directly comparing the ART group with the non-ART group, the hazard ratio for ovarian cancer was 1.02 (95% CI = 0.70 to 1.50), adjusted for age at start and parity (Table 3). Compared with no ART, risk did not increase after more ART cycles (≥5 cycles HR = 1.01, 95% CI = 0.61 to 1.68). For serous and nonserous ovarian cancer, the hazard ratios associated with ART were 1.52 (95% CI = 0.87 to 2.68) and 0.68 (95% CI = 0.40 to 1.15), respectively (Supplementary Table 2, available online). The ovarian hyperstimulation syndrome was not associated with increased risk (HR = 1.40, 95% CI = 0.52 to 3.81). A poor response to the first ART cycle (ie, <4 oocytes, or canceled cycle because of anticipated poor response) was associated with a non-statistically significantly reduced risk of ovarian cancer (HR = 0.60, 95% CI = 0.33 to 1.07). Histologically proven endometriosis was associated with a hazard ratio of 1.93 (95% CI = 1.04 to 3.61) for nonserous cancers (data not shown). Parous women experienced statistically significantly lower risk of ovarian cancer (HR = 0.54, 95% CI = 0.39 to 0.75) than nulliparous women (Table 3). In stratified analyses, risks of ovarian cancer in ART- vs non-ART–treated women were increased at older attained ages (50 years or older HR = 1.76, 95% CI = 1.01 to 3.07) but did not increase after longer follow-up (20 years or more HR = 1.07, 95% CI = 0.57 to 2.02) (Table 4).
Table 3.
Invasive ovarian cancer risk according to fertility treatment characteristics, excluding the first year of follow-up
| Fertility treatment characteristics | No. of ovarian cancers | No. of women | Adj. HR (95% CI)a |
|---|---|---|---|
| ART exposure | |||
| Non-ART | 37 | 9972 | 1 (Referent) |
| ART | 115 | 30 565 | 1.02 (0.70 to 1.50) |
| Total No. of ART cycles | |||
| 0 | 37 | 9972 | 1 (Referent) |
| 1-2 | 50 | 12 474 | 1.18 (0.76 to 1.82) |
| 3-4 | 39 | 11 586 | 0.87 (0.55 to 1.39) |
| ≥5 | 26 | 6505 | 1.01 (0.61 to 1.68) |
| Total No. of IUI and ART cyclesb | |||
| 0 | 19 | 4574 | 1 (Referent) |
| 1-2 | 27 | 9047 | 0.84 (0.46 to 1.53) |
| 3-4 | 36 | 8688 | 1.06 (0.60 to 1.87) |
| 5-6 | 11 | 4515 | 0.63 (0.30 to 1.35) |
| ≥7 | 35 | 8446 | 1.04 (0.59 to 1.84) |
| Missing | 24 | 5267 | 0.98 (0.53 to 1.81) |
| Response at 1st ART cyclec,d | |||
| Normal response | 60 | 14 943 | 1 (Referent) |
| Poor response | 14 | 4340 | 0.60 (0.33 to 1.07) |
| Missing | 41 | 11 282 | 0.92 (0.62 to 1.37) |
| OHSSd,e | |||
| Never | 111 | 29 602 | 1 (Referent) |
| Ever | 4 | 963 | 1.40 (0.52 to 3.81) |
| Clomiphene used | |||
| Never | 33 | 9712 | 1 (Referent) |
| Ever | 25 | 6515 | 0.93 (0.55 to 1.57) |
| Missing | 57 | 14 338 | 0.98 (0.63 to 1.50) |
| Main subfertility diagnosisf | |||
| Male factor | 38 | 11 572 | 1 (Referent) |
| Tubal factor | 51 | 12 952 | 0.98 (0.64 to 1.50) |
| Unexplained or other factor | 63 | 16 013 | 1.08 (0.72 to 1.61) |
| Endometriosisf,g | |||
| No | 132 | 36 936 | 1 (Referent) |
| Yes | 20 | 3601 | 1.47 (0.92 to 2.36) |
| Parityf | |||
| Nulliparous | 78 | 13 295 | 1 (Referent) |
| Parous | 73 | 26 988 | 0.54 (0.39 to 0.75) |
Analyses include 40 537 women, 30 565 ART treated women, and 9972 non-ART–treated women. Each variable was analyzed in a separate model. All analyses are adjusted for age at start of treatment or visit to gynecologist and parity. Adj. HR = adjusted hazard ratio; ART = assisted reproductive technology; CI = confidence interval; IUI = intrauterine insemination; OHSS = ovarian hyperstimulation syndrome.
Includes stimulated and nonstimulated IUI cycles. Based on information from medical records if available and on information from questionnaires if no medical record information was available. Women with missing data about IUI cycles and with a tubal cause of subfertility were categorized in the 0 IUI cycles category.
Poor response includes canceled first cycles because of anticipated poor response and less than 4 oocytes; normal response includes 4 or more oocytes collected in first cycle.
Among 30 565 ART-treated women only; 115 invasive ovarian cancer cases.
OHSS includes women who had had no ovum pick-up because of (anticipated) OHSS.
Additionally adjusted for ART exposure (yes/no).
Histologically proven endometriosis, based on information from The Nationwide Network and Registry of Histo- and Cytopathology in the Netherlands (PALGA).
Table 4.
Invasive ovarian cancer or borderline ovarian tumor risk for ART vs non-ART treatment within risk factor subgroups, excluding first year of follow-up
| Ovarian tumor risk factors | Invasive ovarian cancer |
Borderline ovarian tumor |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ART group |
Non-ART group |
Adj. HR for ART vs non-ART (95% CI)b | P c | ART group |
Non-ART group |
Adj. HR for ART vs non-ART (95% CI)b | P c | |||||
| No. of ovarian cancersa | No. of womena | No. of ovarian cancersa | No. of womena | No. of ovarian tumorsa | No. of womena | No. of ovarian tumorsa | No. of womena | |||||
| Parity | ||||||||||||
| Nulliparous | 60 | 10 787 | 18 | 2508 | 0.93 (0.55 to 1.58) | 38 | 10 787 | 2 | 2508 | 5.54 (1.33 to 22.99) | ||
| Parous | 55 | 19 665 | 18 | 7323 | 1.30 (0.76 to 2.22) | .38 | 41 | 19 665 | 15 | 7323 | 1.19 (0.66 to 2.16) | .05 |
| Start yeard | ||||||||||||
| 1983-1989 | 9 | 1818 | 16 | 3040 | 0.87 (0.38 to 1.99) | 11 | 1818 | 8 | 3040 | 2.25 (0.90 to 5.63) | ||
| 1990-2000 | 106 | 28 747 | 21 | 6932 | 1.09 (0.68 to 1.75) | .64 | 68 | 28 747 | 9 | 6932 | 1.91 (0.95 to 3.85) | .78 |
| Time since 1st ART treatment or 1st visit to the gynecologist, y | ||||||||||||
| <10 | 20 | 29 958 | 8 | 9504 | 0.65 (0.29 to 1.49) | 24 | 29 958 | 3 | 9504 | 2.40 (0.72 to 7.99) | ||
| 10-19 | 65 | 28 996 | 14 | 9654 | 1.27 (0.71 to 2.26) | .20 | 40 | 28 996 | 8 | 9654 | 1.73 (0.80 to 3.72) | .63 |
| ≥20 | 30 | 22 440 | 15 | 8136 | 1.07 (0.57 to 2.02) | .35 | 15 | 22 440 | 6 | 8136 | 1.44 (0.56 to 3.74) | .50 |
| Age at 1st ART treatment or 1st visit to the gynecologist, y | ||||||||||||
| <35 | 53 | 20 438 | 26 | 7697 | 0.81 (0.50 to 1.31) | 57 | 20 438 | 12 | 7697 | 2.18 (1.16 to 4.10) | ||
| ≥35 | 62 | 10 127 | 11 | 2275 | 1.48 (0.76 to 2.85) | .18 | 22 | 10 127 | 5 | 2275 | 1.16 (0.44 to 3.08) | .29 |
| Attained age, ye | ||||||||||||
| <50 | 41 | 30 565 | 21 | 9972 | 0.62 (0.36 to 1.05) | 47 | 30 565 | 8 | 9972 | 2.08 (0.98 to 4.42) | ||
| ≥50 | 74 | 25 060 | 16 | 8243 | 1.76 (1.01 to 3.07) | .01 | 32 | 25 060 | 9 | 8243 | 1.42 (0.67 to 2.99) | .48 |
Not all numbers add up to 100%, because of missing values. Adj. HR = adjusted hazard ratio; ART = assisted reproductive technology; CI = confidence interval.
Cox regression analyses: models with age (in years) as time scale and adjusted for parity (and additionally for tubal subfertility in borderline ovarian tumor analysis). Analyses includes 4537 women, 30 565 ART-treated women, and 9972 non-ART–treated women.
P of interaction terms.
ART treatment regimens before 1990 included gonadotropins with or without clomiphene. After 1989, the most often used ART treatment regimen consisted of gonadotropins in combination with gonadotrophin-releasing hormone agonists.
Analyses for attained age younger than 50 years comprise all person-years of women until their 50th birthday or date of any first cancer diagnosis, date of death, date of completeness of cancer registry (July 1, 2018), date of bilateral oophorectomy, or date of questionnaire completion for women who refused linkage with the Netherlands Cancer Registry, whichever came first. Analyses for attained age 50 years or older comprise only person-years of women who were 50 years or older at end of follow-up, starting at their 50th birthday, until date of any first cancer diagnosis, date of death, date of completeness of cancer registry (July 1, 2018), date of bilateral oophorectomy, or date of questionnaire completion for women who refused linkage with the Netherlands Cancer Registry, whichever came first.
Within the ART group, we assessed potentially differential effects of successful and unsuccessful ART cycles. Ovarian cancer risk decreased in women with a larger number of successful cycles (1 successful ART cycle HR = 0.54, 95% CI = 0.34 to 0.87; ≥2 successful ART cycles = 0.37, 95% CI = 0.18 to 0.73; Ptrend = .001, adjusted for age at start, number of unsuccessful cycles, and spontaneous births). A larger number of unsuccessful ART cycles did not increase ovarian cancer risk (Table 5).
Table 5.
Incidence of invasive ovarian cancer and borderline ovarian tumor according to successful (childbirth) and unsuccessful (no childbirth) ART cycles, excluding the first year of follow-up
| Successful/unsuccessful ART cycles | Invasive ovarian cancer |
Borderline ovarian tumor |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of cancers | No. of women | Adj. HR (95% CI)a | P b | No. of tumors | No. of women | Adj. HR (95% CI)a | P b | |
| Successful ART cycle(s)c | ||||||||
| 0 cycles | 75 | 13 272 | 1 (Referent) | 42 | 13 272 | 1 (Referent) | ||
| 1 cycle | 28 | 9743 | 0.54 (0.34 to 0.87) | 23 | 9743 | 0.79 (0.45 to 1.39) | ||
| ≥2 cycles | 12 | 7550 | 0.37 (0.18 to 0.73) | .001 | 14 | 7550 | 0.67 (0.33 to 1.35) | .21 |
| Unsuccessful ART cycle(s)c | ||||||||
| 0 cycles | 12 | 6005 | 1 (Referent) | 13 | 6005 | 1 (Referent) | ||
| 1 ART cycle | 24 | 5761 | 1.20 (0.57 to 2.53) | 13 | 5761 | 0.87 (0.39 to 1.95) | ||
| 2 ART cycles | 26 | 5323 | 1.29 (0.61 to 2.75) | 12 | 5323 | 0.79 (0.34 to 1.84) | ||
| 3 ART cycles | 21 | 5505 | 0.83 (0.37 to 1.87) | 16 | 5505 | 0.94 (0.41 to 2.17) | ||
| ≥4 ART cycles | 32 | 7971 | 0.88 (0.41 to 1.88) | .25 | 25 | 7971 | 0.92 (0.42 to 2.00) | .69 |
Cox regression analyses incorporating the numbers of successful and unsuccessful ART cycles as separate time-dependent variables into 1 model, using age (in years) as time scale. Analyses are adjusted for age at start treatment or first visit to gynecologist and number of spontaneous births (for borderline tumors additionally for tubal subfertility). Analyses include 30 452 ART-treated women. Adj. HR= adjusted hazard ratio; ART = assisted reproductive technology; CI = confidence interval.
P value of trend test. Trend tests were based on the P value of the category-specific mean as a continuous variable.
Successful ART cycles were defined as cycles leading to the birth of a child, unsuccessful cycles as ART cycles that did not result in a childbirth.
Risk of Borderline Ovarian Tumors
In total, 100 borderline tumors were observed, 74 were obtained from NCR and 26 from PALGA. Of these tumors, 51% were serous, 43.0% mucinous, and 6.0% other or not otherwise specified.
Comparisons With External Reference Rates
Risk of borderline ovarian tumors was statistically significantly increased in both the ART and non-ART group compared with the general population (SIR = 2.20, 95% CI = 1.66 to 2.86; SIR = 1.84, 95% CI = 1.05 to 2.99, respectively) (Table 2). No clear trend in borderline ovarian tumor risk was observed with increasing number of ART cycles (Ptrend = .72). Risk of borderline ovarian tumors in ART-treated women was increased after longer follow-up (≥20 years SIR = 5.04, 95% CI = 2.69 to 8.63; Ptrend = .01). The risk of borderline tumors was increased in ART-treated and non-ART–treated women with tubal subfertility (SIR = 3.69, 95% CI = 2.47 to 5.30; SIR = 3.14, 95% CI = 1.57 to 5.63, respectively) (Table 2; Supplementary Table 1, available online). Borderline ovarian tumor risk was increased in ART-treated women with unexplained subfertility (SIR = 1.78, 95% CI = 1.06 to 2.81).
A sensitivity analysis including the first year of follow-up yielded similar results (ART: SIR = 2.27, 95% CI = 1.72 to 2.93; non-ART: SIR = 1.84, 95% CI = 1.05 to 2.98).
Within Cohort Comparisons
Risk of borderline ovarian tumors was statistically significantly increased in ART-treated women compared with women not receiving ART (HR = 1.84, 95% CI = 1.08 to 3.14), adjusted for age at start, parity, and tubal subfertility (Table 6). However, no trend was observed with more ART cycles (Ptrend = .26). At age 55 years, cumulative incidences in the ART and non-ART groups were 0.3% and 0.2%, respectively.
Table 6.
Borderline ovarian tumor risk according to fertility treatment characteristics, excluding the first year of follow-up
| Fertility treatment characteristics | No. of ovarian tumors | No. of women | Adj. HR (95% CI)a |
|---|---|---|---|
| ART exposure | |||
| Non-ART | 17 | 9972 | 1 (Referent) |
| ART | 79 | 30 565 | 1.84 (1.08 to 3.14) |
| Total No. of ART cycles | |||
| 0 | 17 | 9972 | 1 (Referent) |
| 1-2 | 30 | 12 485 | 1.84 (1.00 to 3.37) |
| 3-4 | 33 | 11 587 | 2.04 (1.12 to 3.71) |
| ≥5 | 16 | 6493 | 1.55 (0.78 to 3.09) |
| Total No. of IUI and ART cyclesb | |||
| 0 | 11 | 4574 | 1 (Referent) |
| 1-2 | 25 | 9047 | 1.62 (0.79 to 3.32) |
| 3-4 | 27 | 8688 | 1.69 (0.83 to 3.45) |
| 5-6 | 11 | 4515 | 1.34 (0.58 to 3.12) |
| ≥7 | 15 | 8446 | 1.07 (0.48 to 2.37) |
| Missing | 7 | 5267 | 0.62 (0.24 to 1.61) |
| Response at 1st ART cyclec,d | |||
| Normal response | 38 | 14 943 | 1 (Referent) |
| Poor response | 14 | 4340 | 1.13 (0.61 to 2.09) |
| Missing | 27 | 11 282 | 1.09 (0.66 to 1.79) |
| Clomiphene used | |||
| Never | 28 | 9712 | 1 (Referent) |
| Ever | 13 | 6515 | 0.62 (0.32 to 1.20) |
| Missing | 38 | 14 338 | 0.80 (0.49 to 1.30) |
| Main subfertility diagnosise | |||
| Male factor | 18 | 11 572 | 1 (Referent) |
| Tubal factor | 51 | 12 952 | 2.47 (1.44 to 4.25) |
| Unexplained or other factor | 27 | 16 013 | 1.07 (0.59 to 1.95) |
| Endometriosise,f | |||
| No | 83 | 36 936 | 1 (Referent) |
| Yes | 13 | 3601 | 1.33 (0.74 to 2.40) |
| Paritye | |||
| Nulliparous | 40 | 13 295 | 1 (Referent) |
| Parous | 56 | 26 988 | 0.76 (0.51 to 1.15) |
Analyses include 40 537 women, 30 565 ART-treated women, and 9972 non-ART–treated women. Each variable was analyzed in a separate model. All analyses are adjusted for age at start treatment or first visit to gynecologist, parity, and tubal subfertility. Adj. HR = adjusted hazard ratio; ART = assisted reproductive technology; CI = confidence interval; IUI = intrauterine insemination.
Includes stimulated and nonstimulated IUI cycles. Based on information from medical records if available and on information from questionnaires if no medical record information was available. Women with missing data about IUI cycles and with a tubal cause of subfertility were categorized in the 0 IUI cycles category.
Poor response includes canceled first cycles because of anticipated poor response and less than 4 oocytes; normal response includes 4 or more oocytes collected in first cycle. Too few women with ovarian hyperstimulation syndrome to perform analysis.
Among 30 565 ART-treated women only; 79 borderline ovarian tumor cases.
Additionally adjusted for ART exposure (yes/no), not adjusted for tubal subfertility.
Histologically proven endometriosis, based on information from the Nationwide Network and Registry of Histo- and Cytopathology in the Netherlands (PALGA).
ART was associated with increased risk of serous borderline ovarian tumors (HR = 3.44, 95% CI = 1.35 to 8.77) but not with nonserous borderline tumors (HR = 1.17, 95% CI = 0.60 to 2.29) (Supplementary Table 2, available online). The risk of borderline ovarian tumors was increased in women with tubal subfertility compared with male subfertility (HR = 2.47, 95% CI = 1.44 to 4.25). Parous women were not at lower risk of borderline tumors than nulliparous women (HR = 0.76, 95% CI = 0.51 to 1.15).
Risks of borderline ovarian tumors in ART-treated women compared with women not receiving ART were not statistically significantly different according to age and start year of treatment, parity, attained age, or follow-up time (Table 4). However, nulliparous ART-treated women had an especially high risk compared with nulliparous women not receiving ART (HR = 5.54, 95% CI = 1.33 to 22.99). In analyses incorporating the outcome of the ART cycle (childbirth), risks associated with successful and unsuccessful ART cycles did not differ (Table 5).
Invasive and Borderline Ovarian Tumors Combined
The risk of ovarian tumors overall (n = 257) was not increased in ART-treated women (HR = 1.18, 95% CI = 0.87 to 1.61) compared with women not receiving ART (Supplementary Table 3, available online).
Discussion
Results from this large, nationwide cohort study, with a median follow-up of 24 years, show that ART-treated women do not have an increased risk of ovarian cancer compared with subfertile women not treated with ART when accounting for the higher frequency of nulliparity in the ART group. The increased risk of ovarian cancer in the ART group compared with the general population appears to be due to nulliparity rather than ART treatment or specific subfertility causes.
In contrast, ART-treated women appear to have an increased risk of borderline ovarian tumors, both when compared with the general population and with subfertile women not treated with ART. However, risks of borderline ovarian tumors did not increase after more ART cycles or after longer follow-up of ART-treated women, challenging a causal explanation of our findings. To our knowledge, this is the first large cohort study with long-term follow-up and a subfertile comparison group investigating the association between ART and risk of both invasive and borderline ovarian tumors. In another large study, Williams et al. examined risk of ovarian tumors in a nationwide study of 255 786 ART-treated British women (264 invasive and 141 borderline tumors). However, they only compared risk with the general population. Results suggested that the increased risks observed for ovarian tumors might be due to underlying patient characteristics rather than ART treatment because increased risks were restricted to women with endometriosis, low parity, or both and were not observed in women treated because of male factor only or unexplained infertility (7). However, in the absence of a subfertile comparison group not treated with ART, the authors could not determine whether the increased risks were due to ART, subfertility, or parity.
The inclusion of a subfertile comparison group not treated with ART and the availability of complete parity data enabled the current study to disentangle the effects of ART, subfertility, and parity. Importantly, we show that the observed increased risk of ovarian cancer compared with the general population is due to nulliparity and not to ART treatment or specific subfertility causes. To date, only 2 other studies included a subfertile comparison group not treated with ART when assessing ovarian cancer risk after ART (8,13). Although based on smaller cohorts, shorter follow-up and less ovarian cancer cases in ART-exposed women (n = 21 and n = 16), neither study found an increased risk of ovarian cancer after ART when compared with no ART (8,13).
A few studies investigated the risk of borderline ovarian tumors after ART compared with a general population reference group (5,7,11), but only one study included a subfertile comparison group (9). Results from our general population comparison agree with those from the largest study to date (7), in that ART-treated women have an increased risk of borderline ovarian tumors compared with the general population and that no clear trend is apparent with increasing number of ART cycles. Our results also confirm those of Stewart et al., who showed in within-cohort analysis a statistically significantly increased risk of borderline ovarian tumors after ART (HR = 2.46, 95% CI = 1.20 to 5.04) (9) but, in contrast to our work, without information about number of ART cycles.
Interpretation of our results with respect to the increased risk of borderline tumors remains speculative. Although increased risks were observed both in comparison with the general population and subfertile women not receiving ART, no clear dose-response relationship emerged. If ART treatment (ie, punctures) increases epithelial damage with concomitant genetic alterations, a higher risk after receiving more ART cycles would be expected. Therefore, we cannot exclude the possibility that the observed increased risk of borderline tumors might be due to unmeasured confounding (eg, by severity of subfertility).
Women with tubal subfertility had an increased risk of borderline ovarian tumors, irrespective of ART. This may be due to pelvic inflammatory disease underlying tubal subfertility (21), because pelvic inflammatory disease is a risk factor for borderline ovarian tumors (22). Our questionnaire data showed that almost 34.1% of women with tubal subfertility reported an inflammation of the fallopian tube and/or ovary in the past.
Our study is the first to examine whether unsuccessful ART cycles carry a different risk of ovarian tumors than successful cycles, hypothesizing that childbirth after ART might counteract any risk increase from ART. Analyses incorporating numbers of successful and unsuccessful cycles showed that ART-treated women with more successful cycles had a statistically significantly reduced risk of ovarian cancer, whereas a larger number of unsuccessful cycles did not increase the risk. This supports our findings that ART does not increase ovarian cancer risk and parity (possibly associated with less severe subfertility) reduces risk in ART-treated women.
Our study has several strengths and limitations. Strengths include long-term and complete follow-up, information on parity and subfertility cause, and the availability of a subfertile comparison group. Our study illustrates that such a comparison group and multivariable analyses are crucial in studies on ovarian cancer risk and ART, because ART-treated women differ from the general population regarding several risk factors for ovarian cancer.
Incidence of ovarian cancer in the population is low before age 50 years and rises exponentially thereafter. Even with our long-term follow-up (median: 24 years), relatively few women had reached the sixth or seventh decade of life (median attained age: 56 years). Consequently, the number of cases in our large cohort was still relatively small, rendering it difficult to draw definitive conclusions.
To examine whether left censoring has affected our results, we performed an analysis restricted to women who entered the cohort after 1988. Results (based on fewer events and shorter follow-up) were quite similar, with hazard ratios associated with ART of 0.96 (95% CI = 0.62 to 1.48) and 1.59 (95% CI = 0.86 to 2.94) for ovarian cancer and borderline tumors, respectively.
Detection bias might have occurred because of diagnostic procedures for subfertility. However, results from analyses including the first year of follow-up showed comparable results, rendering such bias unlikely. Furthermore, in our earlier study, additional data collection showed that almost none of the ovarian cancers were screen detected (4).
Finally, because the NCR is incomplete for borderline ovarian tumors, we also included borderline ovarian tumors from PALGA. Because these tumors were not included in the reference rates of the NCR, they could only be included in the within-cohort analyses. This slightly complicates comparison of results from the within-cohort analysis with the general population comparison.
In conclusion, after a median follow-up of 24 years, ART-treated women do not have an increased risk of ovarian cancer compared with subfertile women not treated with ART. The higher risk of ovarian cancer compared with the general population is likely due to the higher prevalence of nulliparity in ART-treated women. However, ART-treated women had a statistically significantly 1.8-fold higher risk of borderline ovarian tumors than non-ART women. Although lack of a dose-response relationship with ART-treatment cycles does not support a causal association, more research is warranted to examine the role of ART in the etiology of borderline ovarian tumors.
Funding
This work was supported by grants from the Dutch Cancer Society (NKI 2006-3631).
Notes
Role of the funder: The Dutch Cancer Society had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to disclose.
Role of the authors: FEvL, MSp, and AWvdB-D had full access to all of the data in the study and responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: FEvL, CWB, CBL, AWvdB-D, MSp, and MSch. Acquisition, analysis, or interpretation of data: CWB, CBL, HHvB, RS, MK, FJMB, JSEL, EJPvS, DDMB, LAJvdW, BJC, AEPC, JMJS, MMvR, MG, RJTvG, PAMM, CJCMH, GMO, MAG, MSch, AWvdB-D, FEvL, and MSp. Drafting of the manuscript: MSp, AWvdB-D, and FEvL. Critical revision of the manuscript for important intellectual content: CWB, CBL, HHvB, RS, MK, FJMB, JSEL, EJPvS, DDMB, LAJvdW, BJC, AEPC, JMJS, MMvR, MG, RJTvG, PAMM, CJCMH, GMO, MAG, MSch, AWvdB-D, FEvL, and MSp. Statistical analysis: MSp, AWvdB-D, FEvL, and MSch. Obtained funding: FEvL and CWB. Administrative, technical, or material support: GMO, MAG, AWvdB-D, FEvL, and MSp. Study supervision: FEvL, CBL, and CWB.
Acknowledgments: The authors thank the participants of the OMEGA study without whom this study would not have been possible. The authors thank the medical registries of the participating clinics for making patient selection possible and all participating physicians for providing access to their patients’ medical records. The authors thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice. The authors thank the Nationwide Network and Registry of Histo- and Cytopathology in the Netherlands (PALGA) for providing data about borderline ovarian tumors and the Dutch Municipal Personal Records Database for providing data on dates of childbirth for the entire cohort.
Prior presentation: This work has been presented at the European Society of Human Reproduction and Embryology, June 06, 2019, Vienna, Austria.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Fathalla MF. Incessant ovulation and ovarian cancer - a hypothesis re-visited. Facts Views Vis Obgyn. 2013;5(4):292–297. [PMC free article] [PubMed] [Google Scholar]
- 2. Li LL, Zhou J, Qian XJ, et al. Meta-analysis on the possible association between in vitro fertilization and cancer risk. Int J Gynecol Cancer. 2013;23(1):16–24. [DOI] [PubMed] [Google Scholar]
- 3. Reigstad MM, Larsen IK, Myklebust TA, et al. Cancer risk among parous women following assisted reproductive technology. Hum Reprod. 2015;30(8):1952–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Leeuwen FE, Klip H, Mooij TM, et al. Risk of borderline and invasive ovarian tumours after ovarian stimulation for in vitro fertilization in a large Dutch cohort. Hum Reprod. 2011;26(12):3456–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reigstad MM, Storeng R, Myklebust TA, et al. Cancer risk in women treated with fertility drugs according to parity status—a registry-based cohort study. Cancer Epidemiol Biomarkers Prev. 2017;26(6):953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siristatidis C, Sergentanis TN, Kanavidis P, et al. Controlled ovarian hyperstimulation for IVF: impact on ovarian, endometrial and cervical cancer—a systematic review and meta-analysis. Hum Reprod Update. 2013;19(2):105–123. [DOI] [PubMed] [Google Scholar]
- 7. Williams CL, Jones ME, Swerdlow AJ, et al. Risks of ovarian, breast, and corpus uteri cancer in women treated with assisted reproductive technology in Great Britain, 1991-2010: data linkage study including 2.2 million person years of observation. BMJ. 2018;362:k2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stewart LM, Holman CD, Aboagye-Sarfo P, et al. In vitro fertilization, endometriosis, nulliparity and ovarian cancer risk. Gynecol Oncol. 2013;128(2):260–264. [DOI] [PubMed] [Google Scholar]
- 9. Stewart LM, Holman CD, Finn JC, et al. In vitro fertilization is associated with an increased risk of borderline ovarian tumours. Gynecol Oncol. 2013;129(2):372–376. [DOI] [PubMed] [Google Scholar]
- 10. Vassard D, Schmidt L, Glazer CH, et al. Assisted reproductive technology treatment and risk of ovarian cancer-a nationwide population-based cohort study. Hum Reprod. 2019;34(11):2290–2296. doi:10.1093/humrep/dez165. [DOI] [PubMed] [Google Scholar]
- 11. Lundberg FE, Johansson ALV, Rodriguez-Wallberg K, et al. Assisted reproductive technology and risk of ovarian cancer and borderline tumors in parous women: a population-based cohort study. Eur J Epidemiol. 2019;34(11):1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kessous R, Davidson E, Meirovitz M, et al. The risk of female malignancies after fertility treatments: a cohort study with 25-year follow-up. J Cancer Res Clin Oncol. 2016;142(1):287–293. [DOI] [PubMed] [Google Scholar]
- 13. Brinton LA, Trabert B, Shalev V, et al. In vitro fertilization and risk of breast and gynecologic cancers: a retrospective cohort study within the Israeli Maccabi Healthcare Services. Fertil Steril. 2013;99(5):1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rizzuto I, Behrens RF, Smith LA. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database Syst Rev. 2019;(6):Cd008215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van den Belt-Dusebout AW, Spaan M, Lambalk CB, et al. Ovarian stimulation for in vitro fertilization and long-term risk of breast cancer. JAMA. 2016;316(3):300–312. [DOI] [PubMed] [Google Scholar]
- 16. Spaan M, van den Belt-Dusebout AW, Schaapveld M, et al. Melanoma risk after ovarian stimulation for in vitro fertilization. Hum Reprod. 2015;30(5):1216–1228. doi:10.1093/humrep/dev023. [DOI] [PubMed] [Google Scholar]
- 17. Van den Brandt PA, Schouten LJ, Goldbohm RA, et al. Development of a record linkage protocol for use in the Dutch Cancer Registry for Epidemiological Research. Int J Epidemiol. 1990;19(3):553–558. [DOI] [PubMed] [Google Scholar]
- 18.IARC. Cancer incidence in five continents. IARC Sci Publ. 2008;IX(160):1–837. [PubMed] [Google Scholar]
- 19. Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in the Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stata Statistical Software. Stata/SE (version 13.0; StataCorp LP, College Station, TX). Release 13. Stata Corp.; 2013. http://www.stata.com
- 21. Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372(21):2039–2048. [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen CB, Kjaer SK, Albieri V, et al. Pelvic inflammatory disease and the risk of ovarian cancer and borderline ovarian tumors: a pooled analysis of 13 case-control studies. Am J Epidemiol. 2017;185(1):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.